Analysis of Volatile Secondary Metabolites in Ocimum basilicum Cell Suspensions: Inhibition, In Silico Molecular Docking, and an ADMET Analysis against Proteolytic Enzymes of Rhynchophorus ferrugineus
Abstract
1. Introduction
2. Results
2.1. Cell Suspension and Callus Initiation and Total Protein Content
2.2. O. basilicum Volatile Extract’s Formation and Chemical Composition
2.3. Volatile Extract and Pure Components Activity against Adults and Larvae of R. ferrugineus
2.4. O. basilicum Cell Suspension’s Extract Impact on Serine, Cysteine, and Metalloproteinase Particular Activity Assays (In Vitro)
2.5. In Vivo Effect of O. basilicum Volatile Extract and Pure Components on the Serine, Metalloprotease, and Cysteine Protease Activities from Fourth R. ferrugineus Instar Midgut Preparations
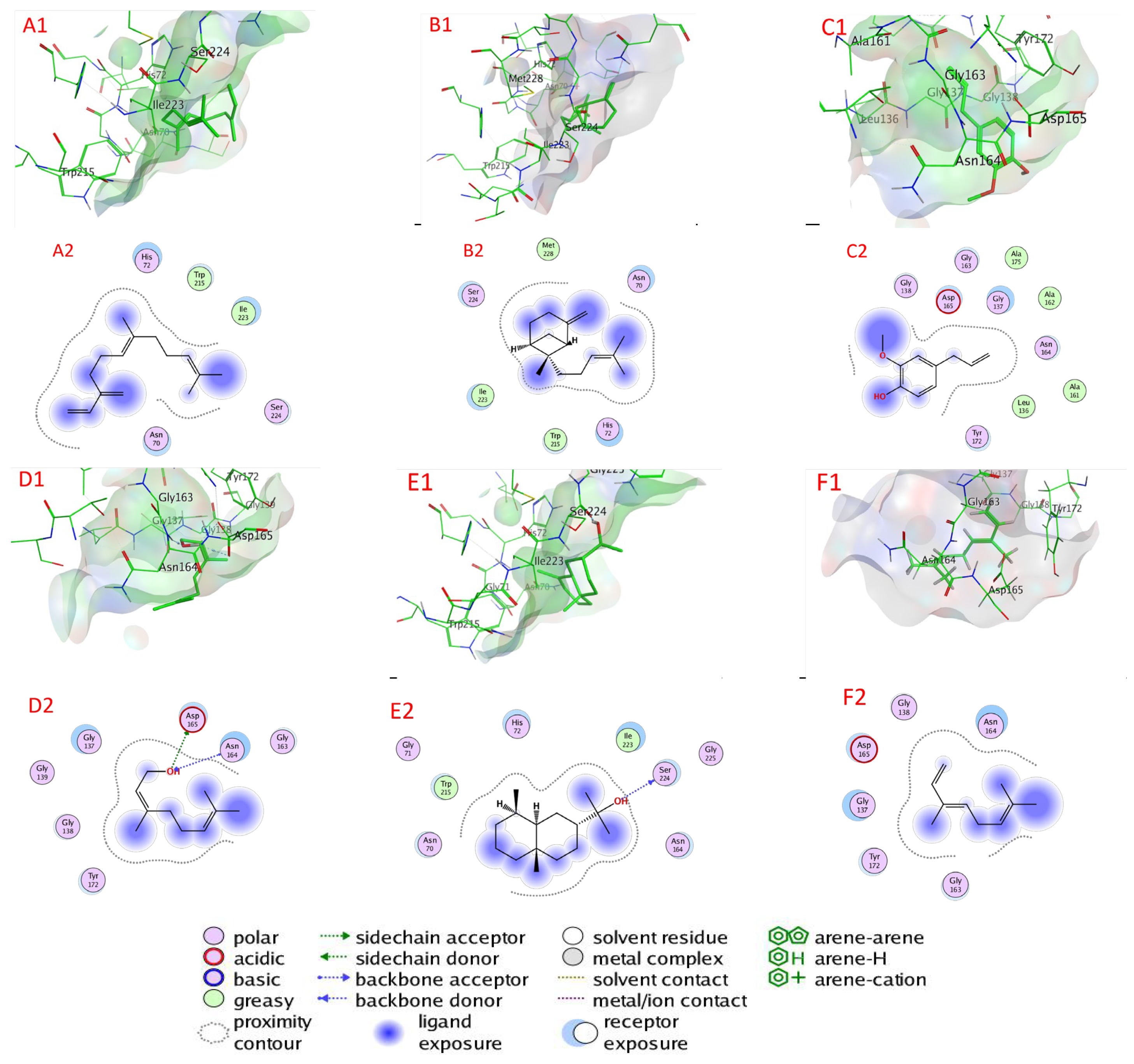
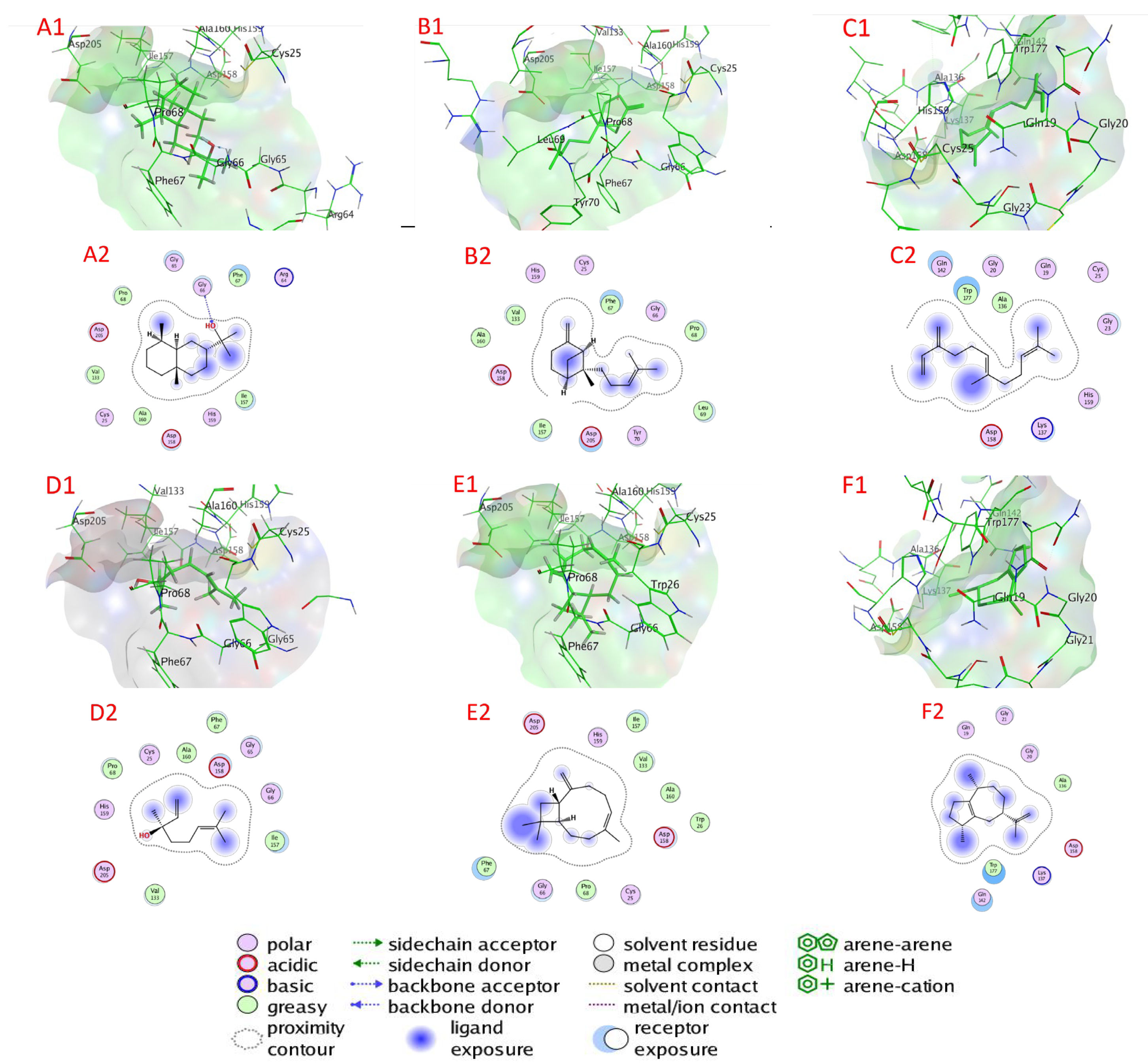
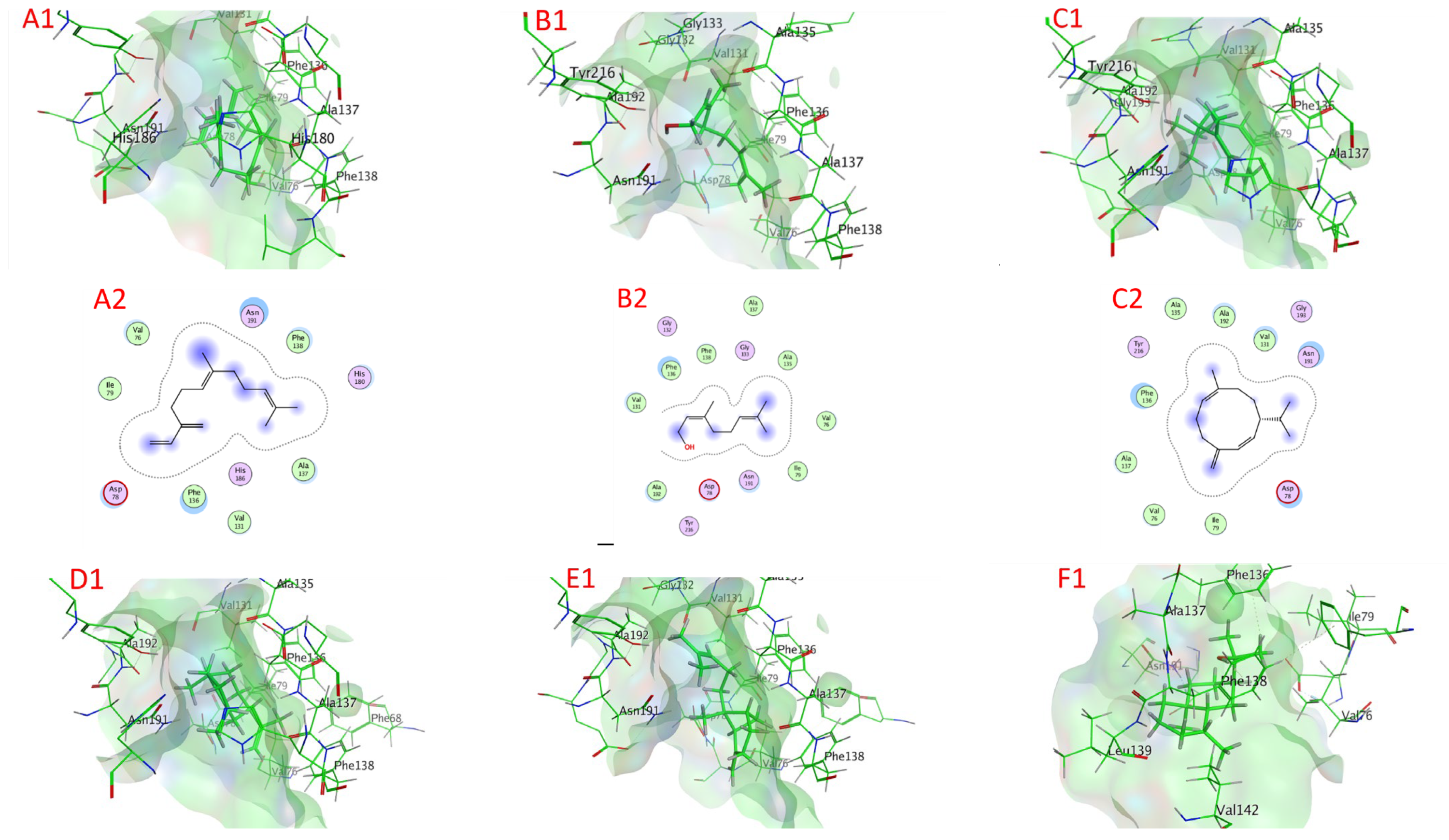
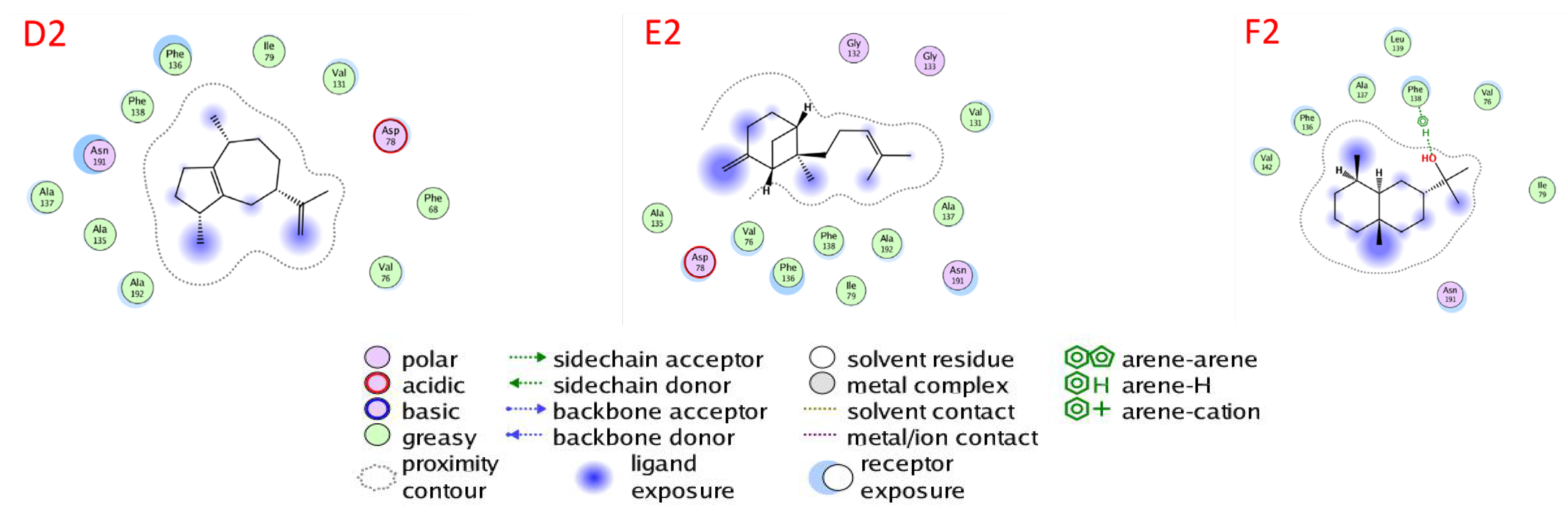
2.6. Docking of Compounds into Proteinase Enzymes
2.6.1. Serine Proteinase Docking
2.6.2. Cysteine Protease Docking
2.6.3. Metalloprotease Docking
2.6.4. Trypsin Proteinase Docking
2.7. ADMET Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Media
4.2. Plant Materials
4.3. O. basilicum Callus and Cell Suspension Initiation with V. dahliae as a Biotic Elicitor
4.4. Total Protein Assay in O. basilicum’s Callus and Cell Suspension
4.5. Characterization Using GC-MS Analysis
4.6. Evaluation of the Extracted Secondary Metabolites’ Contact-Insecticide and Antifeedant Efficacy against R. ferrugineus
4.7. Assessment of O. basilicum Cell Suspension Extract and Pure Components on R. ferrugineus Larvae’s Overall Proteolytic Enzyme Activity (In Vitro)
4.8. Assessment of the Cell Suspension Extract and Components on Serine Proteinase (In Vitro) Specific Activity of R. ferrugineus Larvae
4.9. Assessment of the Cell Suspension Extract and Pure Components on the Specific Activity of Metalloproteinase (In Vitro) of R. ferrugineus Larvae
4.10. Assessment of Cell Suspension Extract and Components on Cysteine Proteinase Activity of R. ferrugineus Larvae (In Vitro)
4.11. Docking of Experimented Compounds into Enzymes
4.12. ADMET Screening
4.13. Statistical Design
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tangpao, T.; Charoimek, N.; Teerakitchotikan, P.; Leksawasdi, N.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Phimolsiripol, Y.; Chaiyaso, T.; Ruksiriwanich, W.; et al. Volatile Organic Compounds from Basil Essential Oils: Plant Taxonomy, Biological Activities, and Their Applications in Tropical Fruit Productions. Horticulturae 2022, 8, 144. [Google Scholar] [CrossRef]
- Singh, D.; Chaudhuri, P.K. A review on phytochemical and pharmacological properties of Holy basil (Ocimum sanctum L.). Ind. Crops Prod. 2018, 118, 367–382. [Google Scholar] [CrossRef]
- Dafni, A.; Petanidou, T.; Vallianatou, I.; Kozhuharova, E.; Blanché, C.; Pacini, E.; Peyman, M.; Stevanovic, Z.D.; Franchi, G.G.; Benítez, G. Myrtle, Basil, Rosemary, and Three-Lobed Sage as Ritual Plants in the Monotheistic Religions: An Historical–Ethnobotanical Comparison. Econ. Bot. 2020, 74, 330–355. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; El-Ansary, D.O.; Al-Mana, F.A.; Mahmoud, E.A. Saudi Rosmarinus officinalis and Ocimum basilicum L. Polyphenols and Biological Activities. Processes 2020, 8, 446. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Albohy, A.; Zahran, E.M.; Abdelmohsen, U.R.; Salem, M.A.; Al-Warhi, T.; Al-Sanea, M.M.; Abelyan, N.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A. Multitarget in silico studies of Ocimum menthiifolium, family Lamiaceae against SARS-CoV-2 supported by molecular dynamics simulation. J. Biomol. Struct. Dyn. 2020, 40, 4062–4072. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdelmohsen, U.R.; Kolkeila, A.; Salem, M.A.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Anti-epileptic potential, metabolic profiling and in silico studies of the aqueous fraction from Ocimum menthiifolium benth, family Lamiaceae. Nat. Prod. Res. 2020, 35, 5972–5976. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdelmohsen, U.R.; Ayoub, A.T.; Salem, M.A.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Metabolic profiling, histopathological anti-ulcer study, molecular docking and molecular dynamics of ursolic acid isolated from Ocimum forskolei Benth. (Family Lamiaceae). S. Afr. J. Bot. 2020, 131, 311–319. [Google Scholar] [CrossRef]
- Darrag, H.M.; Alhajhoj, M.R.; Khalil, H.E. Bio-Insecticide of Thymus vulgaris and Ocimum basilicum Extract from Cell Suspensions and Their Inhibitory Effect against Serine, Cysteine, and Metalloproteinases of the Red Palm Weevil (Rhynchophorus ferrugineus). Insects 2021, 12, 405. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Athar, M.T.; Al-Faraidy, A.A.; Almuhaiza, M.S. Chemical composition of essential oil of Thymus vulgaris collected from Saudi Arabian market. Asian Pac. J. Trop. Biomed. 2017, 7, 147–150. [Google Scholar] [CrossRef]
- Chenni, M.; El Abed, D.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Comparative study of essential oils extracted from Egyptian basil leaves (Ocimum basilicum L.) using hydro-distillation and solvent-free microwave extraction. Molecules 2016, 21, 113. [Google Scholar] [CrossRef] [PubMed]
- Bhavya, M.L.; Chandu, A.G.S.; Devi, S.S. Ocimum tenuiflorum oil, a potential insecticide against rice weevil with anti-acetylcholinesterase activity. Ind. Crops Prod. 2018, 126, 434–439. [Google Scholar] [CrossRef]
- Tripathi, P.; Dubey, N.K.; Banerji, R.; Chansouria, J.P.N. Evaluation of some essential oils as botanical fungitoxicants in management of post-harvest rotting of citrus fruits. World J. Microbiol. Biotechnol. 2004, 20, 317–321. [Google Scholar] [CrossRef]
- Rodríguez-González, Á.; Álvarez-García, S.; González-López, Ó.; Silva, F.D.; Casquero, P.A. Insecticidal Properties of Ocimum basilicum and Cymbopogon winterianus against Acanthoscelides obtectus, Insect Pest of the Common Bean (Phaseolus vulgaris L.). Insects 2019, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Milosavljević, I.; El-Shafie, H.A.; Faleiro, J.R.; Hoddle, C.D.; Lewis, M.; Hoddle, M.S. Palmageddon: The wasting of ornamental palms by invasive palm weevils, Rhynchophorus spp. J. Pest Sci. 2019, 92, 143–156. [Google Scholar] [CrossRef]
- Shehawy, A.A.; Ibrahim, M.T.; Aboutaleb, E.S.; Qari, S.H. Bioactivity and biochemical efficacy of chitinase and Justicia brandegeana extract against Red Palm Weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae). Food Sci. Nutr. 2020, 8, 4625–4636. [Google Scholar] [CrossRef]
- Faleiro, J. A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Insect Sci. 2006, 26, 135–154. [Google Scholar]
- Downer, A.J.; Uchida, J.Y.; Hodel, D.R.; Elliott, M.L. Lethal palm diseases common in the United States. HortTechnology 2009, 19, 710–716. [Google Scholar] [CrossRef][Green Version]
- Wattanapongsiri, A. A Revision of the Genera Rhynchophorus and Dynamis (Coleoptera: Curculionidae). Ph.D. Dissertation, Oregon State University, Corvallis, OR, USA, 1966. [Google Scholar]
- Žďárek, J.; Howard, F.W.; Moore, D.; Giblin-Davis, R.M.; Abad, R.G. Insects on Palms (Ecological Studies 142). Biol. Plant. 2002, 45, 196. [Google Scholar] [CrossRef]
- Fiaboe, K.; Peterson, A.T.; Kairo, M.; Roda, A. Predicting the potential worldwide distribution of the red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) using ecological niche modeling. Fla. Entomol. 2012, 95, 659–673. [Google Scholar] [CrossRef]
- Rugman-Jones, P.F.; Hoddle, C.D.; Hoddle, M.S.; Stouthamer, R. The lesser of two weevils: Molecular-genetics of pest palm weevil populations confirm Rhynchophorus vulneratus (Panzer 1798) as a valid species distinct from R. ferrugineus (Olivier 1790), and reveal the global extent of both. PLoS ONE 2013, 8, e78379. [Google Scholar] [CrossRef] [PubMed]
- Hoddle, M.; Hoddle, C. Palmageddon: The invasion of California by the South American palm weevil is underway. CAPCA Advis. 2017, 20, 40–44. [Google Scholar]
- Padalia, R.; Verma, R.; Chauhan, A.; Chanotiya, C. Changes in aroma profiles of 11 Indian Ocimum taxa during plant ontogeny. Acta Physiol. Plant. 2013, 35, 2567–2587. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. PCTOC 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Darrag, H.M.; Almuhanna, H.T.; Hakami, E.H. Secondary Metabolites in Basil, Bio-Insecticide, Inhibition Effect, and In Silico Molecular Docking against Proteolytic Enzymes of the Red Palm Weevil (Rhynchophorus ferrugineus). Plants 2022, 11, 1087. [Google Scholar] [CrossRef]
- Khalil, H.E.; Ibrahim, H.-I.M.; Darrag, H.M.; Matsunami, K. Insight into Analysis of Essential Oil from Anisosciadium lanatum Boiss.—Chemical Composition, Molecular Docking, and Mitigation of Hepg2 Cancer Cells through Apoptotic Markers. Plants 2021, 11, 66. [Google Scholar] [CrossRef]
- Khalil, H.E.; Alqahtani, N.K.; Darrag, H.M.; Ibrahim, H.-I.M.; Emeka, P.M.; Badger-Emeka, L.I.; Matsunami, K.; Shehata, T.M.; Elsewedy, H.S. Date Palm Extract (Phoenix dactylifera) PEGylated Nanoemulsion: Development, Optimization and Cytotoxicity Evaluation. Plants 2021, 10, 735. [Google Scholar] [CrossRef]
- Ibrahim, H.-I.M.; Darrag, H.M.; Alhajhoj, M.R.; Khalil, H.E. Biomolecule from Trigonella stellata from Saudi Flora to Suppress Osteoporosis via Osteostromal Regulations. Plants 2020, 9, 1610. [Google Scholar] [CrossRef]
- Younis, H.M.; Badawy, M.E.I.; Darrag, H.M.S. Tissue culture of the Egyptian cotton cultivars: Production and morphological heterogeneity of primary callus tissues. FASEB J. 2013, 27, 10141. [Google Scholar] [CrossRef]
- Ross, D.C.; Brown, T.M. Inhibition of larval growth in Spodoptera frugiperda by sublethal dietary concentrations of insecticides. J. Agric. Food Chem. 1982, 30, 193–196. [Google Scholar] [CrossRef]
- Lewis, N.G.; Yamamoto, E. Lignin: Occurrence, biogenesis and biodegradation. Annu. Rev. Plant Biol. 1990, 41, 455–496. [Google Scholar] [CrossRef]
- Bolwell, G.P.; Robbins, M.P.; Dixon, R.A. Metabolic changes in elicitor-treated bean cells: Enzymic responses associated with rapid changes in cell wall components. Eur. J. Biochem. 1985, 148, 571–578. [Google Scholar] [CrossRef]
- Kefeli, V.I.; Kalevitch, M.V.; Borsari, B. Phenolic cycle in plants and environment. J. Cell Mol. Biol. 2003, 2, 13–18. [Google Scholar]
- Arnaldos, T.L.; Muñoz, R.; Ferrer, M.A.; Calderón, A.A. Changes in phenol content during strawberry (Fragaria × ananassa, cv. Chandler) callus culture. Physiol. Plant. 2001, 113, 315–322. [Google Scholar] [CrossRef]
- Mato, M.; Rua, M.; Ferro, A. Changes in levels of peroxidases and phenolics during root formation in Vitis cultured in vitro. Physiol. Plant. 1988, 72, 84–88. [Google Scholar] [CrossRef]
- Pickens, C.L.; Airavaara, M.; Theberge, F.; Fanous, S.; Hope, B.T.; Shaham, Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011, 34, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.O.; Jin, Y.-W.; Lee, E.-K.; Loake, G.J. Plant cell culture strategies for the production of natural products. BMB Rep. 2016, 49, 149. [Google Scholar] [CrossRef]
- Mathew, R.; Sankar, P.D. Comparison of major secondary metabolites quantified in elicited cell cultures, non-elicited cell cultures, callus cultures and field grown plants of Ocimum. Int. J. Pharm. Pharm. Sci. 2014, 6, 102–106. [Google Scholar]
- Michaud, N.R.; Fabian, J.R.; Mathes, K.D.; Morrison, D.K. 14-3-3 is not essential for Raf-1 function identification of Raf-1 proteins that are biologically activated in a 14-3-3-and Ras-independent manner. Mol. Cell. Biol. 1995, 15, 3390–3397. [Google Scholar] [CrossRef]
- Li, H.; Child, M.A.; Bogyo, M. Proteases as regulators of pathogenesis: Examples from the Apicomplexa. Biochim. Biophys. Acta BBA Proteins Proteom. 2012, 1824, 177–185. [Google Scholar] [CrossRef]
- Gomes, M.T.; Teixeira, R.D.; Lopes, M.T.; Nagem, R.A.; Salas, C.E. X-ray crystal structure of CMS1MS2: A high proteolytic activity cysteine proteinase from Carica candamarcensis. Amino Acids 2012, 43, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Nováková, L.; Vildová, A.; Mateus, J.P.; Gonçalves, T.; Solich, P. Development and Application of UHPLC–MS/MS Method for the Determination of Phenolic Compounds in Chamomile Flowers and Chamomile Tea Extracts. Talanta 2010, 82, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.; Li, Y.; Li, X.; Luo, J.; Kong, L. Qualitative and Quantitative Analysis of the Major Constituents in Chinese Medicinal Preparation Guan-Xin-Ning Injection by HPLC–DAD–ESI-MSn. J. Pharm. Biomed. Anal. 2012, 59, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Foo, Y. Rosmarinic Acid Derivatives from Salvia officinalis. Phytochemistry 1999, 51, 91–94. [Google Scholar] [CrossRef]
- Prinsi, B.; Negri, A.S.; Quattrocchio, F.M.; Koes, R.E.; Espen, L. Proteomics of Red and White Corolla Limbs in Petunia Reveals a Novel Function of the Anthocyanin Regulator ANTHOCYANIN1 in Determining Flower Longevity. J. Proteom. 2016, 131, 38–47. [Google Scholar] [CrossRef]
- Abad-García, B.; Garmón-Lobato, S.; Berrueta, L.A.; Gallo, B.; Vicente, F. A Fragmentation Study of Dihydroquercetin Using Triple Quadrupole Mass Spectrometry and Its Application for Identification of Dihydroflavonols in Citrus Juices. Rapid Commun. Mass Spectrom. 2009, 23, 2785–2792. [Google Scholar] [CrossRef]
- Rypniewski, W.; Østergaard, P.; Nørregaard-Madsen, M.; Dauter, M.; Wilson, K.S. Fusarium oxysporum trypsin at atomic resolution at 100 and 283 K: A study of ligand binding. Acta Crystallogr. Sect. D Biol. Crystallogr. 2001, 57, 8–19. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]


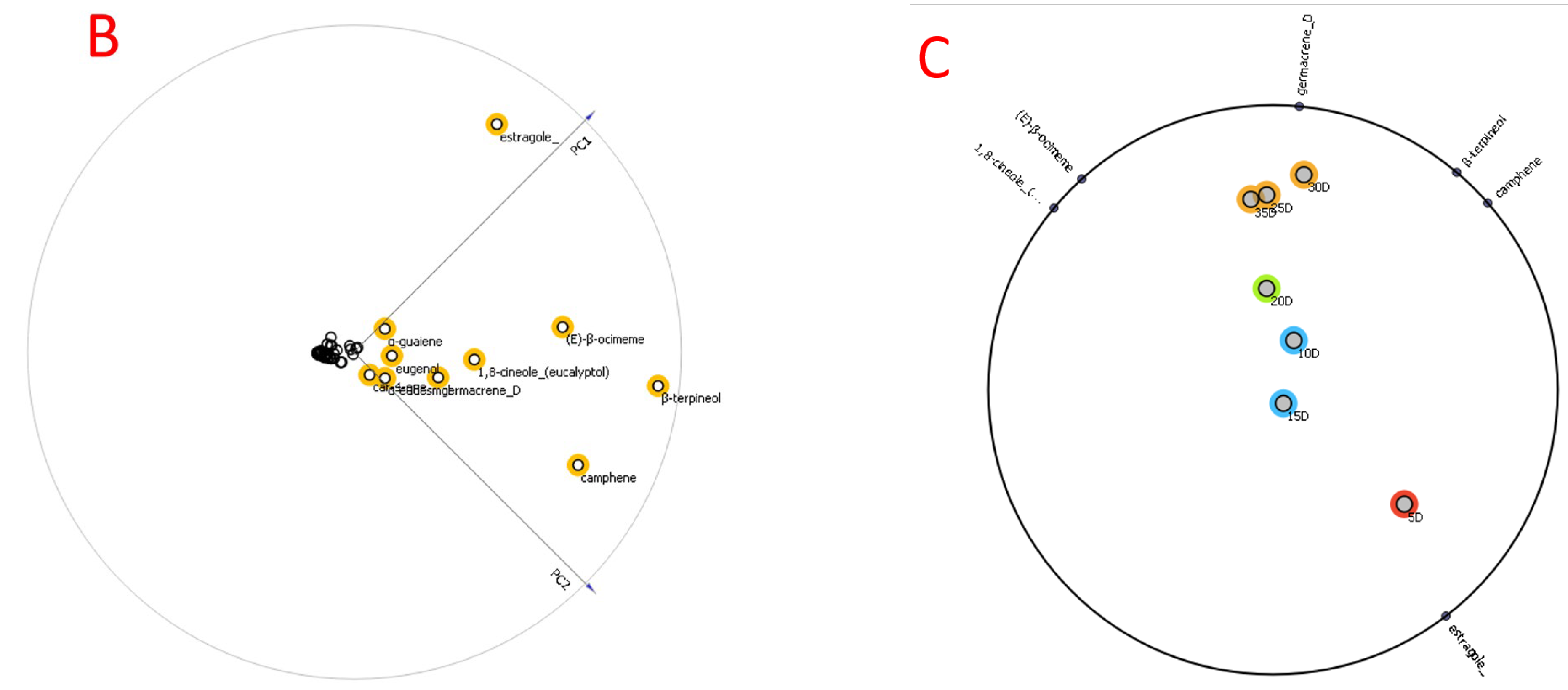


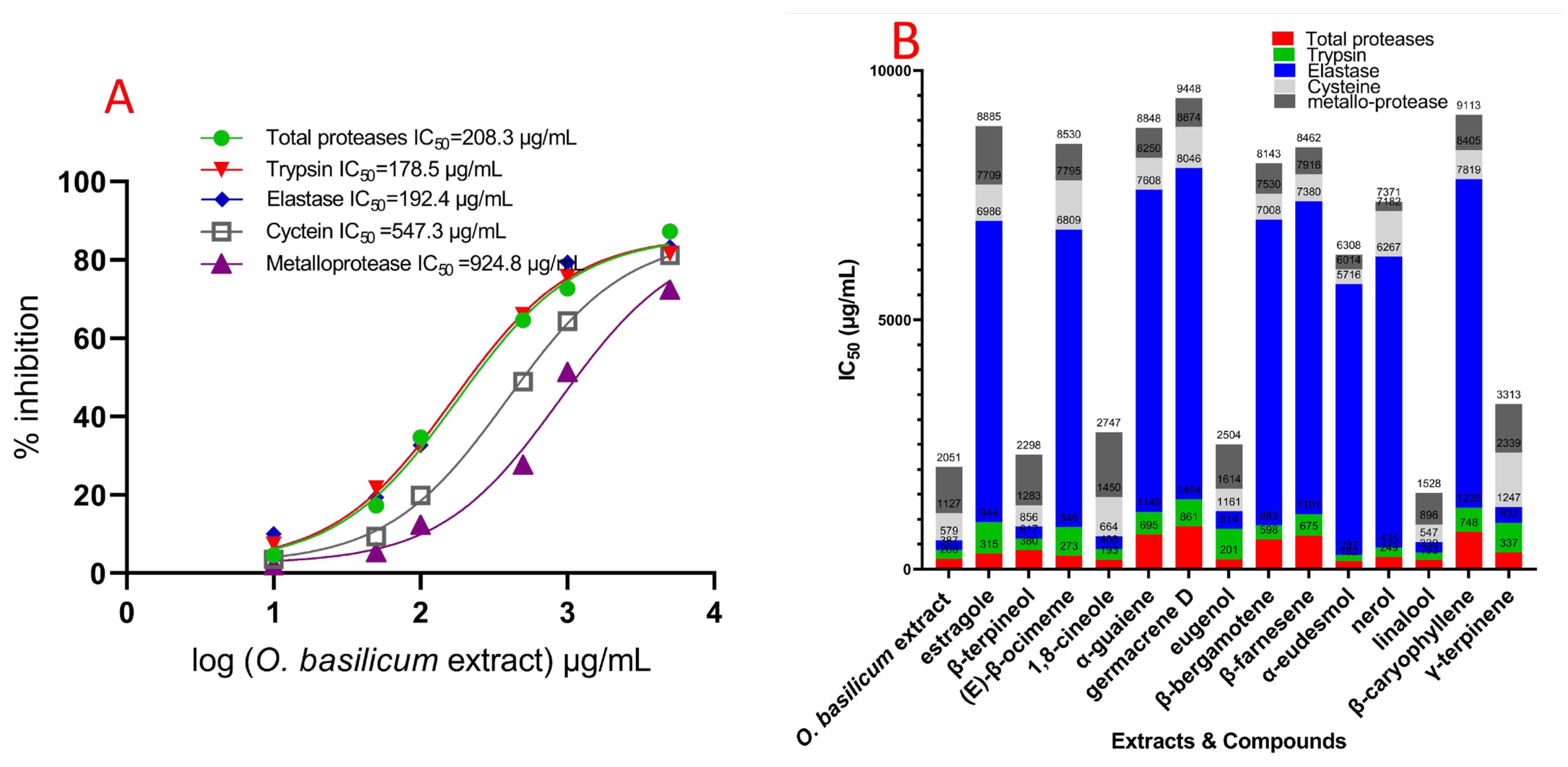

| No. | Compounds | RI (exp) | RI (lit) | Relative Abundance % |
|---|---|---|---|---|
| Monoterpene Hydrocarbons (MH) | ||||
| 1 | α-thujene | 929 | 924 | 0.1± 0.10 |
| 2 | α-pinene | 937 | 932 | 0.1 ± 0.02 |
| 3 | camphene | 952 | 946 | 0.1± 0.02 |
| 4 | sabinene | 973 | 973 | 0.4± 0.06 |
| 5 | β-pinene | 977 | 977 | 1 ± 0.02 |
| 6 | β-myrcene | 991 | 988 | 0.1 ± 0.04 |
| 7 | α-phellandrene | 1005 | 1002 | 0.1 ± 0.04 |
| 8 | car-4-ene | 1009 | 1004 | 1.2 ± 0.1 |
| 9 | α-terpinene | 1017 | 1014 | 0.1 ± 0.1 |
| 10 | limonene | 1030 | 1224 | 0.4 ± 0.1 |
| 11 | (Z)-β-ocimene | 1038 | 1032 | 0.2 ± 0.02 |
| 12 | (E)-β-ocimene | 1049 | 1044 | 11.96 ± 0.2 |
| 13 | γ-terpinene | 1060 | 1067 | 1.0 ± 0.2 |
| 14 | terpinolene | 1088 | 1086 | 0.7 ± 0.1 |
| Total Monoterpene Hydrocarbons (MH) Identified % | 17.46 ± 1.12 | |||
| Oxygenated Monoterpenes (OM) | ||||
| 1 | 1,8-cineole (eucalyptol) | 1031 | 1031 | 7.24 ± 0.4 |
| 2 | linalool (β-linalool) | 1099 | 1095 | 1.2 ± 0.2 |
| 3 | β-terpineol | 1130 | 1130 | 12.37 ± 0.87 |
| 4 | camphor | 1145 | 1141 | 1.4 ± 0.2 |
| 5 | borneol (isoborneol) | 1167 | 1165 | 0.2 ± 0.05 |
| 6 | terpinen-4-ol | 1177 | 1174 | 2 ± 0.3 |
| 7 | α-terpineol | 1189 | 1186 | 0.1 ± 0.04 |
| 8 | estragole | 1199 | 1199 | 22.38 ± 0.7 |
| 9 | fenchyl acetate | 1214 | 1214 | 0.1 ± 0.02 |
| 10 | nerol | 1228 | 1227 | 1.6 ± 0.2 |
| 11 | neral | 1244 | 1244 | 0.3 ± 0.1 |
| 12 | p-mentha-1,8-dien-7-ol | 1261 | 1261 | 0.1 ± 0.04 |
| 13 | bornyl acetate | 1285 | 1284 | 0.5 ± 0.02 |
| 14 | methyl geranate | 1321 | 1319 | 0.3 ± 0.03 |
| 15 | neryl acetate | 1364 | 1359 | 0.1 ± 0.04 |
| Total Oxygenated Monoterpenes (OM) Identified as % | 49.89 ± 2.88 | |||
| Sesquiterpene Hydrocarbons (SH) | ||||
| 1 | α-copaene | 1376 | 1374 | 0.4 ± 0.03 |
| 2 | (E)-β-bourbonene | 1384 | 1387 | 0.1 ± 0.02 |
| 3 | α-cubebene | 1385 | 1387 | 0.5 ± 0.02 |
| 4 | β-cubebene | 1389 | 1387 | 0.7 ± 0.05 |
| 5 | β-elemene | 1391 | 1389 | 0.1 ± 0.03 |
| 6 | 7-epi-sesquithujene | 1402 | 1405 | 0.3 ± 0.04 |
| 7 | α-gurjunene | 1409 | 1409 | 0.1 ± 0.02 |
| 8 | β-caryophyllene | 1424 | 1424 | 1.1 ± 0.1 |
| 9 | β-copaene | 1432 | 1430 | 0.2 ± 0.05 |
| 10 | β-gurjunene (calarene) | 1434 | 1431 | 0.2± 0.04 |
| 11 | trans-α-bergamotene | 1435 | 1432 | 0.2 ± 0.03 |
| 12 | α-guaiene | 1439 | 1437 | 4.8 ± 0.2 |
| 13 | β-bergamotene | 1441 | 1438 | 2.3 ± 0.2 |
| 14 | α-humulene | 1455 | 1452 | 1.52 ± 0.04 |
| 15 | (E)-β-farnesene | 1457 | 1454 | 2.3 ± 0.08 |
| 16 | cis-muurola-4(14),5-diene | 1463 | 1465 | 0.2 ± 0.1 |
| 17 | γ-muurolene | 1477 | 1478 | 0.1 ± 0.03 |
| 18 | germacrene D | 1481 | 1484 | 4.2 ± 0.2 |
| 19 | β-selinene | 1486 | 1489 | 0.2 ± 0.03 |
| 20 | β-bulnesene | 1505 | 1508 | 0.1 ± 0.02 |
| 21 | β-bisabolene | 1509 | 1512 | 0.3 ± 0.06 |
| 22 | γ-cadinene | 1513 | 1513 | 0.7 ± 0.02 |
| 23 | β-sesquiphellandrene | 1524 | 1521 | 0.8 ± 0.03 |
| 24 | δ-cadinene | 1525 | 1522 | 0.4 ± 0.04 |
| 25 | α-cadinene (α-amorphene) | 1538 | 1537 | 0.1 ± 0.02 |
| Total Sesquiterpene Hydrocarbons (SH) Identified as % | 21.92 ± 1.50 | |||
| Oxygenated Sesquiterpenes (OS) | ||||
| 1 | τ-cadinol | 1640 | 1638 | 0.1 ± 0.02 |
| 2 | α-eudesmol | 1653 | 1652 | 1.8 ± 0.1 |
| 3 | α-Bisabolene oxide | 1680 | 1682 | 0.1 ± 0.02 |
| Total Oxygenated Sesquiterpenes (OS) Identified (%) | 2.0 ± 0.14 | |||
| Phenylpropanoids (PP) | ||||
| 1 | chavicol | 1256 | 1247 | 0.1 ± 0.03 |
| 2 | eugenol | 1357 | 1356 | 3.7 ± 0.3 |
| 3 | methyl eugenol | 1406 | 1402 | 0.4 ± 0.02 |
| Total Phenylpropanoids (PP) Identified (%) | 4.2 ± 0.35 | |||
| Non-Terpene Derivatives | ||||
| 1 | ethyl isovalerate | 853 | 856 | 0.3 ± 0.02 |
| 2 | 6-methyl-5-hepten-2-one | 985 | 988 | 0.4 ± 0.02 |
| Total Non-Terpene Derivatives (NT) Identified (%) | 0.7 ± 0.04 | |||
| Total Identified (%) | 96.17 | |||
| Extract | Adult | 4th Larvae | ||||||
|---|---|---|---|---|---|---|---|---|
| LC50 (µg/mL) 95% CF | Slope | Chi-Square | p | LD50 (µg/Larvae) 95% CF | Slope | Chi-Square | p | |
| O. basilicum | 1229 (1041–1392) | 2.54 ± 0.20 | 42.4 | <0.01 | 13.8 (12.01–15.58) | 1.29 ± 0.23 | 42.41 | <0.01 |
| estragole | 3587 (2269–2847) | 2.04 ± 0.22 | 49.1 | 0.009 | 34.8 (31.7–35.6) | 1.08 ± 0.12 | 49.2 | 0.008 |
| β-terpineol | 3924 (2694–4259) | 1.94 ± 0.23 | 49.7 | 0.009 | 42.6 (39.7–44.3) | 1.04 ± 0.11 | 49.3 | 0.009 |
| (E)-β-ocimeme | 3014 (1845–2356) | 2.12 ± 0.23 | 48.2 | 0.009 | 31.2 (27.4–32.8) | 1.12 ± 0.13 | 48.2 | 0.008 |
| 1,8-cineole | 1426 (1197–1594) | 2.69 ± 0.19 | 43.9 | 0.006 | 13.9 (12.8–14.7) | 1.36 ± 0.18 | 41.8 | 0.001 |
| α-guaiene | 1491 (1265–1682) | 2.62 ± 0.21 | 44.8 | 0.007 | 19.2 (17.8–20.7) | 1.24 ± 0.17 | 44.2 | 0.004 |
| germacrene D | 2045 (1825–2314) | 2.48 ± 0.22 | 47.2 | 0.009 | 21.3 (19.7–22.5) | 1.20 ± 0.17 | 44.6 | 0.006 |
| eugenol | 1459 (1226–1647) | 2.66 ± 0.20 | 44.4 | 0.006 | 14.2 (13.1–15.4) | 1.34 ± 0.19 | 42.2 | 0.002 |
| β-bergamotene | 1294 (1086–1418) | 2.76 ± 0.21 | 43.2 | 0.003 | 17.2 (16.1–18.4) | 1.28 ± 0.16 | 43.4 | 0.003 |
| β-farnesene | 1356 (1178–1572) | 2.73 ± 0.20 | 43.7 | 0.004 | 18.4 (17.1–19.7) | 1.26 ± 0.17 | 43.8 | 0.004 |
| α-eudesmol | 1312 (1167–1548) | 2.75 ± 0.19 | 43.6 | 0.004 | 12.4 (11.3–13.1) | 1.39 ± 0.17 | 41.3 | 0.001 |
| nerol | 1947 (1569–2119) | 2.51 ± 0.22 | 46.2 | 0.009 | 15.6 (14.3–16.2) | 1.31 ± 0.18 | 42.3 | 0.002 |
| linalool | 1398 (1176–1583) | 2.71 ± 0.20 | 43.8 | 0.005 | 13.7 (12.6–14.8) | 1.37 ± 0.18 | 41.6 | 0.001 |
| β-caryophyllene | 1523 (1312–1736) | 2.60 ± 0.21 | 45.7 | 0.008 | 20.1 (18.7–21.4) | 1.22 ± 0.17 | 44.3 | 0.005 |
| γ-terpinene | 3841 (2589–4027) | 1.97 ± 0.22 | 49.4 | 0.009 | 38.3 (35.1–40.7) | 1.07 ± 0.14 | 49.8 | 0.008 |
| Compounds | Docking Score ΔG (kcal/mol) | |||
|---|---|---|---|---|
| Serine Proteinase | Cysteine Proteinase | Metallo Proteinase | Trypsin Proteinase | |
| Estragole | −4.3709 (10) | −4.5151 (9) | −4.6842 (12) | −4.3164 (13) |
| β-terpineol | −4.1040 (12) | −4.6980 (7) | −4.6411 (13) | −4.4555 (10) |
| (E)-β-ocimeme | −4.5195 (6) | −4.4243 (13) | −4.9996 (9) | −4.5755 (9) |
| 1,8-cineole | −4.0485 (14) | −4.4759(10) | −4.2706 (14) | −4.2897 (14) |
| α-guaiene | −4.4206 (7) | −4.8005 (6) | −5.3298 (4) | −4.8352 (5) |
| germacrene D | −4.4132 (9) | −4.4563 (11) | −5.4350 (3) | −4.5790 (8) |
| Eugenol | −4.9100 (3) | −4.6507 (8) | −4.8218 (10) | −4.3781 (12) |
| β-bergamotene | −4.9929 (2) | −5.0760 (2) | −5.3167 (5) | −5.2044 (1) |
| β-farnesene | −5.0728 (1) | −5.0141 (3) | −5.6725 (1) | −5.1448 (3) |
| α-eudesmol | −4.6450 (5) | −5.2551 (1) | −5.2549 (6) | −5.1964 (2) |
| nerol | −4.7396 (4) | −4.4430 (12) | −5.5082 (2) | −4.7201 (7) |
| linalool | −4.0872 (13) | −4.9738 (4) | −5.0587 (7) | −4.9127(4) |
| β-caryophyllene | −4.4190 (8) | −4.8315 (5) | −5.0288 (8) | −4.7619 (6) |
| γ-terpinene | −4.1591 (11) | −4.1835 (14) | −4.7142 (11) | −4.4256 (11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darrag, H.M.; Almuhanna, H.T.; Hakami, E.H.; Alhojaily, S.M. Analysis of Volatile Secondary Metabolites in Ocimum basilicum Cell Suspensions: Inhibition, In Silico Molecular Docking, and an ADMET Analysis against Proteolytic Enzymes of Rhynchophorus ferrugineus. Plants 2022, 11, 2949. https://doi.org/10.3390/plants11212949
Darrag HM, Almuhanna HT, Hakami EH, Alhojaily SM. Analysis of Volatile Secondary Metabolites in Ocimum basilicum Cell Suspensions: Inhibition, In Silico Molecular Docking, and an ADMET Analysis against Proteolytic Enzymes of Rhynchophorus ferrugineus. Plants. 2022; 11(21):2949. https://doi.org/10.3390/plants11212949
Chicago/Turabian StyleDarrag, Hossam Moustafa, Hani Taher Almuhanna, Emadaldeen Hamad Hakami, and Sameer M. Alhojaily. 2022. "Analysis of Volatile Secondary Metabolites in Ocimum basilicum Cell Suspensions: Inhibition, In Silico Molecular Docking, and an ADMET Analysis against Proteolytic Enzymes of Rhynchophorus ferrugineus" Plants 11, no. 21: 2949. https://doi.org/10.3390/plants11212949
APA StyleDarrag, H. M., Almuhanna, H. T., Hakami, E. H., & Alhojaily, S. M. (2022). Analysis of Volatile Secondary Metabolites in Ocimum basilicum Cell Suspensions: Inhibition, In Silico Molecular Docking, and an ADMET Analysis against Proteolytic Enzymes of Rhynchophorus ferrugineus. Plants, 11(21), 2949. https://doi.org/10.3390/plants11212949







