Abstract
SEPALLATA transcription factors (SEP TFs) have been extensively studied in angiosperms as pivotal components of virtually all the MADS-box tetrameric complex master regulators of floral organ identities. However, there are published reports that suggest that some SEP members also regulate earlier reproductive events, such as inflorescence meristem determinacy and inflorescence architecture, with potential for application in breeding programs in crops. The SEP subfamily underwent a quite complex pattern of duplications during the radiation of the angiosperms. Taking advantage of the many whole genomic sequences now available, we present a revised and expanded SEP phylogeny and link it to the known functions of previously characterized genes. This snapshot supports the evidence that the major SEP3 clade is highly specialized for the specification of the three innermost floral whorls, while its sister LOFSEP clade is functionally more versatile and has been recruited for diverse roles, such as the regulation of extra-floral bract formation and inflorescence determinacy and shape. This larger pool of angiosperm SEP genes confirms previous evidence that their evolution was driven by whole-genome duplications rather than small-scale duplication events. Our work may help to identify those SEP lineages that are the best candidates for the improvement of inflorescence traits, even in far distantly related crops.
1. Introduction
Several classes of MIKC-type MADS-box TFs are essential for the specification of all floral organs, as described by the ABC model [1]. They function by forming homo- or heterodimers that, based on the quartet model, further combine into tetramers [2,3]. Unique tetrameric combinations of MADS-box TFs specify the identities of each of the floral organs (sepal, petal, stamen, carpel) and ovules, as well as floral meristem determinacy. Although there are plenty of in vitro experiments supporting the quartet model, conclusive proof is still lacking in vivo, where, although most interactions have been confirmed [4], it has not been exactly confirmed that tetramers must form, such that it cannot be excluded that simple dimers might be functional at least in some of the target genes [5,6].
Among these classes of MADS-box TFs, the so-called SEPALLATA (SEP) is the only common component of all the known functional complexes and is thus essential for the identities of all floral organs [7,8,9]. Most plant genomes encode several SEP TFs, which are often functionally redundant; hence, single mutants may display no or only a slight phenotype. However, in the absence of SEP function, flowers lose determinacy and all their organs are reverted to leaf-like structures [10], thus suggesting that all floral organs are, indeed, modified leaves, as proposed by Goethe in 1790 [11]. Although the ABC model was derived from the observation of loss-of-function mutants in Arabidopsis thaliana and Antirrhinum majus and although the possible homology of perianth organs between core eudicots, monocots and other taxa is a highly debated topic [12,13,14], the model seems to largely hold for angiosperms, albeit with some variations ([15,16] and references within).
Most angiosperms produce flowers arranged in diversified clusters termed inflorescences [17,18], which are orchestrated by the inflorescence meristem (IM) and, eventually, by a subsequent hierarchical order of specialized reproductive meristems, such as the branch meristems (BMs) [19,20,21]. The relevant products of most crops and ornamental plants are their fruits and seeds or flowers, respectively. Therefore, the modification of inflorescence architecture is a major goal of breeding programs in crops and ornamental plants [22,23,24,25].
A few works conducted on distantly related angiosperms have shown that some SEP TFs have important roles not only in floral development but also in the regulation of IM function and/or of the other reproductive meristems that derive from it. For example, the SEP genes of tomato (Solanum lycopersicum) JOINTLESS-2 and ENHANCER-OF-JOINTLESS-2 (J2 and EJ2) are two important domestication loci for jointless pedicel and large calyx traits, respectively, but are also important regulators of inflorescence complexity and productivity [26,27]. The loss of OsMADS34/PANICLE PHYTOMER2 (PAP2) function profoundly alters inflorescence development and architecture in rice (Oryza sativa) [28,29,30]. Similar SEP genes have been shown to regulate IM function and determinacy even in the highly modified and specialized capitulum inflorescence of Asteraceae [31].
Within MIKC-type MADS-box genes, SEP forms a well-defined subfamily specific to and ubiquitous in angiosperm plants. It is divided into two major sister clades, SEP3 (AGL9) and LOFSEP (AGL2/3/4) [32,33], whose split coincided with the whole-genome duplication ‘Epsilon’ (WGD-ε) that predated the most recent common ancestor (MRCA) of angiosperms [34,35].
Thanks to the incessant advances in DNA sequencing techniques, thousands of high-quality genomes, and even some pangenomes, are now available for most angiosperm clades, with some agriculturally important families, such as Poaceae in monocots and Solanaceae, Asteraceae, Rosaceae, Cucurbitaceae, Fabaceae and Brassicaceae in core eudicots, being particularly well-represented. The use of annotated high-quality genomes allows more precise assessment of the real numbers of orthologous genes in each species and better tracing of their patterns of duplication, loss and retention and in- and out-paralogous relationships throughout different plant taxa. The MADS-box genes encoding for subunits of tetrameric complexes are supposed to be needed in relatively strict stoichiometric ratios, which might explain why they mostly duplicate by WGDs, while copies originated from segmental or single-gene duplications are preferentially lost ([36] and references therein). This makes it effective to support their sequence-based phylogenies also by studying microsynteny, that is, the conservation of local gene content and order. Despite microsynteny having rarely been studied in plant MADS-box genes so far, such analyses have already contributed substantially to the reconstruction of their evolution and expansion in angiosperms [35,37,38].
Here, we present an updated analysis of SEP subfamily evolution in core eudicots and core monocots (i.e., Petrosaviidae sensu Cantino et al., 2007 [39,40]), including taxa that were previously poorly or not covered by whole genomic and transcriptomic sequencing data. In conjunction with available and future functional data, this phylogenomic snapshot helps to correlate specific SEP lineages with sub- and neo-functionalization processes associated with inflorescence and floral functions in non-model species and crops.
2. Results and Discussion
2.1. Evolution of the SEPALLATA Subfamily in Core Monocots
To better understand the evolution and complexity of the SEP subfamily in core monocots, we took advantage of high-quality genome assemblies currently available from Poales, other commelinids and a few Asparagales (orchids, Asparagus officinalis and Allium cepa), the remaining taxonomic orders being still poorly or not represented. All the SEP gene models that we retrieved from these monocots, as well as those from core eudicots and Amborella trichopoda, had eight exons and seven introns. The few exceptions were clearly due to incomplete or incorrect annotations, showing that the SEP gene structure is highly conserved across angiosperms. By comparison with the protein structure of Arabidopsis SEP3 TF [3], we determined that, in all the SEP genes that we studied, the MADS-box domain is encoded by exon 1, the I (intervening) domain by exon 2, the K (keratin-like) domain by exons 3 to 6, and the less conserved C-terminal region by exons 7 and 8 (data not shown).
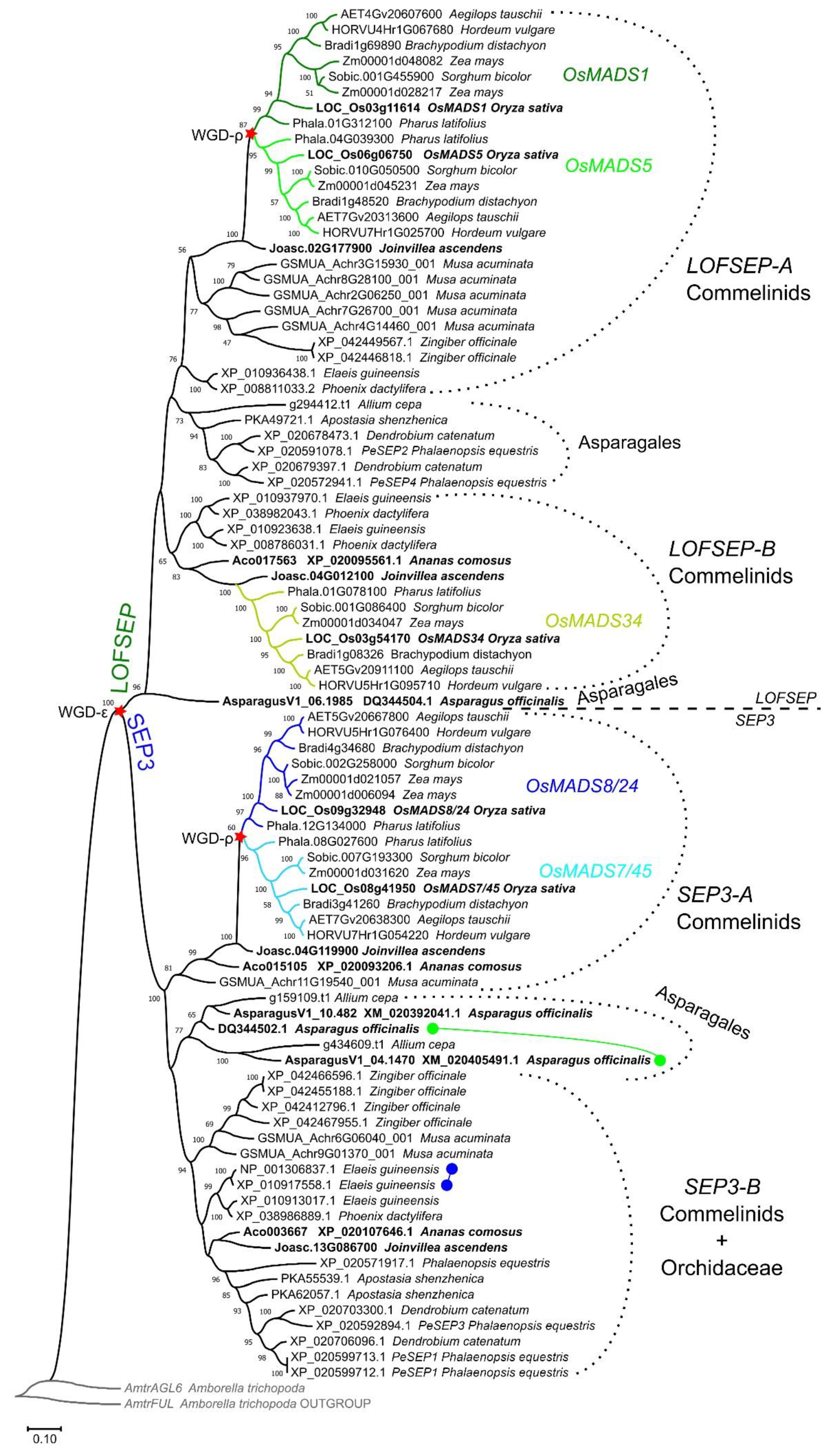
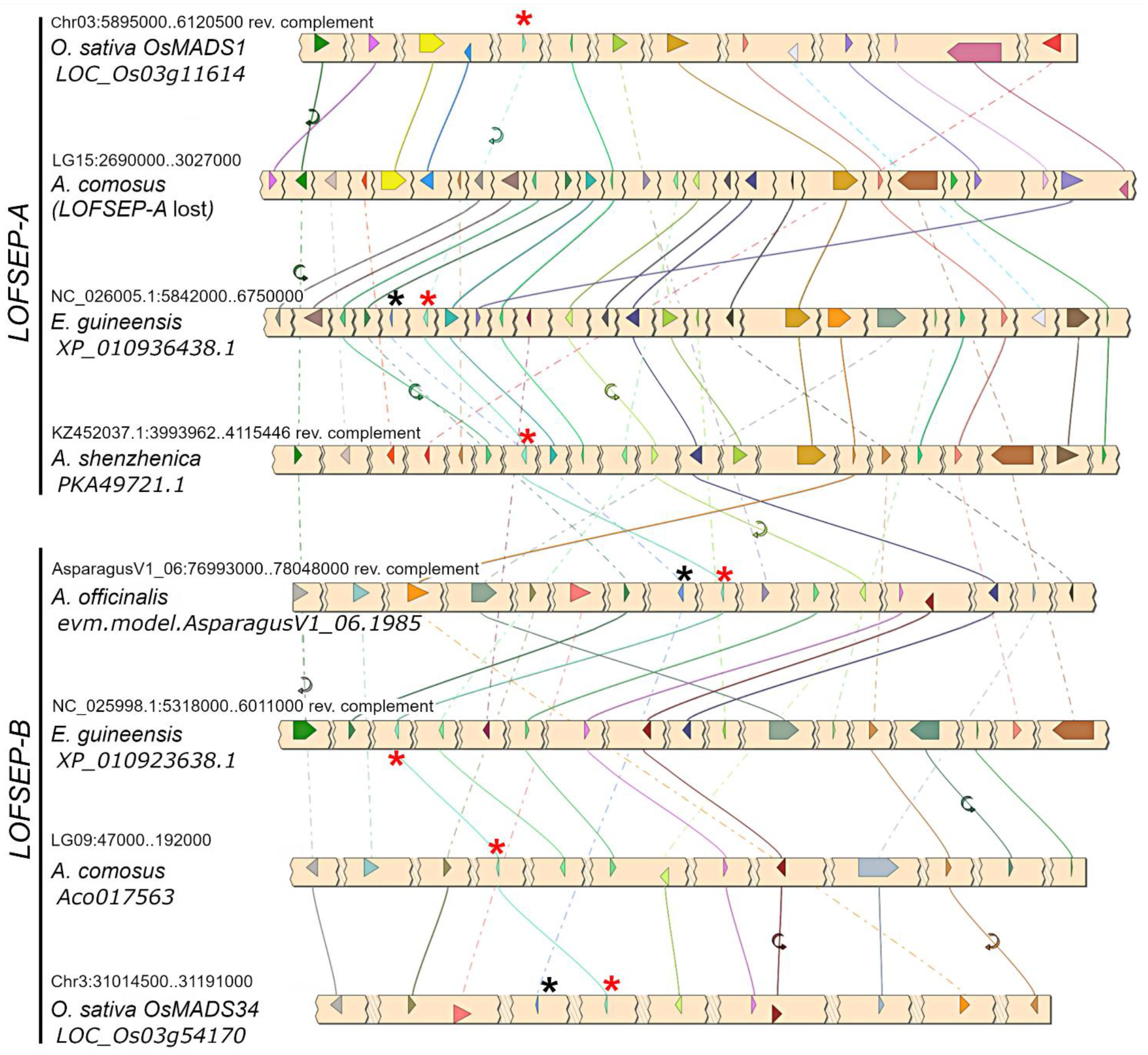
A phylogenetic analysis revealed that LOFSEP formed two large subgroups in commelinid monocots, which we refer to as LOFSEP-A and LOFSEP-B hereafter (Figure 1). Grasses, Joinvillea ascendens (sister to grasses) and palms possessed genes from both clades. The result was supported by microsynteny analysis of representative species (Figure 2), which also compensated for the low bootstrap values in the phylogenetic tree for the palm species Elaeis guineensis and Phoenix dactylifera. As shown in Figure 2, a strong microsynteny is common to each group, A and B, of LOFSEP genes. Interestingly, a lower degree of microsynteny is also shared between A and B, indicating that they originated by an ancient large-scale or whole-genome duplication. Such an event was most likely the ancient WGD-τ that took place before the MRCA of core monocots [41,42]. Although the positions of sequences from Asparagales were unresolved in the phylogenetic tree (Figure 1), the analysis of microsynteny allowed us to assign the LOFSEP sequences of orchids to group A and an orphan gene of Asparagus officinalis (06.1985; Figure 1) to group B (Figure 2).
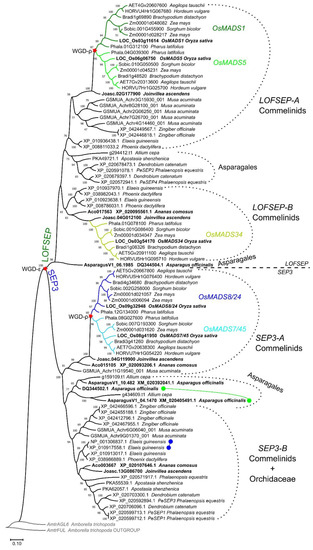
Figure 1.
ML phylogenetic analysis of the SEPALLATA (SEP) subfamily genes from the core monocots commelinids and Asparagales. Dichotomies unequivocally linked to the angiosperm WGD-ε and the grass WGD-ρ events are marked with a red star. The three LOFSEP subclades of grasses, OsMADS1, OsMADS5 and OsMADS34, are marked with different shades of green. The two SEP3 subclades of grasses, OsMADS7/45 and OsMADS8/24, are marked with different shades of blue. Two tandem duplication events of SEP3 genes were detected in Asparagus officinalis and in Elaeis guineensis, which are marked with green and blue connected circles, respectively.
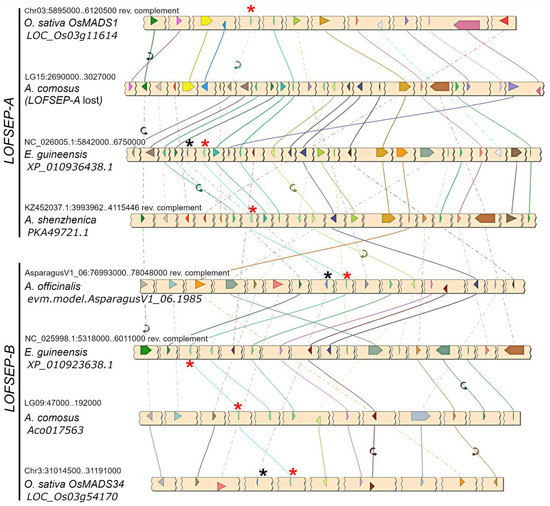
Figure 2.
Microsynteny analysis of LOFSEP genes from representative species of commelinids and Asparagales. Conserved loci are connected by lines of the same color. For simplicity, the non-conserved loci were omitted. In each chromosomal region, the LOFSEP locus is marked with a red asterisk. The LOFSEP-A gene is lost in the conserved region of Ananas comosus, in agreement with the phylogenetic analysis shown in Figure 1. The linked SQUA locus, when present, is marked with a black asterisk.
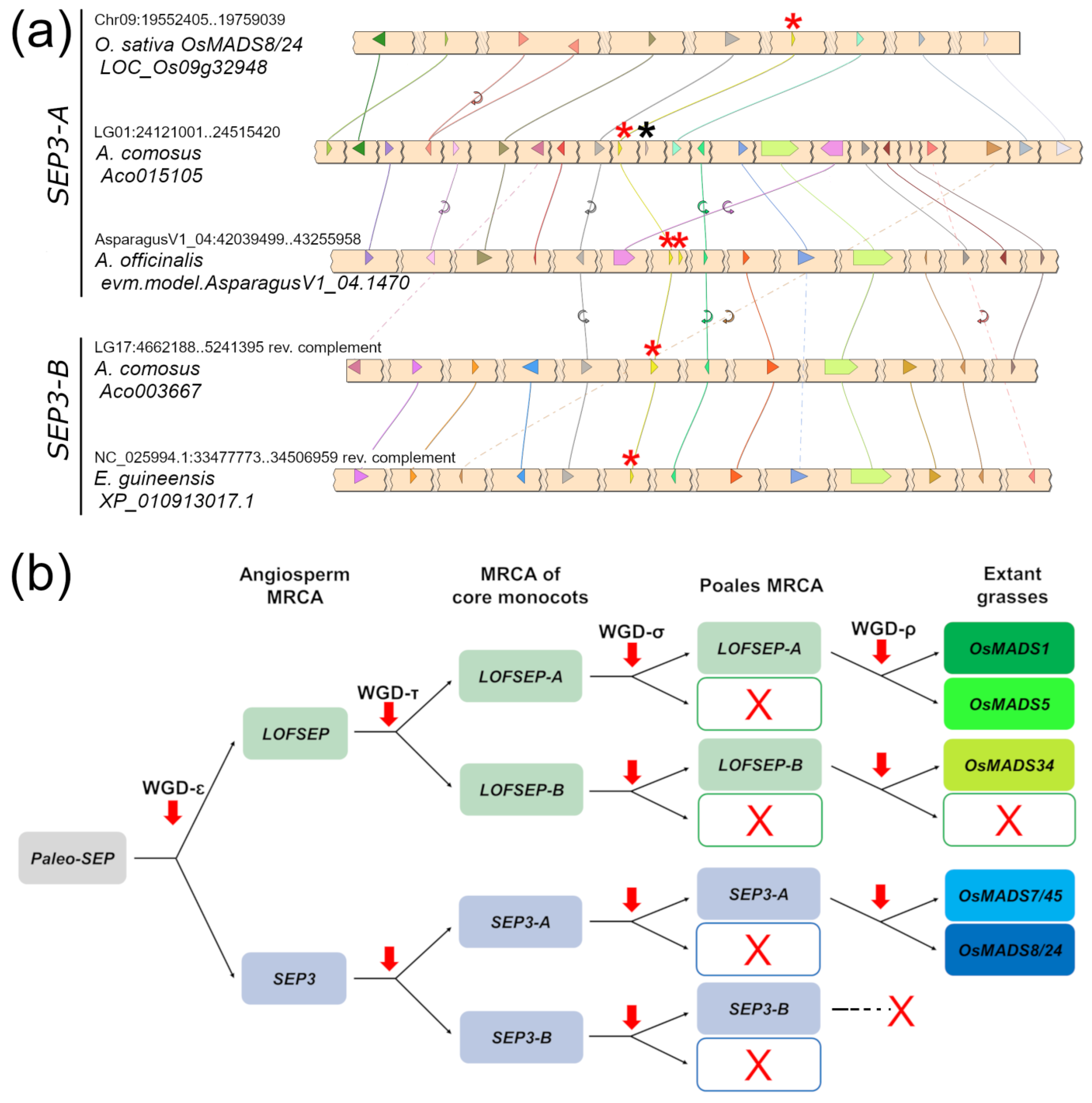
A very similar picture emerged from the analysis of the SEP3 clade, which was also separated into two large ‘A’ and ‘B’ groups (Figure 1). In this case, all the genes from grasses fell in the SEP3-A group, suggesting that grasses lost SEP3-B after their divergence from Joinvillea ascendens, while all orchid genes clustered strongly with several other genes of commelinids to form the SEP3-B group. Pineapple (Ananas comosus), Joinvillea ascendens and banana (Musa acuminata) possessed genes from both clades (Figure 1). Microsynteny results further supported the existence of the two groups (Figure 3). The small unresolved clade of five Allium cepa and Asparagus officinalis genes (Figure 1) likely belong to SEP3-A, based on microsynteny scores (Figure 3a). As exceptions to the evidence that single-gene duplications of MADS-box genes are rapidly lost, we found tandem duplications of SEP3 genes in Asparagus and Elaeis (Figure 1).
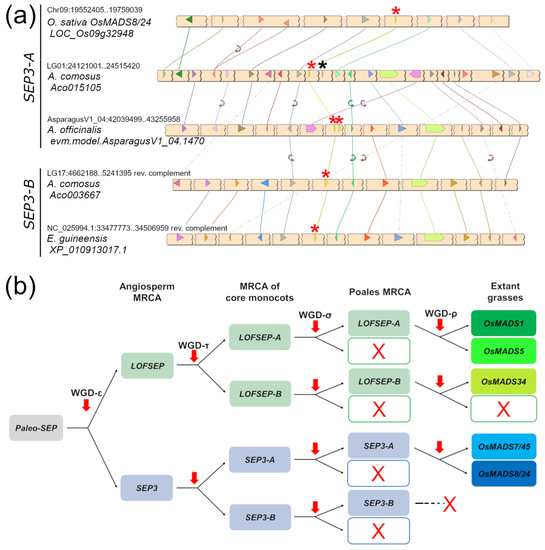
Figure 3.
Evolutionary analysis of the SEPALLATA (SEP) subfamily in core monocots. (a) Microsynteny analysis of SEP3 genes from representative species of commelinids and Asparagales. Conserved loci are connected by lines of the same color. For simplicity, the non-conserved loci were omitted. In each chromosomal region, the SEP3 locus is marked with a red asterisk. The linked FLC locus, when present, is marked with a black asterisk. (b) Representation of the most likely pattern that drove the evolution of the SEP subfamily in extant grasses (Poaceae), based on our analysis and previous works. Based on the phylogeny results shown in Figure 1, the grass lineage lost SEP3-B after its divergence from the sister family Joinvilleaceae.
In conclusion, core monocots are characterized by four main groups of SEP genes: LOFSEP-A, LOFSEP-B, SEP3-A and SEP3-B, which, however, have been differentially retained throughout their radiation. Among the species that we analyzed, only Joinvillea ascendens (Poales, Joinvilleaceae) possessed member genes from all four clades (Figure 1).
Deciphering the Evolution of SEPALLATA Genes along the Lineage That Led to Grasses
Since the phylogenomic data suggested that an early duplication of both LOFSEP and SEP3 occurred in monocots, we sought to reconstruct the subsequent evolutionary path of these genes in Poaceae, the family of true grasses and cereals.
Two more rounds of WGD occurred in the MRCA of Poales (WGD-σ) and then in the MRCA of Poaceae (WGD-ρ) [43], which would predict up to eight SEP3 and eight LOFSEP genes in extant diploid grasses, such as rice, barley (Hordeum vulgare), Brachypodium distachyon, Sorghum bicolor and Pharus latifolius. Instead, only two SEP3 (OsMADS7/45 and OsMADS8/24) and three LOFSEP (OsMADS1, OsMADS5 and OsMADS34) paralogous lineages have been maintained in grasses, respectively (Figure 1, Table 1), which we named after their corresponding genes in rice [44]. These five lineages are highly conserved in diploid grasses (Figure 1, Table 1). Comparison of the relatively ancient allotetraploid maize (Zea mays; [45,46,47]) versus the recent allohexaploid bread wheat (Triticum aestivum; [48]) gives clues as to the speed of the process of selection of SEP homeologous genes after a polyploidization event: while only two out of five duplicated copies have been retained in maize, three homeologs for each gene still exist in bread wheat (Table 1). In addition, atypical local duplications of the OsMADS1- and OsMADS5-like genes were found in the Aegilops–Triticum complex (Table 1), whose existence and functionality are mostly supported by transcriptome assemblies publicly available in NCBI GenBank (data not shown). Therefore, these two well-studied polyploid genomes suggest that the elimination of excessive SEP homeologous genes is quite a long process. In recent polyploids, processes of pseudogenization and epigenetic silencing are likely to take place beforehand [49].

Table 1.
Accessions of all the LOFSEP and SEP3 loci found in the diploid genomes of Oryza sativa (rice), Pharus latifolius, Brachypodium distachyon, Hordeum vulgare (barley), Aegilops tauschii and Sorghum bicolor, in the ancient allotetraploid Zea mays (corn) and in the recent allohexaploid Triticum aestivum (bread wheat).
Grasses are devoid of SEP3-B genes, while their highly homologous OsMADS7/45 and OsMADS8/24 paralogous lineages seem to have emerged by duplication of SEP3-A after their divergence from Joinvilleaceae (Figure 1), suggesting that such duplication coincided with the grass-specific WGD-ρ. This is strongly supported by the observation that the OsMADS7/45 and OsMADS8/24 lineages reside in highly syntenic chromosomes, as can be seen, for example, in synteny maps of rice and barley [50]. Based on our phylogeny results (Figure 1), the origins of the OsMADS1 and OsMADS5 paralogous lineages were likely the same; however, they are located on unrelated chromosomes, and OsMADS5 even lost the microsynteny shared by the other monocot LOFSEP genes (data not shown). This suggests that either OsMADS5 transposed or that major rearrangements of its genomic position occurred in the grass MRCA.
The third and more functionally diverged LOFSEP clade of grasses is OsMADS34, which was believed to exist only in grasses up to now. However, our analysis clarified that it belongs to the LOFSEP-B lineage (Figure 1 and Figure 2), meaning that it very likely diverged from its out-paralogues OsMADS1 and OsMADS5 at the time of the ancient WGD-τ, which occurred before the MRCA of core monocots [41,42]. Since rice OsMADS34/PAP2 is an important regulator of inflorescence architecture [28,29,30,51], this interesting and non-canonical SEP function might exist also in its orthologues within and outside grasses—a matter that requires further research.
Taken together, our data support a precise pattern of SEP subfamily evolution and expansion in grasses, which is summarized in Figure 3b, where the Poales-specific WGD- σ made no contribution.
In general, the rate of sequence divergence seems to be much higher in LOFSEP than in SEP3 TFs, which could already be noticed by comparing the homeologous peptides encoded by bread wheat A, B and D sub-genomes. The SEP3-like homeologous peptides accumulated just 0–4 aminoacidic changes only in the C-terminus (Figures S1–S5), suggesting that SEP3 is under stronger selective constraints.
2.2. Three LOFSEP Sister Clades and a Single SEP3 Clade Evolved in Core Eudicots
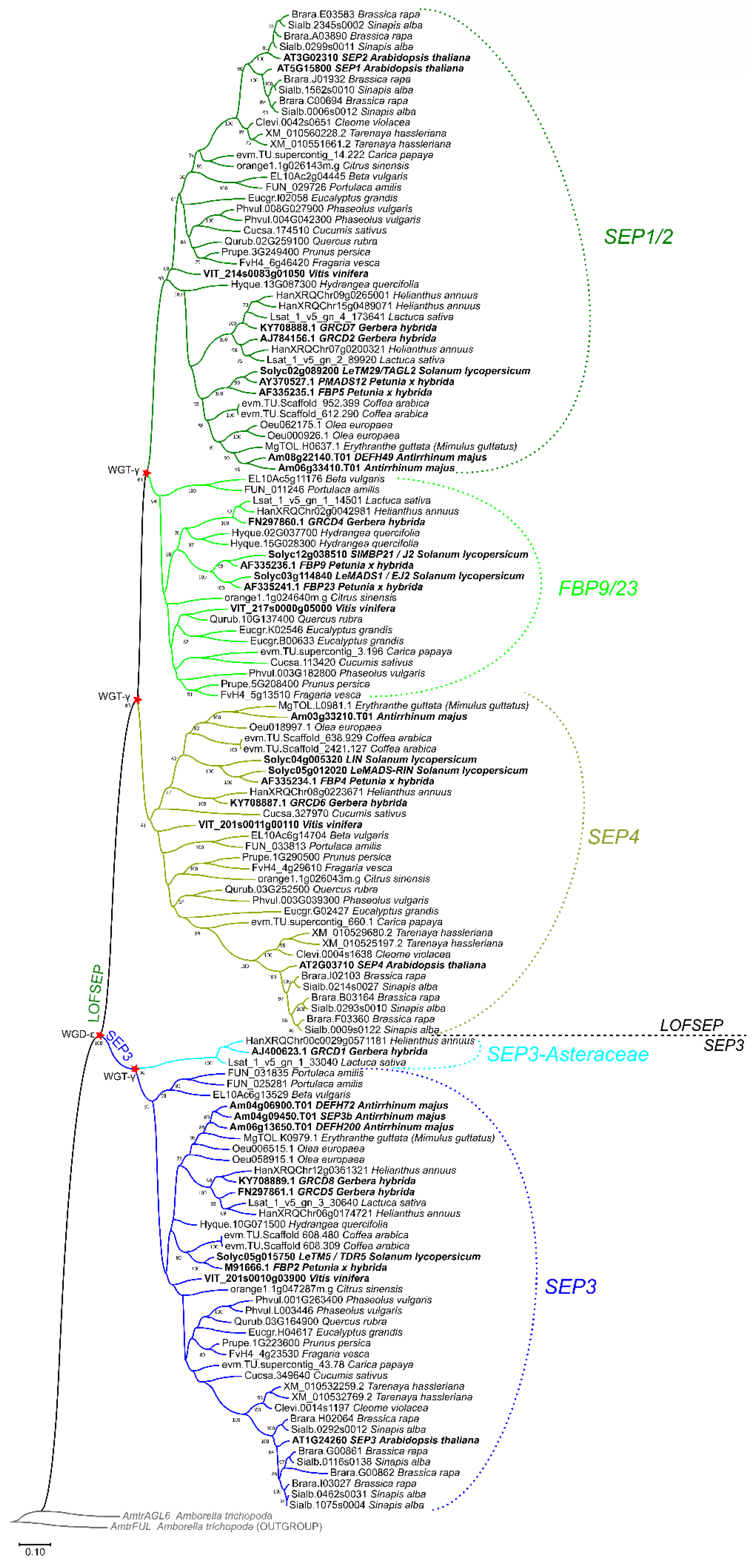
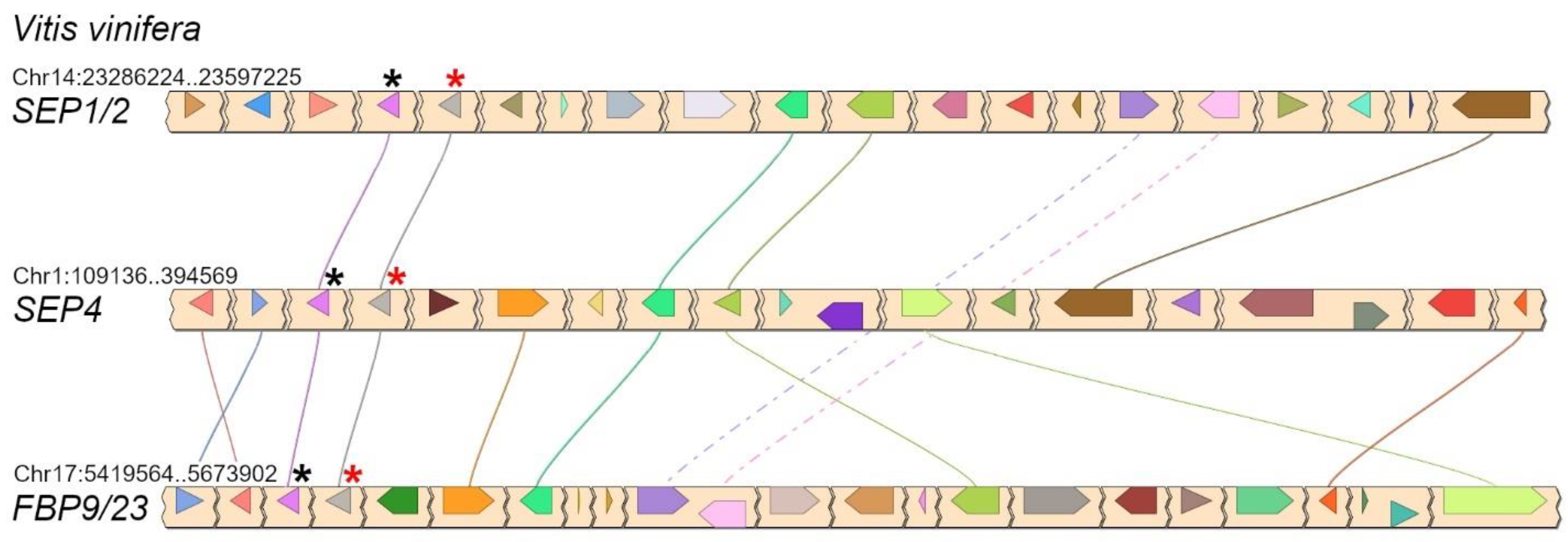
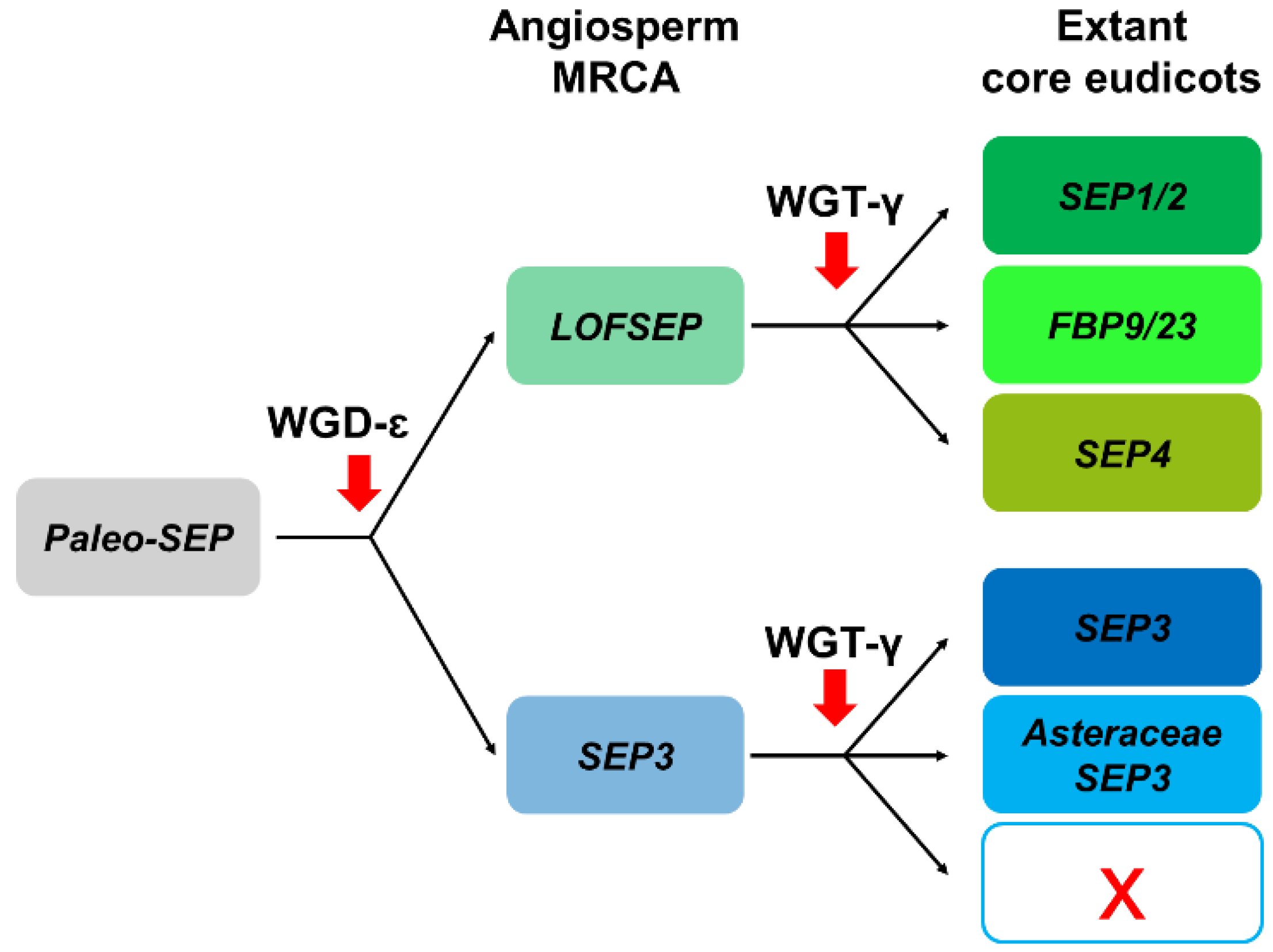
In core eudicots, our analysis confirmed with strong support the existence of three highly conserved LOFSEP clades (Figure 4), which we named SEP1/2, FBP9/23 and SEP4, in agreement with a previous work [32]. Since all extant core eudicots are descendants of a hexaploid MRCA, such expansion of the LOFSEP lineage is likely related to the ancestral whole-genome triplication event known as gamma (WGT-γ) [52,53,54,55,56]. Indeed, our analysis of grape (Vitis vinifera), a model for the study of genome evolution in core eudicots [34,55], revealed that the genomic regions of SEP1/2, FBP9/23 and SEP4 share significant collinearity with each other (Figure 5), in agreement with previous models of the origin of angiosperm-specific MADS-box subfamilies [35]. The FBP9/23 clade was lost in Brassicales after the divergence from Carica papaya and, probably, also in coffee (Coffea arabica) and Lamiales (which are represented by Erythranthe guttata, Antirrhinum majus and Olea europaea in our analysis) (Figure 4). Three MADS-box genes involved in inflorescence complexity in tomato, J2, EJ2 and LONG INFLORESCENCE (LIN), have been reported as SEP4 homologues [26]. However, our phylogenetic analysis unambiguously placed J2 and EJ2 in the FBP9/23 clade, while only LIN and its close homolog RIPENING INHIBITOR (RIN/LeMADS-RIN; [57]) belonged to the SEP4 clade (Figure 4).
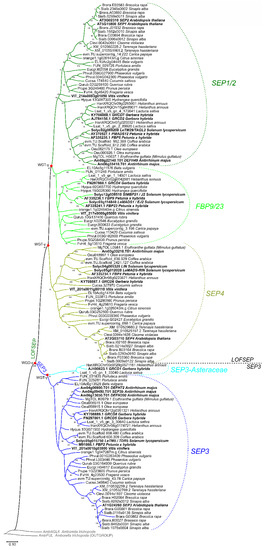
Figure 4.
ML phylogenetic analysis of the SEPALLATA (SEP) subfamily genes in core eudicots. Dichotomies unequivocally linked to the angiosperm WGD-ε and the core eudicot WGT-γ events are marked with a red star. The three LOFSEP subclades of core eudicots, SEP1/2, FBP9/23 and SEP4, are marked with different shades of green. The main SEP3 subclade and the Asteraceae-specific SEP3 clade are marked with different shades of blue.
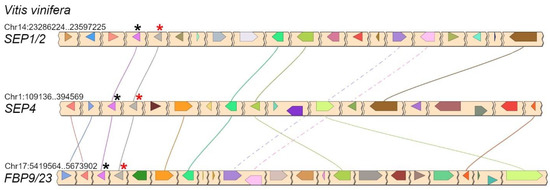
Figure 5.
Conserved microsynteny between the three LOFSEP subclades of core eudicots, visualized in grape (Vitis vinifera L.). In each chromosomal region, the linked LOFSEP and SQUA loci are marked with red and black asterisks, respectively. They form the three tandems SEP1/2–FL, SEP4–EuAP1 and FBP9/23–EuFUL [35].
In striking contrast, we only found one conserved monophyletic SEP3 group in core eudicots, except for an Asteraceae-specific clade (Figure 4) that was already reported by Malcomber and Kellogg [32]. In the Vitis vinifera genome, the only SEP3 locus resides on chromosome 1, orthologous to the whole core eudicot clade. As expected, we have identified two other microsyntenic regions in grape that derived from WGT-γ, on chromosomes 14 and 17 (data not shown), but these have lost their ancestral SEP3 copies. Intriguingly, the genomic location of the Asteraceae-specific SEP3 clade corresponds to the microsyntenic region of grape chromosome 17 (data not shown), revealing that this lineage has ancient origins related to WGT-γ. Considering the evolutionary position of Asteraceae, this implies that recurrent independent losses of this paralogous clade occurred a surprising number of times throughout the radiation of extant core eudicots.
In conclusion, the LOFSEP clade is significantly more expanded than SEP3 in core eudicots, and those genomes that experienced only WGT-γ are predicted to possess a 3:1 ratio of LOFSEP to SEP3 genes (Figure 4 and Figure 6), while further cycles of polyploidizations and gene losses occurred repeatedly and independently in the majority of core eudicot lineages. Our analysis supports the model of SEP subfamily expansion in core eudicots represented in Figure 6.
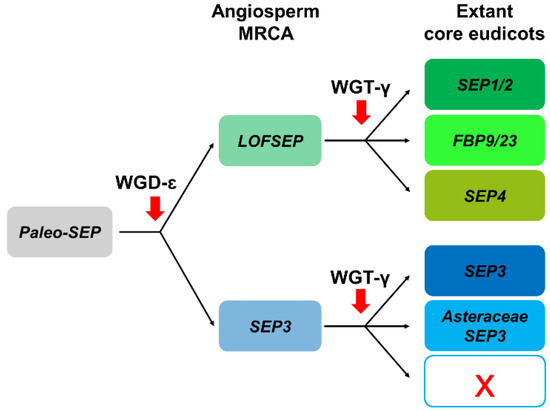
Figure 6.
Representation of the most likely pattern that drove the evolution of the SEPALLATA (SEP) subfamily in extant core eudicots, based on our analysis and previous works.
2.3. Conserved Genetic Linkage between SEPALLATA, SQUAMOSA and FLOWERING LOCUS C Subfamilies
Phylogenomic reconstructions showed that, in the angiosperm MRCA, the ancestral LOFSEP and SQUAMOSA (SQUA) genes formed a close tandem, while the ancestral SEP3 was in tandem with FLOWERING LOCUS C (FLC), and that this configuration has been maintained in many extant angiosperms [35]. In core eudicots, SQUA underwent a process of triplication just as LOFSEP did, which led to three paralogous LOFSEP–SQUA tandems, as clearly shown in the grape genome (Figure 5). All these LOFSEP–SQUA and SEP3–FLC linkage relationships were lost in the lineage of Arabidopsis and other Brassicaceae.
While noticing several such tandems during our analyses of monocots (Figure 2 and Figure 3a and additional data not shown), we found that only one LOFSEP–SQUA tandem, i.e., OsMADS34–OsMADS14, is still conserved in rice (Figure 2), with an intergenic space of just 6 kb, and in other grasses. These two loci act synergistically in floral induction in rice [58], and the latter regulates vernalization-induced flowering in winter cereal crops [59]. An SEP3–FLC tandem is also conserved in rice genomes: OsMADS7/45–OsMADS37 [35,44]. More generally, both LOFSEP and SQUA genes play pivotal and diversified roles in agronomically relevant traits, such as floral induction, vernalization, inflorescence architecture and flower and fruit development. Targeted gene modifications, selection of natural or mutagenesis-induced variants and functional characterizations must be carried out with awareness of these conserved genetic linkage groups, which also hint at possible coregulation mechanisms. In tomato, the misinterpretation of the classic rin (ripening inhibitor; [60]) mutant led to models depicting the SEP4 ortholog RIN (Figure 4) as indispensable to the induction of fruit ripening. Unexpectedly, however, rin is not a knock-out but a gain-of-function mutant encoding a chimeric protein from RIN and from the downstream SQUA gene Macrocalyx (MC), whose new properties as a transcriptional repressor actively repress ripening: RIN, indeed, is not indispensable to the induction of fruit ripening, being only required for the completion of normal ripening [61].
2.4. Patterns of Sub- and neo-Functionalization Associated with Diverged SEPALLATA Lineages
Our analysis provides new insights into the evolutionary history of the SEP subfamily in core monocots and core eudicots. Inferred polyploidization events at the base of both lineages caused a first round of independent amplifications of LOFSEP and SEP3 genes, followed by many others throughout the radiation of these angiosperms. The resulting duplicated genes followed different paths of retention and loss in different taxa. In addition, SEP genes seem to have diverged significantly between commelinids and Asparagales, and even within Asparagales. Here, we were able to bypass the limits of phylogenetic analysis by analyzing microsynteny.
An increasing number of functional studies are clarifying that the concept of full redundancy is misleading and that the several LOFSEP and SEP3 subclades that we have defined are instead specialized to regulate specific functions. Arabidopsis has only one SEP3 gene (Figure 4), which is highly redundant, along with the LOFSEP genes SEP1 and SEP2, in conferring FM determinacy and the identities of the three inner floral whorls. The Arabidopsis sep1 sep2 sep3 triple mutant produces indeterminate flowers made only of sepals [7]. SEP3 in not expressed at early developmental stages in the first whorl domain [62], where the last LOFSEP member of Arabidopsis, SEP4, is expressed instead [10]. In the Arabidopsis sep1 sep2 sep3 sep4 quadruple mutant all the floral organs are converted to leaves, showing that SEP4 alone is sufficient to specify sepal identity in the sep1 sep2 sep3 triple-mutant background [10]. Despite the significant degree of redundancy shown under experimental conditions, mass spectrometry analysis of in vivo formed complexes showed that SEP3 is far more abundant than SEP1 and SEP2 in the petal, stamen and carpel identity MADS-box complexes of Arabidopsis, while SEP4 is absent [4]. Moreover, the transcriptional activation potential of SEP3 exceeds those of SEP1 and SEP2 [63]. Altogether, these data point to SEP3 as the most important SEP TF for floral identity in Arabidopsis. Unfortunately, only partial gene titration experiments on sep mutants have been reported so far [9,10], which include data not shown, yet which support the molecular data. Other studies have suggested that SEP3 and LOFSEP are even more sub-functionalized in other species. The specific or preferential expression of SEP3 genes in the three inner whorls is commonly observed in core eudicots (petal, stamen, gynoecium; [62,64,65,66]) and grasses (lodicule, stamen, gynoecium; [67]). Therefore, cases where SEP3-like genes are involved in first-whorl organ development, as in the orchid Phalaenopsis equestris [68], probably reflect deviations from a conserved and ancestral model which caused the expansion of SEP3 gene activity in the outermost perianth organs or calyx. Interestingly, the sepals of Phalaenopsis are showy and mimic petals. Petunia hybrida has only one SEP3 gene, FLORAL BINDING PROTEIN2 (FBP2), whose loss of function results in the conversion of petals into green sepaloid organs and the development of secondary inflorescences in the third whorl [64,69,70]. In Asteraceae, one SEP3 gene of Gerbera hybrida is GERBERA REGULATOR OF CAPITULUM DEVELOPMENT5 (GRCD5; Figure 4), which also shows unique functions in petal development. Interestingly, GRCD1 belongs to the Asteraceae-specific SEP3 clade (Figure 4) and seems to be sub-functionalized to regulate stamen identity. In orchids, the suppression of one SEP3 gene is also sufficient to trigger a partial loss of floral organ identity [68,71]. The co-suppression of rice OsMADS7/45 and OsMADS8/24 led to serious defects in all the inner three whorls (lodicule, stamen, gynoecium; [67]), suggesting that SEP3 and LOFSEP are even more sub-functionalized in rice than in core eudicot model species. However, the reversion of the flower into leafy, indeterminate, shoot-like structures required the simultaneous suppression of most or all LOFSEP and SEP3 genes in core eudicots and rice [10,31,67,72]. In rice, we have previously shown that the combined mutation of the three LOFSEP genes (OsMADS1, OsMADS5 and OsMADS34) is sufficient to convert the flowers almost completely into indeterminate leafy organs, but it is important to notice that this phenotype was associated with a dramatic decrease in the expression of SEP3 genes (OsMADS7/45 and OsMADS8/24). Therefore, it seems that rice LOFSEP genes positively regulate SEP3 genes, and that the floral phenotypes observed in lofsep triple mutants were caused by global reductions in both LOFSEP and SEP3 function [73]. Rice LOFSEP genes are also important regulators of the bract- and prophyll-like spikelet organs that protect the flower and represent evolutionary innovations: OsMADS1 specifies the identities of lemmas and paleas [74,75,76,77,78], OsMADS34 represses the development of the two lateral sterile lemmas [28,29,79] and, finally, all these organs are converted into leaves in the osmads1 osmads5 osmads34 triple mutant [73].
It is intriguing that LOFSEP genes have been recruited to regulate inflorescence development in several species, mostly by limiting branching and promoting the switch to FM identity, which are functions that temporally precede their well-known and essential functions in flower development. In Solanaceae, the FBP9/23 subclade is the main player, with contributions from SEP4 [26,27,72], while SEP1/2 genes are the main regulators of IM determinacy in the capitulum of Gerbera hybrida [31,80]. Rice OsMADS34 and SQUA-like genes synergistically act to specify IM identity, downstream of the florigen signal [58]. Subsequently, OsMADS34 limits inflorescence primary branching by repressing IM activity [28,29,30]. In addition, OsMADS34 shares functions with OsMADS5 in repressing secondary branching by promoting the maturation of meristems toward the spikelet meristem stage and in promoting the elongation of the inflorescence rachis and branches [30]. As a consequence, osmads34 and osmads5 osmads34 knock-out mutants produce much more branched inflorescence primordia, but several meristems subsequently fail to develop into mature, fertile spikelets [30], similarly to what has been observed in tomato plants defective with respect to J2, EJ2 and LIN functionality [26]. Unfortunately, mild OsMADS34 alleles able to trigger more productive inflorescences, which could be beneficial for breeding programs, have not emerged so far. The function of OsMADS34 in inflorescence architecture is likely conserved in other grasses [81,82]. Our analysis reveals that genes similar to OsMADS34 exist in other core monocots, which opens new perspectives for future functional studies, especially in monocot crops with complex inflorescences, such as pineapple and palms.
Given the fact that different LOFSEP subclades have been recruited for similar inflorescence functions in rice, Solanaceae and Asteraceae reveal their ancestral potential in regulating inflorescence development, which then was lost or retained during evolution.
3. Conclusions
We found both LOFSEP and SEP3 genes ubiquitously in core eudicot and monocot species, which suggests that each clade has specific essential functions besides their shared roles in FM and floral organ identity. The strong conservation of SEP3 genes, in terms of sequences and expression patterns, suggests that their major role in petal, stamen and carpel identity complexes was established and fixed before the MRCA of monocots + eudicots, while LOFSEP genes appear to have enjoyed more functional flexibility to allow their neo-functionalization, acquiring diversified roles in different angiosperm families, such as the regulation of bract identity, pedicel abscission zone, calyx size and inflorescence architecture. Therefore, besides their relevance for understanding angiosperm evolution, some SEPALLATA genes are major players in agronomically relevant traits. While SEP3 or other MADS-box homeotic mutants are potentially useful in the creation of ornamental floral oddities and flowers less attractive to insect pests [71,83], the biotechnological manipulation of LOFSEP genes or their network shows promise with respect to the improvement of inflorescence characters, such as numbers of flowers and fruits. To this aim, genes from the FBP9/23 and SEP4 clades are promising candidates for further studies in asterid species with branched inflorescences, while homologues of rice OsMADS34 are likely the main players in grasses and, perhaps, even in other core monocots.
4. Materials and Methods
All the SEPALLATA genes used in this study were identified through BLAST analysis of the following databases: NCBI Genome (Tarenaya hassleriana, Gerbera hybrida, Petunia x hybrida, Zingiber officinale, Elaeis guineensis, Phoenix dactylifera, Apostasia shenzhenica, Dendrobium catenatum, Phalaenopsis equestris), Gramene (Aegilops tauschii and Triticum aestivum), www.oniongenome.wur.nl (Allium cepa), the Snapdragon Genome Database (http://bioinfo.sibs.ac.cn/Am/index.php; Antirrhinum majus) and Phytozome 13 (all the other species). Genes from Asparagus officinalis and Ananas comosus were identified from both the NCBI and Phytozome 13 databases, and incomplete or incorrect annotations were eventually corrected by searching the NCBI Transcriptome Shotgun Assembly (TSA) database. Accession numbers are available in Table 1 and Table S1. Protein sequences were aligned using MAFFT (https://mafft.cbrc.jp/alignment/server/), checked manually and then back-translated to nucleotide alignments with PAL2NAL (http://www.bork.embl.de/pal2nal/).
Phylogenetic trees were calculated with MEGA 11 [84]. Evolutionary history was inferred using the Maximum Likelihood (ML) method and the Tamura–Nei model [85]. The model was accepted based on the high consistency of the resulting topologies with respect to previously published clades and genes. The trees with the highest log likelihoods were shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying the Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Tamura–Nei model and then selecting the topology with a superior log likelihood value. The trees were drawn to scale, with branch lengths measured as the number of substitutions per site. Codon positions included were 1st + 2nd + 3rd.
Microsynteny was calculated and scored using SynFind [86] on the CoGe platform (https://genomevolution.org/coge/). Then, selected genomic regions and genes were downloaded from Phytozome 13 Phytomine and NCBI Genomes. Gene homology was confirmed manually with BLAST analysis. The final images shown in this work were generated with Simple Synteny online (https://www.dveltri.com/simplesynteny/; [87]). All the databases and online tools were accessed between November 2021 and July 2022.
Images were edited with InkScape 0.92 (https://inkscape.org/) and GIMP 2.10.32 (https://www.gimp.org/).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants11212934/s1, Figure S1: Alignment of all the LOFSEP, OsMADS1-like proteins of Triticum aestivum (bread wheat), Figure S2: Alignment of all the LOFSEP, OsMADS5-like proteins of Triticum aestivum (bread wheat), Figure S3: Alignment of the three LOFSEP, OsMADS34-like homeolog proteins of Triticum aestivum (bread wheat), Figure S4: Alignment of the three SEP3, OsMADS7/45-like homeolog proteins of Triticum aestivum (bread wheat), Figure S5: Alignment of the three SEP3, OsMADS8/24-like homeolog proteins of Triticum aestivum (bread wheat), Table S1: Accession codes for all the genes reported in this study.
Author Contributions
L.D. conceived the original research plan, performed the analysis and wrote the manuscript; C.F. supervised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Commission through a Marie Sklodowska–Curie Individual Fellowship (RI no. 661678 ‘GainGrain’) awarded to L.D. and by CSIC (Spanish National Research Council) through the fellowship ‘convocatoria extensión MSCA IF ERC’ awarded to L.D.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Coen, E.; Meyerowitz, E. The War of the Whorls: Genetic Interactions Controlling Flower Development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Theißen, G.; Saedler, H. Floral Quartets. Nature 2001, 409, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Puranik, S.; Acajjaoui, S.; Conn, S.; Costa, L.; Conn, V.; Vial, A.; Marcellin, R.; Melzer, R.; Brown, E.; Hart, D.; et al. Structural Basis for the Oligomerization of the MADS Domain Transcription Factor SEPALLATA3 in Arabidopsis. Plant Cell 2014, 26, 3603–3615. [Google Scholar] [CrossRef] [PubMed]
- Smaczniak, C.; Immink, R.G.H.; Muiño, J.M.; Blanvillain, R.; Busscher, M.; Busscher-Lange, J.; Dinh, Q.D.; Liu, S.; Westphal, A.H.; Boeren, S.; et al. Characterization of MADS-Domain Transcription Factor Complexes in Arabidopsis Flower Development. Proc. Natl. Acad. Sci. USA 2012, 109, 1560–1565. [Google Scholar] [CrossRef]
- Hugouvieux, V.; Silva, C.S.; Jourdain, A.; Stigliani, A.; Charras, Q.; Conn, V.; Conn, S.J.; Carles, C.C.; Parcy, F.; Zubieta, C. Tetramerization of MADS Family Transcription Factors SEPALLATA3 and AGAMOUS Is Required for Floral Meristem Determinacy in Arabidopsis. Nucleic Acids Res. 2018, 46, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Theißen, G.; Melzer, R.; Ruümpler, F. MADS-Domain Transcription Factors and the Floral Quartet Model of Flower Development: Linking Plant Development and Evolution. Development 2016, 143, 3259–3271. [Google Scholar] [CrossRef]
- Pelaz, S.; Ditta, G.S.; Baumann, E.; Wisman, E.; Yanofsky, M.F. B and C Floral Organ Identity Functions Require SEPALLATA MADS-Box Genes. Nature 2000, 405, 200–203. [Google Scholar] [CrossRef]
- Pelaz, S.; Tapia-López, R.; Alvarez-Buylla, E.R.; Yanofsky, M.F. Conversion of Leaves into Petals in Arabidopsis. Curr. Biol. 2001, 11, 182–184. [Google Scholar] [CrossRef]
- Favaro, R.; Pinyopich, A.; Battaglia, R.; Kooiker, M.; Borghi, L.; Ditta, G.; Yanofsky, M.F.; Kater, M.M.; Colombo, L. MADS-Box Protein Complexes Control Carpel and Ovule Development in Arabidopsis. Plant Cell 2003, 15, 2603–2611. [Google Scholar] [CrossRef]
- Ditta, G.; Pinyopich, A.; Robles, P.; Pelaz, S.; Yanofsky, M.F. The SEP4 Gene of Arabidopsis Thaliana Functions in Floral Organ and Meristem Identity. Curr. Biol. 2004, 14, 1935–1940. [Google Scholar] [CrossRef]
- Goethe, J.W. Versuch Die Metamorphose Der Pflanzen Zu Erklären; Ettingersche Buchhandlung: Gotha, Germany, 1790. [Google Scholar]
- Irish, V.F. Variations on a Theme: Flower Development and Evolution. Genome Biol. 2000, 1, reviews1015.1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ronse De Craene, L.P.; Brockington, S.F. Origin and Evolution of Petals in Angiosperms. Plant Ecol. Evol. 2013, 146, 5–25. [Google Scholar] [CrossRef]
- Kellogg, E. A Evolutionary History of the Grasses. Plant Physiol. 2001, 125, 1198–1205. [Google Scholar] [CrossRef]
- Causier, B.; Schwarz-Sommer, Z.; Davies, B. Floral Organ Identity: 20 Years of ABCs. Semin. Cell Dev. Biol. 2010, 21, 73–79. [Google Scholar] [CrossRef]
- Chanderbali, A.S.; Berger, B.A.; Howarth, D.G.; Soltis, P.S.; Soltis, D.E. Evolving Ideas on the Origin and Evolution of Flowers: New Perspectives in the Genomic Era. Genetics 2016, 202, 1255–1265. [Google Scholar] [CrossRef]
- Endress, P.K. Disentangling Confusions in Inflorescence Morphology: Patterns and Diversity of Reproductive Shoot Ramification in Angiosperms. J. Syst. Evol. 2010, 48, 225–239. [Google Scholar] [CrossRef]
- Claßen-Bockhoff, R.; Bull-Hereñu, K. Towards an Ontogenetic Understanding of Inflorescence Diversity. Ann. Bot. 2013, 112, 1523–1542. [Google Scholar] [CrossRef]
- Kellogg, E.A. Genetic Control of Branching Patterns in Grass Inflorescences. Plant Cell 2022, 34, 2518–2533. [Google Scholar] [CrossRef]
- Bartlett, M.E.; Thompson, B. Meristem Identity and Phyllotaxis in Inflorescence Development. Front. Plant Sci. 2014, 2014, 508. [Google Scholar] [CrossRef]
- Bommert, P.; Whipple, C. Grass Inflorescence Architecture and Meristem Determinacy. Semin. Cell Dev. Biol. 2018, 79, 37–47. [Google Scholar]
- Kyozuka, J. Chapter Seven—Grass Inflorescence: Basic Structure and Diversity. In Advances in Botanical Research; Academic Press; Elsevier: Oxford, UK, 2014; Volume 72, pp. 191–219. [Google Scholar]
- Zhang, D.; Yuan, Z. Molecular Control of Grass Inflorescence Development. Annu. Rev. Plant Biol. 2014, 65, 553–578. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Hibrand-Saint Oyant, L.; Crespel, L.; Thouroude, T.; Lalanne, D.; Foucher, F. Quantitative Trait Loci for Flowering Time and Inflorescence Architecture in Rose. Theor. Appl. Genet. 2011, 122, 661–675. [Google Scholar] [CrossRef]
- Benlloch, R.; Berbel, A.; Ali, L.; Gohari, G.; Millán, T.; Madueño, F. Genetic Control of Inflorescence Architecture in Legumes. Front. Plant Sci. 2015, 6, 543. [Google Scholar] [CrossRef]
- Soyk, S.; Lemmon, Z.H.; Oved, M.; Fisher, J.; Liberatore, K.L.; Park, S.J.; Goren, A.; Jiang, K.; Ramos, A.; van der Knaap, E.; et al. Bypassing Negative Epistasis on Yield in Tomato Imposed by a Domestication Gene. Cell 2017, 169, 1142–1155. [Google Scholar] [CrossRef]
- Soyk, S.; Lemmon, Z.H.; Sedlazeck, F.J.; Jiménez-Gómez, J.M.; Alonge, M.; Hutton, S.F.; Van Eck, J.; Schatz, M.C.; Lippman, Z.B. Duplication of a Domestication Locus Neutralized a Cryptic Variant That Caused a Breeding Barrier in Tomato. Nat. Plants 2019, 5, 471–479. [Google Scholar] [CrossRef]
- Kobayashi, K.; Maekawa, M.; Miyao, A.; Hirochika, H.; Kyozuka, J. PANICLE PHYTOMER2 (PAP2), Encoding a SEPALLATA Subfamily MADS-Box Protein, Positively Controls Spikelet Meristem Identity in Rice. Plant Cell Physiol. 2010, 51, 41–51. [Google Scholar] [CrossRef]
- Gao, X.; Liang, W.; Yin, C.; Ji, S.; Wang, H.; Su, X.; Guo, C.; Kong, H.; Xue, H.; Zhang, D. The SEPALLATA-like Gene OsMADS34 Is Required for Rice Inflorescence and Spikelet Development. Plant Physiol. 2010, 110, 728–740. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, L.; Wu, D.; Meng, Q.; Deng, X.; Huang, G.; Zhang, J.; Chen, X.; Ferrándiz, C.; Liang, W.; et al. Rice SEPALLATA Genes OsMADS5 and OsMADS34 Cooperate to Limit Inflorescence Branching by Repressing the TERMINAL FLOWER1-like Gene RCN4. New Phytol. 2022, 233, 1682–1700. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Juntheikki, I.; Mouhu, K.; Broholm, S.K.; Rijpkema, A.S.; Kins, L.; Lan, T.; Albert, V.A.; Teeri, T.H.; et al. Dissecting Functions of SEPALLATA-like MADS Box Genes in Patterning of the Pseudanthial Inflorescence of Gerbera Hybrida. New Phytol. 2017, 216, 939–954. [Google Scholar] [CrossRef]
- Malcomber, S.T.; Kellogg, E.A. SEPALLATA Gene Diversification: Brave New Whorls. Trends Plant Sci. 2005, 10, 427–435. [Google Scholar] [CrossRef]
- Zahn, L.M.; Kong, H.; Leebens-Mack, J.H.; Kim, S.; Soltis, P.S.; Landherr, L.L.; Soltis, D.E.; DePamphilis, C.W.; Ma, H. The Evolution of the SEPALLATA Subfamily of MADS-Box Genes: A Preangiosperm Origin with Multiple Duplications throughout Angiosperm History. Genetics 2005, 169, 2209–2223. [Google Scholar] [CrossRef] [PubMed]
- DePamphilis, C.W.; Palmer, J.D.; Rounsley, S.; Sankoff, D.; Schuster, S.C.; Ammiraju, J.S.S.; Barbazuk, W.B.; Chamala, S.; Chanderbali, A.S.; Determann, R.; et al. The Amborella Genome and the Evolution of Flowering Plants. Science 2013, 342, 1241089. [Google Scholar] [CrossRef]
- Ruelens, P.; De Maagd, R.A.; Proost, S.; Theißen, G.; Geuten, K.; Kaufmann, K. FLOWERING LOCUS C in Monocots and the Tandem Origin of Angiosperm-Specific MADS-Box Genes. Nat. Commun. 2013, 4, 2280. [Google Scholar] [CrossRef]
- Airoldi, C.A.; Davies, B. Gene Duplication and the Evolution of Plant MADS-Box Transcription Factors. J. Genet. Genomics 2012, 39, 157–165. [Google Scholar] [CrossRef]
- Causier, B.; Castillo, R.; Xue, Y.; Schwarz-Sommer, Z.; Davies, B. Tracing the Evolution of the Floral Homeotic B-and C-Function Genes through Genome Synteny. Mol. Biol. Evol. 2010, 27, 2651–2664. [Google Scholar] [CrossRef]
- Zhao, T.; Holmer, R.; De Bruijn, S.; Angenent, G.C.; Van Den Burg, H.A.; Eric Schranz, M. Phylogenomic Synteny Network Analysis of MADS-Box Transcription Factor Genes Reveals Lineage-Specific Transpositions, Ancient Tandem Duplications, and Deep Positional Conservation. Plant Cell 2017, 29, 1278–1292. [Google Scholar] [CrossRef]
- Cantino, P.D.; Doyle, J.A.; Graham, S.W.; Judd, W.S.; Olmstead, R.G.; Soltis, D.E.; Soltis, P.S.; Donoghue, M.J. Towards a Phylogenetic Nomenclature of Tracheophyta. Taxon 2007, 56, E1–E44. [Google Scholar] [CrossRef]
- Coiffard, C.; Kardjilov, N.; Manke, I.; Bernardes-de-Oliveira, M.E.C. Fossil Evidence of Core Monocots in the Early Cretaceous. Nat. Plants 2019, 5, 691–696. [Google Scholar] [CrossRef]
- Ming, R.; VanBuren, R.; Wai, C.M.; Tang, H.; Schatz, M.C.; Bowers, J.E.; Lyons, E.; Wang, M.L.; Chen, J.; Biggers, E.; et al. The Pineapple Genome and the Evolution of CAM Photosynthesis. Nat. Genet. 2015, 47, 1435–1442. [Google Scholar] [CrossRef]
- Xu, Q.; Jin, L.; Zhang, Y.; Zhang, X.; Zheng, C.; Leebens-Mack, J.H.; Sankoff, D. Ancestral Flowering Plant Chromosomes and Gene Orders Based on Generalized Adjacencies and Chromosomal Gene Co-Occurrences. J. Comput. Biol. 2021, 28, 1156–1179. [Google Scholar] [CrossRef]
- Tang, H.; Bowers, J.E.; Wang, X.; Paterson, A.H. Angiosperm Genome Comparisons Reveal Early Polyploidy in the Monocot Lineage. Proc. Natl. Acad. Sci. USA 2010, 107, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Agarwal, P.; Ray, S.; Singh, A.K.; Singh, V.P.; Tyagi, A.K.; Kapoor, S. MADS-Box Gene Family in Rice: Genome-Wide Identification, Organization and Expression Profiling during Reproductive Development and Stress. BMC Genomics 2007, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Messing, J. The Polyploid Origin of Maize. In Handbook of Maize: Genetics and Genomics; Springer: New York, NY, USA, 2009; pp. 221–238. ISBN 978-0-387-77863-1. [Google Scholar]
- Swigoňová, Z.; Lai, J.; Ma, J.; Ramakrishna, W.; Llaca, V.; Bennetzen, J.L.; Messing, J. Close Split of Sorghum and Maize Genome Progenitors. Genome Res. 2004, 14, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Schnable, P.S.; Ware, D.; Fulton, R.S.; Stein, J.C.; Wei, F.; Pasternak, S.; Liang, C.; Zhang, J.; Fulton, L.; Graves, T.A.; et al. The B73 Maize Genome: Complexity, Diversity, and Dynamics. Science 2009, 326, 1112–1115. [Google Scholar] [CrossRef]
- Matsuoka, Y. Evolution of Polyploid Triticum Wheats under Cultivation: The Role of Domestication, Natural Hybridization and Allopolyploid Speciation in Their Diversification. Plant Cell Physiol. 2011, 52, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Shitsukawa, N.; Tahira, C.; Kassai, K.I.; Hirabayashi, C.; Shimizu, T.; Takumi, S.; Mochida, K.; Kawaura, K.; Ogihara, Y.; Muraia, K. Genetic and Epigenetic Alteration among Three Homoeologous Genes of a Class E MADS Box Gene in Hexaploid Wheat. Plant Cell 2007, 19, 1723–1737. [Google Scholar] [CrossRef]
- Thiel, T.; Graner, A.; Waugh, R.; Grosse, I.; Close, T.J.; Stein, N. Evidence and Evolutionary Analysis of Ancient Whole-Genome Duplication in Barley Predating the Divergence from Rice. BMC Evol. Biol. 2009, 9, 209. [Google Scholar] [CrossRef]
- Meng, Q.; Li, X.; Zhu, W.; Yang, L.; Liang, W.; Dreni, L.; Zhang, D. Regulatory Network and Genetic Interactions Established by OsMADS34 in Rice Inflorescence and Spikelet Morphogenesis. J. Integr. Plant Biol. 2017, 59, 693–707. [Google Scholar] [CrossRef]
- Vekemans, D.; Proost, S.; Vanneste, K.; Coenen, H.; Viaene, T.; Ruelens, P.; Maere, S.; Van De Peer, Y.; Geuten, K. Gamma Paleohexaploidy in the Stem Lineage of Core Eudicots: Significance for MADS-BOX Gene and Species Diversification. Mol. Biol. Evol. 2012, 29, 3793–3806. [Google Scholar] [CrossRef]
- Jiao, Y.; Leebens-Mack, J.; Ayyampalayam, S.; Bowers, J.E.; McKain, M.R.; McNeal, J.; Rolf, M.; Ruzicka, D.R.; Wafula, E.; Wickett, N.J.; et al. A Genome Triplication Associated with Early Diversification of the Core Eudicots. Genome Biol. 2012, 13, R3. [Google Scholar] [CrossRef]
- Chanderbali, A.S.; Jin, L.; Xu, Q.; Zhang, Y.; Zhang, J.; Jian, S.; Carroll, E.; Sankoff, D.; Albert, V.A.; Howarth, D.G.; et al. Buxus and Tetracentron Genomes Help Resolve Eudicot Genome History. Nat. Commun. 2022, 13, 643. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, O.; Aury, J.M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. The Grapevine Genome Sequence Suggests Ancestral Hexaploidization in Major Angiosperm Phyla. Nature 2007, 449, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Chanderbali, A.S.; Berger, B.A.; Howarth, D.G.; Soltis, D.E.; Soltis, P.S. Evolution of Floral Diversity: Genomics, Genes and Gamma. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20150509. [Google Scholar] [CrossRef]
- Vrebalov, J.; Ruezinsky, D.; Padmanabhan, V.; White, R.; Medrano, D.; Drake, R.; Schuch, W.; Giovannoni, J. A MADS-Box Gene Necessary for Fruit Ripening at the Tomato Ripening-Inhibitor (Rin) Locus. Science 2002, 296, 343–346. [Google Scholar] [CrossRef]
- Kobayashi, K.; Yasuno, N.; Sato, Y.; Yoda, M.; Yamazaki, R.; Kimizu, M.; Yoshida, H.; Nagamura, Y.; Kyozukaa, J. Inflorescence Meristem Identity in Rice Is Specified by Overlapping Functions of Three AP1/FUL-Like MADS Box Genes and PAP2, a SEPALLATA MADS Box Gene. Plant Cell 2012, 24, 1848–1859. [Google Scholar] [CrossRef] [PubMed]
- Trevaskis, B.; Bagnall, D.J.; Ellis, M.H.; Peacock, W.J.; Dennis, E.S. MADS Box Genes Control Vernalization-Induced Flowering in Cereals. Proc. Natl. Acad. Sci. USA 2003, 100, 13099–13104. [Google Scholar] [CrossRef]
- Robinson, R.; Tomes, M. Ripening Inhibitor: A Gene with Multiple Effects on Ripening. Rep. Tomato Genet. Coop. 1968, 18, 36–37. [Google Scholar]
- Ito, Y.; Nishizawa-Yokoi, A.; Endo, M.; Mikami, M.; Shima, Y.; Nakamura, N.; Kotake-Nara, E.; Kawasaki, S.; Toki, S. Re-Evaluation of the Rin Mutation and the Role of RIN in the Induction of Tomato Ripening. Nat. Plants 2017, 3, 866–874. [Google Scholar] [CrossRef]
- Mandel, M.A.; Yanofsky, M.F. The Arabidopsis AGL9 MADS Box Gene Is Expressed in Young Flower Primordia. Sex Plant Reprod. 1998, 11, 22–28. [Google Scholar] [CrossRef]
- Honma, T.; Goto, K. Complexes of MADS-Box Proteins Are Sufficient to Convert Leaves into Floral Organs. Nature 2001, 409, 525–529. [Google Scholar] [CrossRef]
- Ferrario, S.; Immink, R.G.H.; Shchennikova, A.; Busscher-Lange, J.; Angenent, G.C. The MADS Box Gene FBP2 Is Required for SEPALLATA Function in Petunia. Plant Cell 2003, 15, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Pnueli, L.; Hareven, D.; Broday, L.; Hurwitz, C.; Lifschitz, E. The TM5 MADS Box Gene Mediates Organ Differentiation in the Three Inner Whorls of Tomato Flowers. Plant Cell 1994, 6, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.; Egea-Cortines, M.; de Andrade Silva, E.; Saedler, H.; Sommer, H. Multiple Interactions amongst Floral Homeotic MADS Box Proteins. EMBO J. 1996, 15, 4330–4343. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Han, J.; Zhao, S.; Su, K.; Wu, F.; Du, X.; Xu, Q.; Chong, K.; Theißen, G.; Meng, Z. Functional Conservation and Diversification of Class e Floral Homeotic Genes in Rice (Oryza sativa). Plant J. 2010, 61, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.J.; Chen, Y.Y.; Du, J.S.; Chen, Y.Y.; Chung, M.C.; Tsai, W.C.; Wang, C.N.; Chen, H.H. Flower Development of Phalaenopsis Orchid Involves Functionally Divergent SEPALLATA-like Genes. New Phytol. 2014, 202, 1024–1042. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, M.; Zethof, J.; Souer, E.; Koes, R.; Tornielli, G.B.; Pezzotti, M.; Ferrario, S.; Angenent, G.C.; Gerats, T. Toward the Analysis of the Petunia MADS Box Gene Family by Reverse and Forward Transposon Insertion Mutagenesis Approaches: B, C, and D Floral Organ Identity Functions Require SEPALLATA-like MADS Box Genes in Petunia. Plant Cell 2003, 15, 2680–2693. [Google Scholar] [CrossRef]
- Angenent, G.C.; Franken, J.; Busscher, M.; Weiss, D.; Van Tunen, A.J. Co-suppression of the Petunia Homeotic Gene Fbp2 Affects the Identity of the Generative Meristem. Plant J. 1994, 5, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Mitoma, M.; Kanno, A. The Greenish Flower Phenotype of Habenaria Radiata (Orchidaceae) Is Caused by a Mutation in the SEPALLATA-like MADS-Box Gene HrSEP-1. Front. Plant Sci. 2018, 9, 831. [Google Scholar] [CrossRef]
- Morel, P.; Chambrier, P.; Boltz, V.; Chamot, S.; Rozier, F.; Bento, S.R.; Trehin, C.; Monniaux, M.; Zethof, J.; Vandenbussche, M. Divergent Functional Diversification Patterns in the SEP/AGL6/AP1 MADS-Box Transcription Factor Superclade. Plant Cell 2019, 31, 3033–3056. [Google Scholar] [CrossRef]
- Wu, D.; Liang, W.; Zhu, W.; Chen, M.; Ferrándiz, C.; Burton, R.A.; Dreni, L.; Zhang, D. Loss of LOFSEP Transcription Factor Function Converts Spikelet to Leaf-like Structures in Rice. Plant Physiol. 2018, 176, 1646–1664. [Google Scholar] [CrossRef]
- Jeon, J.S.; Jang, S.; Lee, S.; Nam, J.; Kim, C.; Lee, S.H.; Chung, Y.Y.; Kim, S.R.; Lee, Y.H.; Cho, Y.G.; et al. Leafy Hull Sterile 1 Is a Homeotic Mutation in a Rice MADS Box Gene Affecting Rice Flower Development. Plant Cell 2000, 12, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Parameswaran, S.; Vijayraghavan, U. OsMADS1, a Rice MADS-Box Factor, Controls Differentiation of Specific Cell Types in the Lemma and Palea and Is an Early-Acting Regulator of Inner Floral Organs. Plant J. 2005, 43, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Sriram, P.; Santhosh Kumar, C.; Kushalappa, K.; Vijayraghavan, U. Ectopic Expression of Rice OsMADS1 Reveals a Role in Specifying the Lemma and Palea, Grass Floral Organs Analogous to Sepals. Dev. Genes Evol. 2001, 211, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.K.; Abe, K.; Yamazaki, M.; Miyao, A.; Hirochika, H. Conservation of the E-Function for Floral Organ Identity in Rice Revealed by the Analysis of Tissue Culture-Induced Loss-of-Function Mutants of the OsMADS1 Gene. Plant Mol. Biol. 2005, 59, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liang, W.; Yin, C.; Yang, X.; Ping, B.; Li, A.; Jia, R.; Chen, M.; Luo, Z.; Cai, Q.; et al. Interactions of OsMADS1 with Floral Homeotic Genes in Rice Flower Development. Mol. Plant 2015, 8, 1366–1384. [Google Scholar] [CrossRef]
- Lin, X.; Wu, F.; Du, X.; Shi, X.; Liu, Y.; Liu, S.; Hu, Y.; Theißen, G.; Meng, Z. The Pleiotropic SEPALLATA-like Gene OsMADS34 Reveals That the “empty Glumes” of Rice (Oryza Sativa) Spikelets Are in Fact Rudimentary Lemmas. New Phytol. 2014, 202, 689–702. [Google Scholar] [CrossRef]
- Uimari, A.; Kotilainen, M.; Elomaa, P.; Yu, D.; Albert, V.A.; Teeri, T.H. Integration of Reproductive Meristem Fates by a SEPALLATA-like MADS-Box Gene. Proc. Natl. Acad. Sci. USA 2004, 101, 15817–15822. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Tian, C.; Sajjad, M.; Gao, C.; Tong, Y.; Wang, X.; Jiao, Y. Transcriptome Association Identifies Regulators of Wheat Spike Architecture. Plant Physiol. 2017, 175, 746–757. [Google Scholar] [CrossRef]
- Hussin, S.H.; Wang, H.; Tang, S.; Zhi, H.; Tang, C.; Zhang, W.; Jia, G.; Diao, X. SiMADS34, an E-Class MADS-Box Transcription Factor, Regulates Inflorescence Architecture and Grain Yield in Setaria Italica. Plant Mol. Biol. 2021, 105, 419–434. [Google Scholar] [CrossRef]
- Kater, M.M.; Franken, J.; Inggamer, H.; Gretenkort, M.; Van Tunen, A.J.; Mollema, C.; Angenent, G.C. The Use of Floral Homeotic Mutants as a Novel Way to Obtain Durable Resistance to Insect Pests. Plant Biotechnol. J. 2003, 1, 123–127. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Bomhoff, M.D.; Briones, E.; Zhang, L.; Schnable, J.C.; Lyons, E. SynFind: Compiling Syntenic Regions across Any Set of Genomes on Demand. Genome Biol. Evol. 2015, 7, 3286–3298. [Google Scholar] [CrossRef]
- Veltri, D.; Wight, M.M.; Crouch, J.A. SimpleSynteny: A Web-Based Tool for Visualization of Microsynteny across Multiple Species. Nucleic Acids Res. 2016, 44, W41–W45. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).