Eucalyptus cinerea and E. nicholii by-Products as Source of Bioactive Compounds for Agricultural Applications
Abstract
1. Introduction
2. Results
2.1. Micromorphological Characterization
2.2. Chemical Composition of Essential Oils
2.3. Phytotoxic Activity
2.4. Antimicrobial Activity
2.5. Antifungal Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Macro- and Micromorphological Analyses
4.4. Extraction of Essential Oils
4.5. GC-FID Analyses
4.6. GC/MS Analyses
4.7. Identification of the Essential Oil Components
4.8. Phytotoxic Activity
4.9. Plant Pathogens
4.10. Antimicrobial Activity
4.11. Antifungal Activity
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Littardi, C. German Botanists and Naturalist on the Riviera between the End of the 19th Century and the First Half of the 20th. The Origin of the Industrial Floriculture. BMIB Boll. Dei Musei E Degli Ist. Biol. 2017, 79, 166. [Google Scholar]
- Danna, C.; Cornara, L.; Smeriglio, A.; Trombetta, D.; Amato, G.; Aicardi, P.; De Martino, L.; De Feo, V.; Caputo, L. Eucalyptus gunnii and Eucalyptus pulverulenta “Baby Blue” Essential Oils as Potential Natural Herbicides. Molecules 2021, 26, 6749. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Montero, G.; Coronado, M.A.; Valdez, B.; Stoytcheva, M.; Rosas, N.; Torres, R.; Sagaste, C.A. Valorization of Eucalyptus Leaves by Essential Oil Extraction as an Added Value Product in Mexico. Waste Biomass Valorization 2017, 8, 1187–1197. [Google Scholar] [CrossRef]
- Mediavilla, I.; Guillamón, E.; Ruiz, A.; Esteban, L.S. Essential oils from residual foliage of forest tree and shrub species: Yield and antioxidant capacity. Molecules 2021, 26, 3257. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Smeriglio, A.; Trombetta, D.; Cornara, L.; Trevena, G.; Valussi, M.; Fratianni, F.; De Feo, V.; Nazzaro, F. Chemical Composition and Biological Activities of the Essential Oils of Leptospermum petersonii and Eucalyptus gunnii. Front. Microbiol. 2020, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Pauzer, M.S.; Borsato, T.D.O.; de Almeida, V.P.; Raman, V.; Justus, B.; Pereira, C.B.; Flores, T.B.; Maia, B.H.L.N.S.; Meneghetti, E.K.; Kanunfre, C.C.; et al. Eucalyptus cinerea: Microscopic Profile, Chemical Composition of Essential Oil and its Antioxidant, Microbiological and Cytotoxic Activities. Braz. Arch. Biol. Technol. 2021, 64, 1–19. [Google Scholar] [CrossRef]
- Barbosa, L.C.A.; Filomeno, C.A.; Teixeira, R.R. Chemical variability and biological activities of Eucalyptus spp. essential oils. Molecules 2016, 21, 1671. [Google Scholar] [CrossRef]
- Zhou, L.; Li, J.; Kong, Q.; Luo, S.; Wang, J.; Feng, S.; Yuan, M.; Chen, T.; Yuan, S.; Ding, C. Chemical composition, antioxidant, antimicrobial, and phytotoxic potential of Eucalyptus grandis × E. urophylla leaves essential oils. Molecules 2021, 26, 1450. [Google Scholar] [CrossRef]
- De Lillo, E.; Simoni, S.; Rapetti, S.; Repetto, L.; Odasso, M.; Bozzano, G.; Restuccia, P.; Martini, P. Un nuovo acaro eriofide dannoso per l’eucalipto da fronda in Italia e prime esperienze di lotta. ATTI Giornate Fitopatol. 2016, 1, 413–416. [Google Scholar]
- De Brito, P.S.; Sabedotti, C.; Flores, T.B.; Raman, V.; Bussade, J.E.; Farago, P.V.; Manfron, J. Light and Scanning Electron Microscopy, Energy Dispersive X-Ray Spectroscopy, and Histochemistry of Eucalyptus tereticornis. Microsc. Microanal. 2021, 27, 1295–1303. [Google Scholar] [CrossRef]
- Kahla, Y.; Zouari-Bouassida, K.; Rezgui, F.; Trigui, M.; Tounsi, S. Efficacy of Eucalyptus Cinerea as a Source of Bioactive Compounds for Curative Biocontrol of Crown Gall Caused by Agrobacterium Tumefaciens Strain B6. Biomed Res. Int. 2017, 2017. Available online: https://riviste.unige.it/index.php/BMIB/issue/view/22 (accessed on 29 September 2022). [CrossRef] [PubMed]
- Soliman, F.M.; Fathy, M.M.; Salama, M.M.; Saber, F.R. Chemical composition and bioactivity of the volatile oil from leaves and stems of Eucalyptus cinerea. Pharm. Biol. 2014, 52, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.S.; Kiran Babu, G.D.; Guleria, S.; Singh, B. Comparison of Eucalyptus cinerea essential oils produced by hydrodistillation and supercritical carbon dioxide extraction. Nat. Prod. Commun. 2011, 6, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.M.; Abe, S.Y.; Murakami, F.S.; Frensch, G.; Marques, F.A.; Nakashima, T. Essential oils from different plant parts of Eucalyptus cinerea F. Muell. ex Benth. (Myrtaceae) as a source of 1,8-Cineole and their bioactivities. Pharmaceuticals 2011, 4, 1535–1550. [Google Scholar] [CrossRef]
- Heikal, A.A.E.M. Variation in the Essential Oil Content and its Composition in Eucalyptus cinerea Leaves and its Relation to Some Environmental Factors. J. Essent. Oil-Bear. Plants 2017, 20, 995–1005. [Google Scholar] [CrossRef]
- Maleknia, S.D.; Vail, T.M.; Cody, R.B.; Sparkman, D.O.; Bell, T.L.; Adams, M.A. Temperature-dependent release of volatile organic compounds of eucalypts by direct analysis in real time (DART) mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 2241–2246. [Google Scholar] [CrossRef]
- Amini, J.; Farhang, V.; Javadi, T.; Nazemi, J. Antifungal Effect of Plant Essential Oils on Controlling Phytophthora Species. Plant Pathol. J. 2016, 32, 16–24. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- De Martino, L.; Mancini, E.; De Almeida, L.F.R.; De Feo, V. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef]
- Koul, O.; Walia, S.; Dhaliwal, G.S.; Nagar, P. Essential oils as green pesticides potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Chu, C.; Mortimer, P.E.; Wang, H.; Wang, Y.; Liu, X.; Yu, S. Allelopathic effects of Eucalyptus on native and introduced tree species. For. Ecol. Manag. 2014, 323, 79–84. [Google Scholar] [CrossRef]
- Kaur, S.; Pal Singh, H.; R Batish, D.; Kumar Kohli, R. Role of Monoterpenes in Eucalyptus Communities. Curr. Bioact. Compd. 2012, 8, 101–107. [Google Scholar] [CrossRef]
- Singh, D.; Kohli, R.K.; Saxena, D.B. Effect of eucalyptus oil on germination and growth of Phaseolus aureus Roxb. Plant Soil 1991, 137, 223–227. [Google Scholar] [CrossRef]
- Kohli, R.K.; Singh, D. Allelopathic impact of volatile components from Eucalyptus on crop plants. Biol. Plant. 1991, 33, 475–483. [Google Scholar] [CrossRef]
- Silva, E.R.; Igartuburu, J.M.; Overbeck, G.E.; Soares, G.L.G.; MacÍas, F.A. Are phytotoxic effects of Eucalyptus saligna (Myrtaceae) essential oil related to its major compounds? Aust. J. Bot. 2021, 69, 174–183. [Google Scholar] [CrossRef]
- Qiu, X.; YU, S.; Wang, Y.; Fang, B.; Cai, C.; Li, S. Identification and allelopathic effects of 1,8-cineole from Eucalyptus urophylla on lettuce. Allelopath. J. 2010, 26, 255–264. [Google Scholar]
- Zhou, S.; Wei, C.; Zhang, C.; Han, C.; Kuchkarova, N.; Shao, H. Chemical composition, phytotoxic, antimicrobial and insecticidal activity of the essential oils of dracocephalum integrifolium. Toxins 2019, 11, 598. [Google Scholar] [CrossRef]
- Muller, C.H. The Role of Chemical Inhibition (Allelopathy) in Vegetational Composition. Bull. Torrey Bot. Club 1966, 93, 332. [Google Scholar] [CrossRef]
- Muller, W.H.; Lorber, P.; Haley, B. Volatile Growth Inhibitors Produced by Salvia leucophylla: Effect on Seedling Growth and Respiration. Bull. Torrey Bot. Club 1968, 95, 415. [Google Scholar] [CrossRef]
- Romagni, J.G.; Duke, S.O.; Dayan, F.E. Inhibition of plant asparagine synthetase by monoterpene cineoles. Plant Physiol. 2000, 123, 725–732. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barton, A.F.M.; Dell, B.; Knight, A.R. Herbicidal activity of cineole derivatives. J. Agric. Food Chem. 2010, 58, 10147–10155. [Google Scholar] [CrossRef]
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 2015, 48, 1–5. [Google Scholar] [CrossRef]
- Elaissi, A.; Salah, K.H.; Mabrouk, S.; Larbi, K.M.; Chemli, R.; Harzallah-Skhiri, F. Antibacterial activity and chemical composition of 20 Eucalyptus species’ essential oils. Food Chem. 2011, 129, 1427–1434. [Google Scholar] [CrossRef]
- Aleksic Sabo, V.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crops Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Hąc-Wydro, K.; Flasiński, M.; Broniatowski, M.; Sołtys, M. Studies on the Behavior of Eucalyptol and Terpinen-4-ol-Natural Food Additives and Ecological Pesticides-in Model Lipid Membranes. Langmuir 2017, 33, 6916–6924. [Google Scholar] [CrossRef]
- Połeć, K.; Wójcik, A.; Flasiński, M.; Wydro, P.; Broniatowski, M.; Hąc-Wydro, K. The influence of terpinen-4-ol and eucalyptol—The essential oil components-on fungi and plant sterol monolayers. Biochim. Biophys. Acta-Biomembr. 2019, 1861, 1093–1102. [Google Scholar] [CrossRef]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6290–6304. [Google Scholar]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 4531. [Google Scholar] [CrossRef]
- Sales, A.; Felipe, L.D.O.; Bicas, J.L. Production, Properties, and Applications of α-Terpineol. Food Bioprocess Technol. 2020, 13, 1261–1279. [Google Scholar] [CrossRef]
- Chowdhury, S.; Kumar, S. Alpha-terpinyl acetate: A natural monoterpenoid from Elettaria cardamomum as multi-target directed ligand in Alzheimer’s disease. J. Funct. Foods 2020, 68, 103892. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Marshall, M.R.; Wei, C.I. Antibacterial Activity of Some Essential Oil Components against Five Foodborne Pathogens. J. Agric. Food Chem. 1995, 43, 2839–2845. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, S.Y.; Hardie, W.J.; Smith, J.P. Stomatal density of grapevine leaves (Vitis vinifera L.) responds to soil temperature and atmospheric carbon dioxide. Aust. J. Grape Wine Res. 2011, 17, 147–152. [Google Scholar] [CrossRef]
- Santos, L.D.T.; Thadeo, M.; Iarema, L.; Meira, R.M.S.A.; Ferreira, F.A. Foliar anatomy and histochemistry in seven species of Eucalyptus. Rev. Arvore 2008, 32, 769–779. [Google Scholar] [CrossRef][Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Chieco, C.; Rotondi, A.; Morrone, L.; Rapparini, F.; Baraldi, R. An ethanol-based fixation method for anatomical and micro-morphological characterization of leaves of various tree species. Biotech. Histochem. 2013, 88, 109–119. [Google Scholar] [CrossRef]
- Pathan, A.K.; Bond, J.; Gaskin, R.E. Sample preparation for scanning electron microscopy of plant surfaces-Horses for courses. Micron 2008, 39, 1049–1061. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Allured Publ. Corp. 2017, 8, 804. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Goodner, K.L. Practical retention index models of OV-101, DB-1, DB-5, and DB-Wax for flavor and fragrance compounds. LWT 2008, 41, 951–958. [Google Scholar] [CrossRef]
- Jennings, W.G.; Shibamoto, T. Qualitative analysis of flavor and fragrance volatiles by glass capillary gas chromatography. Jennings Walter Shibamoto Takayuki 1980, 26, 472. [Google Scholar]
- Wiley, J. Wiley Registry of Mass Spectral Data, with NIST Spectral Data CD Rom, 7th ed.; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Bewley, J.D. Seed Germination and Dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Bachir, R.G.; Benali, M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2012, 2, 739. [Google Scholar] [CrossRef]
- Montone, A.M.I.; Papaianni, M.; Malvano, F.; Capuano, F.; Capparelli, R.; Albanese, D. Lactoferrin, quercetin, and hydroxyapatite act synergistically against pseudomonas fluorescens. Int. J. Mol. Sci. 2021, 22, 9247. [Google Scholar] [CrossRef]
- Vitale, S.; Di Pietro, A.; Turrà, D. Autocrine pheromone signalling regulates community behaviour in the fungal pathogen Fusarium oxysporum. Nat. Microbiol. 2019, 4, 1443–1449. [Google Scholar] [CrossRef]

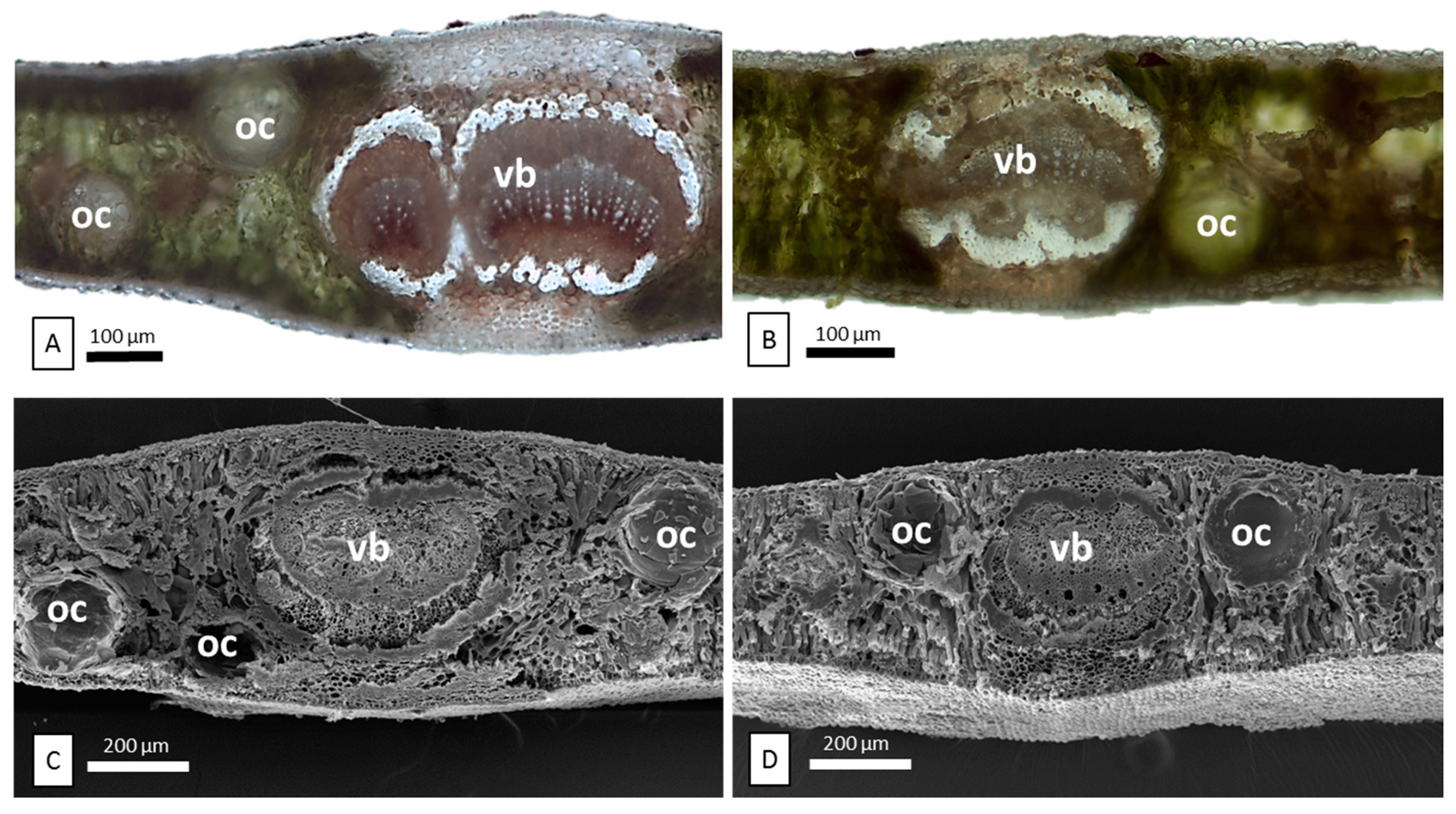
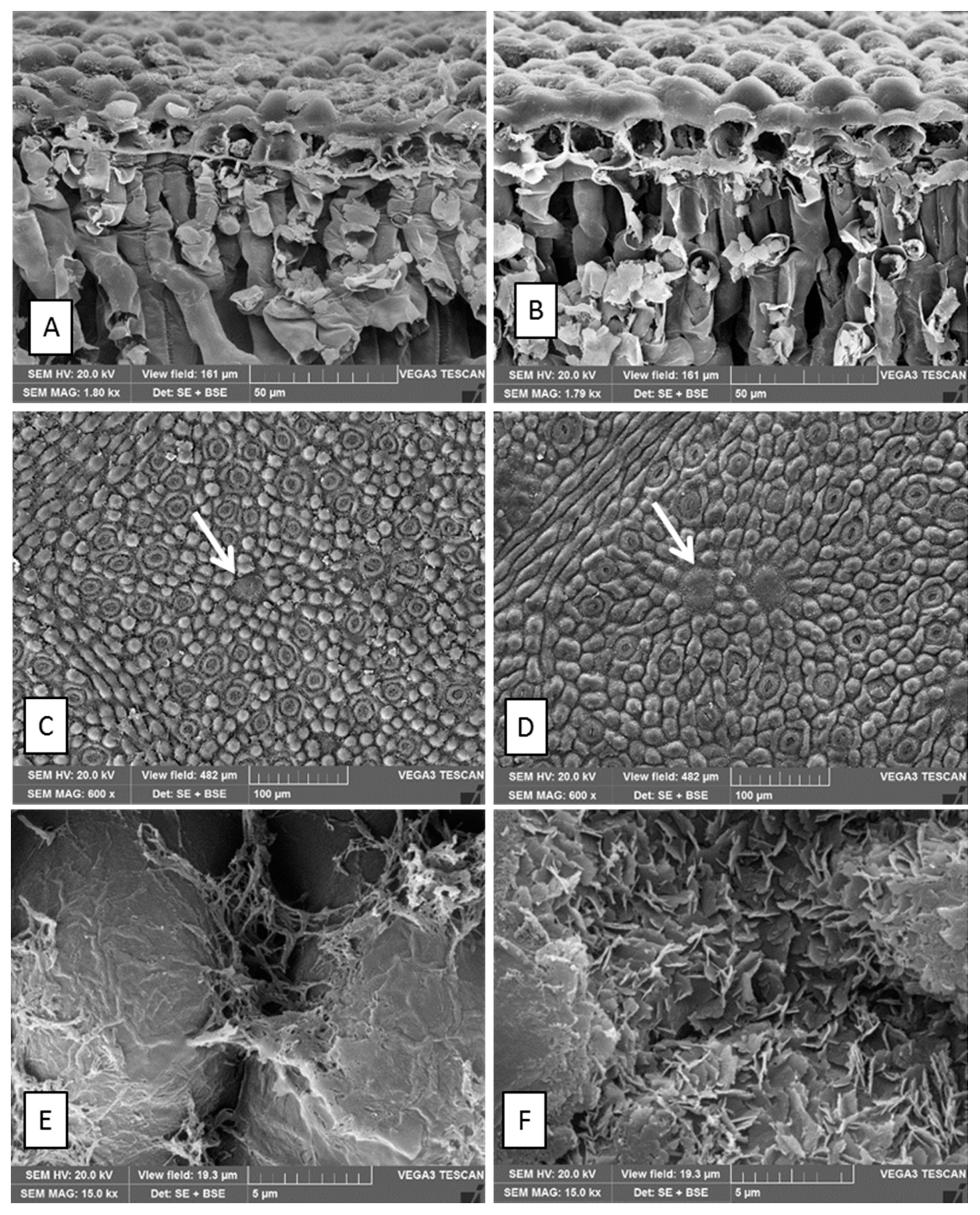



| Species | Oil Gland Mean Density (No. Glands per mm2 ± DS) | %Yield of Essential Oil | |
|---|---|---|---|
| Abaxial | Adaxial | ||
| E. cinerea | 9.6 ± 3.2 | 12.2 ± 2.4 | 2.56 |
| E. nicholii | 22.1 ± 4.9 | 18.3 ± 4.2 | 3.67 |
| Compound | EC % | EN % | Ki a | Ki b | Identification c |
|---|---|---|---|---|---|
| α-Pinene | 7.3 | 3.7 | 858 | 1028 | 1,2,3 |
| Camphene | 0.2 | 0.2 | 869 | 1075 | 1,2,3 |
| β-Pinene | 0.3 | 0.2 | 893 | 1105 | 1,2,3 |
| 3-p-Menthene | 0.1 | 0.2 | 903 | 1,2 | |
| β-Myrcene | 0.2 | 0.1 | 913 | 1174 | 1,2,3 |
| α-Phellandrene | 0.8 | 0.8 | 921 | 1176 | 1,2,3 |
| α-Terpinene | 3.3 | 3.3 | 934 | 1188 | 1,2,3 |
| Eucalyptol (1,8-cineole) | 67.7 | 79.5 | 949 | 1213 | 1,2,3 |
| γ-Terpinene | 1.2 | 0.9 | 971 | 1254 | 1,2,3 |
| p-Mentha-3,8-diene | 0.2 | 0.1 | 982 | 1259 | 1,2 |
| Terpinolene | 3.5 | 2.5 | 998 | 1265 | 1,2,3 |
| Linalool | 0.2 | 0.3 | 1018 | 1553 | 1,2,3 |
| allo-Ocimene | 0.1 | - | 1039 | 1409 | 1,2 |
| trans-Pinocarveol | - | 0.3 | 1041 | 1641 | 1,2 |
| neo-allo-Ocimene | 0.3 | - | 1042 | 1,2 | |
| Pinocarvone | - | 0.1 | 1063 | 1587 | 1,2 |
| Borneol | 0.1 | 0.1 | 1067 | 1715 | 1,2,3 |
| neoiso-Pulegol | 0.2 | 0.1 | 1070 | 1,2 | |
| Terpinen-4-ol | 0.6 | 0.7 | 1079 | 1705 | 1,2,3 |
| α-Terpineol | 3.9 | 3.9 | 1093 | 1720 | 1,2,3 |
| Linalyl acetate | 0.1 | - | 1219 | 1565 | 1,2 |
| δ-EIemene | - | 0.1 | 1220 | 1480 | 1,2,3 |
| α-Terpinyl acetate | 5.2 | - | 1234 | 1687 | 1,2 |
| α-Copaene | 0.3 | - | 1270 | 1498 | 1,2,3 |
| α-Gurjunene | 0.1 | - | 1287 | 1529 | 1,2 |
| (E)-Caryophyllene | 0.5 | 0.3 | 1295 | 1575 | 1,2,3 |
| Aromadendrene | 0.3 | - | 1308 | 1628 | 1,2 |
| α-Humulene | 0.1 | - | 1323 | 1651 | 1,2 |
| allo-Aromadendrene | 0.1 | 0.2 | 1330 | 1661 | 1,2 |
| β-Selinene | 0.2 | - | 1347 | 1697 | 1,2 |
| Viridiflorene | 0.5 | 0.1 | 1366 | 1713 | 1,2 |
| Total | 97.6 | 97.7 | |||
| Monoterpene hydrocarbons | 17.5 | 12.0 | |||
| Oxigenated monoterpenes | 78.0 | 85.0 | |||
| Sesquiterpene hydrocarbons | 2.1 | 0.7 | |||
| Oxigenated sesquiterpenes | 0 | 0 |
| Number of Germinated Seeds | |||||||
|---|---|---|---|---|---|---|---|
| L. multiflorum | S. alba | C. sativus | L. sativa | P. sativum | R. sativus | R. graveolens | |
| Control | 6.3 ± 1.5 | 8.3 ± 1.5 | 9.3 ± 1.2 | 7.3 ± 1.5 | 9.7 ± 0.6 | 6.3 ± 1.2 | 6.3 ± 2.5 |
| Treatment (µg/mL) | |||||||
| 100 | 3.3 ± 2.1 | 6.0 ± 1.7 | 8.7 ± 0.6 | 8.0 ± 2.0 | 8.7 ± 2.3 | 2.0 ± 1.0 ** | 1.0 ± 1.0 ** |
| 250 | 8.0 ± 1.0 | 1.0 ± 1.0 **** | 9.7 ± 0.6 | 9.0 ± 1.0 | 8.7 ± 1.5 | 2.0 ± 2.0 ** | 0.3 ± 0.6 *** |
| 500 | 6.0 ± 3.6 | 0.7 ± 1.2 **** | 10.0 ± 0.0 | 7.3 ± 1.5 | 8.7 ± 1.5 | 0.0 ± 0.0 *** | 2.0 ± 1.0 ** |
| 1000 | 6.7 ± 1.5 | 0.0 ± 0.0 **** | 8.7 ± 1.2 | 8.0 ± 0.0 | 8.0 ± 1.0 | 0.3 ± 0.6 *** | 0.3 ± 0.6 *** |
| Radicle Length (cm) | |||||||
| L. multiflorum | S. alba | C. sativus | L. sativa | P. sativum | R. sativus | R. graveolens | |
| Control | 1.2 ± 0.6 | 0.4 ± 0.2 | 6.3 ± 0.9 | 3.2 ± 1.2 | 6.0 ± 1.8 | 3.1 ± 1.0 | 1.7 ± 0.8 |
| Treatment (µg/mL) | |||||||
| 100 | 0.4 ± 0.0 | 0.2 ± 0.1 | 3.4 ± 0.7 *** | 1.4 ± 0.5 | 3.5 ± 1.3 | 2.2 ± 0.0 ** | 0.0 ± 0.0 ** |
| 250 | 0.9 ± 0.4 | 0.0 ± 0.0 ** | 2.3 ± 0.5 **** | 2.5 ± 0.7 | 2.4 ± 0.8 * | 0.0 ± 0.0 ** | 0.0 ± 0.0 ** |
| 500 | 1.1 ± 0.5 | 0.0 ± 0.0 ** | 2.3 ± 0.4 **** | 1.8 ± 0.7 | 2.9 ± 0.9 * | 0.0 ± 0.0 ** | 0.9 ± 0.0 |
| 1000 | 0.8 ± 0.4 | 0.0 ± 0.0 ** | 2.3 ± 0.8 **** | 2.3 ± 0.8 | 2.8 ± 1.0 * | 0.0 ± 0.0 ** | 0.0 ± 0.0 ** |
| Number of Germinated Seeds | |||||||
|---|---|---|---|---|---|---|---|
| L. multiflorum | S. alba | C. sativus | L. sativa | P. sativum | R. sativus | R. graveolens | |
| Control | 5.3 ± 2.1 | 9.7 ± 0.6 | 9.0 ± 1.0 | 8.7 ± 0.6 | 10.0 ± 0.0 | 6.0 ± 1.0 | 8.0 ± 2.0 |
| Treatment (µg/mL) | |||||||
| 100 | 5.7 ± 1.5 | 6.0 ± 1.0 *** | 10.0 ± 0.0 | 8.0 ± 0.0 | 9.3 ± 0.6 | 2.0 ± 1.0 ** | 4.7 ± 3.5 |
| 250 | 4.3 ± 1.2 | 4.0 ± 1.0 **** | 10.0 ± 0.0 | 8.7 ± 0.6 | 9.0 ± 0.0 | 2.0 ± 1.0 ** | 1.0 ± 1.0 ** |
| 500 | 5.3 ± 3.2 | 0.0 ± 0.0 **** | 9.0 ± 1.0 | 9.0 ± 1.7 | 9.7 ± 0.6 | 0.7 ± 0.6 *** | 1.0 ± 1.0 ** |
| 1000 | 5.7 ± 2.3 | 0.0 ± 0.0 **** | 10.0 ± 0.0 | 8.0 ± 1.0 | 9.0 ± 1.0 | 0.7 ± 1.2 *** | 0.0 ± 0.0 ** |
| Radicle Length (cm) | |||||||
| L. multiflorum | S. alba | C. sativus | L. sativa | P. sativum | R. sativus | R. graveolens | |
| Control | 3.4 ± 0.8 | 0.6 ± 0.2 | 6.7 ± 0.9 | 2.7 ± 1.0 | 6.3 ± 1.9 | 2.8 ± 1.1 | 1.2 ± 0.5 |
| Treatment (µg/mL) | |||||||
| 100 | 0.9 ± 0.3 *** | 0.4 ± 0,2 | 2.6 ± 0.7 *** | 2.6 ± 0.8 | 3.4 ± 0.9 * | 1.3 ± 0.0 ** | 0.8 ± 0.0 |
| 250 | 0.6 ± 0.2 **** | 0.2 ± 0.1 * | 2.7 ± 0.5 *** | 1.5 ± 0.5 | 2.9 ± 0.7 * | 1.9 ± 0.0 | 0.0 ± 0.0 *** |
| 500 | 0.6 ± 0.2 **** | 0.0 ± 0.0 *** | 1.9 ± 0.6 **** | 2.6 ± 0.9 | 3.7 ± 1.2 | 0.0 ± 0.0 **** | 0.0 ± 0.0 *** |
| 1000 | 0.9 ± 0.4 *** | 0.0 ± 0.0 *** | 2.0 ± 0.9 **** | 2.4 ± 0.8 | 2.9 ± 1.2 * | 0.0 ± 0.0 **** | 0.0 ± 0.0 *** |
| Treatment | MIC (% v/v) | ||
|---|---|---|---|
| Xanthomonas campestris pv. campestris | Enterobacter cloacae | Citrobacter freundii | |
| EO of E. cinerea (EC) | 0.01% | 0.01% | 0.01% |
| EO of E. nicholii (EN) | 0.01% | 0.01% | 0.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaspina, P.; Papaianni, M.; Ranesi, M.; Polito, F.; Danna, C.; Aicardi, P.; Cornara, L.; Woo, S.L.; De Feo, V. Eucalyptus cinerea and E. nicholii by-Products as Source of Bioactive Compounds for Agricultural Applications. Plants 2022, 11, 2777. https://doi.org/10.3390/plants11202777
Malaspina P, Papaianni M, Ranesi M, Polito F, Danna C, Aicardi P, Cornara L, Woo SL, De Feo V. Eucalyptus cinerea and E. nicholii by-Products as Source of Bioactive Compounds for Agricultural Applications. Plants. 2022; 11(20):2777. https://doi.org/10.3390/plants11202777
Chicago/Turabian StyleMalaspina, Paola, Marina Papaianni, Marta Ranesi, Flavio Polito, Cristina Danna, Pierluca Aicardi, Laura Cornara, Sheridan L. Woo, and Vincenzo De Feo. 2022. "Eucalyptus cinerea and E. nicholii by-Products as Source of Bioactive Compounds for Agricultural Applications" Plants 11, no. 20: 2777. https://doi.org/10.3390/plants11202777
APA StyleMalaspina, P., Papaianni, M., Ranesi, M., Polito, F., Danna, C., Aicardi, P., Cornara, L., Woo, S. L., & De Feo, V. (2022). Eucalyptus cinerea and E. nicholii by-Products as Source of Bioactive Compounds for Agricultural Applications. Plants, 11(20), 2777. https://doi.org/10.3390/plants11202777









