Extracts from Frangula alnus Mill. and Their Effects on Environmental and Probiotic Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extraction Procedure
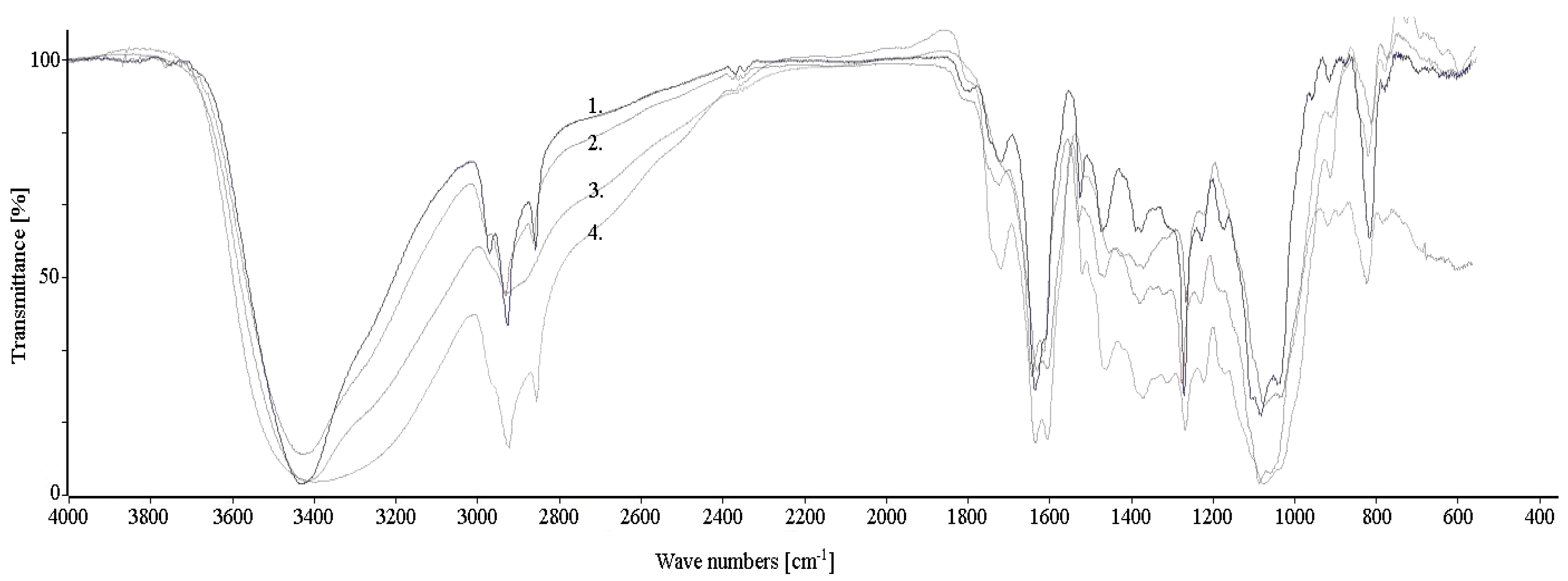
2.3. Extracts Analysis
2.4. Biological Activity of Extracts
2.5. Statistical Analysis
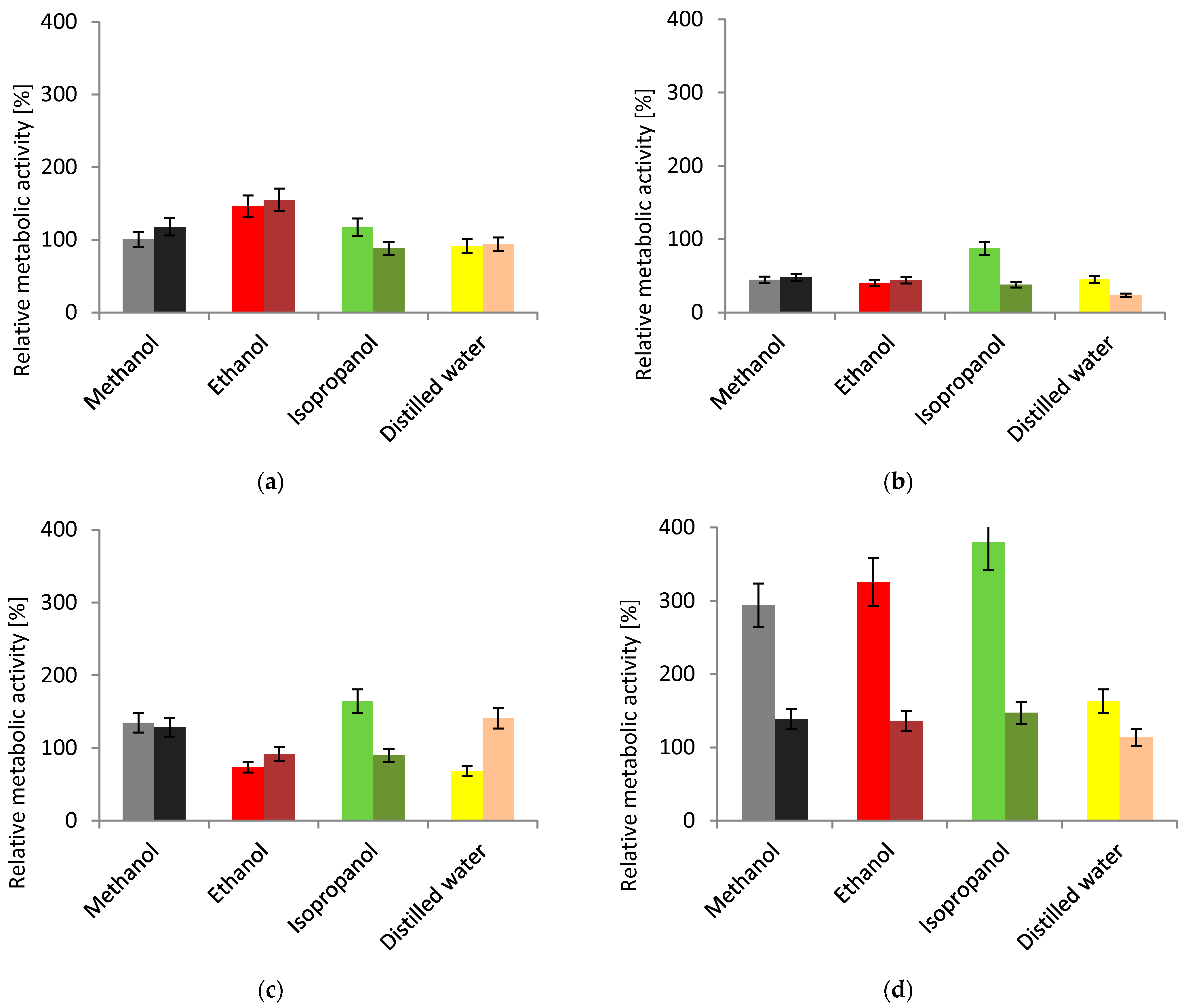
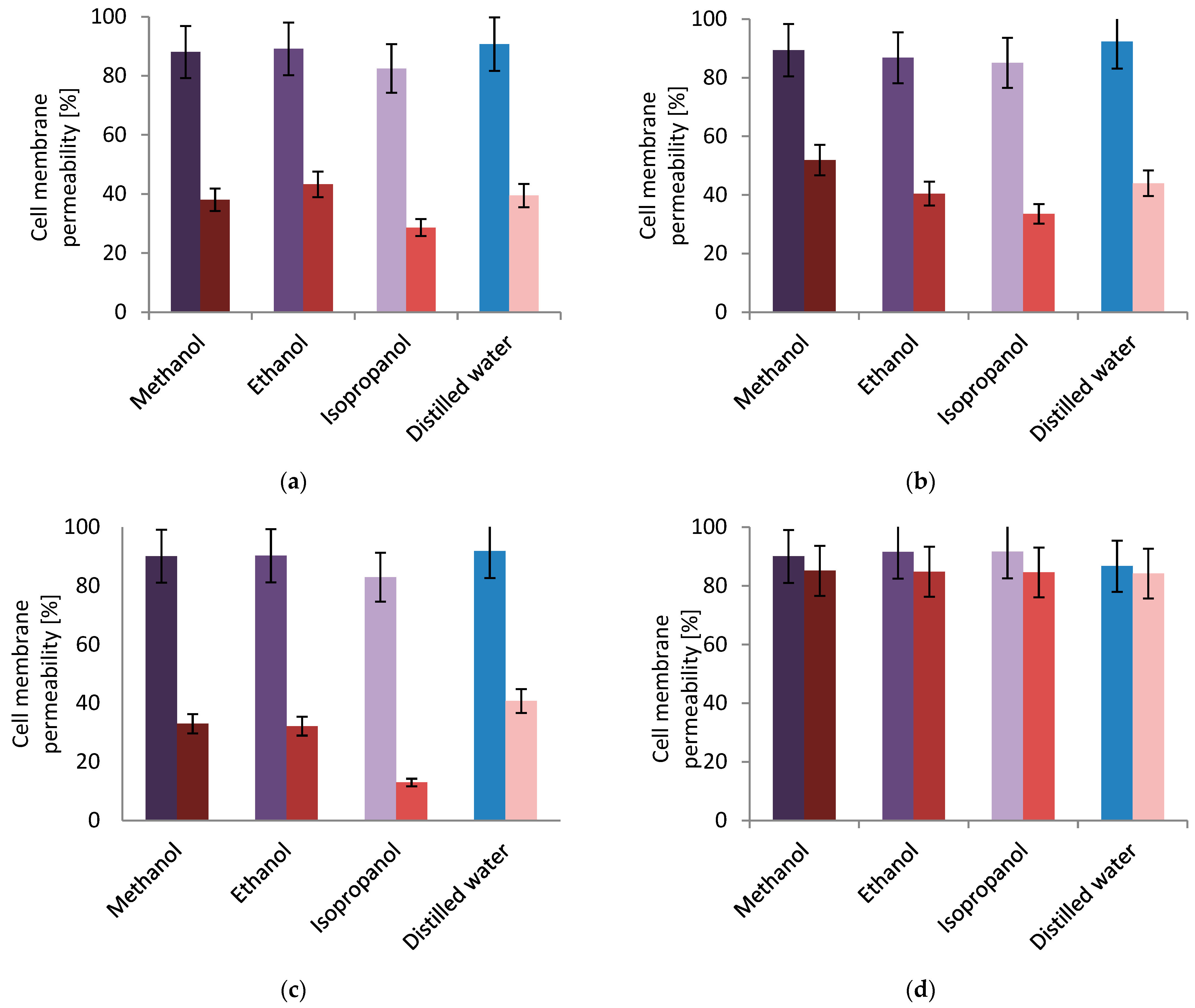
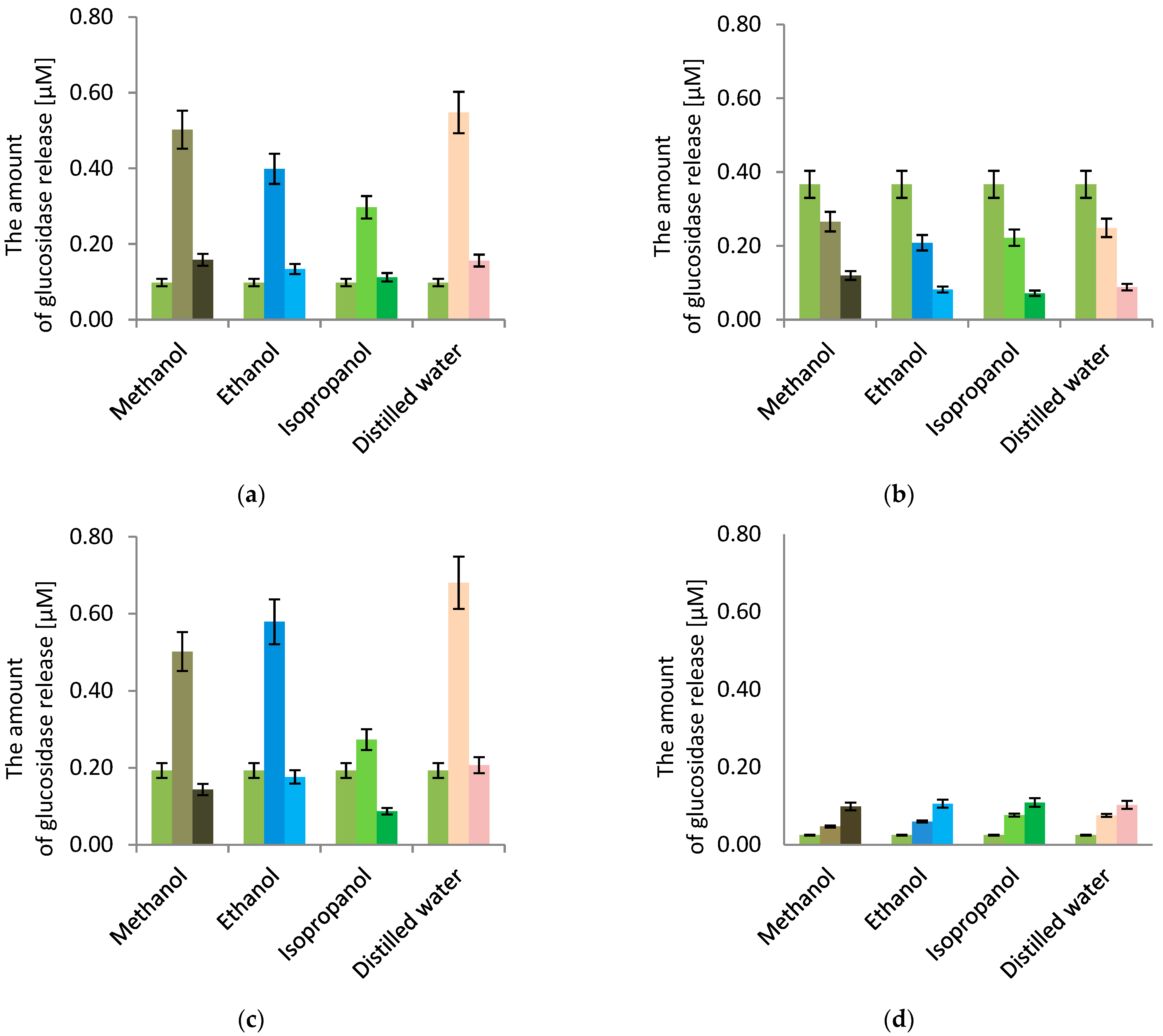
3. Results
3.1. The Efficiency of the Extraction Process from the Bark of F. alnus Mill
3.2. Extracts Composition
3.3. Impact of F. alnus Extracts on Bacteria
3.3.1. Cell Viability
3.3.2. Cell Membrane Permeability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowalczewski, P.Ł.; Olejnik, A.; Świtek, S.; Bzducha-Wróbel, A.; Kubiak, P.; Kujawska, M.; Lewandowicz, G. Bioactive compounds of potato (Solanum tuberosum L.) juice: From industry waste to food and medical applications. CRC. Crit. Rev. Plant Sci. 2022, 41, 52–89. [Google Scholar] [CrossRef]
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Ariño, A.; Batool, A.; Tariq, R.M.; Azam, M.; Akhtar, S. Phytochemicals in Daucus carota and Their Health Benefits—Review Article. Foods 2019, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, A.; Zafar, U.; Ahmed, W.; Shabbir, M.A.; Sameen, A.; Sahar, A.; Bhat, Z.F.; Kowalczewski, P.Ł.; Jarzębski, M.; Aadil, R.M. Applications of Cannabis sativa L. in Food and Its Therapeutic Potential: From a Prohibited Drug to a Nutritional Supplement. Molecules 2021, 26, 7699. [Google Scholar] [CrossRef] [PubMed]
- Khalid, W.; Arshad, M.S.; Ranjha, M.M.A.N.; Różańska, M.B.; Irfan, S.; Shafique, B.; Rahim, M.A.; Khalid, M.Z.; Abdi, G.; Kowalczewski, P.Ł. Functional constituents of plant-based foods boost immunity against acute and chronic disorders. Open Life Sci. 2022, 17, 1075–1093. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Kosalec, I.; Locatelli, M.; Epifano, F.; Genovese, S.; Carlucci, G.; Zovko Končić, M. Anthraquinone profiles, antioxidant and antimicrobial properties of Frangula rupestris (Scop.) Schur and Frangula alnus Mill. bark. Food Chem. 2012, 131, 1174–1180. [Google Scholar] [CrossRef]
- Ţebrencu, C.E.; Creţu, R.M.; Mitroi, G.R.; Iacob, E.; Ionescu, E. Phytochemical evaluation and HPTLC investigation of bark and extracts of Rhamnus Frangula Linn. Phytochem. Rev. 2015, 14, 613–621. [Google Scholar] [CrossRef]
- Oybak Dönmez, E.; Akyol, A.A.; Karadağ, R.; Torgan, E.; İren, K. Ancient plant remains with special reference to buckthorn, Frangula alnus Mill., pyrenes from Dascyleum, Balıkesir, NW Turkey. Acta Soc. Bot. Pol. 2016, 86, 3520. [Google Scholar] [CrossRef]
- Zecchin, B.; Caudullo, G.; de Rigo, D. Frangula alnus in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Durrant, T.H., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; ISBN 9789279367403. [Google Scholar]
- Kozlowska, W.; Wagner, C.; Moore, E.M.; Matkowski, A.; Komarnytsky, S. Botanical Provenance of Traditional Medicines From Carpathian Mountains at the Ukrainian-Polish Border. Front. Pharmacol. 2018, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Cenic-Milosevic, D.; Tambur, Z.; Ivancajic, S.; Bokonjic, D.; Cukovic, A.; Stanojkovic, T.; Grozdanic, N.; Kulisic, Z.; Juranic, Z. Antiproliferative effects of Camellia sinensis, Frangula alnus and Rosmarinus officinalis. Arch. Biol. Sci. 2013, 65, 885–891. [Google Scholar] [CrossRef]
- Maleš, Ž.; Kremer, D.; Randić, Z.; Randić, M.; Pilepić, K.; Bojić, M. Quantitative Analysis of Glucofrangulins and Phenolic Compounds in Croatian Rhamnus and Frangula Species. Acta Biol. Cracoviensia Ser. Bot. 2010, 52, 108–113. [Google Scholar] [CrossRef]
- Serdar, B.; Çoşkunçelebi, K.; Terzioğlu, S.; Hampe, A. Anatomical notes on Turkish Frangula alnus Mill. (Rhamnaceae). Plant Biosyst.—An Int. J. Deal. with all Asp. Plant Biol. 2007, 141, 69–74. [Google Scholar] [CrossRef]
- Shukla, V.; Asthana, S.; Gupta, P.; Dwivedi, P.D.; Tripathi, A.; Das, M. Toxicity of Naturally Occurring Anthraquinones. In Advances in Molecular Toxicology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–50. [Google Scholar]
- Brkanac, S.R.; Gerić, M.; Gajski, G.; Vujčić, V.; Garaj-Vrhovac, V.; Kremer, D.; Domijan, A.-M. Toxicity and antioxidant capacity of Frangula alnus Mill. bark and its active component emodin. Regul. Toxicol. Pharmacol. 2015, 73, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe vera (L.) Webb.: Natural Sources of Antioxidants—A Review. Plant Foods Hum. Nutr. 2019, 74, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Manojlovic, N.T.; Solujic, S.; Sukdolak, S.; Milosev, M. Antifungal activity of Rubia tinctorum, Rhamnus frangula and Caloplaca cerina. Fitoterapia 2005, 76, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Alimova, S.; Baishanbo, A.; Yessimsiitova, Z.; Ablaikhanova, N.; Ydyrys, A. A brief overview on medicinal plant Frangula alnus. Eurasian J. Ecol. 2017, 4, 4–11. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Siejak, P.; Smułek, W.; Nowak-Karnowska, J.; Dembska, A.; Neunert, G.; Polewski, K. Bird Cherry (Prunus padus) Fruit Extracts Inhibit Lipid Peroxidation in PC Liposomes: Spectroscopic, HPLC, and GC–MS Studies. Appl. Sci. 2022, 12, 7820. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Kędzierski, B.; Głowniak, K. Influence of extrahent on antioxidant capacity of Aesculus hippocastanum seeds. Nat. Prod. Res. 2015, 29, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Horhammer, L.; Wagner, H.; Bittner, G. Rhamnus Frangula. Kurze Orig. 1964, 13, 311. [Google Scholar]
- Zdarta, A.; Smułek, W.; Pacholak, A.; Kaczorek, E. Environmental Aspects of the Use of Hedera helix Extract in Bioremediation Process. Microorganisms 2019, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Marchlewicz, A.; Guzik, U.; Smułek, W.; Wojcieszyńska, D. Exploring the Degradation of Ibuprofen by Bacillus thuringiensis B1(2015b): The New Pathway and Factors Affecting Degradation. Molecules 2017, 22, 1676. [Google Scholar] [CrossRef]
- Smułek, W.; Bielan, Z.; Pacholak, A.; Zdarta, A.; Zgoła-Grześkowiak, A.; Zielińska-Jurek, A.; Kaczorek, E. Nitrofurazone Removal from Water Enhanced by Coupling Photocatalysis and Biodegradation. Int. J. Mol. Sci. 2021, 22, 2186. [Google Scholar] [CrossRef]
- Pacholak, A.; Burlaga, N.; Guzik, U.; Kaczorek, E. Investigation of the bacterial cell envelope nanomechanical properties after long-term exposure to nitrofurans. J. Hazard. Mater. 2021, 407, 124352. [Google Scholar] [CrossRef] [PubMed]
- Smułek, W.; Pacholak, A.; Kaczorek, E. Modification of the Bacterial Cell Wall—Is the Bioavailability Important in Creosote Biodegradation? Processes 2020, 8, 147. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L. Extraction, Isolation And Characterization Of Bioactive Compounds From Plants’ Extracts. African J. Tradit. Complement. Altern. Med. 2010, 8, 1–10. [Google Scholar] [CrossRef]
- ONG, E. Extraction methods and chemical standardization of botanicals and herbal preparations. J. Chromatogr. B 2004, 812, 23–33. [Google Scholar] [CrossRef]
- Nyiredy, S. Separation strategies of plant constituents–current status. J. Chromatogr. B 2004, 812, 35–51. [Google Scholar] [CrossRef]
- Rosenthal, I.; Wolfram, E.; Peter, S.; Meier, B. Validated Method for the Analysis of Frangulins A and B and Glucofrangulins A and B Using HPLC and UHPLC. J. Nat. Prod. 2014, 77, 489–496. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Lee, S.; Kang, S.-S.; Shin, H.-S. Selected commercial plants: A review of extraction and isolation of bioactive compounds and their pharmacological market value. Trends Food Sci. Technol. 2018, 82, 89–109. [Google Scholar] [CrossRef]
- Li, R.; Xia, Z.; Li, B.; Tian, Y.; Zhang, G.; Li, M.; Dong, J. Advances in Supercritical Carbon Dioxide Extraction of Bioactive Substances from Different Parts of Ginkgo biloba L. Molecules 2021, 26, 4011. [Google Scholar] [CrossRef]
- Bellachioma, L.; Marini, E.; Magi, G.; Pugnaloni, A.; Facinelli, B.; Rocchetti, G.; Martinelli, E.; Lucini, L.; Morresi, C.; Bacchetti, T.; et al. Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas. Open Chem. 2022, 20, 431–443. [Google Scholar] [CrossRef]
- Noman, O.M.; Nasr, F.A.; Alqahtani, A.S.; Al-zharani, M.; Cordero, M.A.W.; Alotaibi, A.A.; Bepari, A.; Alarifi, S.; Daoud, A. Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia. Open Chem. 2021, 19, 408–416. [Google Scholar] [CrossRef]
- Stahl, E.; Schild, W. Pharmazeutische Biologie; Springer: Berlin/Heidelberg, Germany, 2013; pp. 102–103. [Google Scholar]
- Abubacker, M.N.; Devi, P.K. In Vitro Antifungal Potentials of Bioactive Compound Oleic Acid, 3-(Octadecyloxy) Propyl Ester Isolated from Lepidagathis Cristata Willd. (Acanthaceae) Inflorescence. Asian Pac. J. Trop. Med. 2014, 7, S190–S193. [Google Scholar] [CrossRef]
- Papke, R.L.; Horenstein, N.A.; Stokes, C. Nicotinic Activity of Arecoline, the Psychoactive Element of “Betel Nuts”, Suggests a Basis for Habitual Use and Anti-Inflammatory Activity. PLoS ONE 2015, 10, e0140907. [Google Scholar] [CrossRef]
- Gaitan, E.; Cooksey, R.C.; Legan, J.; Lindsay, R.H. Antithyroid Effects in Vivo and in Vitro of Vitexin: A C-Glucosylflavone in Millet. J. Clin. Endocrinol. Metab. 1995, 80, 1144–1147. [Google Scholar] [CrossRef]
- Numazawa, S.; Honma, Y.; Yamamoto, T.; Yoshida, T.; Kuroiwa, Y. A Cardiotonic Steroid Bufalin-like Factor in Human Plasma Induces Leukemia Cell Differentiation. Leuk. Res. 1995, 19, 945–953. [Google Scholar] [CrossRef]
- Dalbeth, N.; Lauterio, T.J.; Wolfe, H.R. Mechanism of Action of Colchicine in the Treatment of Gout. Clin. Ther. 2014, 36, 1465–1479. [Google Scholar] [CrossRef]
- Ariyoshi, T.; Eishi, K.; Sakamoto, I.; Matsukuma, S.; Odate, T. Effect of Etidronic Acid on Arterial Calcification in Dialysis Patients: Clin. Drug Investig. 2006, 26, 215–222. [Google Scholar] [CrossRef]
- Wen, M.; Han, Z.; Cui, Y.; Ho, C.-T.; Wan, X.; Zhang, L. Identification of 4-O-p-Coumaroylquinic Acid as Astringent Compound of Keemun Black Tea by Efficient Integrated Approaches of Mass Spectrometry, Turbidity Analysis and Sensory Evaluation. Food Chem. 2022, 368, 130803. [Google Scholar] [CrossRef]
- Yu, C.P.; Gerlei, K.Z.; Rágyanszki, A.; Jensen, S.J.K.; Viskolcz, B.; Csizmadia, I.G. Reactivity of Ala-Gly Dipeptide with β-Turn Secondary Structure. Chem. Phys. Lett. 2018, 692, 402–406. [Google Scholar] [CrossRef]
- Czyżewska, U.; Konończuk, J.; Teul, J.; Drągowski, P.; Pawlak-Morka, R.; Surażyński, A.; Miltyk, W. Verification of Chemical Composition of Commercially Available Propolis Extracts by Gas Chromatography–Mass Spectrometry Analysis. J. Med. Food 2015, 18, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.P.; Choi, D.W. Na+-Ca2+ Exchange Currents in Cortical Neurons: Concomitant Forward and Reverse Operation and Effect of Glutamate. Eur. J. Neurosci. 1997, 9, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
| Extractant | Polarity of the Extractant | m [g] | E [%] | λmax1 [nm] | Amax1 [−] | λmax2 [nm] | Amax2 [−] |
|---|---|---|---|---|---|---|---|
| Ethanol | 0.654 | 0.99 | 19.78 | 221.5 | 0.34 | 265 | 0.20 |
| Isopropanol | 0.617 | 0.48 | 9.61 | 216 | 0.31 | 263 | 0.12 |
| Methanol | 0.762 | 0.96 | 19.23 | 217.5 | 0.31 | 265 | 0.18 |
| Distilled water | 1.000 | 0.61 | 12.22 | 219.5 | 0.33 | 265 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kledecka, A.; Siejak, P.; Pratap-Singh, A.; Kowalczewski, P.Ł.; Fathordoobady, F.; Jarzębski, M.; Smułek, W. Extracts from Frangula alnus Mill. and Their Effects on Environmental and Probiotic Bacteria. Plants 2022, 11, 2719. https://doi.org/10.3390/plants11202719
Kledecka A, Siejak P, Pratap-Singh A, Kowalczewski PŁ, Fathordoobady F, Jarzębski M, Smułek W. Extracts from Frangula alnus Mill. and Their Effects on Environmental and Probiotic Bacteria. Plants. 2022; 11(20):2719. https://doi.org/10.3390/plants11202719
Chicago/Turabian StyleKledecka, Agata, Przemysław Siejak, Anubhav Pratap-Singh, Przemysław Łukasz Kowalczewski, Farahnaz Fathordoobady, Maciej Jarzębski, and Wojciech Smułek. 2022. "Extracts from Frangula alnus Mill. and Their Effects on Environmental and Probiotic Bacteria" Plants 11, no. 20: 2719. https://doi.org/10.3390/plants11202719
APA StyleKledecka, A., Siejak, P., Pratap-Singh, A., Kowalczewski, P. Ł., Fathordoobady, F., Jarzębski, M., & Smułek, W. (2022). Extracts from Frangula alnus Mill. and Their Effects on Environmental and Probiotic Bacteria. Plants, 11(20), 2719. https://doi.org/10.3390/plants11202719











