Arbuscular Mycorrhizal Fungi Promote Physiological and Biochemical Advantages in Handroanthus serratifolius Seedlings Submitted to Different Water Deficits
Abstract
1. Introduction
2. Results
2.1. Growth and Colonization
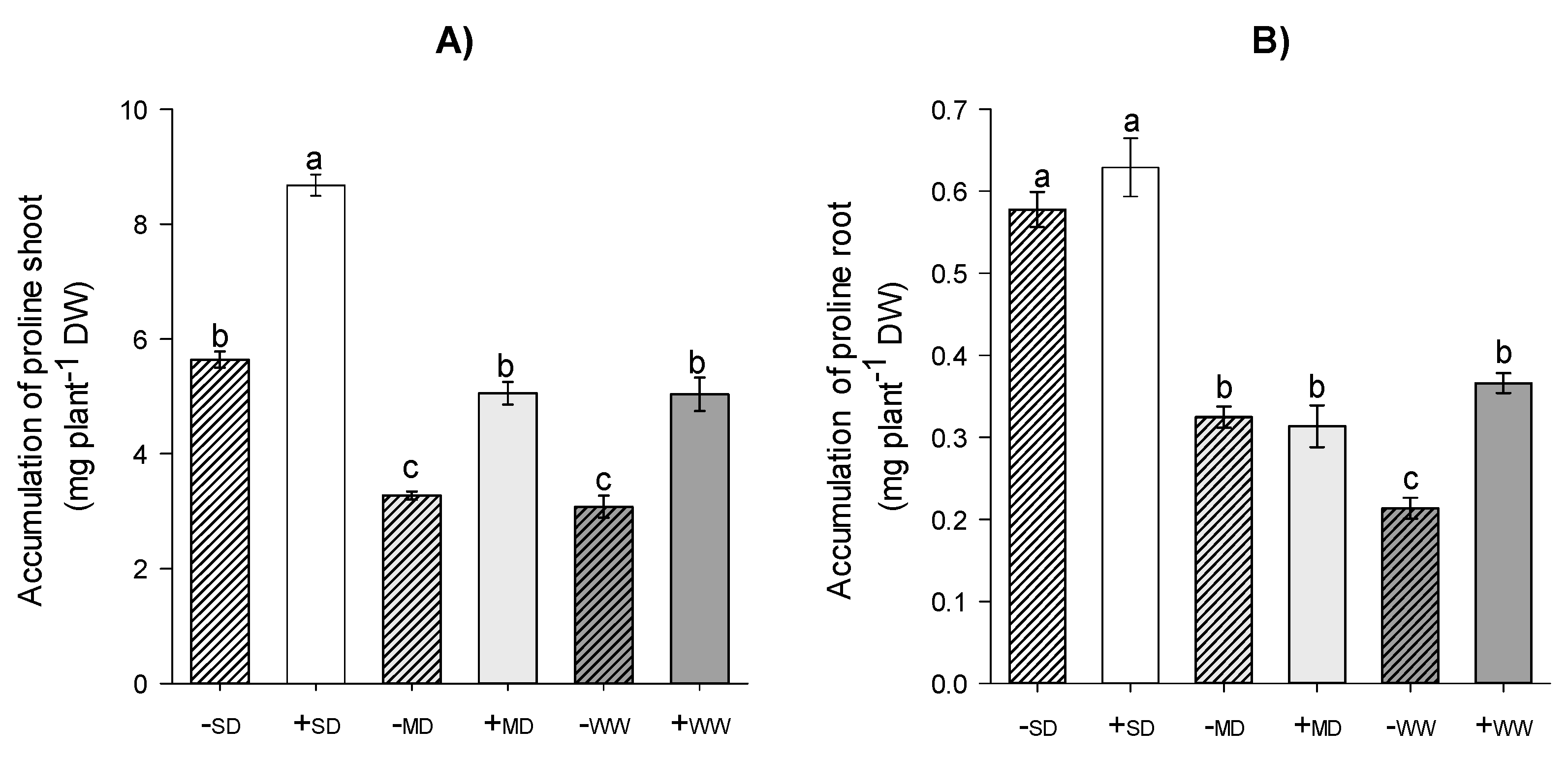
2.2. Nitrogen Metabolism
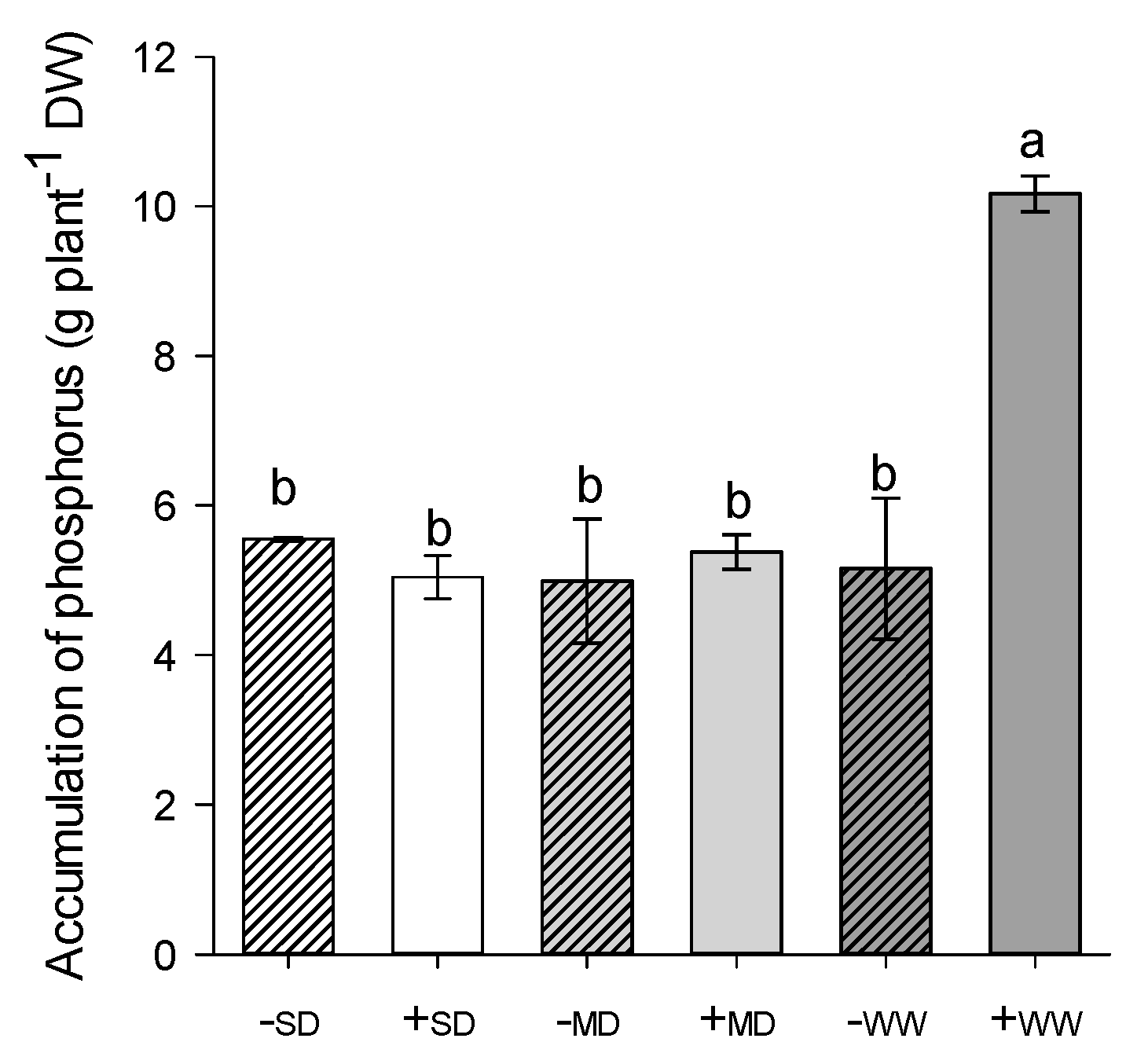
2.3. Phosphorus
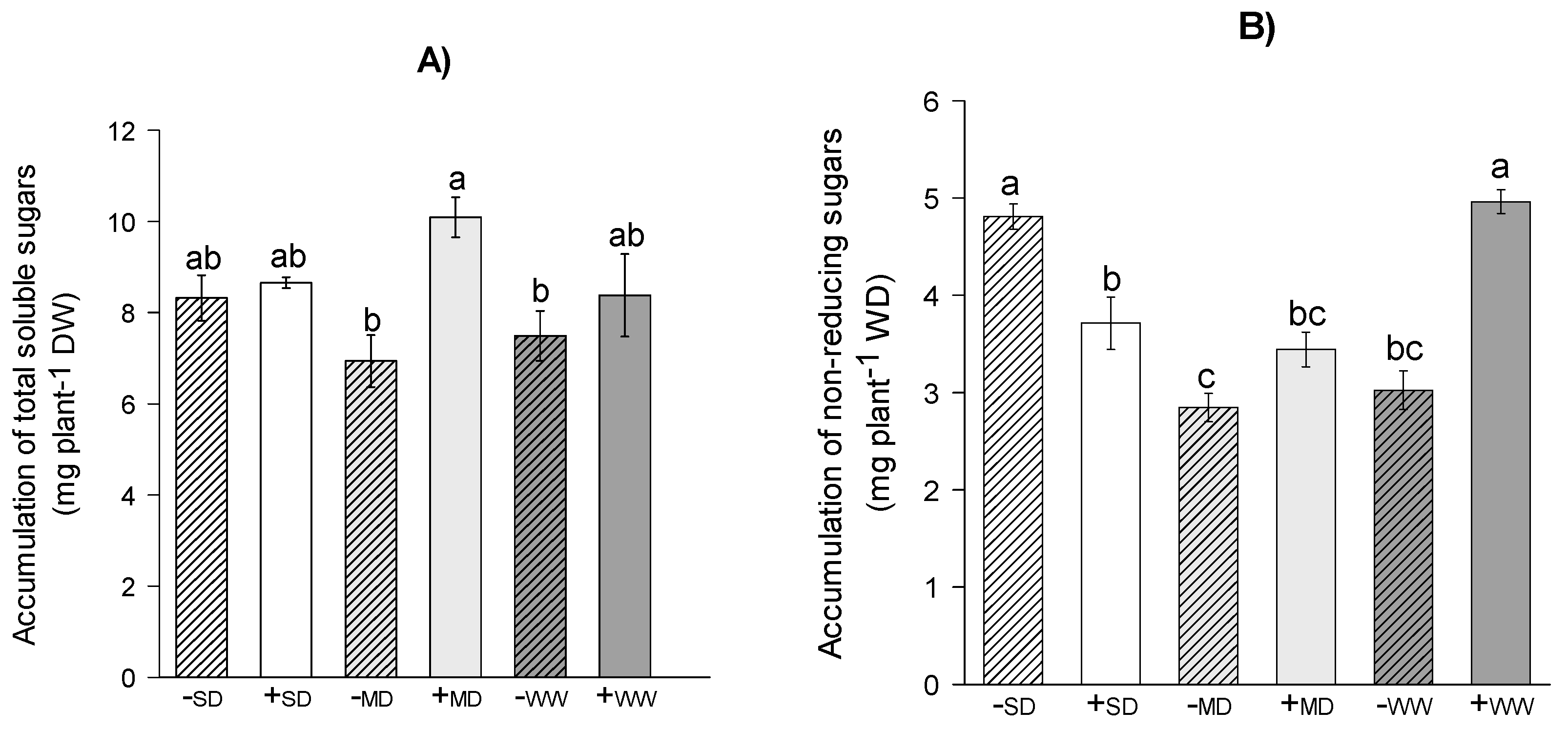
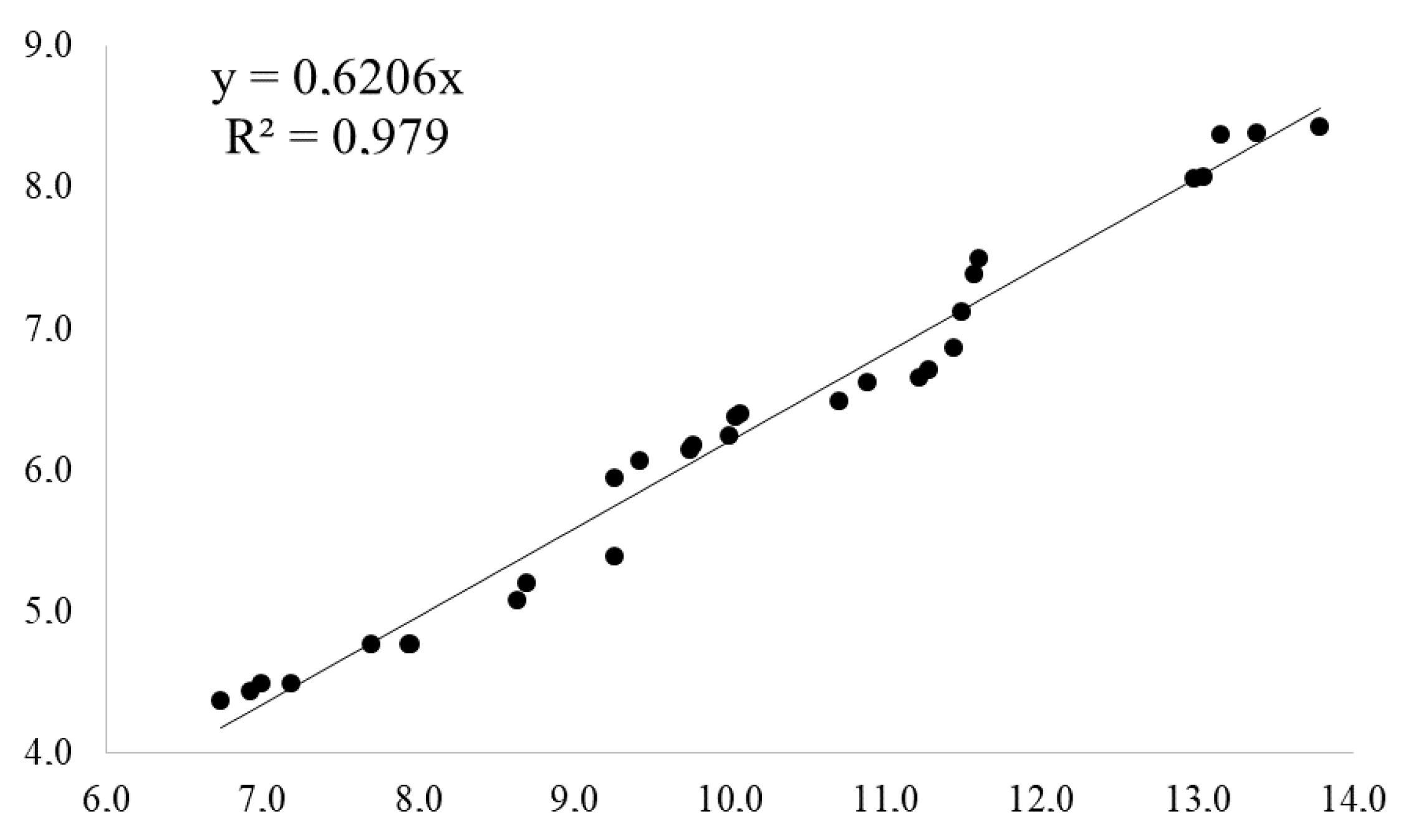
2.4. Carbohdrate Metabolism
3. Discussion
4. Materials and Methods
4.1. Location and Experiment Design
4.2. Observations and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.-H.; Zou, Y.-N.; Rahman, M.M.; Ni, Q.-D.; Wu, Q.-S. Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci. Rep. 2017, 7, 42389. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Chiarelli, D.D.; Rulli, M.C.; Dell’Angelo, J.; D’Odorico, P. Global agricultural economic water scarcity. Sci. Adv. 2020, 6, eaaz6031. [Google Scholar] [CrossRef] [PubMed]
- Stovall, A.E.L.; Shugart, H.; Yang, X. Tree height explains mortality risk during an intense drought. Nat. Commun. 2019, 10, 1–6. [Google Scholar] [CrossRef]
- Phillips, O.L.; Aragão, L.E.O.C.; Lewis, S.L.; Fisher, J.B.; Lloyd, J.; López-González, G.; Malhi, Y.; Monteagudo, A.; Peacock, J.; Quesada, C.A.; et al. Drought Sensitivity of the Amazon Rainforest. Science 2009, 323, 1344–1347. [Google Scholar] [CrossRef]
- da Silva, A.P.M.; Schweizer, D.; Marques, H.R.; Cordeiro Teixeira, A.M.; Dos Santos, T.V.M.N.; Sambuichi, R.H.R.; Badari, C.G.; Gaudare, U.; Brancalion, P.H.S. Can current native tree seedling production and infrastructure meet an increasing forest restoration demand in Brazil? Restor. Ecol. 2017, 25, 509–515. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Caron, B.O.; Elli, E.F.; Monteiro, G.C.; Schwerz, F.; Basso, C.J.; Manfron, P.A. Production and quality of Caesalpinia pluviosa seedlings in different substrates. Científica 2017, 45, 1–8. [Google Scholar] [CrossRef][Green Version]
- Andrade, G.K.O.; Ferreira, R.A.; Fernandes, M.M.; Da Silva, T.R.; Souza, I.B.A.; Magalhães, J.S. Regeneração natural em área de reflorestamento misto com espécies nativas no município de Laranjeiras, SE. Rev. Ciências Agrárias 2018, 61, 1–9. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, B.-R.; La, V.H.; Mamun, A.; Bae, D.-W.; Kim, T.-H. Characterization of salicylic acid- and abscisic acid-mediated photosynthesis, Ca2+ and H2O2 accumulation in two distinct phases of drought stress intensity in Brassica napus. Environ. Exp. Bot. 2021, 186, 104434. [Google Scholar] [CrossRef]
- Bourbia, I.; Carins-Murphy, M.R.; Gracie, A.; Brodribb, T.J. Xylem cavitation isolates leaky flowers during water stress in pyrethrum. New Phytol. 2020, 227, 146–155. [Google Scholar] [CrossRef]
- Brodribb, T.; Brodersen, C.R.; Carriqui, M.; Tonet, V.; Dominguez, C.R.; McAdam, S. Linking xylem network failure with leaf tissue death. New Phytol. 2021, 232, 68–79. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Barroso, D.G.; Fiqueiredo, F.A.M.M.D.A. Fungos micorrízicos arbusculares no crescimento e na nutrição mineral de mudas de Tectona grandis L. F. Cienc. Florest. 2018, 28, 25–34. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Paredes-Jácome, J.R.; Hernández-Montiel, L.G.; Robledo-Torres, V.; González-Fuentes, J.A.; Chiquito-Contreras, R.G.; Mendoza-Villarreal, R. Arbuscular mycorrhizal fungus and organics substrates effect on bean plant morphology and minerals. Rev. Terra Latinoam. 2022, 40, e1012. [Google Scholar] [CrossRef]
- Pereira, J.; Barreto-Garcia, P.; Scoriza, R.; Júnior, O.S.; Gomes, V.D.S. Forest science (ciências florestais) Arbuscular mycorrhizal fungi in soils of arboreal Caatinga submitted to forest management. Rev. Bras. Cienc. Agrar. 2018, 13, 1–6. [Google Scholar] [CrossRef]
- da Silva, E.P.; Ferreira, P.A.A.; Furtini-Neto, A.E.; Soares, C.R.F.S. Micorrizas arbusculares e fosfato no desenvolvimento de mudas de cedro-australiano. Cienc. Florest. 2017, 27, 1269–1281. [Google Scholar] [CrossRef]
- Merwad, A.-R.M.A.; Desoky, E.-S.M.; Rady, M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. CMLS 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Zappi, D.C.; Filardi, F.L.R.; Leitman, P.; Souza, V.C.; Walter, B.M.T.; Pirani, J.R.; Morim, M.P.; Queiroz, L.P.; Cavalcanti, T.; Mansano, V.; et al. Growing knowledge: An overview of Seed Plant diversity in Brazil. Rodriguesia 2015, 66, 1085–1113. [Google Scholar] [CrossRef]
- Lima, P.A.F.; Gatto, A.; De Albuquerque, L.B.; Malaquias, J.V.; Aquino, F.D.G. Native species seedling growth in the ecological restoration of riparian forest. Neotrop. Biol. Conserv. 2016, 11, 72–79. [Google Scholar] [CrossRef]
- Macêdo, F.A.d.A.; Coêlho, M.L.; Ribeiro, M.C.; Barros, E.D.S.; Gomes, J.P.D.S.; Batista, C.L.; Lima, L.K.F.; de Sousa, R.P. Análise das atividades farmacológicas da Handroanthus serratifolius (Vahl) S.Grose. Res. Soc. Dev. 2022, 11, e5611222891. [Google Scholar] [CrossRef]
- Lentini, M.; Carvalho, T.; Nunes, F.; Cerignoni, F. A exploração do ipê (Handroanthus spp.) em florestas naturais da amazônia brasileira: Desafios e oportunidades para a conservação e o manejo responsável. Bol. Timberflow 2021, 4, 1–10. [Google Scholar]
- Scabora, M.H.; Maltoni, K.L.; Cassiolato, A.M.R. Crescimento, fosfatase ácida e micorrização de espécies arbóreas, em solo de cerrado degradado. Bragantia 2010, 69, 445–451. [Google Scholar] [CrossRef]
- Souza, R.G.; Silva, D.K.A.; Oliveira, J.R.G.; Goto, B.T.; Silva, F.S.B.; Sampaio, E.V.; Maia, L.C. Use of mycorrhizal seedlings on recovery of mined dunes in northeastern Brazil. Pedobiol. Int. J. Soil Biol. 2012, 55, 303–309. [Google Scholar] [CrossRef]
- Zou, Y.-N.; Wu, Q.-S.; Huang, Y.-M.; Ni, Q.-D.; He, X.-H. Mycorrhizal-Mediated Lower Proline Accumulation in Poncirus trifoliata under Water Deficit Derives from the Integration of Inhibition of Proline Synthesis with Increase of Proline Degradation. PLoS ONE 2013, 8, e80568. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Xu, G.; Zhou, L.; Li, Y. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Zenia insignis seedlings under drought stress. New For. 2019, 50, 593–604. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Huang, Y. Effects of arbuscular mycorrhizal fungi on the drought tolerance of Cyclobalanopsis glauca seedlings under greenhouse conditions. New For. 2014, 45, 545–556. [Google Scholar] [CrossRef]
- Vieira, D.A.; Toro-Herrera, M.A.; Mendonça, A.M.D.C.; Barbosa, J.P.R.A.D. Do brassinosteroids alleviate water stress in seedlings of Handroanthus serratifolius ? Ann. Appl. Biol. 2022, 180, 151–162. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean Ornamental Plants to Drought Stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef]
- Huang, L.; Li, M.; Zhou, K.; Sun, T.; Hu, L.; Li, C.; Ma, F. Uptake and metabolism of ammonium and nitrate in response to drought stress in Malus prunifolia. Plant Physiol. Biochem. 2018, 127, 185–193. [Google Scholar] [CrossRef]
- Urgiles, N.; Loján, P.; Aguirre, N.; Blaschke, H.; Günter, S.; Stimm, B.; Kottke, I. A aplicação de raízes micorrízicas melhora o crescimento de mudas de árvores tropicais no viveiro: Um passo para o reflorestamento com espécies nativas nos Andes do Equador. New For. 2009, 38, 229–239. [Google Scholar] [CrossRef]
- Schüßler, A.; Kruger, C.; Urgiles, N. Phylogenetically diverse AM fungi from Ecuador strongly improve seedling growth of native potential crop trees. Mycorrhiza 2016, 26, 199–207. [Google Scholar] [CrossRef]
- Frosi, G.; Barros, V.A.; Oliveira, M.T.; Santos, M.; Ramos, D.G.; Maia, L.C.; Santos, M.G. Symbiosis with AMF and leaf Pi supply increases water deficit tolerance of woody species from seasonal dry tropical forest. J. Plant Physiol. 2016, 207, 84–93. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved Drought Tolerance by AMF Inoculation in Maize (Zea mays) Involves Physiological and Biochemical Implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef]
- Ozturk, M.; Unal, B.T.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-S.; Srivastava, A.K.; Zou, Y.-N. AMF-induced tolerance to drought stress in citrus: A review. Sci. Hortic. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Oliveira, J.J.F.; Sousa, R.F.; Carneiro, R.F.V.; Fonseca, J.M. Crescimento inicial de plantas de leucena frente à inoculação micorrízica e adubação orgânica Initial growth plant leucaena forward to mycorrhizal inoculation and organic fertilization. Rev. Bras. Agroecol. 2013, 8, 212–220. [Google Scholar]
- Baslam, M.; Antolín, M.; Gogorcena, Y.; Muñoz, F.; Goicoechea, N. Changes in alfalfa forage quality and stem carbohydrates induced by arbuscular mycorrhizal fungi and elevated atmospheric CO2. Ann. Appl. Biol. 2014, 164, 190–199. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Gopi, R.; Somasundaram, R.; Panneerselvam, R. Induction of drought stress tolerance by ketoconazole in Catharanthus roseus is mediated by enhanced antioxidant potentials and secondary metabolite accumulation. Colloids Surf. B Biointerfaces 2007, 60, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Yooyongwech, S.; Phaukinsang, N.; Cha-Um, S.; Supaibulwatana, K. Arbuscular mycorrhiza improved growth performance in Macadamia tetraphylla L. grown under water deficit stress involves soluble sugar and proline accumulation. Plant Growth Regul. 2013, 69, 285–293. [Google Scholar] [CrossRef]
- Al-Arjani, A.-B.F.; Hashem, A.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi modulates dynamics tolerance expression to mitigate drought stress in Ephedra foliata Boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef]
- Chun, S.C.; Paramasivan, M.; Chandrasekaran, M. Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbiol. 2018, 9, 2525. [Google Scholar] [CrossRef]
- Chen, S.; Jin, W.; Liu, A.; Zhang, S.; Liu, D.; Wang, F.; Lin, X.; He, C. Arbuscular mycorrhizal fungi (AMF) increase growth and secondary metabolism in cucumber subjected to low temperature stress. Sci. Hortic. 2013, 160, 222–229. [Google Scholar] [CrossRef]
- Sharma, M.; Prasad, R. The Quorum-Sensing Molecule Farnesol Is a Modulator of Drug Efflux Mediated by ABC Multidrug Transporters and Synergizes with Drugs in Candida albicans. Antimicrob. Agents Chemother. 2011, 55, 4834–4843. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Hashem, A.; Abd-Allah, E.F.; Ahmad, P. Arbuscular Mycorrhiza in Crop Improvement under Environmental Stress. Emerg. Technol. Manag. Crop Stress Toler. 2014, 2, 69–95. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Aldubise, A.; Egamberdieva, D. Arbuscular mycorrhizal fungi enhances salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J. Plant Interact. 2015, 10, 230–242. [Google Scholar] [CrossRef]
- Miller, A.J.; Cramer, M.D. Root Nitrogen Acquisition and Assimilation. Plant Soil 2005, 274, 1–36. [Google Scholar] [CrossRef]
- Rani, B.; Madan, S.; Sharma, K.; Ja, P.; Berwal, M.K.; Kumar, A. Effect of Mycorrhizal Colonization on Nitrogen and Phosphorous Metabolism in Wheat (Triticum aestivum L.) under Water Deficit Stress. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 916–929. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant Nitrogen Assimilation and Use Efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Crawford, N.M. Nitrate: Nutrient and signal for plant growth. Plant Cell 1995, 7, 859–868. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, D.; Yapa, N. Arbuscular mycorrhizal fungi inoculation enhances drought stress tolerance of plants. Groundw. Sustain. Dev. 2018, 7, 490–494. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Samphumphuang, T.; Tisarum, R.; Theerawitaya, C.; Cha-Um, S. Arbuscular mycorrhizal fungi (AMF) improved water deficit tolerance in two different sweet potato genotypes involves osmotic adjustments via soluble sugar and free proline. Sci. Hortic. 2016, 198, 107–117. [Google Scholar] [CrossRef]
- Tisarum, R.; Samphumphuang, T.; Yooyoungwech, S.; Singh, H.P.; Cha-Um, S. Arbuscular mycorrhizal fungi modulate physiological and morphological adaptations in para rubber tree (Hevea brasiliensis) under water deficit stress. Biologia 2022, 77, 1723–1736. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Hashem, A.; Rasool, S.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D.; Jan, S.; Anjum, N.A.; Ahmad, P. Arbuscular mycorrhizal symbiosis and abiotic stress in plants: A review. J. Plant Biol. 2016, 59, 407–426. [Google Scholar] [CrossRef]
- Grace, E.J.; Cotsaftis, O.; Tester, M.; Smith, F.A.; Smith, S.E. Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytol. 2009, 181, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, A.; Magurno, F.; Bonfante, P.; Lanfranco, L. Expression profiles of a phosphate transporter gene (GmosPT) from the endomycorrhizal fungus Glomus mosseae. Mycorrhiza 2005, 15, 620–627. [Google Scholar] [CrossRef]
- Fiorilli, V.; Lanfranco, L.; Bonfante, P. The expression of GintPT, the phosphate transporter of Rhizophagus irregularis, depends on the symbiotic status and phosphate availability. Planta 2013, 237, 1267–1277. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Xia, R.-X. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J. Plant Physiol. 2006, 163, 417–425. [Google Scholar] [CrossRef]
- Ouledali, S.; Ennajeh, M.; Zrig, A.; Gianinazzi, S.; Khemira, H. Estimating the contribution of arbuscular mycorrhizal fungi to drought tolerance of potted olive trees (Olea europaea). Acta Physiol. Plant. 2018, 40, 81. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Yang, R.; Zheng, J.; Liu, C.; Li, H.; Ma, J.; Zhang, Y.; Wei, C.; Zhang, X. Regulation of Plant Growth, Photosynthesis, Antioxidation and Osmosis by an Arbuscular Mycorrhizal Fungus in Watermelon Seedlings under Well-Watered and Drought Conditions. Front. Plant Sci. 2016, 7, 644. [Google Scholar] [CrossRef] [PubMed]
- Zarik, L.; Meddich, A.; Hijri, M.; Hafidi, M.; Ouhammou, A.; Ouahmane, L.; Duponnois, R.; Boumezzough, A. Utilisation de champignons mycorhiziens arbusculaires en vue de l’amélioration de la tolérance à la sécheresse de Cupressus atlantica G. Comptes Rendus. Biol. 2016, 339, 185–196. [Google Scholar] [CrossRef]
- Zhang, H.; Sonnewald, U. Differences and commonalities of plant responses to single and combined stresses. Plant J. Cell Mol. Biol. 2017, 90, 839–855. [Google Scholar] [CrossRef]
- Murakeozy, E.P.; Nagy, Z.; Duhazé, C.; Bouchereau, A.; Tuba, Z. Seasonal changes in the levels of compatible osmolytes in three halophytic species of inland saline vegetation in Hungary. J. Plant Physiol. 2003, 160, 395–401. [Google Scholar] [CrossRef]
- Karimi, S.; Rahemi, M.; Rostami, A.A.; Sedaghat, S. Drought Effects on Growth, Water Content and Osmoprotectants in Four Olive Cultivars with Different Drought Tolerance. Int. J. Fruit Sci. 2018, 18, 254–267. [Google Scholar] [CrossRef]
- Zhang, F.; He, J.-D.; NI, Q.-D.; Wu, Q.-S.; Zou, Y.-N. Enhancement of Drought Tolerance in Trifoliate Orange by Mycorrhiza: Changes in Root Sucrose and Proline Metabolisms. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 46, 270–276. [Google Scholar] [CrossRef]
- Fraser, L.H.; Greenall, A.; Carlyle, C.; Turkington, R.; Friedman, C.R. Adaptive phenotypic plasticity of Pseudoroegneria spicata: Response of stomatal density, leaf area and biomass to changes in water supply and increased temperature. Ann. Bot. 2009, 103, 769–775. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Nutrition and Growth: New Paradigms from Cellular to Ecosystem Scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Santos, H.G.D.; Jacomine, P.K.T.; Anjos, L.H.C.d.; Oliveira, V.Á.D.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.d.; Filho, J.C.A.; Oliveira, J.B.d.; Cunha, T.J. Sistema brasileiro de classificação de solos. Embrapa 2018, 5, 356. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1094003 (accessed on 5 August 2022).
- Dos, S.J.A.; Silva, L.T.; Santos, C.T.; da Silva, S.L. Influência da densidade de esporos de fungos micorrízicos arbusculares nativos da Savana no desenvolvimento do milho (Zea mays). Rev. Bras. De Ciências Da Amaz. 2020, 9, 21–28. [Google Scholar] [CrossRef]
- Santos, C.S.; Lara, T.S.; Santana, M.D.F.; Junior, A.I.S. Soil sterilization for arbuscular mycorrhizal fungi and the influence of remaining spores on maize plants growth. Sci. Amazon. 2021, 10, 10–18. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A. Manual de métodos de análise de solos. Embrapa Solos–Livro Técnico 2017, 3, 573. [Google Scholar]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2006, 11, 249–258. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Dische, Z.; Whistler, R.L.; Wolfram, M.L. General color reactions. Carbohydrate chemistry. Academic 1962, 1, 477–520. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- van Handel, E. Direct microdetermination of sucrose. Anal. Biochem. 1968, 22, 280–283. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The determination of amino-acids with ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Carmo, C.A.F.S.; Araújo, W.S.; Bernardi, A.C.C.; Saldanha, M.F.C. EMBRAPA. Métodos de Análises de Tecidos Vegetais Utilizados na Embrapa Solos; Embrapa Solos: Rio de Janeiro, Brazil, 2000; pp. 41–88. [Google Scholar]
- Ferreira, D.F. SISVAR: A computer analysis system to fixed effects split plot type designs: Sisvar. Braz. J. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]



| Water Regime | Inoculation | Shoot Dry Mass (g) | Shoot Height (cm) | LA (cm2) | TLA (cm2) | RV (cm2) | %C |
|---|---|---|---|---|---|---|---|
| SD | -AMF | 82.3 b | 10.6 ab | 5.41 a | 37.7 a | 0.27 c | - |
| +AMF | 86.7 ab | 9.99 ab | 5.23 a | 38.0 a | 0.37 bc | 17.3 c | |
| MD | -AMF | 81.1 b | 10.5 ab | 5.50 a | 40.0 a | 0.32 bc | - |
| +AMF | 93.6 a | 10.8 a | 6.83 a | 47.8 a | 0.33 bc | 29.8 b | |
| WW | -AMF | 79.9 b | 9.53 b | 5.36 a | 38.9 a | 0.53 a | - |
| +AMF | 94.9 a | 10.7 a | 6.88 a | 44.7 a | 0.44 ab | 57.7 a |
| pH | Al | Ca | Mg | H + Al | H | P | K | Fe | Mg | MO | CO | Areia | Argila | Silte |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cmolc/dm3 | mg/dm3 | dag/dm3 | % | |||||||||||
| 5 | 2.42 | 1.03 | 0.53 | 5.3 | 2.9 | 22 | 102 | 65.1 | 6.3 | 5.47 | 3.18 | 42.9 | 41.5 | 15.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, T.S.; Lara, T.S.; Santos, J.A.d.; Sousa, L.d.S.; Santana, M.D.F. Arbuscular Mycorrhizal Fungi Promote Physiological and Biochemical Advantages in Handroanthus serratifolius Seedlings Submitted to Different Water Deficits. Plants 2022, 11, 2731. https://doi.org/10.3390/plants11202731
Correia TS, Lara TS, Santos JAd, Sousa LdS, Santana MDF. Arbuscular Mycorrhizal Fungi Promote Physiological and Biochemical Advantages in Handroanthus serratifolius Seedlings Submitted to Different Water Deficits. Plants. 2022; 11(20):2731. https://doi.org/10.3390/plants11202731
Chicago/Turabian StyleCorreia, Tatiane Santos, Túlio Silva Lara, Jéssica Aires dos Santos, Ludyanne da Silva Sousa, and Marcos Diones Ferreira Santana. 2022. "Arbuscular Mycorrhizal Fungi Promote Physiological and Biochemical Advantages in Handroanthus serratifolius Seedlings Submitted to Different Water Deficits" Plants 11, no. 20: 2731. https://doi.org/10.3390/plants11202731
APA StyleCorreia, T. S., Lara, T. S., Santos, J. A. d., Sousa, L. d. S., & Santana, M. D. F. (2022). Arbuscular Mycorrhizal Fungi Promote Physiological and Biochemical Advantages in Handroanthus serratifolius Seedlings Submitted to Different Water Deficits. Plants, 11(20), 2731. https://doi.org/10.3390/plants11202731






