Abstract
Schistosomiasis and soil-transmitted helminths are some of the priority neglected tropical diseases (NTDs) targeted for elimination by the World Health Organization (WHO). They are prevalent in Botswana and although Botswana has begun mass drug administration with the hope of eliminating soil-transmitted helminths as a public health problem, the prevalence of schistosomiasis does not meet the threshold required to warrant large-scale interventions. Although Botswana has a modern healthcare system, many people in Botswana rely on traditional medicine to treat worm infections and schistosomiasis. In this study, ten plant species used by traditional health practitioners against worm infections were collected and tested against Ancylostoma ceylanicum (zoonotic hookworm), Heligmosomoides polygyrus (roundworm of rodents), Necator americanus (New World hookworm), Schistosoma mansoni (blood fluke) [adult and newly transformed schistosomula (NTS)], Strongyloides ratti (threadworm) and Trichuris muris (nematode parasite of mice) in vitro. Extracts of two plants, Laphangium luteoalbum and Commiphora pyaracanthoides, displayed promising anthelmintic activity against NTS and adult S. mansoni, respectively. L. luteoalbum displayed 85.4% activity at 1 μg/mL against NTS, while C. pyracanthoides displayed 78.5% activity against adult S. mansoni at 10 μg/mL.
1. Introduction
Neglected tropical diseases (NTDs) include diverse groups of communicable diseases that globally infect or affect more than 2.7 billion of the most impoverished populations living in low- to middle-income countries of Africa, Asia and Latin America [1]. In Africa, 90% of the disease burden of NTDs is in Sub-Saharan Africa due to widespread poverty and the suitability of specific climates in Africa for some NTDs to thrive [2]. In Botswana, schistosomiasis and soil-transmitted helminth infections are some of the priority NTDs targeted for elimination [3,4]. Schistosomiasis and soil-transmitted helminth infections are prevalent in Botswana, particularly in the North-West Inland Wetland, the Okavango Delta, and along the Chobe River, both regions of major tourist attraction. Botswana has begun mass drug administration of albendazole in the hope of eliminating soil-transmitted helminths as a public health problem [4]. Although the prevalence of schistosomiasis does not meet the threshold required to warrant large-scale interventions, there has been an increase in the prevalence of Schistosoma species in Botswana between 2010 and 2019 [5], and it was predicted that there may be future epidemics of schistosomiasis based on the correlation between the flow of the rivers in the Okavango Delta and prevalence patterns of snail populations [6,7]. Mass drug administration may be an effective control measure against soil-transmitted helminth infections and schistosomiasis; however, the reliance on praziquantel for the treatment of schistosomiasis in mass drug administration control programs presents a constant threat of the development of drug resistance [8]. An example of this can be seen in Uganda, where the efficacy of praziquantel has been shown to be lower in schools that have a longer duration of mass drug administration [9]. The limited number of anthelmintic drugs and their prolonged use will inevitably lead to anthelmintic drug resistance. This is a well-documented issue in animal populations [10] and the resistance to anthelmintic drugs in veterinary species serves as a reference for how anthelmintic resistance may increase within the human population [11]. Mutations associated with benzimidazole resistance have been identified in eggs from human stools [12]. There is therefore a need for new anthelmintic drugs and the plants used in traditional medicine against parasitic helminths could provide promising leads.
The role of traditional medicine in health systems was re-affirmed when the WHO declared 2011–2020 as the Second Decade of African Traditional Medicine and extended this by formulating the WHO traditional medicine strategy 2014–2023. Traditional medicine plays an important role in the diagnosis, prevention or elimination of physical, mental and social illnesses. In Africa and the Diaspora, traditional medicine usage has been, and remains, a significant contributor to primary healthcare delivery [13,14].
In Botswana, the public healthcare system is made up primarily of modern biomedical formal structures. These include hospitals, clinics and outreach health posts and this ensures that every inhabitant lives no more than 15 km away from a health facility. In spite of their close proximity to modern biomedical structures, many people in Botswana still seek healthcare from traditional health practitioners. This is because it is familiar to them as it is ingrained in their culture and due to the fact that public health facilities are often overwhelmed and are not always well equipped. It can also be argued that access to traditional health practitioners is usually easier, faster and cheaper than finding a modern healthcare facility [15]. Seeking help from traditional health practitioners is also perceived as more personalized and confidential. An example of this is the fact that although there was an increase in the accessibility of combination anti-retroviral therapy, individuals with moderate and advanced HIV infection continued to use traditional medicine [16].
Traditional medicine is widely used in Africa in the management of worm infections and schistosomiasis [17,18,19,20]. Patient observations by traditional health practitioners therefore might lead us to botanicals useful for the treatment/management of worm infections and schistosomiasis. Previously, the main screening approaches used for the discovery of new anthelmintics were animal-based, target-based and phenotypic methods [21]. Animal-based methods are low throughput, time consuming and require a large amount of the extract or compound, and therefore in vitro screening methods (target-based and phenotypic) are now used [22]. Target-based methods screen extracts and compounds against one or more molecules with essential functions in the parasite metabolism [23]. These methods, however, do not consider the bioavailability of the compound to the parasite. Phenotypic screening employs whole parasites in vitro and quantitatively measures the phenotypic features after treatment of the parasites with extracts or compounds [21].
Natural products from botanicals might increase the available pool of potential new anthelmintics with unique structures and/or unique modes of actions, and hence delay or prevent resistance [24,25].
In this study, therefore, we documented traditional medical knowledge relating to worm infections and schistosomiasis in one area of high prevalence, the Ngamiland District in Botswana, and we determined the anthelmintic bioactivities of medicinal plants administered by local traditional health practitioners against a battery of hookworm, roundworm, nematode and Schistosoma species. To the best of our knowledge, this is the first comprehensive characterization of the anthelmintic bioactivities of traditional medicinal plants in Botswana.
2. Results
2.1. Traditional Medicinal Plants for Treatment/Management of Worm Infections
During our interaction with three traditional health practitioners, the medicinal plant uses of ten plant species were documented (Table 1).

Table 1.
Traditional medicinal anthelmintic plants used in North West District, Botswana.
With the exception of the A. ferox and A. zebrina species, preparations were made mostly from dried plant parts. The majority of the samples were leaves (55%), followed by roots (18%). Seeds, fruit and stem bark were each 9%. Roots are normally used in traditional medicine in Botswana since secondary metabolites are usually stored there, so it is interesting to note the majority of samples are leaves. Most of the samples are boiled (55%) and some are infused with cold, warm or hot water. Two samples are mixed with milk and this may be in order to reduce the amount of bioavailable compounds from the plant sample as milk is known to reduce the bioavailability of certain compounds. Milk, however, may also increase or decrease the excretion of certain metabolites in the extract [35]. The dosage for the majority of the samples was three times a day (60%) and only one sample (S. panduriforme) is taken once a day. To the best of our knowledge there is no supporting literature on the use of B. albitrunca, C. pyracanthoides and L. luteoalbum as anthelmintics, making this investigation the first to report their use to treat helminth infections. This is also the first in vitro bioactivity report on anthelmintic activity for A. zebrina, T. sericea, C. mopane, B. albitrunca, C. pyracanthoides, S. panduriforme and L. luteoalbum.
2.2. Bioactivities of Medicinal Plant Extracts against Parasites
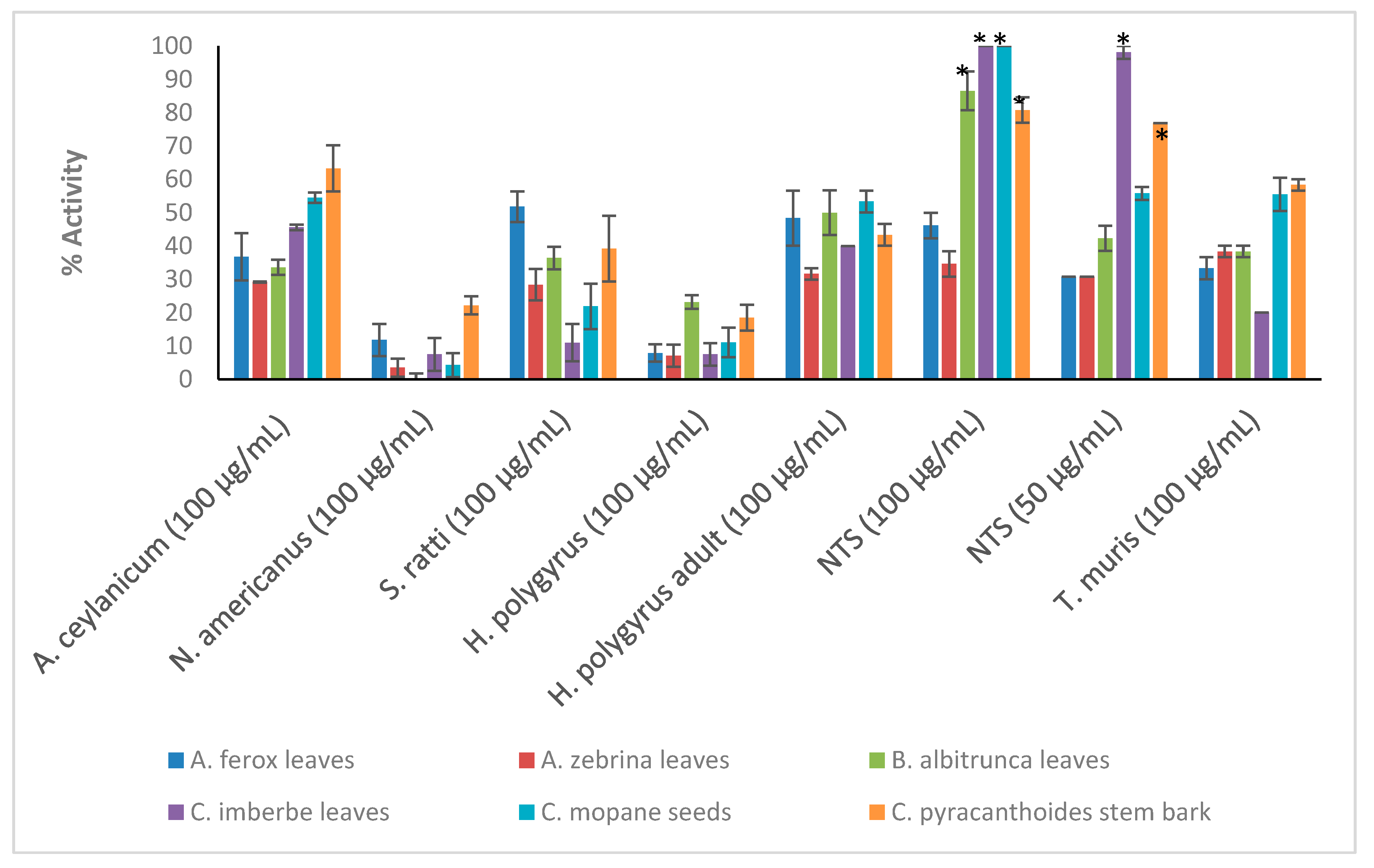
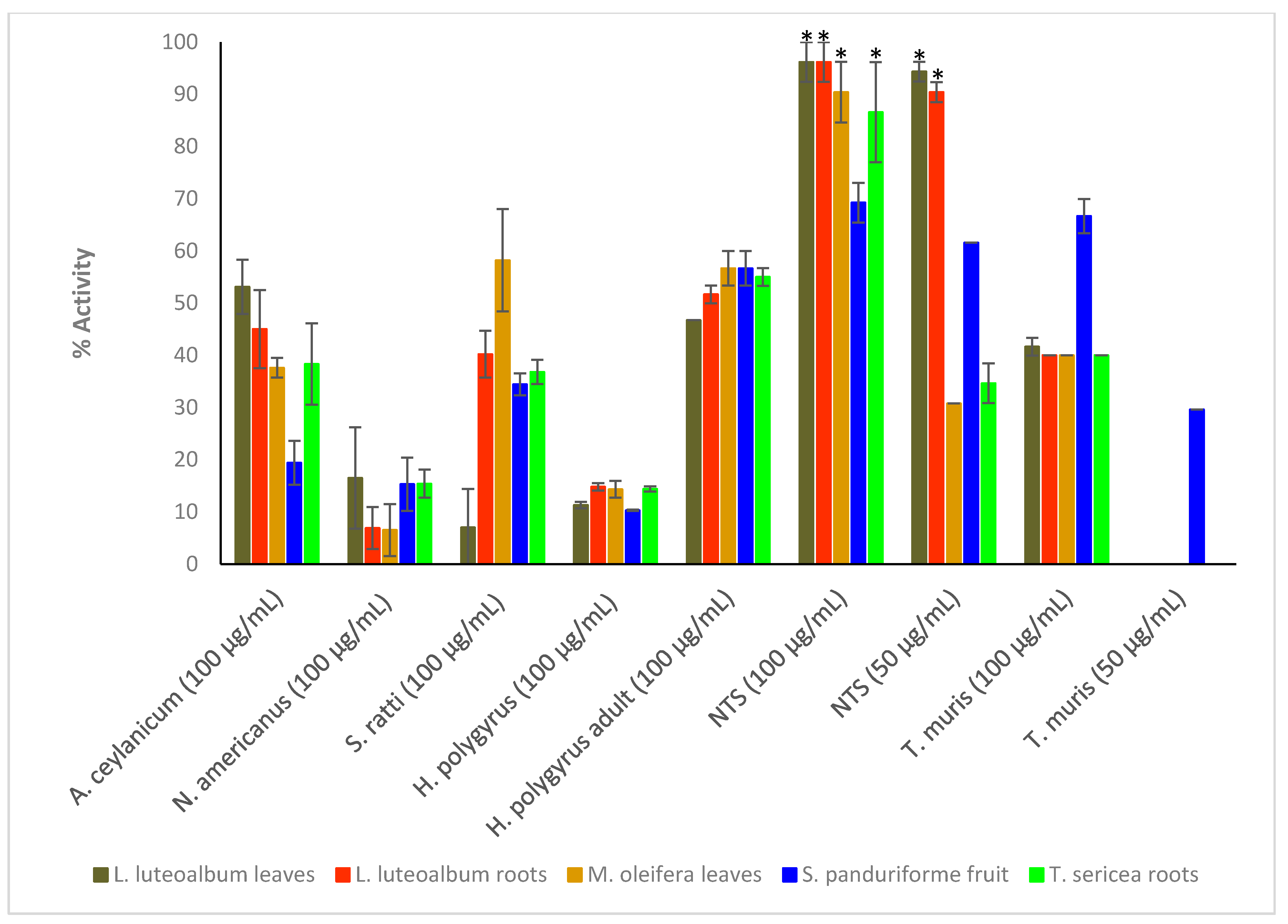
Crude extracts prepared from the ten plant species were tested at the starting concentration of 100 μg/mL against the larvae (L3) of Ancylostoma ceylanicum, Necator americanus, Strongyloides ratti, Heligmosomoides polygyrus (adult and L3 larvae), Trichuris muris (adult worms) and NTS (Figure 1; see Tables S1 and S2 in Supplementary Materials for numerical data). Only the most active extracts against NTS (threshold 70% at 50 μg/mL) (Figure 2) were further tested against adult Schistosoma mansoni beginning at 50 μg/mL (Figure 3). Activity above 50% at a concentration of 100 μg/mL for a plant extract is considered relevant [36,37]. Therefore, activity at a concentration of 100 μg/mL above 65% was considered ‘good’, between 30–65% ‘moderate’ and below 30% ‘weak’.
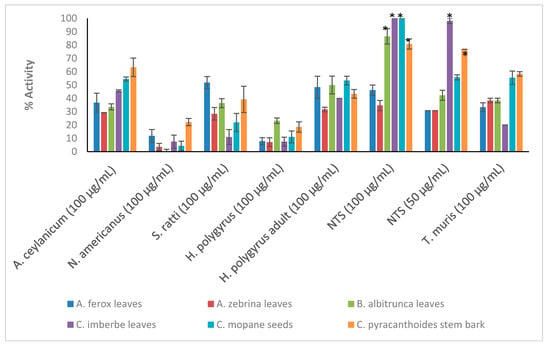
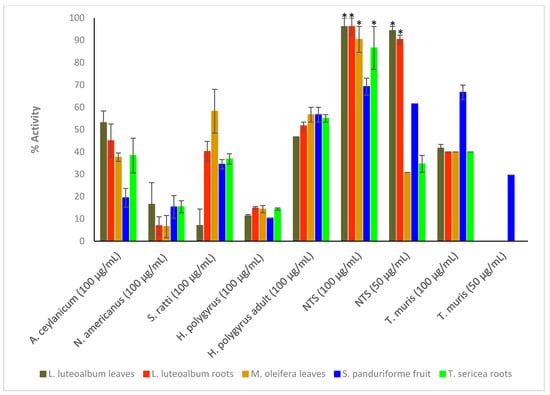
Figure 1.
Anthelmintic activity of the plant extracts against the parasitic organisms. Activity based on three replicates. Statistical analysis (one way ANOVA, p ≤ 0.001, and an all pairwise multiple comparison procedure (Tukey Test), * extract activities determined to be significant) was performed using SigmaPlot 14.0 (Supplementary Materials, Table S3).
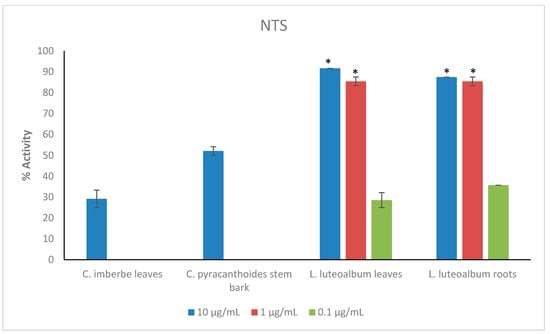
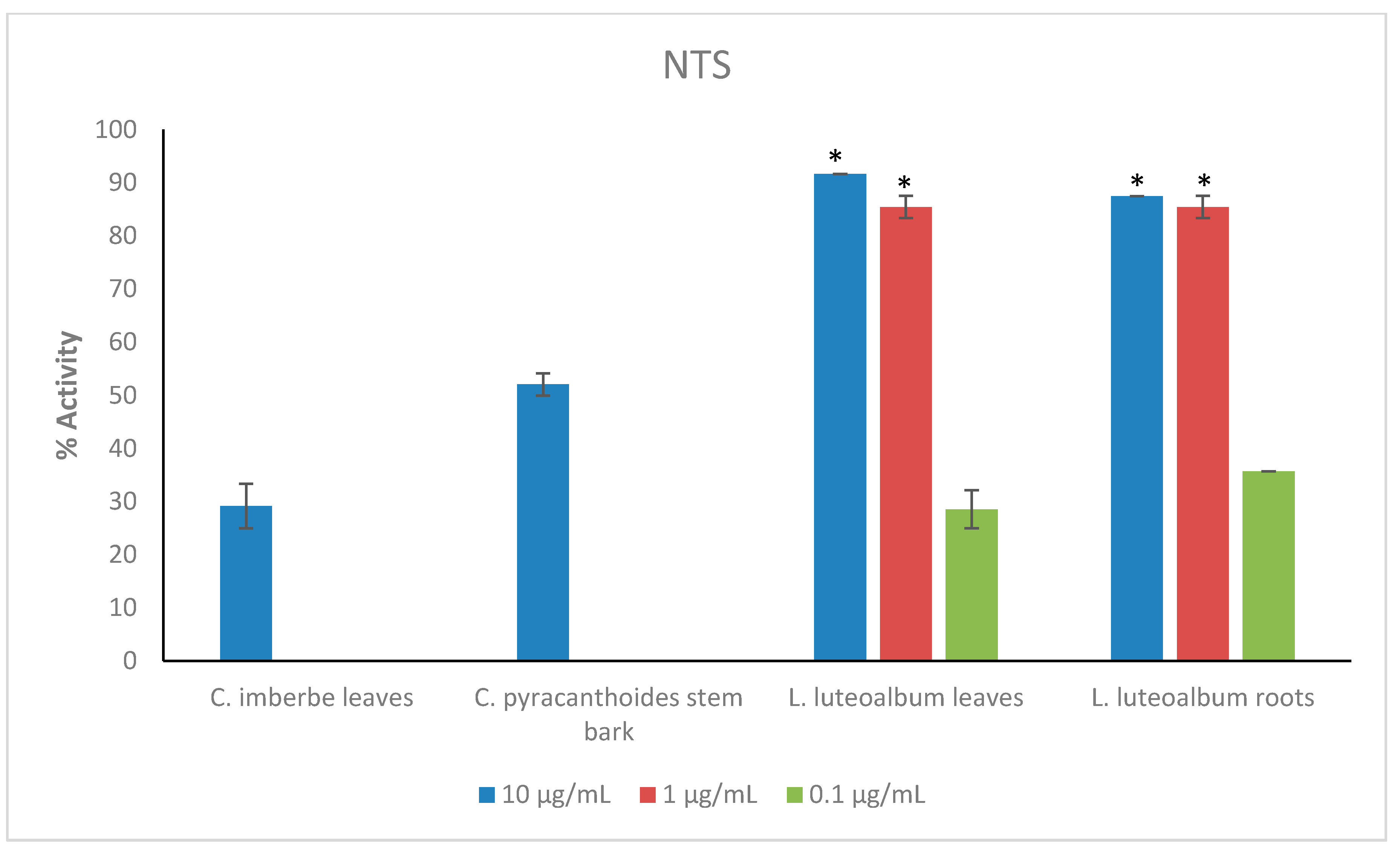
Figure 2.
Anthelmintic activity of the most active plant extracts at reduced concentrations against newly transformed schistosomula (NTS). Activity based on three replicates. Statistical analysis (one way ANOVA, p ≤ 0.001, and an all pairwise multiple comparison procedure (Tukey Test), * extract activities determined to be significant) was performed using SigmaPlot 14.0 (Supplementary Materials, Table S4).
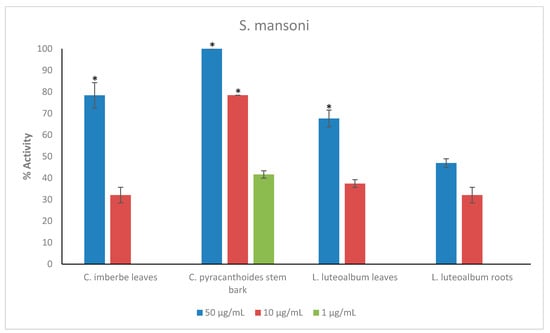
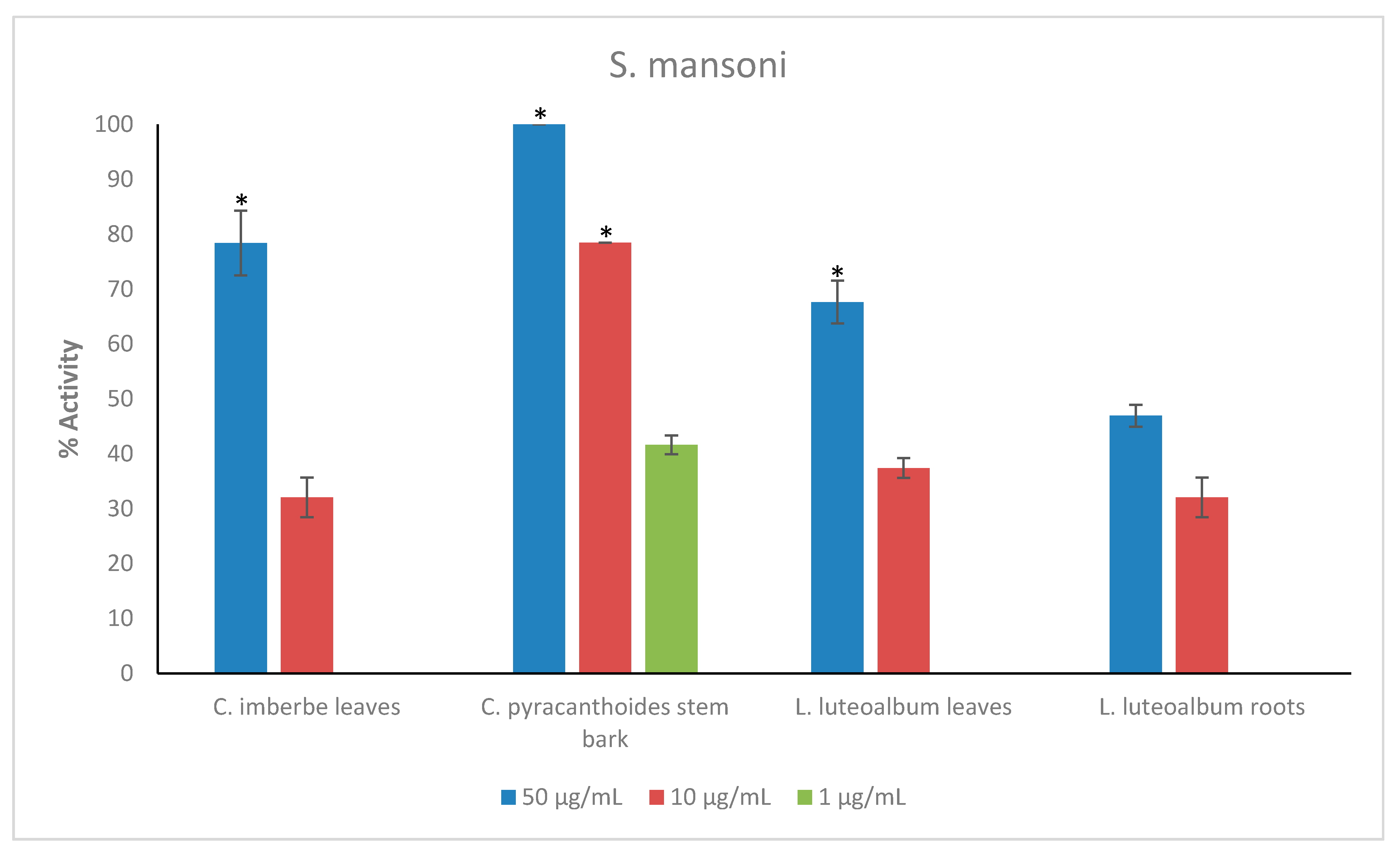
Figure 3.
Anthelmintic activity of the plant extracts against S. mansoni. Activity based on three replicates. Statistical analysis (one way ANOVA, p ≤ 0.001, and an all pairwise multiple comparison procedure (Tukey Test), * extract activities determined to be significant) was performed using SigmaPlot 14.0 (Supplementary Materials, Table S5).
The leaves and roots of L. luteoalbum showed the highest activity against NTS with activity above 90% at 1 μg/mL. At 0.1 μg/mL, the activity was 28.6 ± 3.6% for L. luteoalbum leaves and 35.7 ± 0% for L. luteoalbum roots. C. imberbe leaves had 29.2 ± 4.2% activity, while C. pyracanthoides stem bark displayed 52.1 ± 2.1% activity. Both plant extracts were investigated at the test concentration of 10 μg/mL (Figure 2).
C. pyracanthoides stem bark (63.3 ± 6.9%) had the highest activity against A. ceylanicum, followed by C. mopane seeds (54.5 ± 1.9%) and L. luteoalbum leaves (53.1 ± 5.2%). The other plant extracts exhibited activity between 19.4 ± 4.2% and 45.6 ± 0.8%. M. oleifera leaves (58.2 ± 9.8%) exhibited the highest activity against S. ratti larvae. All plant extracts showed low activity against N. americanus larvae with activity between 0.3 ± 1.5% and 22.2 ± 2.7%. The plant extracts displayed activity between 31.7 ± 1.7% and 56.7 ± 3.3% against adult H. polygyrus worms but showed lower activity against the larvae (between 7.1 ± 3.3% and 23.2 ± 2%). M. oleifera leaves and S. panduriforme fruit had the highest activity (both at 56.7 ± 3.3%) against adult H. polygyrus worms, while B. albitrunca leaves (23.2 ± 2%) had the highest activity against H. polygyrus larvae. S. panduriforme fruit (66.7 ± 3.3%) was the only extract that showed activity above 65% against adult T. muris and it was therefore tested at a lower concentration of 50 μg/mL, at which it displayed reduced activity (29.6 ± 0%). The plant extracts generally showed the highest activity against NTS (between 69.2 ± 3.8% and 100 ± 0%, except for A. ferox leaves, 46.1 ± 3.8%, and A. zebrina leaves, 34.6 ± 3.8%) and they were tested at the reduced concentration of 50 μg/mL, where four plant extracts (C. imberbe seeds (98.1 ± 1.9%), C. pyracanthoides stem bark (76.9 ± 0%) and L. luteoalbum leaves (94.3 ± 1.9%) and roots (90.4 ± 1.9%)) displayed the highest activity. These four plant extracts were then tested at further reduced concentrations against NTS (Figure 2).
C. pyracanthoides exhibited the highest activity against adult S. mansoni, displaying 78.5 ± 0% activity at 10 μg/mL and 41.7 ± 1.7% activity at the lower concentration of 1 μg/mL. C. imberbe leaves displayed 78.4 ± 5.9% activity at 50 μg/mL, while L. luteoalbum leaves displayed 67.7 ± 3.9% activity also at 50 μg/mL (Figure 3).
3. Discussion
In this investigation, extracts from medicinal plants that are used as anthelmintics in traditional medicine by the traditional health practitioners from North-Western Botswana were tested for their in vitro anthelmintic activity against various helminth parasites. Conventional drug screening usually uses compound libraries to test for various biological activities in vitro, and the active compounds are then further tested in vivo. However, there is a very high failure rate with this approach, as often compounds that gave good in vitro activity can be inactive when tested in animal models [37] or can be toxic. About 60% of failures for potential new therapeutic drugs are due to lack of efficacy and toxicity, making these the two main causes for lack of success in drug development [38]. The approach of testing herbal extracts which are used in traditional medicine is referred to as reverse pharmacology [39]. The idea is that if a plant is already widely used in traditional medicine without reported toxicity, then the likelihood of the plant-based remedy being safe and effective is high [37]. Heinrich [40] stated, however, that working with a plant extract brings the major challenge that it is a mixture of active, partially active and inactive compounds whose activity is often not on a single target. Some of the compounds in the mixture can also be prodrugs, meaning that they need to be converted to an active form, usually by the gut microflora. Flavonoids are a group of compounds for which there is increasing evidence that they could possibly act as prodrugs. For example, flavonols are metabolized by the intestinal microflora to their corresponding hydroxyphenylacetic acids [41,42,43,44]. The presence of prodrugs may lead to lack of activity in in vitro assays as the conditions required for the prodrug to become active are not available. The biological activity of plant extracts can result from the overall effect of compounds with synergistic, additive or antagonistic activity, and this can lead to loss of activity when fractionation is done in an effort to isolate the active principle [45]. Studies have also shown that disease resistance is less likely to occur when an extract with many compounds is used rather than a single active compound [46,47]. Plenty of plants used in traditional medicine are now registered and marketed as botanical drugs in their crude form without having the active ingredients isolated [48]. A remarkable example is the phytopharmaceutical preparation Iberogast®, sold in Germany, which consists of nine different plant extracts. The preparation is used in the treatment of dyspepsia and showed therapeutic equivalence when it was compared with the synthetic drugs cisapride and metoclopramide, but it had fewer side effects than the synthetic drugs [49]. The plant extracts in our study are used by traditional health practitioners against helminths as extracts and were therefore tested in vitro as extracts. The plant extracts all showed anthelmintic activity to varying degrees.
The overall best anthelmintic activity was exhibited by C. pyracanthoides stem bark extract against S. mansoni adult worms, A. ceylanicum L3 and N. americanus L3. Only S. panduriforme fruit extracts had better activity against T. muris than C. pyracanthoides stem bark extract, while B. albitrunca leaves extract was the only plant extract with better anthelmintic activity against H. polygyrus L3 than C. pyracanthoides extract. Plant extracts and compounds with broad spectrum anthelmintic activity are desirable as multiple parasitic helminths are often endemic in the same regions. A plant extract showing anthelmintic activity against several target species across the Nematoda and Platyhelminthes phyla could be helpful in the isolation of compounds with the potential to be broad spectrum therapeutic agents [50]. Commiphora (Burseraceae) species are often used in traditional medicine in southern Africa for various ailments, including malaria where the stem is used and stomach aches where the bark, resin and leaf are used. Investigations have shown that C. pyracanthoides essential oil extract has various biological properties including anti-inflammatory (5-LOX enzyme inhibition), anti-cancer (against HT-29, MCF-7 and SF-268 cell lines), antimicrobial (Bacillus cereus) and antioxidant activity (ABTS and DPPH assays) [51]. C. pyracanthoides was not cytotoxic against kidney epithelial cells, indicating that it has selective activity against cancer cells.
L. luteoalbum leaves and root extract exhibited excellent activity against NTS with both the roots and leaves showing similar activity against the parasite. The plant originates from Europe and was introduced to southern Africa by early settlers, and it is now widespread and known as a winter weed in maize lands [52]. The plant has shown antifungal activity and the compounds responsible for the antifungal activity did not show cytotoxic activity when tested against the Vero cell line [53]. Although no cytotoxic effects were observed against the Vero cell line, further toxicological studies need to be done to ensure the safety of the plant. This is because plants sometimes produce toxic secondary metabolites to act as defense compounds against pathogens and herbivores [54]. The promising activity against schistosomes makes L. luteoalbum an ideal candidate for further investigations as it is a weed, meaning it grows easily even in unfavorable conditions and obtaining adequate biomass would not be a challenge [55].
M. oleifera leaves exhibited the best anthelmintic activity against adult H. polygyrus and S. ratti L3 larvae. M. oleifera is a fast-growing woody plant whose seeds, leaves and flowers have broad spectrum therapeutic applications [56]. Among the biological properties investigated, the leaves have shown anthelmintic activity reducing the worm burdens of H. contortus, T. colubriforms and O. columbianum in goats [33]. The M. oleifera leaf extract has also exhibited anthelmintic properties against Trichuris sp. and Ostertagia sp. The bioactive compounds thought to be responsible for the anthelmintic activity are heneicosane, di(2-ethylhexyl)phthalate (as 1,2-benzenedicarboxylic acid in [34]), heptacosane pentatriacontane and hexadecanoic acid ethyl ester [34].
S. panduriforme fruits had the highest activity against adult T. muris and also shared the best activity against adult H. polygyrus with M. oleifera leaves. T. muris has low cure rates using benzimidazole drugs and therefore new approaches are needed to eliminate morbidity from trichuriasis [50]. Further investigations could be carried out on the fruits of S. panduriforme in order to isolate the active principle. S. panduriforme, C. imberbe and T. sericea are some of the plants used by traditional health practitioners in the treatment/management of HIV-related opportunistic infections in Ngamiland District in Northern Botswana [57]. S. panduriforme and T. sericea are also used in South Africa for the treatment of tuberculosis and a study carried out by Green et al. [58] showed that T. sericea bark extract (MIC 25 μg/mL) had better activity against Mycobacterium tuberculosis than S. panduriforme leaves, although S. panduriforme leaves showed activity at a concentration higher than 100 μg/mL. The roots of S. panduriforme are also used for the treatment of oral diseases [59] and the plant is used for skin infections, wounds and ulcers [56]. The leaves of S. panduriforme have antiplasmodial activity against Plasmodium falciparum, although Solanum nigrum not S. panduriforme is reported to be used against malaria in traditional medicine [60].
The roots, leaves and bark of T. sericea are widely used in South Africa to prepare remedies used in ethnoveterinary medicine for the treatment of wounds, ticks and diarrhea [61]. T. sericea is a multipurpose medicinal plant used to treat many ailments and the plant contains various biological activities including anti-HIV, antifungal, antibacterial, antiparasitic, anticancer, wound-healing, antioxidant and anti-inflammatory activity [30].
A. ferox is used in traditional medicine in South Africa to treat intestinal worm infections [62] and several studies have shown the in vitro and in vivo anthelmintic activity of the plant against H. contortus and H. gallinarum [26,27,28]. A. zebrina is used in southern Africa for the treatment of myiasis and in vitro studies have shown that the leaf extract reduces pupation rate and pupal mass of Lucilia cuprina and Chrysomya marginalis [63]. B. albitrunca is widely used as a medicinal plant in southern Africa. Its uses include the treatment of constipation, diarrhea and epilepsy. In Botswana, it is used for the treatment of skin diseases, haemorrhoids and in ethnoveterinary medicine [64]. Antibacterial and antifungal activities have been reported for leaf and fruit extracts of B. albitrunca [65,66]. C. mopane is a dominant tree occurring in the dry regions of southern Africa, and it is used in traditional medicine for the treatment of tapeworms [31], syphilis, dysentery, diarrhea, inflamed eyes [67] and is also used in ethnoveterinary medicine [68]. C. imberbe is widely used in Africa to treat bacterial infections [69], sexually transmitted infections [70] and also in ethnoveterinary medicine [71]. The leaf extract of the plant has shown in vitro biological activity against S. haematobium [32], and anti-inflammatory activity [72]. Isolated compounds were active against Mycobacterium fortuitum and Staphylococcus aureus [73].
Overall, the plant extracts showed the best activity against NTS and only the best four were tested against adult S. mansoni. The plant extracts also showed moderate activity against adult H. polygyrus and adult T. muris. Activity of the plant extracts against A. ceylanicum L3 and S. ratti L3 was reduced. N. americanus and H. polygyrus L3 were the worms for which the plant extracts had the least effect with activity ranging between 0.3 and 23.2%. The anthelmintic activity shown by the plant samples shows the importance of traditional medicine, as traditional health practitioners have knowledge about which plants to use against various ailments. Further investigations need to be carried out in order to identify the active principles in the plant extracts and validate the safety of the plant extracts through toxicological studies. Once the active principles are identified, then crude extracts can be standardized with identified marker molecules.
4. Materials and Methods
4.1. Study Site
The study was conducted in the Ngamiland District in the North-West of Botswana, which is the site of a unique ecosystem, the Okavango Delta, where over 95% of its inhabitants depend on wetland resources to sustain their livelihoods. It is furthermore a hotspot for biodiversity in Botswana, which attracts traditional health practitioners to procure and use medicinal plants from the area. Historically, S. mansoni transmission is known to occur in the Okavango Delta due to the abundance of Biomphalaria pfeifferi, the snail intermediate host for the parasite [74]. Many Okavango Delta inhabitants from poorer sections of the society, and in rural villages around the Okavango Delta, are subsistence farmers who are engaging in flood recession farming (‘Molapo-Farming’). This farming practice utilizes wetland flooding patterns for planting. Unfortunately, the risk of exposure of farmers to Schistosoma is high as flood waters bring the snails serving as vectors. Usually, whole families are involved in Molapo-Farming, which has led in the past to schistosomiasis in school students, which seriously affected their performance. Two sites in the Okavango served as sampling origins, the Ngamiland District capital, Maun, and the village of Sehithwa, 80 km from Maun (Figure 4). Both villages are home to traditional health practitioners who collaborated with us in this study.
Figure 4.
Map of Botswana showing areas where plants where collected (adapted from [74]).
4.2. Study Design, Data Collection Methods and Ethical Considerations
The study design followed an exploratory, mixed-methods approach [75,76] to solicit traditional medical knowledge and therefore was of qualitative nature. Three traditional health practitioners collaborated with us in this study, the late Mrs. Tshwanelo Seputhe, Mr. Nkaelang Seputhe, both from Maun, and Mrs. Tsholofelo Tiroyakgosi based in Sehithwa. All three health practitioners have collaborated with one of us (K. Andrae-Marobela) for over fifteen years and have demonstrated reliable, in-depth traditional medical knowledge. Both female traditional health practitioners were/are involved in Molapo-Farming contributing to their livelihood, which is representative of many Okavango Delta inhabitants [77]. Initial traditional medical knowledge about worm infections was shared by the health practitioners during an ethno-survey undertaken between 2008 and 2010 [78], which included in-depth interviews and informal discussions conducted after obtaining community consent and individual prior informed consent. We also used informal conversations to discover categories of meaning [79] during the celebrations of the African Traditional Medicine Day in 2013 in Toteng, Ngamiland District, Botswana, which was organized by the Ministry of Health, Botswana. These data were supplemented by recent (January 2019), subsequent, semi-structured and unstructured conversations with the three traditional health practitioners to obtain more detailed knowledge and to confirm previously generated data to enhance credibility. Though conversations were unstructured, the interviewer had a guideline in mind to focus on knowledge of worm infections and schistosomiasis, but the idea was to let respondents express themselves freely on their own terms [80]. Data were also collected through informal participant observations of traditional health practitioners outside of their homes while accompanying them during medicinal plant collection. These participant observations provided nuances of subjective meaning and valuable narratives of health practitioners’ experiences. Ethical approval and a research permit were granted by the Ministry of Infrastructure, Science & Technology, Botswana (Permit no.: ETH 5 (1)), and by the Ministry of Health (Permit no.: PPME: 13/18/1 Vol VIII (354); HPDME 13/18/1).
4.3. Plant Collection and Extract Preparation
The traditional medicinal plants investigated in this study were collected together with the traditional health practitioners to avoid misidentification. The plant species were taxonomically identified using the dichotomous key in Coates Palgrave and Ellery and Ellery [81,82] and authenticated species were deposited in the University of Botswana Herbarium. Correct botanical names were counterchecked using the WFO plant list (www.wfoplantlist.org). The plant samples were air dried indoors at room temperature and ground to a fine powder using a blender, and 1 g of each powdered sample was extracted by sonication for 15 min with 10 mL of 80% MeOH (methanol and water have proved to be the solvents with the highest extraction efficiency, Ref [83]) at room temperature. The resulting solutions were evaporated to dryness under reduced pressure using a rotary evaporator maintained at 40 °C to afford crude extracts. A total of 10 mg of each crude extract was tested at the Swiss Tropical and Public Health Institute (Swiss TPH) to investigate their anthelmintic activities.
4.4. Anthelmintic Assays
In vitro studies using parasitic helminths were carried out in accordance with Swiss national and cantonal regulations on animal welfare under the permission number 2070. The anthelmintic assays to test the activity of the plant extracts against A. ceylanicum (zoonotic hookworm), H. polygyrus (roundworm of rodents), N. americanus (New World hookworm), S. mansoni (blood fluke) [adult and newly transformed schistosomula (NTS)], S. ratti (threadworm) and T. muris (nematode parasite of mice) were carried out as described previously [84,85,86]. Three-week-old female NMRI mice were obtained from Charles River (Sulzfeld, Germany). Three-week-old female C57BL/6NRj mice and three-week-old male Syrian golden hamsters were purchased from Janvier Laboratories (Le Genest-Saint-Isle, France). Rodents were kept in types 3 and 4 macrolon cages under environmentally controlled conditions (temperature: 25 °C, humidity: 70%, light/dark cycle 12 h/12 h) and had free access to water (municipal tap water supply) and rodent food. Rodents were allowed to acclimatize for 1 week before infection. Statistical analysis (one way ANOVA, p ≤ 0.001) was performed using SigmaPlot 14.0.
4.4.1. In Vitro Tests on A. ceylanicum, H. polygyrus, N. americanus, S. ratti and T. muris
The life cycles of the assayed nematodes are maintained at the Swiss TPH. Hamsters were infected per os with 140 A. ceylanicum L3 or subcutaneously with 150 N. americanus L3. The feces of infected hamsters were filtered to obtain A. ceylanicum and N. americanus eggs which were then cultivated on an agar plate for 8–10 days in the dark at 24 °C to obtain larvae (L3), while mice were used to obtain H. polygyrus larvae (L3) following the same procedure. S. ratti L3 were acquired as summarized by Garcia and Bruckner [87]. For the drug assay, 30–40 L3 were placed in each well of a 96-well plate for each extract. Larvae were incubated in 198 μL culture medium with the test samples at a concentration of 100 μg/mL. RPMI 1640 (Gibco, Waltham MA, USA) medium supplemented with 5% amphotericin B (250 μg/mL, Sigma-Aldrich, Buchs, Switzerland) and 1% penicillin 10,000 U/mL and streptomycin 10 mg/mL solution (Sigma-Aldrich) was used for the assays with H. polygyrus L3. Phosphate-buffered saline (PBS, Sigma-Aldrich) supplemented with 1% penicillin (10,000 U/mL) and streptomycin (10 mg/mL) solution was used to incubate S. ratti L3. A. ceylanicum and N. americanus L3 stages were incubated in Hanks’ balanced salt solution (HBSS; Gibco, Waltham MA, USA) supplemented with 10% amphotericin B and 1% penicillin (10,000 U/mL) and streptomycin (10 mg/mL) solution. Larvae were kept in the dark at room temperature for 72 h, except A. ceylanicum, which were incubated at 37 °C and 5% CO2, after which the effect of the extract was evaluated. For this, the total number of L3 per well was determined. Then, 50–80 μL of hot water (≈80 °C) was added to each well and the larvae that responded (the moving worms) were counted. The proportion of larval death was determined and the percentage of survival was determined by the ratio of moving larvae to the total number of larvae present in the well. The N. americanus L3 assay was an exception as the wells were stimulated by vigorous up and down pipetting. The in vitro tests on adult H. polygyrus were carried out by first infecting female NMRI mice with 88 H. polygyrus L3. Mice were dissected two weeks post infection and three hookworm adult pairs were placed in each well of a 24-well plate at a volume 1980 μL and exposed to the test extracts at a concentration of 100 μg/mL. For the in vitro assay with T. muris adult worms, female C57BL/6NRj mice were infected with 200 embryonated T. muris eggs. Seven weeks post infection, T. muris adult worms were collected from the intestines. Three T. muris adult worms were placed in each well of a 24-well plate containing 1980 μL culture medium and the test extracts at a concentration of 100 μg/mL. The adult worms of H. polygyrus and T. muris were scored microscopically based on their phenotype, using a viability scale ranging from 3 to 0 (3: good motility and no morphological changes; 2: low motility and light changes in morphology; 1: very low motility and morphologically impaired; and 0: death). In case the adult worms did not move enough for a clear scoring, they were stimulated with hot water at the last evaluation time point. The reference compounds were abamectin 10 μM (for A. ceylanicum L3), levamisole 10 μM (for N. americanus L3, H. polygyrus L3, S. ratti L3) and tribendimidine 10 μM (for adult H. polygyrus and adult T. muris). The negative control was 1% DMSO.
4.4.2. In Vitro Tests Using S. mansoni
To obtain NTS, cercariae were collected from infected Biomphalaria glabrata snails (maintained at Swiss TPH) and were mechanically transformed. Briefly, 5–6 weeks post infection, infected snails were each placed in a single well of a 24-well plate. The snails were left under a neon lamp for 3–4 h. After removal of the snails, the plate was examined for cercariae. The cercariae were collected using a Pasteur pipette and the cercarial suspension was poured through a 100 μm filter into a 50 mL tube. The cercariae were transformed to NTS by placing 7 mL of cercarial suspension in a 10 mL syringe and connecting each syringe to a Luer Lok. After connecting another empty syringe to the opposite side of the Luer Lok, the liquid was pushed back and forth three to four times vigorously. The suspensions were then poured into 15 mL tubes and placed on ice in the dark for 7 min. The supernatants were removed and discarded by slowly pipetting, leaving the sedimented NTS. The NTS were kept in the incubator (37 °C and 5% CO2) in medium M199, supplemented with 5% FCS and 1% penicillin/streptomycin and 1% (v/v) antibacterial/antifungal solution39 until usage.
In order to obtain adult S. mansoni, cercariae were collected following the same steps mentioned above. The cercarial concentration was adjusted to 100 cercariae/100 μL and 100 μL aliquots were aspirated with a 1 mL syringe ensuring there were no air bubbles in the aspirates. The cercarial suspensions were then each injected subcutaneously into the necks of mice. After infection, the mice were kept at 25 °C with a 12 h day/night cycle. After 7 weeks post infection, mice were euthanized with CO2 for 5 min. The mesenteric veins of the infected mice were then dissected in order to collect adult S. mansoni worms. For adult S. mansoni and NTS, transparent flat-bottom 96- and 24-well plates were used, respectively (Sarstedt, Switzerland). A total of 30–40 NTS were incubated with the test extract (0.1, 1, 10, 50 and 100 μg/mL) in 198–199.8 μL of M199 medium (Gibco, New York, NY, USA) supplemented with 5% (v/v) FCS (Bioconcept AG, Allschwil, Switzerland), 1% (v/v) penicillin/streptomycin solution (Sigma–Aldrich, Buchs, Switzerland) and 1% (v/v) antibacterial/antifungal solution for up to 72 h at 37 °C and 5% CO2. The experiment was conducted in triplicate. For the adult S. mansoni assay, at least three worms (both sexes) were incubated in a final volume of 1980 μL–1998 μL RPMI 1640 supplemented with 5% (v/v) FCS and 1% (v/v) penicillin/streptomycin at 37 °C and 5% CO2 for 72 h and the test extract at 1, 10 and 50 μg/mL. The experiment was conducted in duplicate. Adult worms and NTS were judged via microscopic readout 72 h after incubation; they were scored according to motility, morphology and granularity (scores from 0 to 3) [84]. The reference compounds were auronofin 10 μM (for NTS) and praziquantel 10 μM (for adult S. mansoni). The negative control was 1% DMSO.
5. Conclusions
In this study, we report for the first time the use as anthelmintics and the in vitro anthelmintic activity of B. albitrunca, C. pyracanthoides and L. luteoalbum. We also report for the first time the in vitro anthelmintic activity of A. zebrine, T. sericea, C. mopane and S. panduriforme. The promising antischistosomal activity exhibited by C. pyracanthoides stem bark, as well as the leaves and roots of L. luteoalbum, warrant further investigation of the plants as potential sources of compounds with antischistosomal properties. The overall anthelmintic activity exhibited by the different plant species, especially C. pyracanthoides, require further investigations to identify the active anthelmintic principles and also to perform cytotoxicity studies to give evidence for the safety of the plants. Our investigation confirms the importance of indigenous knowledge and further interviews should be held with traditional health practitioners in order to tap into the vast knowledge of medicinal plants which they have. These plants could be promising leads for the discovery of much needed new therapeutic agents against soil-transmitted helminth infections and schistosomiasis. Additionally, the possibility of using the plants for veterinary applications may also be studied.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11212945/s1, Table S1: Anthelmintic activity against parasitic helminths; Table S2: Most active plants against S. mansoni and NTS; Table S3: Statistical table for one way ANOVA using Sigma Plot 14.0. ANOVA table for data in Figure 1; Table S4: Statistical table for one way ANOVA using Sigma Plot 14.0. ANOVA table for data in Figure 2; Table S5: Statistical table for one way ANOVA using Sigma Plot 14.0. ANOVA table for data in Figure 3.
Author Contributions
Conceptualization, K.A.-M., M.D. and N.A.; traditional medical knowledge for A. ferox, A. zebrina, T. sericea, C. mopane, B. albitrunca, N.S.; for L. luteoalbum, M. oleifera, T.T.; methodology, K.A.-M., M.D., B.R., B.S., C.H. and J.K.; validation, B.S.; formal analysis, M.D., K.A.-M., N.A. and J.K.; writing—original draft preparation, M.D., B.R. and K.A.-M.; writing—review and editing, K.A.-M., N.A., J.K. and P.I.; supervision, N.A. and P.I.; project administration, P.I.; funding acquisition, P.I. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the TRISUSTAIN project (https://trisustain.uni-halle.de, accessed on 1 February 2022) “Economic, ecological and therapeutic sustainability in the development of phytopharmaceuticals for Sub-Saharan Africa” funded by the Federal Ministry of Education and Research (BMBF grant number 01DG17008B) and the German Academic Exchange Service (DAAD grant number 57369155 and grant number 57566179). JK is grateful to the Swiss National Science Foundation for financial support (No: 320030_175585/1).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Ministry of Health Botswana (HPDME 13/18/1 Vol.X (380).
Informed Consent Statement
Prior informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the contribution of the traditional medical knowledge of Mme Tshwanelo Seputhe, who passed away before the compilation of this manuscript. K.A.-M. and B.R. dedicate this publication to her in loving memory. Furthermore, the authors are indebted to Lucie Moeller for administrative support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Herricks, J.R.; Hotez, P.J.; Wanga, V.; Coffeng, L.E.; Haagsma, J.A.; Basañez, M.G.; Buckle, G.; Budke, C.M.; Carabin, H.; Fèvre, E.M.; et al. The global burden of disease study 2013: What does it mean for the NTDs? PLoS Negl. Trop. Dis. 2017, 11, e0005424. [Google Scholar] [CrossRef] [PubMed]
- Ochola, E.A.; Karanja, D.M.S.; Elliott, S.J. The impact of Neglected Tropical Diseases (NTDs) on health and wellbeing in sub-Saharan Africa (SSA): A case study of Kenya. PLoS Negl. Trop. Dis. 2021, 15, e0009131. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Supports Botswana to Strengthen System Operations towards Malaria and Other Vector Borne Disease Elimination. Available online: https://www.afro.who.int/news/who-supports-botswana-strengthen-system-operations-towards-malaria-and-other-vector-borne (accessed on 23 June 2022).
- World Health Organization. Towards Eliminating Intestinal Worms in Primary School-Going Children in Botswana. Available online: https://www.afro.who.int/news/towards-eliminating-intestinal-worms-primary-school-going-children-botswana (accessed on 1 March 2022).
- Kokaliaris, C.; Garba, A.; Matuska, M.; Bronzan, R.N.; Colley, D.G.; Dorkenoo, A.M.; Ekpo, U.F.; Fleming, F.M.; French, M.D.; Kabore, A.; et al. Effect of preventive chemotherapy with praziquantel on schistosomiasis among school-aged children in sub-Saharan Africa: A spatiotemporal modelling study. Lancet Infect. Dis. 2022, 22, 136–149. [Google Scholar] [CrossRef]
- Chimbari, M.J.; Kalinda, C.; Siziba, N. Changing patterns of Schistosoma host snail population densities in Maun, Botswana. Afr. J. Aquat. Sci. 2020, 45, 493–499. [Google Scholar] [CrossRef]
- Appleton, C.C.; Ellery, W.N.; Byskov, J.; Mogkweetsinyana, S.S. Epidemic transmission of intestinal schistosomiasis in the seasonal part of the Okavango Delta, Botswana. Ann. Trop. Med. Parasitol. 2008, 102, 611–623. [Google Scholar] [CrossRef]
- Molehin, A.J.; McManus, D.P.; You, H. Vaccines for human schistosomiasis: Recent progress, new developments and future prospects. Int. J. Mol. Sci. 2022, 23, 2255. [Google Scholar] [CrossRef]
- Crellen, T.; Walker, M.; Lamberton, P.H.; Kabatereine, N.B.; Tukahebwa, E.M.; Cotton, J.A.; Webster, J.P. Reduced efficacy of praziquantel against Schistosoma mansoni is associated with multiple rounds of mass drug administration. Clin. Infect. Dis. 2016, 63, 1151–1159. [Google Scholar] [CrossRef]
- Redman, E.; Whitelaw, F.; Tait, A.; Burgess, C.; Bartley, Y.; Skuce, P.J.; Jackson, F.; Gilleard, J.S. The emergence of resistance to the benzimidazole anthlemintics in parasitic nematodes of livestock is characterised by multiple independent hard and soft selective sweeps. PLoS Negl. Trop. Dis. 2015, 9, e0003494. [Google Scholar] [CrossRef]
- Sharpton, T.J.; Combrink, L.; Arnold, H.K.; Gaulke, C.A.; Kent, M. Harnessing the gut microbiome in the fight against anthelminthic drug resistance. Curr. Opin. Microbiol. 2020, 53, 26–34. [Google Scholar] [CrossRef]
- Rashwan, N.; Bourguinat, C.; Keller, K.; Gunawardena, N.K.; de Silva, N.; Prichard, R. Isothermal diagnostic assays for monitoring single nucleotide polymorphisms in Necator americanus associated with benzimidazole drug resistance. PLoS Negl. Trop. Dis. 2016, 10, e0005113. [Google Scholar] [CrossRef]
- World Health Organization. WHO Traditional Medicine Strategy 2014–2023. Available online: https://apps.who.int/iris/rest/bitstreams/434690/retrieve (accessed on 23 June 2022).
- Andrae-Marobela, K.; Ngwenya, B.N.; Okatch, H.; Monyatsi, K.N.; Masizana-Katongo, A.; Muzila, M. An insight into patient management and health outcome monitoring by traditional healers in Botswana. J. Herb. Med. 2021, 29, 100462. [Google Scholar] [CrossRef]
- Okatch, H.; Andrae-Marobela, K.; Monyatsi, K.N.; Masizana-Katongo, A.; Ngwenya, B.N.; Muzila, M. Perceptions of safety and efficacy of traditional medicines by community members in Botswana. Public Health 2013, 6, 143–157. [Google Scholar] [CrossRef]
- Tietjen, I.; Ngwenya, B.N.; Fotso, G.; Williams, D.E.; Simonambango, S.; Ngadjui, B.T.; Andersen, R.J.; Brockman, M.A.; Brumme, Z.L.; Andrae-Marobela, K. The Croton megalobotrys Müll Arg. traditional medicine in HIV/AIDS management: Documentation of patient use, in vitro activation of latent HIV-1 provirus, and isolation of active phorbol esters. J. Ethnopharmacol. 2018, 211, 267–277. [Google Scholar] [CrossRef]
- Mhlongo, L.S.; Van Wyk, B.-E. Zulu medicinal ethnobotany: New records from the Amandawe area of KwaZulu-Natal, South Africa. S. Afr. J. Bot. 2019, 122, 266–290. [Google Scholar] [CrossRef]
- Odhiambo, G.O.; Musuva, R.M.; Odiere, M.R.; Mwinzi, P.N. Experiences and perspectives of community health workers from implementing treatment for schistosomiasis using the community directed intervention strategy in an informal settlement in Kisumu City, western Kenya. BMC Public Health 2016, 16, 986. [Google Scholar] [CrossRef]
- Bah, S.; Diallo, D.; Dembélé, S.; Paulsen, B.S. Ethnopharmacological survey of plants used for the treatment of schistosomiasis in Niono District, Mali. J. Ethnopharmacol. 2006, 105, 387–399. [Google Scholar] [CrossRef]
- Allan, L.A.; Kutima, H.L.; Muya, S.; Ayonga, D.; Yole, D. The efficacy of a herbal drug, Schitozim over praziquantel in the management of Schistosoma mansoni infection in BALB/c mice. J. Biol. Agric. Health Care 2014, 4, 77–87. [Google Scholar]
- Geary, T.G.; Sakanari, J.A.; Caffrey, C.R. Anthelmintic drug discovery: Into the future. J. Parasitol. 2015, 101, 125–133. [Google Scholar] [CrossRef]
- Geary, T.G.; Thompson, D.P. Development of antiparasitic drugs in the 21st century. Vet. Parasitol. 2003, 115, 167–184. [Google Scholar] [CrossRef]
- Zheng, W.; Thorne, N.; McKew, J.C. Phenotypic screens as a renewed approach for drug discovery. Drug Discov. Today 2013, 18, 1067–1073. [Google Scholar] [CrossRef]
- Jayawardene, K.L.T.D.; Palombo, E.A.; Boag, P.R. Natural products are a promising source for anthelmintic drug discovery. Biomolecules 2021, 11, 1457. [Google Scholar] [CrossRef]
- Liu, M.; Panda, S.K.; Luyten, W. Plant-based natural products for the discovery and development of novel anthelmintics againsts nematodes. Biomolecules 2020, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Laing, M.; Nsahlai, I. In vitro anthelmintic activity of crude extracts of selected medicinal plants against Haemonchus contortus from sheep. J. Helminthol. 2013, 87, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Maphosa, V.; Masika, P.J.; Bizimenyera, E.S.; Eloff, J.N. In-vitro anthelminthic activity of crude aqueous extracts of Aloe ferox, Leonotis leonurus and Elephantorrhiza elephantina against Haemonchus contortus. Trop. Anim. Health Prod. 2010, 42, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Mwale, M.; Masika, P.J. In vivo anthelmintic efficacy of Aloe ferox, Agave sisalana, and Gunnera perpensa in village chickens naturally infected with Heterakis gallinarum. Trop. Anim. Health Prod. 2015, 47, 131–138. [Google Scholar] [CrossRef]
- Bossard, E. La Médecine Traditionnelle au Centre et à l’ouest de l’Angola; Ministério da Ciênciae da Tecnologia: Lisboa, Portugal, 1996. [Google Scholar]
- Mongalo, N.I.; McGaw, L.J.; Segapelo, T.V.; Finnie, J.F.; Van Staden, J. Ethnobotany, phytochemistry, toxicology and pharmacological properties of Terminalia sericea Burch. ex DC. (Combretaceae)—A review. J. Ethnopharmacol. 2016, 194, 789–802. [Google Scholar] [CrossRef]
- Mashabane, L.G.; Wessels, D.C.J.; Potgieter, M.J. The utilisation of Colophospermum mopane by the Vatsonga in the Gazankulu region (eastern Northern Province, South Africa). S. Afr. J. Bot. 2001, 67, 199–205. [Google Scholar] [CrossRef]
- Aremu, A.O.; Finnie, J.F.; Van Staden, J. Potential of South African medicinal plants used as anthelmintics—Their efficacy, safety concerns and reappraisal of current screening methods. S. Afr. J. Bot. 2012, 82, 134–150. [Google Scholar] [CrossRef]
- Moyo, B.; Masika, P.J.; Muchenje, V. Effects of supplementing cross-bred Xhosa lop eared goats with Moringa oleifera Lam. on helminth load and corresponding body condition score, packed cell volume. Afr. J. Agric. Res. 2013, 8, 5327–5335. [Google Scholar]
- Pedraza-Hernández, J.; Elghandour, M.M.M.Y.; Khusro, A.; Salem, M.Z.M.; Camacho-Diaz, L.M.; Barbabosa-Pliego, A.; Salem, A.Z.M. Assessment on bioactive role of Moringa oleifera leaves as anthelmintic agent and improved growth performance in goats. Trop. Anim. Health Prod. 2021, 53, 318. [Google Scholar] [CrossRef]
- Reis, A.; Joaquim, J. Drug Interaction With Milk and the Relevance of Acidifying/Alkalizing Nature of Food. Clin. Ther. 2015, 37, e67–e68. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Butterweck, V.; Nahrstedt, A. What is the best strategy for preclinical testing of botanicals? A critical perspective. Planta Med. 2012, 78, 747–754. [Google Scholar] [CrossRef]
- Chen, B.; Wild, D.; Guha, R. PubChem as a source of polypharmacology. J. Chem. Inf. Model 2009, 49, 2044–2055. [Google Scholar] [CrossRef]
- Takenaka, T. Classical vs. reverse pharmacology in drug discovery. BJU Int. 2001, 88 (Suppl. S2), 7–10. [Google Scholar] [CrossRef]
- Heinrich, M. Ethnopharmacology in the 21st century—Grand challenges. Front. Pharmacol. 2010, 1, 8. [Google Scholar] [CrossRef]
- Aura, A.M.; OʼLeary, K.A.; Williamson, G.; Ojala, M.; Bailey, M.; Puupponen-Pimia, R.; Nuutila, A.M.; Oksman-Caldentey, K.M.; Poutanen, K. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. J. Agric. Food Chem. 2002, 50, 1725–1730. [Google Scholar] [CrossRef]
- Blaut, M.; Schoefer, L.; Braune, A. Transformation of flavonoids by intestinal microorganisms. Int. J. Vitam. Nutr. Res. 2003, 73, 79–87. [Google Scholar] [CrossRef]
- Griffiths, L.A.; Smith, G.E. Metabolism of myricetin and related compounds in the rat. Metabolite formation in vivo and by the intestinal microflora in vitro. Biochem. J. 1972, 130, 141–151. [Google Scholar] [CrossRef]
- Scalbert, A.; Morand, C.; Manach, C.; Remesy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [PubMed]
- van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies--methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Burfield, T.; Reekie, S.-L. Mosquitoes, malaria and essential oils. Int. J. Aromather. 2005, 15, 30–41. [Google Scholar] [CrossRef]
- Wagner, H. Synergy research: Approaching a new generation of phytopharmaceuticals. Fitoterapia 2011, 82, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Allescher, H.D. Multitarget therapy in functional dyspepsia. Phytomedicine 2006, 13 (Suppl. V), 1–130. [Google Scholar] [CrossRef]
- Partridge, F.A.; Bataille, C.J.R.; Forman, R.; Marriott, A.E.; Forde-Thomas, J.; Häberli, C.; Dinsdale, R.L.; O’Sullivan, J.D.B.; Willis, N.J.; Wynne, G.M.; et al. Structural Requirements for dihydrobenzoxazepinone anthelmintics: Actions against medically important and model parasites: Trichuris muris, Brugia malayi, Heligmosomoides polygyrus, and Schistosoma mansoni. ACS Infect. Dis. 2021, 7, 1260–1274. [Google Scholar] [CrossRef]
- Paraskeva, M.P.; van Vuuren, S.F.; van Zyl, R.L.; Davids, H.; Viljoen, A.M. The in vitro biological activity of selected South African Commiphora species. J. Ethnopharmacol. 2008, 119, 673–679. [Google Scholar] [CrossRef]
- Bromilow, C. Problem Plants and Alien Weeds of South Africa, 3rd ed.; Briza Publications: Pretoria, South Africa, 2010. [Google Scholar]
- Aderogba, M.A.; McGaw, L.J.; Bagla, V.P.; Eloff, J.N.; Abegaz, B.M. In vitro antifungal activity of the acetone extract and two isolated compounds from the weed, Pseudognaphalium luteoalbum. S. Afr. J. Bot. 2014, 94, 74–78. [Google Scholar] [CrossRef]
- Wittstock, U.; Gershenzon, J. Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr. Opin. Plant Biol. 2002, 5, 300–307. [Google Scholar] [CrossRef]
- Mdee, L.K.; Masoko, P.; Eloff, J.N. The activity of extracts of seven common invasive plant species on fungal phytopathogens. S. Afr. J. Bot. 2009, 75, 375–379. [Google Scholar] [CrossRef]
- Mahfuz, S.; Piao, X.S. Application of Moringa (Moringa oleifera) as natural feed supplement in poultry diets. Animals 2019, 9, 431. [Google Scholar] [CrossRef]
- Okatch, H.; Ngwenya, B.; Raletamo, K.M.; Andrae-Marobela, K. Determination of potentially toxic heavy metals in traditionally used medicinal plants for HIV/AIDS opportunistic infections in Ngamiland District in Northern Botswana. Anal. Chim. Acta 2012, 730, 42–48. [Google Scholar] [CrossRef]
- Green, E.; Samie, A.; Obi, C.L.; Bessong, P.O.; Ndip, R.N. Inhibitory properties of selected South African medicinal plants against Mycobacterium tuberculosis. J. Ethnopharmacol. 2010, 130, 151–157. [Google Scholar] [CrossRef]
- More, G.; Tshikalange, T.E.; Lall, N.; Botha, F.; Meyer, J.J.M. Antimicrobial activity of medicinal plants against oral microorganisms. J. Ethnopharmacol. 2008, 119, 473–477. [Google Scholar] [CrossRef]
- Prozesky, E.A.; Meyer, J.J.M.; Louw, A.I. In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected South African plants. J. Ethnopharmacol. 2001, 76, 239–245. [Google Scholar] [CrossRef]
- Selogatwe, K.M.; Asong, J.A.; Struwig, M.; Ndou, R.V.; Aremu, A.O. A review of ethnoveterinary knowledge, biological activities and secondary metabolites of medicinal woody plants used for managing animal health in South Africa. Vet. Sci. 2021, 8, 228. [Google Scholar] [CrossRef]
- Wintola, O.A.; Afolayan, A.J. An inventory of indigenous plants used as anthelmintics in Amathole district municipality of the Eastern Cape province, South Africa. Afr. J Tradit. Complement. Altern. Med. 2015, 12, 112–121. [Google Scholar] [CrossRef][Green Version]
- Mukandiwa, L.; McGaw, L.J.; Eloff, J.N.; Naidoo, V. Extracts of four plant species used traditionally to treat myiasis influence pupation rate, pupal mass and adult blowfly emergence of Lucilia cuprina and Chrysomya marginalis (Diptera: Calliphoridae). J. Ethnopharmacol. 2012, 143, 812–818. [Google Scholar] [CrossRef]
- Maroyi, A. Boscia albitrunca: Review of its botany, medicinal uses, phytochemistry, and biological activities. Asian J. Pharm. Clin. Res. 2019, 12, 51–56. [Google Scholar] [CrossRef]
- Pendota, S.C.; Aderogba, M.A.; Van Staden, J. In vitro antimicrobial activity of extracts and an isolated compound from Boscia albitrunca leaves. S. Afr. J. Bot. 2015, 96, 91–93. [Google Scholar] [CrossRef]
- Tshikalange, T.E.; Modishane, D.C.; Tabit, F.T. Antimicrobial, antioxidant, and cytotoxicity properties of selected wild edible fruits of traditional medicinal plants. J. Herbs Spices Med. Plants 2017, 23, 68–76. [Google Scholar] [CrossRef]
- Du, K.; De Mieri, M.; Neuburger, M.; Zietsman, P.C.; Marston, A.; Van Vuuren, S.F.; Ferreira, D.; Hamburger, M.; Van der Westhuizen, J.H. Labdane and clerodane diterpenoids from Colophospermum mopane. J. Nat. Prod. 2015, 78, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Syakalima, M.; Simuunza, M.; Zulu, V.C. Ethnoveterinary treatments for common cattle diseases in four districts of the Southern Province, Zambia. Vet. World 2018, 11, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Angeh, J.E.; Huang, X.; Sattler, I.; Swan, G.E.; Dahse, H.; Härtl, A.; Eloff, J.N. Antimicrobial and anti-inflammatory activity of four known and one new triterpenoid from Combretum imberbe (Combretaceae). J. Ethnopharmacol. 2007, 110, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Chinsembu, K.C. Ethnobotanical study of medicinal flora utilised by traditional healers in the management of sexually transmitted infections in Sesheke District, Western Province, Zambia. Rev. Bras. Farmacogn. 2016, 26, 268–274. [Google Scholar] [CrossRef]
- Chinsembu, K.C.; Negumbo, J.; Likando, M.; Mbangu, A. An ethnobotanical study of medicinal plants used to treat livestock diseases in Onayena and Katima Mulilo, Namibia. S. Afr. J. Bot. 2014, 94, 101–107. [Google Scholar] [CrossRef]
- McGaw, L.J.; Rabe, T.; Sparg, S.G.; Jäger, A.K.; Eloff, J.N.; van Staden, J. An investigation on the biological activity of Combretum species. J. Ethnopharmacol. 2001, 75, 45–50. [Google Scholar] [CrossRef]
- Katerere, D.R.; Gray, A.I.; Nash, R.J.; Waigh, R.D. Antimicrobial activity of pentacyclic triterpenes isolated from African Combretaceae. Phytochemistry 2003, 63, 81–88. [Google Scholar] [CrossRef]
- Available online: https://austria-forum.org/af/Geography/Africa/Botswana/Maps/Botswana (accessed on 7 March 2022).
- Creswell, J.W.; Clarke, V.L.P. Designing and Conducting Mixed Method Research, 2nd ed.; SAGE: London, UK, 2011. [Google Scholar]
- Creswell, J.W. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches, 2nd ed.; SAGE: London, UK, 2003. [Google Scholar]
- Ngwenya, B.N.; Thakadu, O.T.; Magole, L.; Chimbari, M.J. Memories of environmental change and local adaptations among molapo farming communities in the Okavango Delta, Botswana—A gender perspective. Acta Trop. 2017, 175, 31–41. [Google Scholar] [CrossRef]
- Andrae-Marobela, K.; Ngwenya, B.N.; Monyatsi, K.N.; Okatch, H.; Masizana, A.; Muzila, M. Documentation and promotion of indigenous knowledge-based solutions for Botswana—An ethnosurvey, CESRIKI Research Report. In Gaborone: Center for Scientific Research, Indigenous Knowledge and Innovation; University of Botswana: Gaborone, Botswana, 2010. [Google Scholar]
- Fetterman, D.M. Ethnography: Step by Step; Sage Publications: California, CA, USA, 1989. [Google Scholar]
- Bernard, H.R. Research Methods in Anthropology—Qualitative and Quantitative Approaches, 6th ed.; The Rowmann and Littlefield Publishing Group: Lanham, MD, USA, 2018. [Google Scholar]
- Coates Palgrave, K. Trees of Southern Africa, 3rd ed.; Struik Publishers: Capetown, South Africa, 1983; pp. 24–73, 214–286. [Google Scholar]
- Ellery, K.; Ellery, W. Plants of the Okavango Delta: A Field Guide; Tsaro Publishers: Durban, South Africa, 1997; pp. 34–49. [Google Scholar]
- Borges, A.; José, H.; Homem, V.; Simões, M. Comparison of Techniques and Solvents on the Antimicrobial and Antioxidant Potential of Extracts from Acacia dealbata and Olea europaea. Antibiotics 2020, 9, 48. [Google Scholar] [CrossRef]
- Lombardo, F.C.; Pasche, V.; Panic, G.; Endriss, Y.; Keiser, J. Life cycle maintenance and drug-sensitivity assays for early drug discovery in Schistosoma mansoni. Nat. Protoc. 2019, 14, 461–481. [Google Scholar] [CrossRef]
- Keiser, J.; Haeberli, C. Evaluation of commercially available anthelmintics in laboratory models of human intestinal nematode infections. ACS Infect. Dis. 2021, 7, 1177–1185. [Google Scholar] [CrossRef]
- Dube, M.; Saoud, M.; Rennert, R.; Fotso, G.W.; Andrae-Marobela, K.; Imming, P.; Häberli, C.; Keiser, J.; Arnold, N. Anthelmintic Activity and Cytotoxic Effects of Compounds Isolated from the Fruits of Ozoroa insignis Del. (Anacardiaceae). Biomolecules 2021, 11, 1893. [Google Scholar] [CrossRef]
- Garcia, L.S.; Bruckner, D.A. Diagnostic Medical Parasitology, 3rd ed.; ASM Press: Washington, DC, USA, 1997. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).