Genetic Structure of the Liriope muscari Polyploid Complex and the Possibility of Its Genetic Disturbance in Japan
Abstract
1. Introduction
2. Results
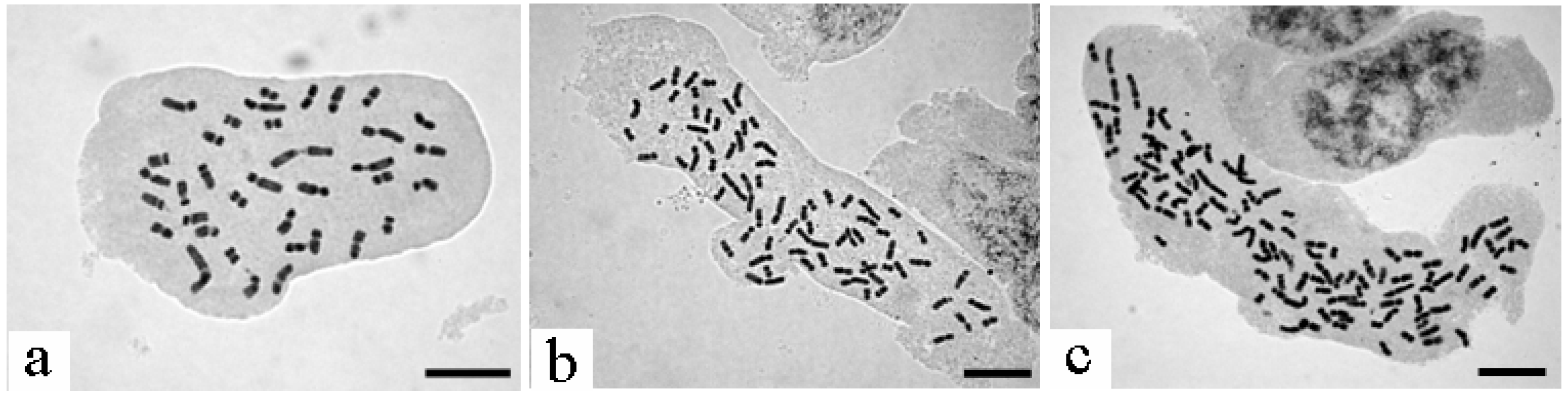
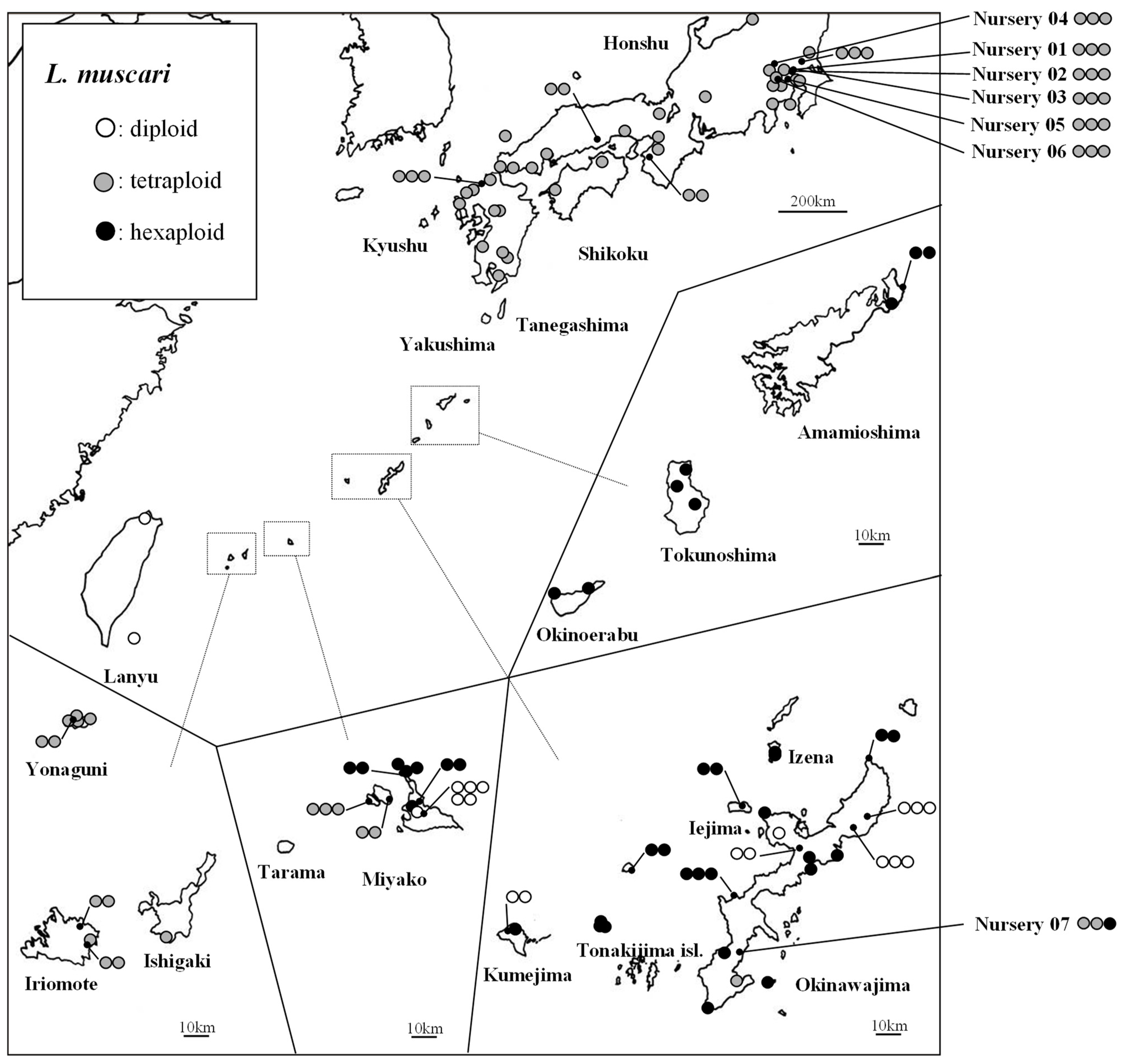
2.1. Polyploidy Level
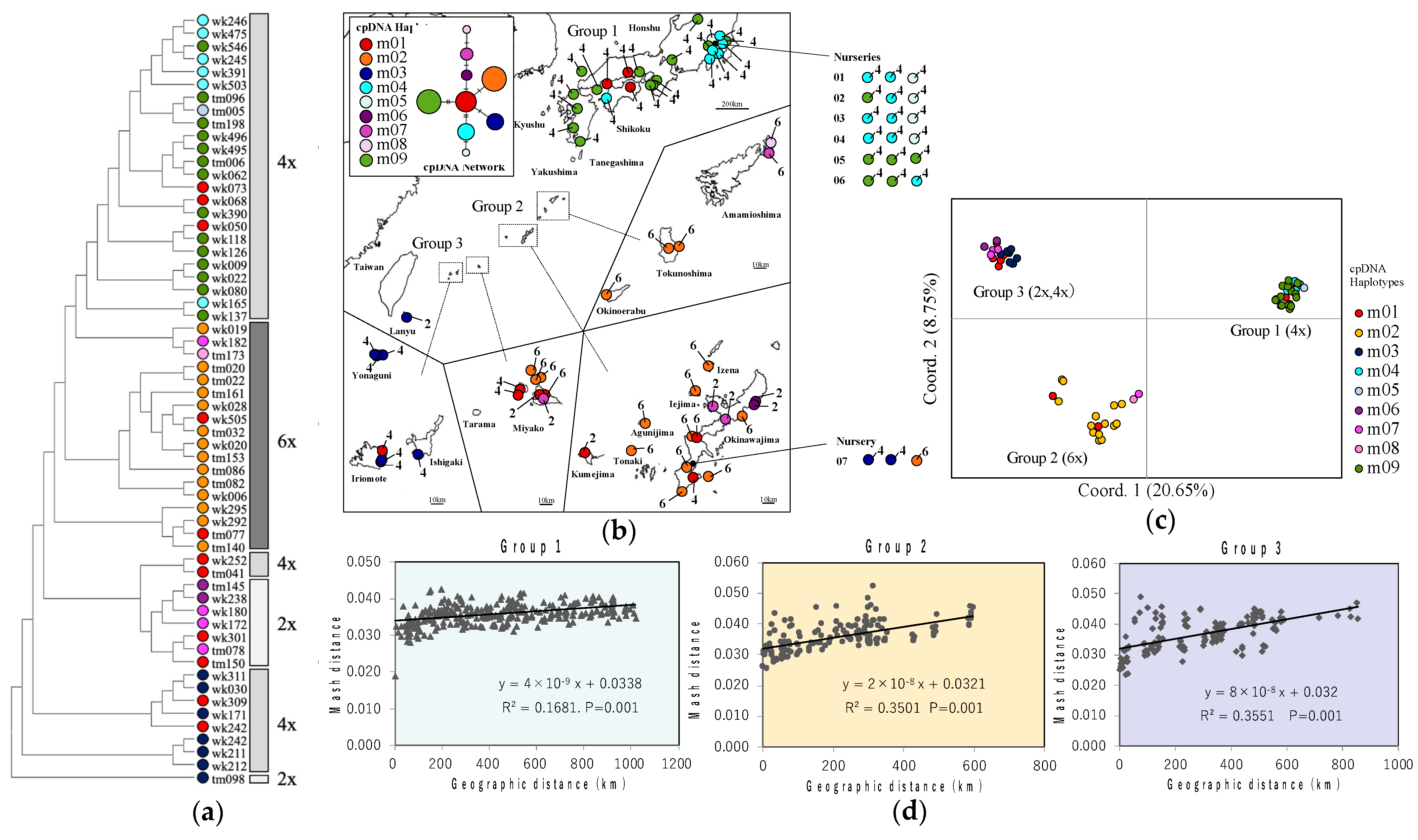
2.2. Chloroplast DNA Haplotypes
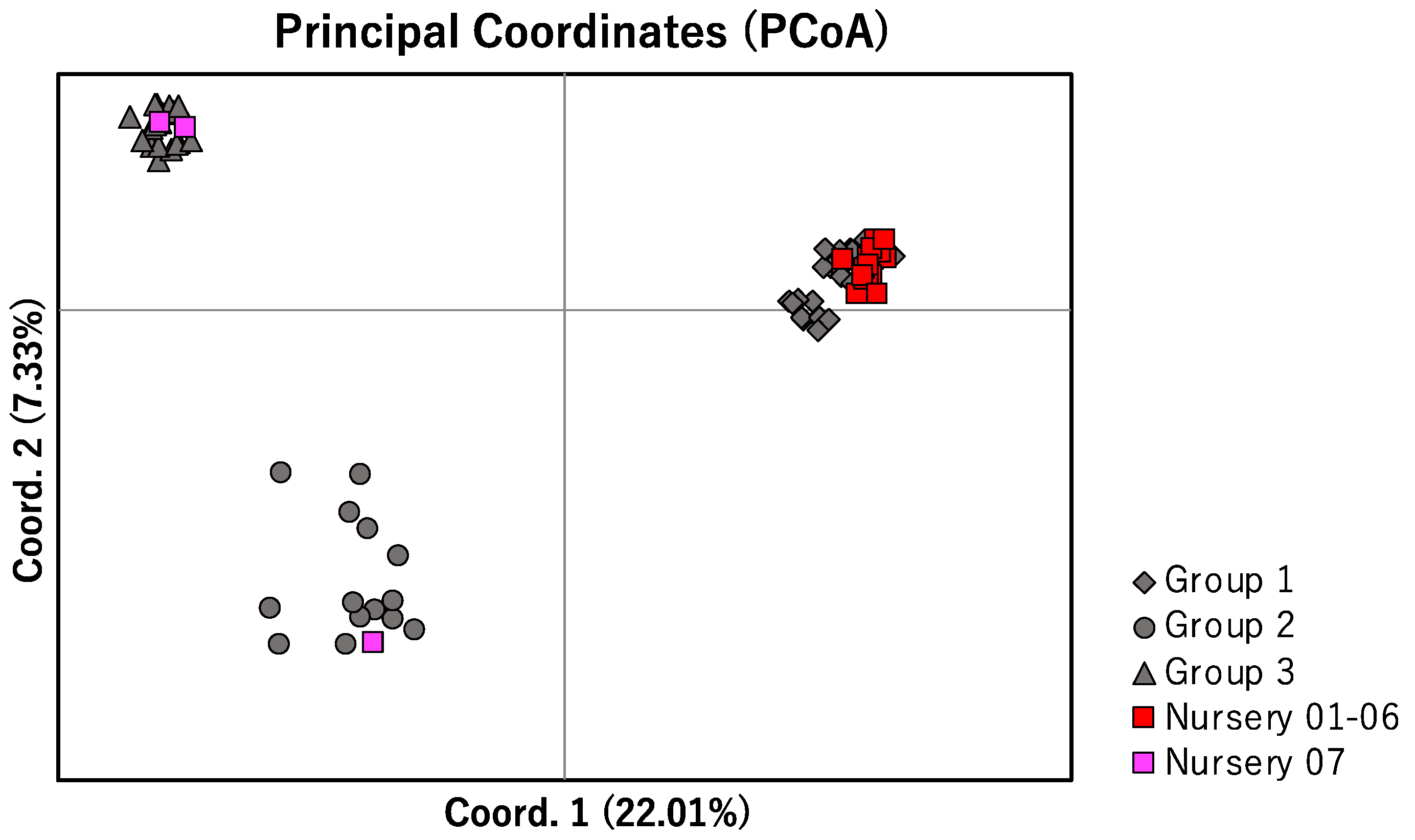
2.3. Nuclear DNA
2.4. Genetic Characteristics of Commercially Produced L. muscari
3. Discussion
3.1. Distribution of Polyploidy Complex
3.2. Genetic Structure of L. muscari in Japan
3.3. Taxonomic Confusion
3.4. Potential of Anthropogenic Disturbance and Countermeasures
4. Materials and Methods
4.1. Collection of Materials
4.2. Determination of Polyploid Level
4.3. Chloroplast DNA Analysis
4.4. Nuclear DNA Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eckert, C.G.; Samis, K.E.; Lougheed, S.C. Genetic Variation across Species’ Geographical Ranges: The Central–Marginal Hypothesis and Beyond. Mol. Ecol. 2008, 17, 1170–1188. [Google Scholar] [CrossRef] [PubMed]
- Etterson, J.R.; Shaw, R.G. Constraint to Adaptive Evolution in Response to Global Warming. Science 2001, 294, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Lenormand, T. Gene Flow and the Limits to Natural Selection. Trends Ecol. Evol. 2002, 17, 183–189. [Google Scholar] [CrossRef]
- Tsumura, Y.; Iwata, H. Considering Genetic Diversity and Differentiation in Revegetation. J. Jpn. Soc. Reveg. Technol. 2003, 28, 470–475. [Google Scholar] [CrossRef]
- Azpilicueta, M.M.; Gallo, L.A.; Van Zonneveld, M.; Thomas, E.; Moreno, C.; Marchelli, P. Management of Nothofagus Genetic Resources: Definition of Genetic Zones Based on a Combination of Nuclear and Chloroplast Marker Data. For. Ecol. Manag. 2013, 302, 414–424. [Google Scholar] [CrossRef]
- Tomita, M.; Kobayashi, S.; Abe, S.; Hanai, T.; Kawazu, K.; Tsuda, S. Phylogeography of Ten Native Herbaceous Species in the Temperate Region of Japan: Implication for the Establishment of Seed Transfer Zones for Revegetation Materials. Landsc. Ecol. Eng. 2017, 13, 33–44. [Google Scholar] [CrossRef]
- Tsumura, Y. Genetic Guidelines for Tree Species and Perspectives on the Conservation and Sustainable Use of Forests. J. For. Res. 2022, 27, 83–95. [Google Scholar] [CrossRef]
- Barker, M.S.; Arrigo, N.; Baniaga, A.E.; Li, Z.; Levin, D.A. On the Relative Abundance of Autopolyploids and Allopolyploids. New Phytol. 2016, 210, 391–398. [Google Scholar] [CrossRef]
- Comai, L. The Advantages and Disadvantages of Being Polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Paape, T.; Briskine, R.V.; Halstead-Nussloch, G.; Lischer, H.E.L.; Shimizu-Inatsugi, R.; Hatakeyama, M.; Tanaka, K.; Nishiyama, T.; Sabirov, R.; Sese, J.; et al. Patterns of Polymorphism and Selection in the Subgenomes of the Allopolyploid Arabidopsis Kamchatica. Nat. Commun. 2018, 9, 3909. [Google Scholar] [CrossRef]
- Akagi, T.; Shirasawa, K.; Nagasaki, H.; Hirakawa, H.; Tao, R.; Comai, L.; Henry, I.M. The Persimmon Genome Reveals Clues to the Evolution of a Lineage-Specific Sex Determination System in Plants. PLoS Genet. 2020, 16, e1008566. [Google Scholar] [CrossRef]
- Baack, E.J. Cytotype Segregation on Regional and Microgeographic Scales in Snow Buttercups (Ranunculus Adoneus: Ranunculaceae). Am. J. Bot. 2004, 91, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, C.A.; Hunter, K.L.; Betancourt, J.L. Creosote Bush (Larrea tridentata) Ploidy History along Its Diploid-Tetraploid Boundary in Southeastern Arizona-Southwestern New Mexico, USA. J. Arid Environ. 2019, 164, 7–11. [Google Scholar] [CrossRef]
- Karbstein, K.; Tomasello, S.; Hodač, L.; Lorberg, E.; Daubert, M.; Hörandl, E. Moving beyond Assumptions: Polyploidy and Environmental Effects Explain a Geographical Parthenogenesis Scenario in European Plants. Mol. Ecol. 2021, 30, 2659–2675. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, L.M.; Young, A.G.; Thrall, P.H.; Murray, B.G. Sourcing Seed for Acacia Acinacea, a Key Revegetation Species in South Eastern Australia. Conserv. Genet. 2006, 7, 49–63. [Google Scholar] [CrossRef]
- Schmidt-Lebuhn, A.N.; Marshall, D.J.; Dreis, B.; Young, A.G. Genetic Rescue in a Plant Polyploid Complex: Case Study on the Importance of Genetic and Trait Data for Conservation Management. Ecol. Evol. 2018, 8, 5153–5163. [Google Scholar] [CrossRef]
- Oinuma, T. Further Studies on Chromosomes of Ophiopogonaceae. Jpn. J. Genet. 1949, 24, 29–34. [Google Scholar]
- Westfall, J. Aneuploidy in Liriope-Muscari Bailey. Am. J. Bot. 1950, 37, 667–668. [Google Scholar]
- Hasegawa, H.K. Cytotaxonomic Studies on the Genera Liriope and Ophiopogon in Japan. J. Jpn. Bot. 1968, 43, 141–155. [Google Scholar]
- Fu, C.-X.; Hong, D.-Y. Cytotaxonomical Studies on Liliaceae (Sl):(2) Report on Chromosome Numbers and Karyotypes of 8 Species of 8 Genera from Zhejiang, China. J. Syst. Evol. 1989, 27, 439. [Google Scholar]
- Nishikawa, T. Chromosome Atlas of Flowering Plants in Japan; National Museum of Nature and Science: Tokyo, Japan, 2008; Volume 37, pp. 1–461. [Google Scholar]
- Kim, H.J.; Park, S.Y.; Kim, D.G.; Park, S.-H.; Lee, H.; Hwang, D.Y.; Jung, M.H.; Ha, K.-T.; Kim, B.J. Effects of the Roots of Liriope Platyphylla Wang Et Tang on Gastrointestinal Motility Function. J. Ethnopharmacol. 2016, 184, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Lu, M.; Li, F.; Yang, B.; Hu, Z.-T. Citric Acid-Assisted Phytoextraction of Trace Elements in Composted Municipal Sludge by Garden Plants. Environ. Pollut. 2021, 288, 117699. [Google Scholar] [CrossRef] [PubMed]
- Japan Nurserymen’s Association. Available online: https://www.ueki.or.jp/media/niwa_navi/20211026_1454_56_0520.xlsx (accessed on 4 October 2022).
- Nakanishi, H. Fruit Color and Fruit Size of Bird-Disseminated Plants in Japan. Vegetatio 1996, 123, 207–218. [Google Scholar] [CrossRef]
- Hirasawa, M.; Kanda, E.; Takatsuki, S. Seasonal Food Habits of the Raccoon Dog at a Western Suburb of Tokyo. Mammal Study 2006, 31, 9–14. [Google Scholar] [CrossRef]
- Sang, Y.-W. A Taxonomic Study of Ophiopogoneae Engler (Liliaceae) of Taiwan. Master’s Thesis, National Taiwan Normal University, Taipei, Taiwan, 1995. [Google Scholar]
- Segraves, K.A.; Thompson, J.N.; Soltis, P.S.; Soltis, D.E. Multiple Origins of Polyploidy and the Geographic Structure of Heuchera Grossulariifolia. Mol. Ecol. 1999, 8, 253–262. [Google Scholar] [CrossRef]
- Wu, L.-L.; Cui, X.-K.; Milne, R.I.; Sun, Y.-S.; Liu, J.-Q. Multiple Autopolyploidizations and Range Expansion of Allium przewalskianum Regel. (Alliaceae) in the Qinghai-Tibetan Plateau. Mol. Ecol. 2010, 19, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Wiley, E.O.; Lieberman, B.S. Phylogenetics: Theory and Practice of Phylogenetic Systematics; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-1-118-01787-6. [Google Scholar]
- Rieseberg, L.; Soltis, D. Phylogenetic Consequences of Cytoplasmic Gene Flow in Plants. Evol. Trends Plants 1991, 5, 65–84. [Google Scholar]
- Tsitrone, A.; Kirkpatrick, M.; Levin, D.A. A Model for Chloroplast Capture. Evolution 2003, 57, 1776–1782. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, F.; Pérez-Barrales, R.; Ojeda, F.; Vargas, P.; Arroyo, J. The Strait of Gibraltar as a Melting Pot for Plant Biodiversity. Quat. Sci. Rev. 2008, 27, 2100–2117. [Google Scholar] [CrossRef]
- Pilot, M.; Jedrzejewski, W.; Branicki, W.; Sidorovich, V.E.; Jedrzejewska, B.; Stachura, K.; Funk, S.M. Ecological Factors Influence Population Genetic Structure of European Grey Wolves. Mol. Ecol. 2006, 15, 4533–4553. [Google Scholar] [CrossRef]
- Nakamura, K.; Suwa, R.; Denda, T.; Yokota, M. Geohistorical and Current Environmental Influences on Floristic Differentiation in the Ryukyu Archipelago, Japan. J. Biogeogr. 2009, 36, 919–928. [Google Scholar] [CrossRef]
- Gao, J. Dominant Plant Speciation Types. A Commentary on: ‘Plant Speciation in the Age of Climate Change’. Ann. Bot. 2019, 124, iv–vi. [Google Scholar] [CrossRef] [PubMed]
- Guillot, G.; Leblois, R.; Coulon, A.; Frantz, A.C. Statistical Methods in Spatial Genetics. Mol. Ecol. 2009, 18, 4734–4756. [Google Scholar] [CrossRef] [PubMed]
- Ohwi, J. Synbolae Ad Floram Asiae Orientalis 13. Acta Phytotaxon. Geobot. 1936, 5, 51–57. [Google Scholar] [CrossRef]
- Yang, Y.P.; Li, H.; Liu, X.Z. Karyotype Study on the Genus Ophiopogon in Yunnan. Plant Divers. 1990, 12, 1–3. [Google Scholar]
- Levin, D.A. Minority Cytotype Exclusion in Local Plant Populations. Taxon 1975, 24, 35–43. [Google Scholar] [CrossRef]
- Pahlich, E.; Gerlitz, C. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochemistry 1980, 19, 11–13. [Google Scholar] [CrossRef]
- Setoguchi, H.; Ohba, H. Phylogenetic Relationships InCrossostylis (Rhizophoraceae) Inferred from Restriction Site Variation of Chloroplast DNA. J. Plant Res. 1995, 108, 87–92. [Google Scholar] [CrossRef]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal Primers for Amplification of Three Non-Coding Regions of Chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef]
- Denda, T.; Yokota, M. Hybrid Origins of Ixeris nakazonei (Asteraceae, Lactuceae) in the Ryukyu Archipelago, Japan: Evidence from Molecular Data. Bot. J. Linn. Soc. 2003, 141, 379–387. [Google Scholar] [CrossRef]
- Nakamura, K.; Chung, S.-W.; Kokubugata, G.; Denda, T.; Yokota, M. Phylogenetic Systematics of the Monotypic Genus Hayataella (Rubiaceae) Endemic to Taiwan. J. Plant Res. 2006, 119, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Liston, A.; Kadereit, J.W. Chloroplast DNA Evidence for Introgression and Long Distance Dispersal in the Desert Annual Senecio flavus (Asteraceae). Plant Syst. Evol. 1995, 197, 33–41. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: New York, NY, USA, 1987; ISBN 978-0-231-88671-0. [Google Scholar]
- Clement, M.; Snell, Q.; Walke, P.; Posada, D.; Crandall, K. TCS: Estimating Gene Genealogies. In Proceedings of the 16th International Parallel and Distributed Processing Symposium, Ft. Lauderdale, FL, USA, 15–19 April 2002; IEEE: Ft. Lauderdale, FL, USA, 2002; p. 7. [Google Scholar]
- Suyama, Y.; Hirota, S.K.; Matsuo, A.; Tsunamoto, Y.; Mitsuyuki, C.; Shimura, A.; Okano, K. Complementary Combination of Multiplex High-Throughput DNA Sequencing for Molecular Phylogeny. Ecol. Res. 2022, 37, 171–181. [Google Scholar] [CrossRef]
- Suyama, Y.; Matsuki, Y. MIG-Seq: An Effective PCR-Based Method for Genome-Wide Single-Nucleotide Polymorphism Genotyping Using the next-Generation Sequencing Platform. Sci. Rep. 2015, 5, 16963. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Katz, L.S.; Griswold, T.; Morrison, S.S.; Caravas, J.A.; Zhang, S.; den Bakker, H.C.; Deng, X.; Carleton, H.A. Mashtree: A Rapid Comparison of Whole Genome Sequence Files. J. Open Source Softw. 2019, 4, 1762. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Zhang, H.; Tao, Q.; Zhang, Y.; Dang, Z.; Zhang, F.; Luo, Z. Sequence Coverage Required for Accurate Genotyping by Sequencing in Polyploid Species. Mol. Ecol. Resour. 2022, 22, 1417–1426. [Google Scholar] [CrossRef]
- VanWallendael, A.; Alvarez, M. Alignment-Free Methods for Polyploid Genomes: Quick and Reliable Genetic Distance Estimation. Mol. Ecol. Resour. 2022, 22, 612–622. [Google Scholar] [CrossRef]
- Rochette, N.C.; Rivera-Colon, A.G.; Catchen, J.M. Stacks 2: Analytical Methods for Paired-End Sequencing Improve RADseq-Based Population Genomics. Mol. Ecol. 2019, 28, 4737–4754. [Google Scholar] [CrossRef] [PubMed]
- VanWallendael, A.; Alvarez, M.; Franks, S.J. Patterns of Population Genomic Diversity in the Invasive Japanese Knotweed Species Complex. Am. J. Bot. 2021, 108, 857–868. [Google Scholar] [CrossRef] [PubMed]
| Locality | Na | 2n | Hap | Accession Numbers | ||||
|---|---|---|---|---|---|---|---|---|
| cpDNA | nDNA | |||||||
| trnK 5′ Intron | trnT-trnL | trnL-trnF | atpB-rbcL | |||||
| Natural distribution area | ||||||||
| Mainland Japan and Ryukyu Islands | ||||||||
| Kakudayama, Niigata City, Niigata Pref. | wk390 | 72 | m09 | LC730908 | LC731009 | LC731090 | LC731171 | DRR412610 |
| Mt. Tsukuba, Tsukuba City, Ibaraki Pref. | tm096 | 72 | m09 | LC730909 | LC731010 | LC731091 | LC731172 | DRR412611 |
| tm001 | 72 | |||||||
| tm179 | 72 | |||||||
| Mt. Mayumi, Hitachiota City, Ibaraki Pref. | wk270 | 72 | ||||||
| Arakawa river, Nagatoro Town, Saitama Pref. | tm005 | 72 | m05 | LC730910 | LC731011 | LC731092 | LC731173 | DRR412612 |
| Kamitanadare, Kisai Town, Saitama Pref. | wk018 | 72 | ||||||
| Sendabori, Matsudo City, Chiba Pref. | wk246 | 72 | m04 | LC730911 | LC731012 | LC731093 | LC731174 | DRR412613 |
| Horiuchinai, Ichikawa City, Chiba Pref. | wk503 | 72 | m04 | LC730912 | LC731013 | LC731094 | LC731175 | DRR412614 |
| Mogusa, Tama City, Tokyo Pref. | tm003 | 72 | ||||||
| Hane, Hamura City, Tokyo Pref. | wk546 | 72 | m09 | LC730913 | LC731014 | LC731095 | LC731176 | DRR412615 |
| Motohachioji, Hachioji City, Tokyo Pref. | wk475 | 72 | m04 | LC730914 | LC731015 | LC731096 | LC731177 | DRR412616 |
| Horiuchi, Hayama Town, Kanagawa Pref. | wk391 | 72 | m04 | LC730915 | LC731016 | LC731097 | LC731178 | DRR412617 |
| Hakone, Hakone Town, Kanagawa Pref. | wk245 | 72 | m04 | LC730916 | LC731017 | LC731098 | LC731179 | DRR412618 |
| Siokawa, Kani City, Gifu Pref. | tm198 | 72 | m09 | LC730917 | LC731018 | LC731099 | LC731180 | DRR412619 |
| Imodani, Hashimoto City, Wakayama Pref. | tm006 | 72 | m09 | LC730918 | LC731019 | LC731100 | LC731181 | DRR412620 |
| Hasemiya, Kimino Town, Wakayama Pref. | wk495 | 72 | m09 | LC730919 | LC731020 | LC731101 | LC731182 | DRR412621 |
| wk496 | 72 | m09 | LC730920 | LC731021 | LC731102 | LC731183 | DRR412622 | |
| Mt. Kurama, Sakyou Ward, Kyoto Pref. | wk278 | 72 | ||||||
| Mt. Takao, Kashiwara City, Osaka Pref. | tm008 | 72 | ||||||
| Higashiune, Akou City, Hyogo Pref. | wk062 | 72 | m09 | LC730921 | LC731022 | LC731103 | LC731184 | DRR412623 |
| Asagoe, Okayama City, Okayama Pref. | wk067 | 72 | ||||||
| wk068 | 72 | m01 | LC730922 | LC731023 | LC731104 | LC731185 | DRR412624 | |
| Mt. Ogonzan, Hiroshima City, Hiroshima Pref. | wk073 | 72 | m01 | LC730923 | LC731024 | LC731105 | LC731186 | DRR412625 |
| Chuocho, Hikari City, Yamaguchi Pref. | wk080 | 72 | m09 | LC730924 | LC731025 | LC731106 | LC731187 | DRR412626 |
| Tyuzankei, Shimonoseki City, Yamaguchi Pref. | tm012 | 72 | ||||||
| Nagaonohana, Hagi City, Yamaguchi Pref. | wk022 | 72 | m09 | LC730925 | LC731026 | LC731107 | LC731188 | DRR412627 |
| Onoyama, Sanyo Onoda City, Yamaguchi Pref. | wk024 | 72 | ||||||
| Kishinoue, Mannou Town, Kagawa Pref. | wk050 | 72 | m01 | LC730926 | LC731027 | LC731108 | LC731189 | DRR412628 |
| Sugeta, Ohzu City, Ehime Pref. | wk165 | 72 | m04 | LC730927 | LC731028 | LC731109 | LC731190 | DRR412629 |
| Nagahama seashore, Hukuoka City, Fukuoka Pref. | wk009 | 72 | m09 | LC730928 | LC731029 | LC731110 | LC731191 | DRR412630 |
| wk010 | 72 | |||||||
| wk011 | 72 | |||||||
| Mt. Ihara, Maebaru City, Fukuoka Pref. | wk013 | 72 | ||||||
| Senbutsudo, Kokura City, Fukuoka Pref. | tm014 | 72 | ||||||
| Mt. Kagamiyama, Karatsu City, Saga Pref. | wk015 | 72 | ||||||
| Hae, Tano Town, Miyazaki Pref. | wk255 | 72 | ||||||
| Okutsu, Kobayashi City, Miyazaki Pref. | wk256 | 72 | ||||||
| Tatara, Ozu City, Kumamoto Pref. | wk134 | 72 | ||||||
| Ino, Kikuchi City, Kumamoto Pref. | wk137 | 72 | m09 | LC730929 | LC731030 | LC731111 | LC731192 | DRR412631 |
| Mt. Tokozan, Izumi City, Kagoshima Pref. | wk126 | 72 | m09 | LC730930 | LC731031 | LC731112 | LC731193 | DRR412632 |
| Hatimanjinja, Kanoya City, Kagoshima Pref. | wk118 | 72 | m09 | LC730931 | LC731032 | LC731113 | LC731194 | DRR412633 |
| Tomori, Amami City, Kagoshima Pref. | tm169 | 108 | ||||||
| tm173 | 108 | m08 | LC730932 | LC731033 | LC731114 | LC731195 | DRR412634 | |
| Oazasetsuta, Amami City, Kagoshima Pref. | wk182 | 108 | m07 | LC730933 | LC731034 | LC731115 | LC731196 | DRR412635 |
| Mt. Amagi, Amagi Town, Kagoshima Pref. | tm022 | 108 | m02 | LC730934 | LC731035 | LC731116 | LC731197 | DRR412636 |
| Syoda, Tokunoshima Town, Kagoshima Pref. | tm019 | 108 | m02 | LC730935 | LC731036 | LC731117 | LC731198 | DRR412637 |
| San, Tokunoshima Town, Kagoshima Pref. | wk548 | 108 | ||||||
| Kibiru, Wadomari Town, Kagoshima Pref. | tm021 | 108 | ||||||
| Taminazaki, China Town, Kagoshima Pref. | tm020 | 108 | m02 | LC730936 | LC731037 | LC731118 | LC731199 | DRR412638 |
| Rikugidara, Izena Vil., Okinawa Pref. | tm168 | 108 | ||||||
| Mt. Chizin, Izena Vil., Okinawa Pref. | tm086 | 108 | m02 | LC730937 | LC731038 | LC731119 | LC731200 | DRR412639 |
| Mt. Gusuku, Ie Vil., Okinawa Pref. | wk028 | 108 | m02 | LC730938 | LC731039 | LC731120 | LC731201 | DRR412640 |
| wk029 | 108 | |||||||
| Cape Hedo, Kunigami Vil., Okinawa Pref. | tm038 | 108 | ||||||
| tm144 | 108 | |||||||
| Uka river, Kunigami Vil., Okinawa Pref. | tm037 | 36 | ||||||
| tm145 | 36 | m06 | LC730939 | LC731040 | LC731121 | LC731202 | DRR412641 | |
| tm146 | 36 | |||||||
| Haramata river, Higashi Vil., Okinawa Pref. | wk236 | 36 | ||||||
| wk237 | 36 | |||||||
| wk238 | 36 | m06 | LC730940 | LC731041 | LC731122 | LC731203 | DRR412642 | |
| Bise, Motobu Town, Okinawa Pref. | tm149 | 108 | ||||||
| Mt. Awa, Motobu Town, Okinawa Pref. | wk172 | 36 | m07 | LC730941 | LC731042 | LC731123 | LC731204 | DRR412643 |
| Kushi, Nago City, Okinawa Pref. | tm029 | 108 | ||||||
| Henoko, Nago City, Okinawa Pref. | tm148 | 108 | ||||||
| Mt. Nago, Nago City, Okinawa Pref. | wk179 | 36 | ||||||
| wk180 | 36 | m07 | LC730942 | LC731043 | LC731124 | LC731205 | DRR412644 | |
| Cape Maeda, Onna Vil, Okinawa Pref. | tm031 | 108 | ||||||
| tm032 | 108 | m02 | LC730943 | LC731044 | LC731125 | LC731206 | DRR412645 | |
| wk505 | 108 | m01 | LC730944 | LC731045 | LC731126 | LC731207 | DRR412646 | |
| Ojana, Ginowan City, Okinawa Pref. | wk006 | 108 | m02 | LC730945 | LC731046 | LC731127 | LC731208 | DRR412647 |
| Sashikisinzato, Nanjo City, Okinawa Pref. | wk242 | 72 | m01 | LC730946 | LC731047 | LC731128 | LC731209 | DRR412648 |
| Cape Kyan, Itoman City, Okinawa Pref. | wk020 | 108 | m02 | LC730947 | LC731048 | LC731129 | LC731210 | DRR412649 |
| Ugu seashore, Aguni Vil., Okinawa Pref. | tm152 | 108 | ||||||
| tm153 | 108 | m02 | LC730948 | LC731049 | LC731130 | LC731211 | DRR412650 | |
| West side of Gityuyama, Tonaki Vil., Okinawa Pref. | tm092 | 108 | ||||||
| Iri, Tonaki Vil., Okinawa Pref. | tm095 | 108 | ||||||
| Womozaki, Tonaki Vil., Okinawa Pref. | tm161 | 108 | m02 | LC730949 | LC731050 | LC731131 | LC731212 | DRR412651 |
| Mt. Ara, Kumejima Town, Okinawa Pref. | tm150 | 36 | m01 | LC730950 | LC731051 | LC731132 | LC731213 | DRR412652 |
| wk368 | 36 | |||||||
| Hiyajo, Kumejima Town, Okinawa Pref. | tm151 | 108 | ||||||
| Ishiki seashore, Nanjo City, Okinawa Pref. | tm082 | 108 | m02 | LC730951 | LC731052 | LC731133 | LC731214 | DRR412653 |
| Hiraraogami, Miyakojima City, Okinawa Pref. | wk295 | 108 | m02 | LC730952 | LC731053 | LC731134 | LC731215 | DRR412654 |
| Maesato, Miyakojima City, Okinawa Pref. | tm140 | 108 | m02 | LC730953 | LC731054 | LC731135 | LC731216 | DRR412655 |
| Nishihennazaki, Miyakojima City, Okinawa Pref. | tm142 | 108 | ||||||
| wk297 | 108 | |||||||
| Setozaki, Miyakojima City, Okinawa Pref. | wk292 | 108 | m02 | LC730954 | LC731055 | LC731136 | LC731217 | DRR412656 |
| Onosanrin, Miyakojima City, Okinawa Pref. | tm077 | 108 | m01 | LC730955 | LC731056 | LC731137 | LC731218 | DRR412657 |
| wk291 | 108 | |||||||
| Otakikoen, Miyakojima City, Okinawa Pref. | tm076 | 36 | ||||||
| tm078 | 36 | m07 | LC730956 | LC731057 | LC731138 | LC731219 | DRR412658 | |
| tm079 | 36 | |||||||
| tm143 | 36 | |||||||
| wk298 | 36 | |||||||
| Nobarudake, Miyakojima City, Okinawa Pref. | wk301 | 36 | m01 | LC730957 | LC731058 | LC731139 | LC731220 | DRR412659 |
| Umarezatonoutaki, Miyakojima City, Okinawa Pref. | wk334 | 108 | ||||||
| Mt. Makiyama, Miyakojima City, Okinawa Pref. | tm111 | 72 | ||||||
| wk289 | 72 | |||||||
| Kuninakautaki, Miyakojima City, Okinawa Pref. | wk252 | 72 | m01 | LC730958 | LC731059 | LC731140 | LC731221 | DRR412660 |
| Toriike, Miyakojima City, Okinawa Pref. | tm041 | 72 | m01 | LC730959 | LC731060 | LC731141 | LC731222 | DRR412661 |
| tm044 | 72 | |||||||
| tm134 | 72 | |||||||
| Misakiutaki, Ishigaki City, Okinawa Pref. | wk171 | 72 | m03 | LC730960 | LC731061 | LC731142 | LC731223 | DRR412662 |
| Yutsun river, Taketomi Town, Okinawa Pref. | tm117 | 72 | ||||||
| wk309 | 72 | m01 | LC730961 | LC731062 | LC731143 | LC731224 | DRR412663 | |
| Komi, Taketomi Town, Okinawa Pref. | wk311 | 72 | m03 | LC730962 | LC731063 | LC731144 | LC731225 | DRR412664 |
| wk312 | 72 | |||||||
| Aira river, Taketomi Town, Okinawa Pref. | wk030 | 72 | m03 | LC730963 | LC731064 | LC731145 | LC731226 | DRR412665 |
| Thindahanata, Yonaguni Town, Okinawa Pref. | tm115 | 72 | ||||||
| Agarizaki, Yonaguni Town, Okinawa Pref. | wk215 | 72 | m03 | LC730964 | LC731065 | LC731146 | LC731227 | DRR412666 |
| Mt. Kubura, Yonaguni Town, Okinawa Pref. | tm113 | 72 | ||||||
| tm114 | 72 | |||||||
| Nama seashore, Yonaguni Town, Okinawa Pref. | wk211 | 72 | m03 | LC730965 | LC731066 | LC731147 | LC731228 | DRR412667 |
| Yonaguni, Yonaguni Town, Okinawa Pref. | wk212 | 72 | m03 | LC730966 | LC731067 | LC731148 | LC731229 | DRR412668 |
| Taiwan | ||||||||
| Chingching-tsaoyan, Lanyu, Taitung | tm098 | 36 | m03 | LC730967 | LC731068 | LC731149 | LC731230 | DRR412669 |
| Nurseries | ||||||||
| Nursery 01, Kawaguchi City, Saitama Pref. | wk460 | 72 | m05 | LC730968 | LC731069 | LC731150 | LC731231 | DRR412670 |
| wk461 | 72 | m04 | LC730969 | LC731070 | LC731151 | LC731232 | DRR412671 | |
| wk462 | 72 | m04 | LC730970 | LC731071 | LC731152 | LC731233 | DRR412672 | |
| Nursery 02, Kawaguchi City, Saitama Pref. | wk463 | 72 | m09 | LC730971 | LC731072 | LC731153 | LC731234 | DRR412673 |
| wk464 | 72 | m04 | LC730972 | LC731073 | LC731154 | LC731235 | DRR412674 | |
| wk465 | 72 | m05 | LC730973 | LC731074 | LC731155 | LC731236 | DRR412675 | |
| Nursery 03, Kawaguchi City, Saitama Pref. | wk466 | 72 | m05 | LC730974 | LC731075 | LC731156 | LC731237 | DRR412676 |
| wk467 | 72 | m04 | LC730975 | LC731076 | LC731157 | LC731238 | DRR412677 | |
| wk468 | 72 | m04 | LC730976 | LC731077 | LC731158 | LC731239 | DRR412678 | |
| Nursery 04, Yorii Town, Saitama Pref. | wk469 | 72 | m04 | LC730977 | LC731078 | LC731159 | LC731240 | DRR412679 |
| wk470 | 72 | m04 | LC730978 | LC731079 | LC731160 | LC731241 | DRR412680 | |
| wk471 | 72 | m05 | LC730979 | LC731080 | LC731161 | LC731242 | DRR412681 | |
| Nursery 05, Musashimurayama City, Tokyo Pref. | wk500 | 72 | m09 | LC730980 | LC731081 | LC731162 | LC731243 | DRR412682 |
| wk501 | 72 | m04 | LC730981 | LC731082 | LC731163 | LC731244 | DRR412683 | |
| wk502 | 72 | m09 | LC730982 | LC731083 | LC731164 | LC731245 | DRR412684 | |
| Nursery 06, Chohu City, Tokyo Pref. | wk472 | 72 | m09 | LC730983 | LC731084 | LC731165 | LC731246 | DRR412685 |
| wk473 | 72 | m09 | LC730984 | LC731085 | LC731166 | LC731247 | DRR412686 | |
| wk474 | 72 | m09 | LC730985 | LC731086 | LC731167 | LC731248 | DRR412687 | |
| Nursery 07, Nishihara City, Okinawa Pref. | wk497 | 72 | m03 | LC730986 | LC731087 | LC731168 | LC731249 | DRR412688 |
| wk498 | 72 | m03 | LC730987 | LC731088 | LC731169 | LC731250 | DRR412689 | |
| wk499 | 108 | m02 | LC730988 | LC731089 | LC731170 | LC731251 | DRR412690 | |
| Groups | Polyploidy | N | NH | h | π |
|---|---|---|---|---|---|
| Natural distribution area | |||||
| Group 1: Mainland Japan | 4x | 24 | 3 | 0.583 | 0.00062 |
| Group 2: Ryukyu Islands | 6x | 18 | 4 | 0.399 | 0.00030 |
| Group 3: Ryukyu Islands | 2x, 4x | 18 | 4 | 0.739 | 0.00041 |
| Group 3–1 | 2x | 8 | 4 | 0.821 | 0.00044 |
| Group 3–2 | 4x | 10 | 2 | 0.533 | 0.00018 |
| Nurseries | |||||
| Nursery 01–06: Mainland Japan | 4x | 18 | 2 | 0.471 | 0.00063 |
| Nursery 07: Ryukyu Islands | 4x | 2 | 1 | 0.000 | 0.00000 |
| Nursery 07: Ryukyu Islands | 6x | 1 | 1 | - | - |
| Groups | Polyploidy | N | He | Ho | FIS | π |
|---|---|---|---|---|---|---|
| Natural distribution area | ||||||
| Group 1: Mainland Japan | 4x | 24 | 0.00262 | 0.00283 | −0.00052 | 0.00268 |
| Group 2: Ryukyu Islands | 6x | 18 | 0.00377 | 0.00382 | 0.00061 | 0.00390 |
| Group 3: Ryukyu Islands | 2x, 4x | 18 | 0.00348 | 0.00256 | 0.00374 | 0.00360 |
| Group 3-1 | 2x | 8 | 0.00178 | 0.00104 | 0.00294 | 0.00196 |
| Group 3-2 | 4x | 10 | 0.00254 | 0.00299 | −0.00093 | 0.00262 |
| Nurseries | ||||||
| Nursery 01-06: Mainland Japan | 4x | 18 | 0.00254 | 0.00299 | −0.00093 | 0.00262 |
| Nursery 07: Ryukyu Islands | 4x | 2 | 0.00234 | 0.00371 | −0.00018 | 0.00359 |
| Nursery 07: Ryukyu Islands | 6x | 1 | 0.00301 | 0.00602 | 0 | 0.00602 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, K.; Yaneshita, M.; Denda, T.; Yokota, M.; Hirota, S.K.; Suyama, Y.; Tsumura, Y. Genetic Structure of the Liriope muscari Polyploid Complex and the Possibility of Its Genetic Disturbance in Japan. Plants 2022, 11, 3015. https://doi.org/10.3390/plants11223015
Watanabe K, Yaneshita M, Denda T, Yokota M, Hirota SK, Suyama Y, Tsumura Y. Genetic Structure of the Liriope muscari Polyploid Complex and the Possibility of Its Genetic Disturbance in Japan. Plants. 2022; 11(22):3015. https://doi.org/10.3390/plants11223015
Chicago/Turabian StyleWatanabe, Keita, Makoto Yaneshita, Tetsuo Denda, Masatsugu Yokota, Shun K. Hirota, Yoshihisa Suyama, and Yoshihiko Tsumura. 2022. "Genetic Structure of the Liriope muscari Polyploid Complex and the Possibility of Its Genetic Disturbance in Japan" Plants 11, no. 22: 3015. https://doi.org/10.3390/plants11223015
APA StyleWatanabe, K., Yaneshita, M., Denda, T., Yokota, M., Hirota, S. K., Suyama, Y., & Tsumura, Y. (2022). Genetic Structure of the Liriope muscari Polyploid Complex and the Possibility of Its Genetic Disturbance in Japan. Plants, 11(22), 3015. https://doi.org/10.3390/plants11223015






