Exercise Training and Verbena officinalis L. Affect Pre-Clinical and Histological Parameters
Abstract
1. Introduction
2. Results
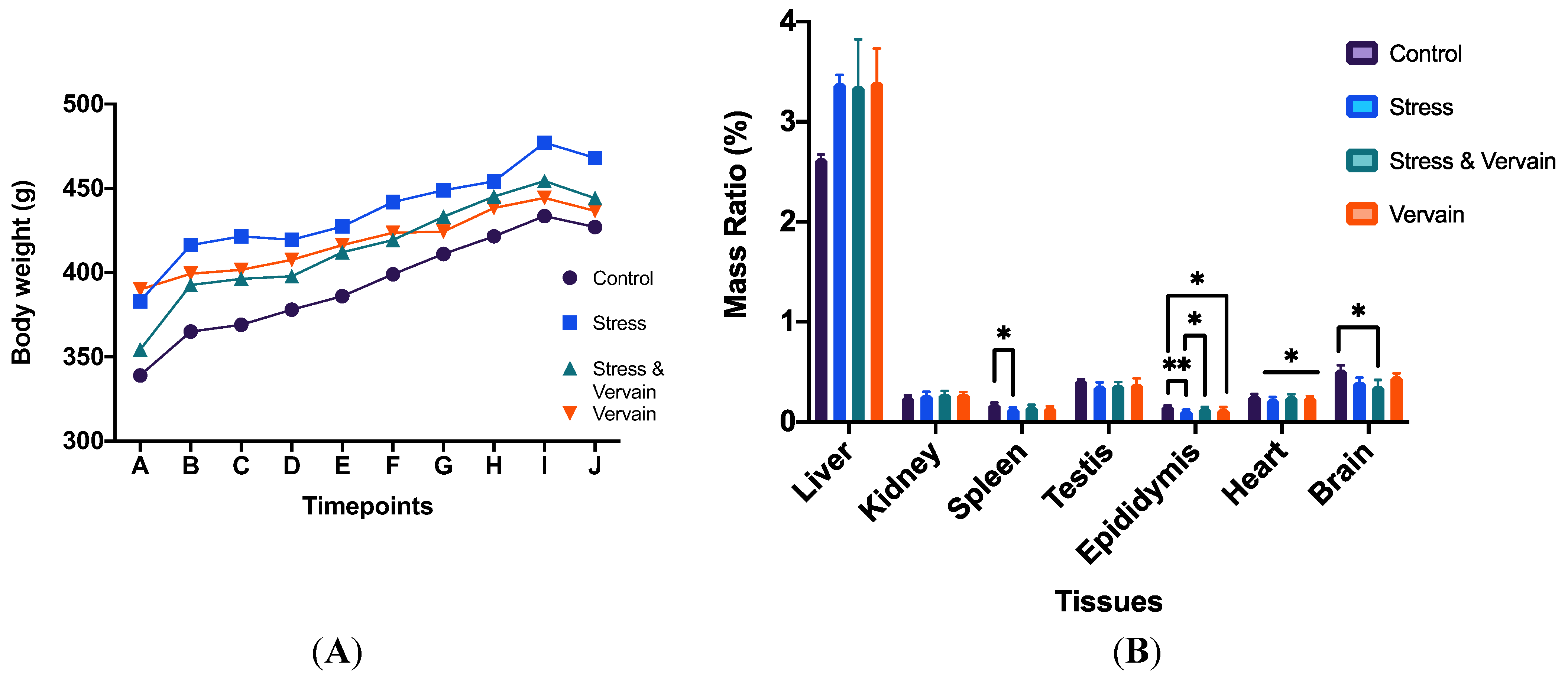
2.1. Survival, Body Weight, and Organ Ratios
2.2. Hematological Assays
2.2.1. Blood Smear
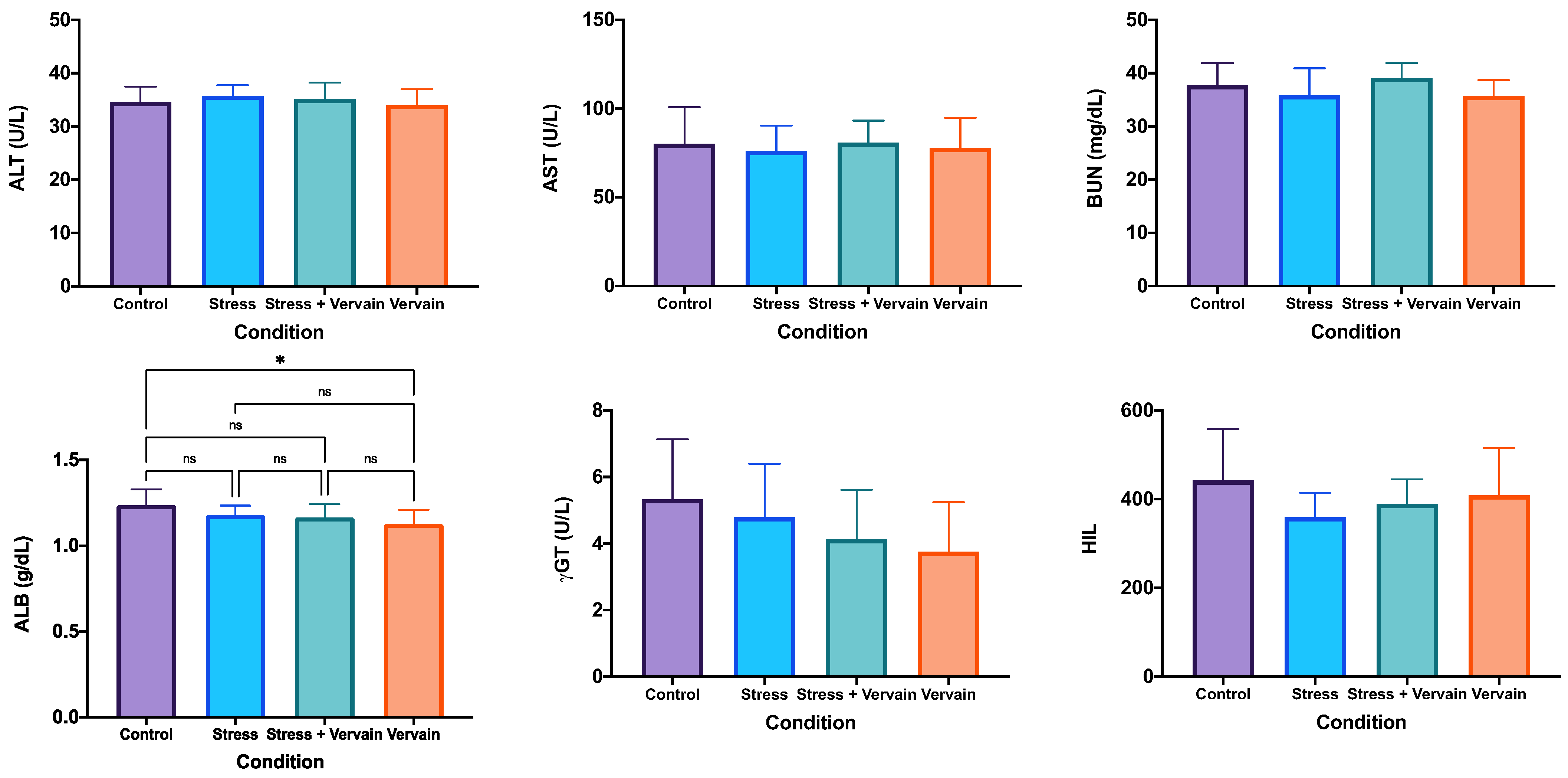
2.2.2. Blood Biochemistry
2.3. Biochemistry and Antioxidant Status Analysis
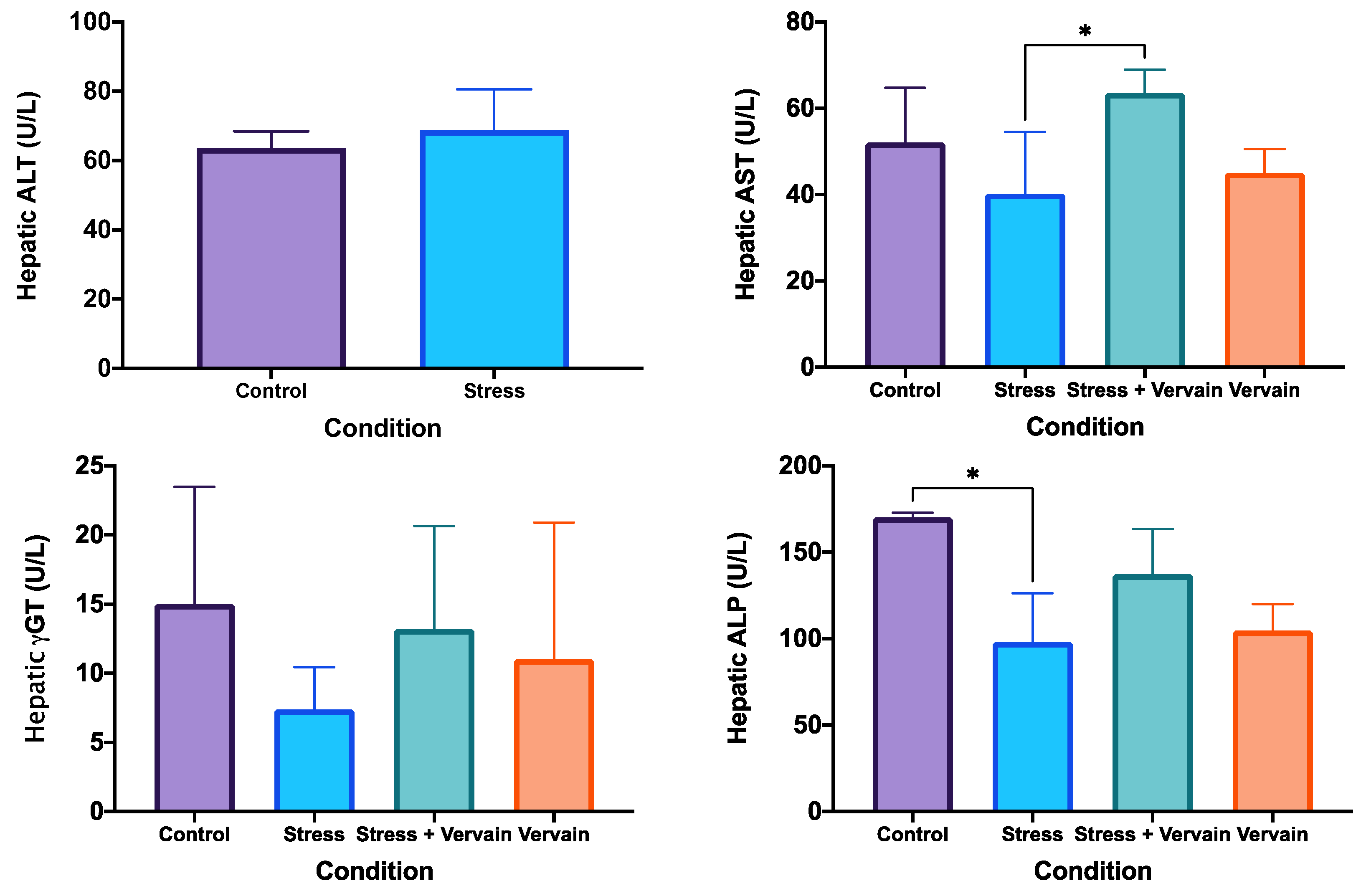
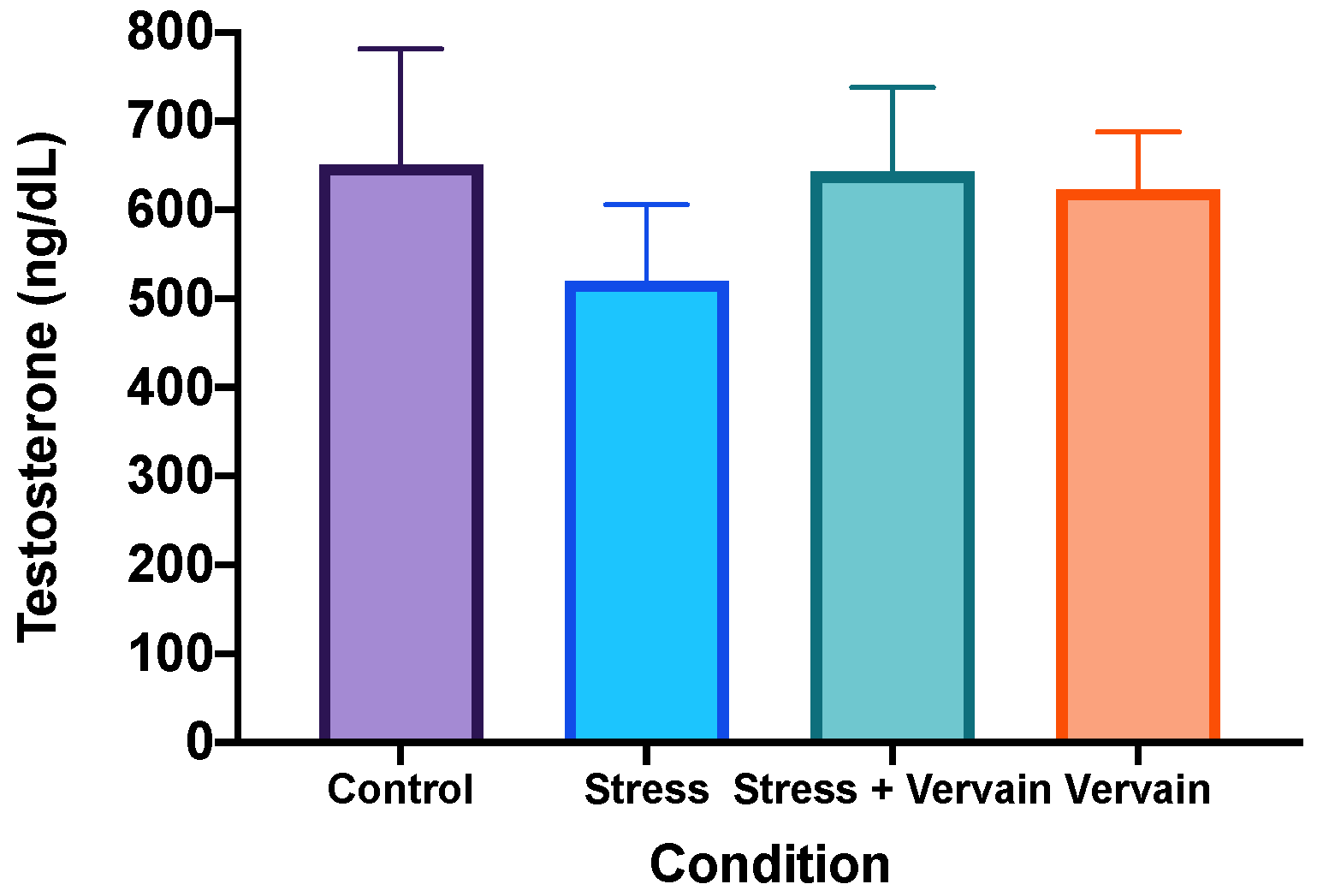
2.3.1. Biochemical Status of the Liver and Testosterone Levels in the Testis
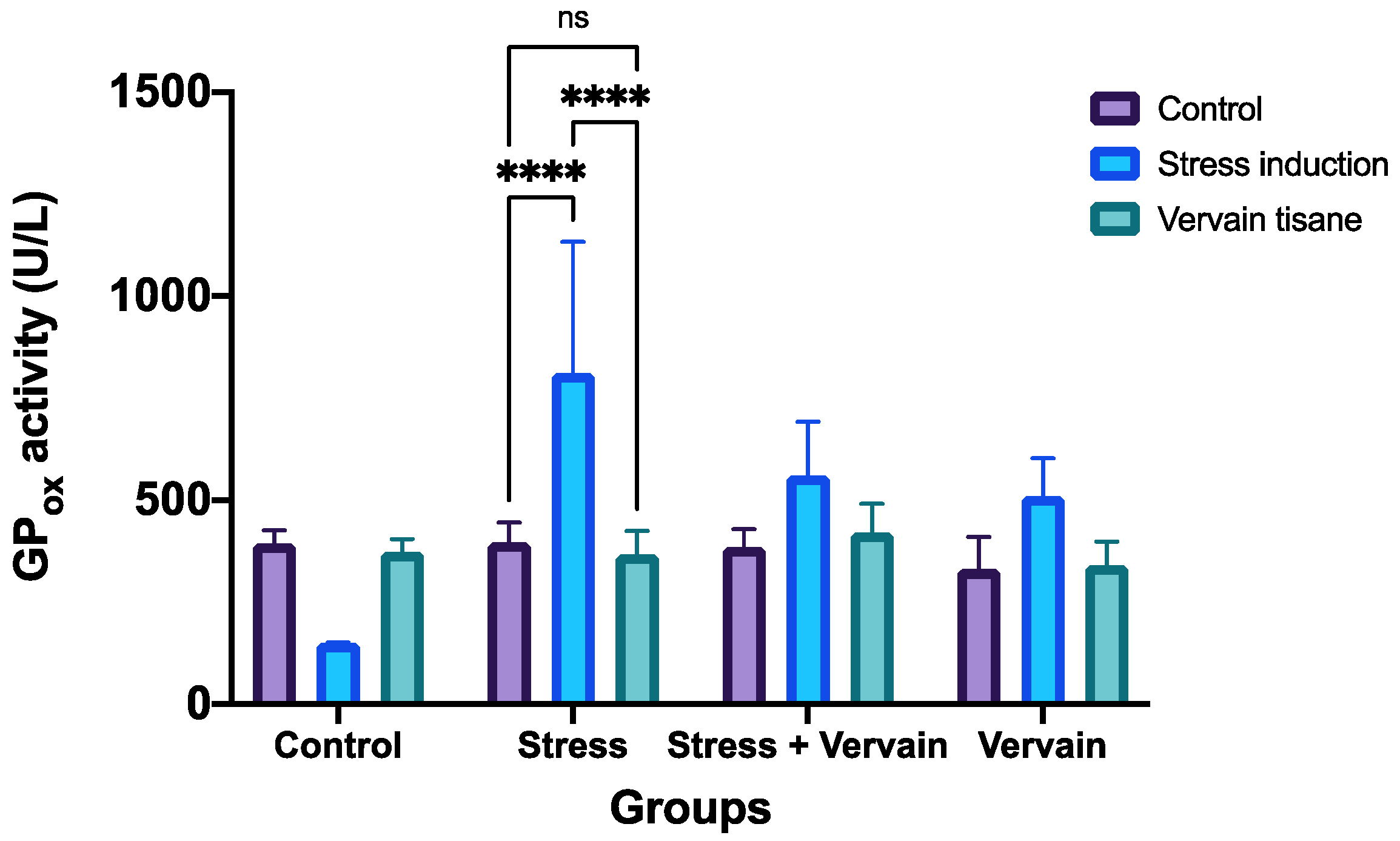
2.3.2. Antioxidant Status of Liver and Kidney
2.4. Histological Studies
2.4.1. Liver
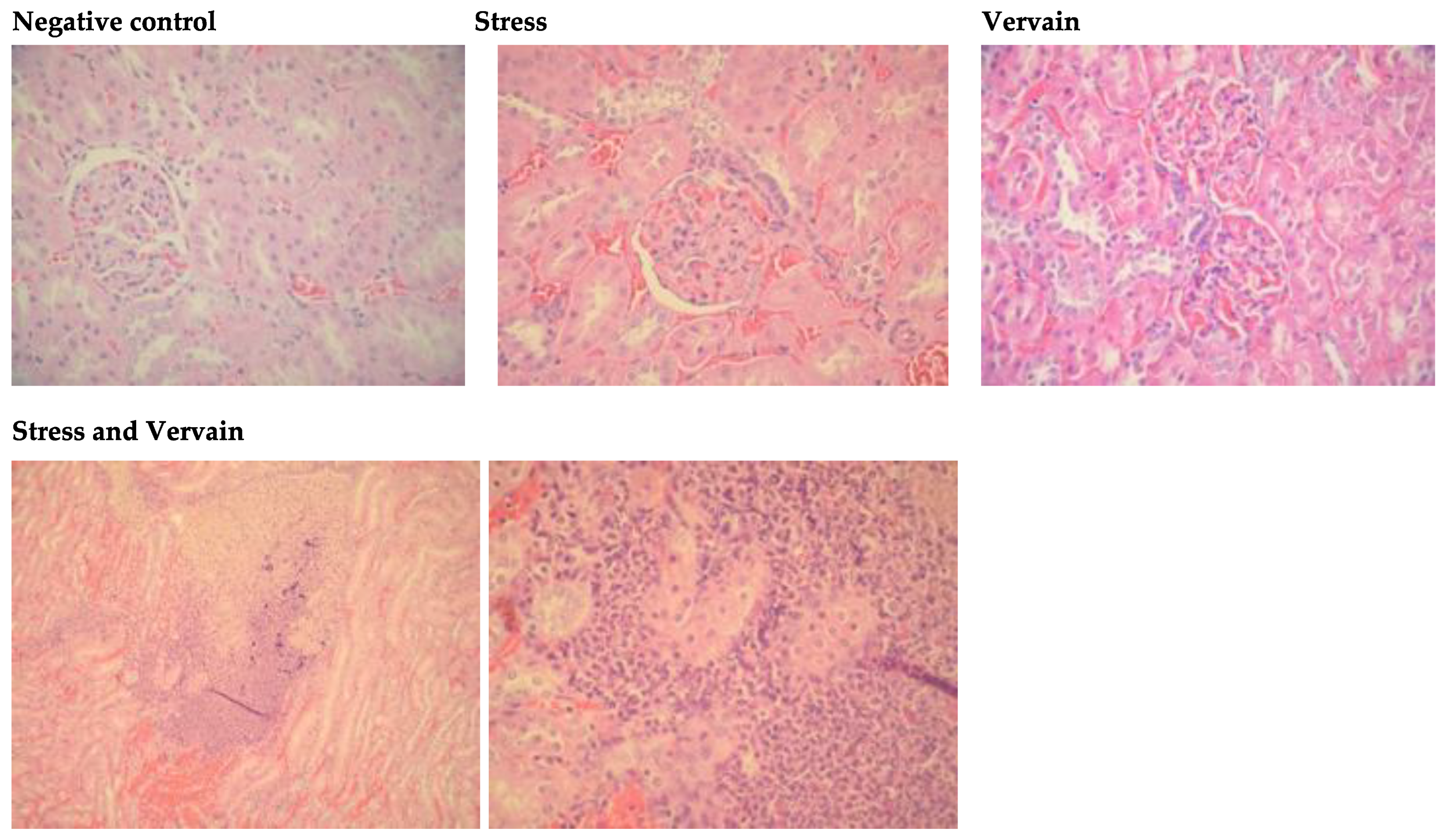
2.4.2. Kidney
2.4.3. Spleen, Brain, and Heart
2.4.4. Testis and Epididymis
3. Discussion
4. Materials and Methods
4.1. Plant Material
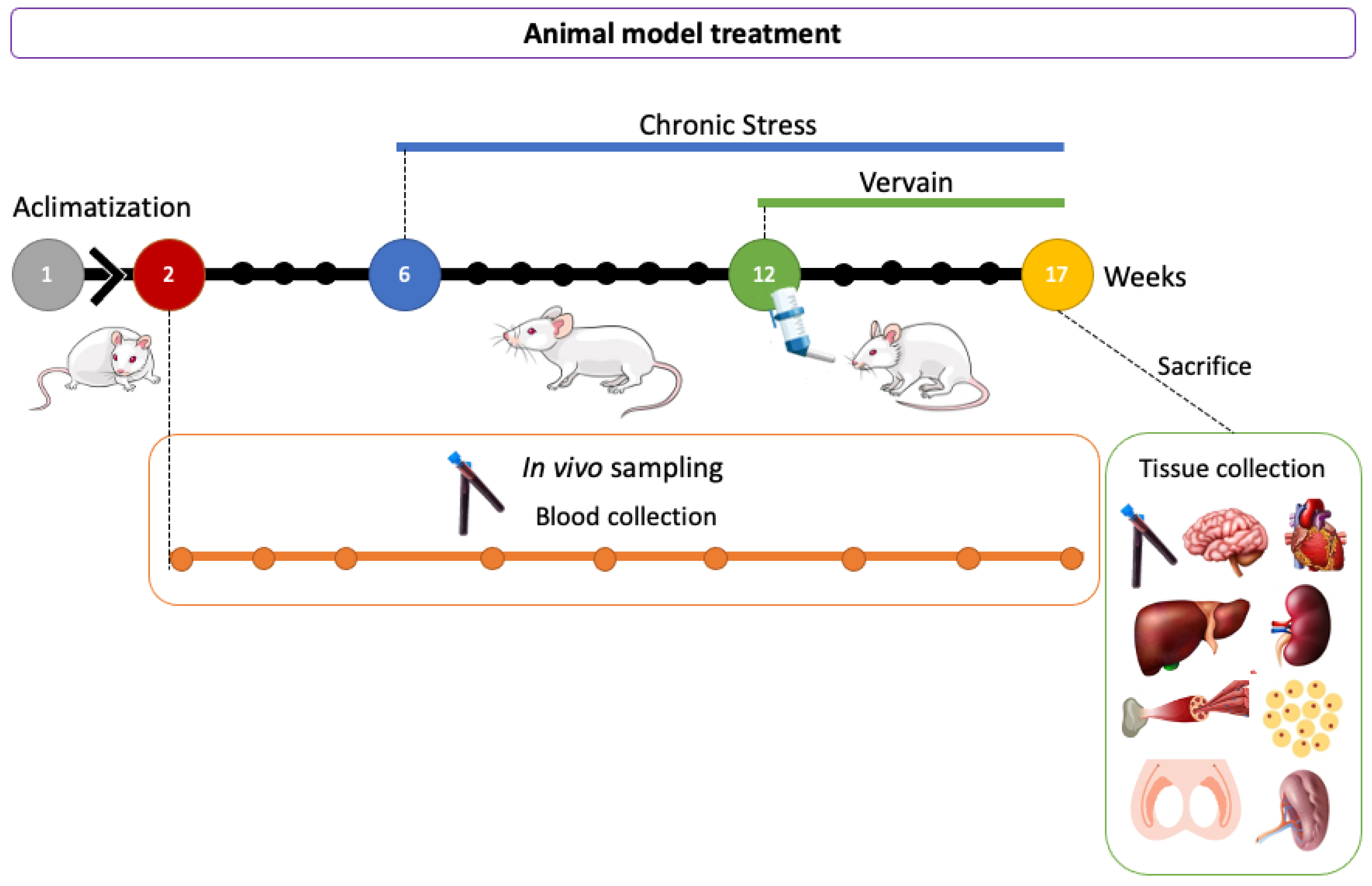
4.2. Animals and Experimental Protocol
4.3. Blood Hematology and Biochemistry Assays
Antioxidant Status Investigation
4.4. Liver and Kidney Analysis
4.4.1. GST Activity
4.4.2. Total Protein
4.5. Histological Studies
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kubica, P.; Szopa, A.; Dominiak, J.; Luczkiewicz, M.; Ekiert, H. Verbena officinalis (Common Vervain)—A Review on the Investigations of This Medicinally Important Plant Species. Planta Med. 2020, 86, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Speroni, E.; Cervellati, R.; Costa, S.; Guerra, M.; Utan, A.; Govoni, P.; Berger, A.; Müller, A.; Stuppner, H. Effects of Differential Extraction of Verbena officinalis on Rat Models of Inflammation, Cicatrization and Gastric Damage. Planta Med. 2007, 73, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.I. Anti-Inflammatory and Analgesic Activity of the Topical Preparation of Verbena officinalis L. J. Ethnopharmacol. 2006, 107, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M.; Forti, G.; Marignoli, S. Ethnobotanical and Ethnomedicinal Uses of Plants in the District of Acquapendente (Latium, Central Italy). J. Ethnopharmacol. 2005, 96, 429–444. [Google Scholar] [CrossRef]
- Popova, A.; Mihaylova, D.; Spasov, A. Plant-Based Remedies with Reference to Respiratory Diseases—A Review. Open Biotechnol. J. 2021, 15, 46–58. [Google Scholar] [CrossRef]
- Popovich, V.I.; Beketova, H.V. Results of a Randomised Controlled Study on the Efficacy of a Combination of Saline Irrigation and Sinupret Syrup Phytopreparation in the Treatment of Acute Viral Rhinosinusitis in Children Aged 6 to 11 Years. Clin. Phytosci. 2018, 4, 21. [Google Scholar] [CrossRef]
- Cavero, R.Y.; Akerreta, S.; Calvo, M.I. Pharmaceutical Ethnobotany in the Middle Navarra (Iberian Peninsula). J. Ethnopharmacol. 2011, 137, 844–855. [Google Scholar] [CrossRef]
- Mendieta, M.; Heck, R.M.; Ceolin, S.; de Souza, A.D.Z.; Vargas, N.R.C.; Piriz, M.A.; Borges, A.M. Plantas Medicinais Indicadas Para Gripes e Resfriados No Sul Do Brasil. Rev. Eletr. Enf. 2015, 17. [Google Scholar] [CrossRef]
- Lai, S.-W.; Yu, M.-S.; Yuen, W.-H.; Chang, R.C.-C. Novel Neuroprotective Effects of the Aqueous Extracts from Verbena officinalis Linn. Neuropharmacology 2006, 50, 641–650. [Google Scholar] [CrossRef]
- Khan, A.W.; Khan, A.; Ahmed, T. Anticonvulsant, Anxiolytic, and Sedative Activities of Verbena officinalis. Front. Pharmacol. 2016, 7, 499. [Google Scholar] [CrossRef][Green Version]
- Rashidian, A.; Kazemi, F.; Mehrzadi, S.; Dehpour, A.R.; Mehr, S.E.; Rezayat, S.M. Anticonvulsant Effects of Aerial Parts of Verbena officinalis Extract in Mice: Involvement of Benzodiazepine and Opioid Receptors. J. Evid. Based Complement. Altern. Med. 2017, 22, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Zaida, F.; Bureau, F.; Guyot, S.; Sedki, A.; Lekouch, N.; Arhan, P.; Bouglé, D. Iron Availability and Consumption of Tea, Vervain and Mint during Weaning in Morocco. Ann. Nutr. Metab. 2006, 50, 237–241. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; D’Arena, G.; Minervini, M.M.; Deaglio, S.; Fusco, B.M.; Cascavilla, N.; De Feo, V. Verbena officinalis Essential Oil and Its Component Citral as Apoptotic-Inducing Agent in Chronic Lymphocytic Leukemia. Int. J. Immunopathol. Pharmacol. 2009, 22, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Liu, X.; Ma, D.; Hua, X.; Jin, N. Optimization of Polysaccharides Extracted from Verbena officinalis L and Their Inhibitory Effects on Invasion and Metastasis of Colorectal Cancer Cells. Trop. J. Pharm. Res. 2017, 16, 2387–2394. [Google Scholar] [CrossRef]
- Encalada, M.A.; Rehecho, S.; Ansorena, D.; Astiasarán, I.; Cavero, R.Y.; Calvo, M.I. Antiproliferative Effect of Phenylethanoid Glycosides from Verbena officinalis L. on Colon Cancer Cell Lines. LWT Food Sci. Technol. 2015, 63, 1016–1022. [Google Scholar] [CrossRef]
- Kou, W.-Z.; Yang, J.; Yang, Q.-H.; Wang, Y.; Wang, Z.-F.; Xu, S.-L.; Liu, J. Study on In-Vivo Anti-Tumor Activity of Verbena officinalis Extract. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 512–517. [Google Scholar] [CrossRef][Green Version]
- British Pharmacopoeia Commission. British Pharmacopoeia 2008; The Stationery Office: London, UK, 2008; ISBN 978-0-11-322799-0. [Google Scholar]
- Xu, W.; Xin, F.; Sha, Y.; Fang, J.; Li, Y.-S. Two New Secoiridoid Glycosides from Verbena officinalis. J. Asian Nat. Prod. Res. 2010, 12, 649–653. [Google Scholar] [CrossRef]
- Aldred, E. Terpenes. In Pharmacology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 167–174. ISBN 978-0-443-06898-0. [Google Scholar]
- Bilia, A.R.; Giomi, M.; Innocenti, M.; Gallori, S.; Vincieri, F.F. HPLC–DAD–ESI–MS Analysis of the Constituents of Aqueous Preparations of Verbena and Lemon Verbena and Evaluation of the Antioxidant Activity. J. Pharm. Biomed. Anal. 2008, 46, 463–470. [Google Scholar] [CrossRef]
- Vaidya, H.B.; Ahmed, A.A.; Goyal, R.K.; Cheema, S.K. Glycogen Phosphorylase-a Is a Common Target for Anti-Diabetic Effect of Iridoid and Secoiridoid Glycosides. J. Pharm. Pharm. Sci. 2013, 16, 530–540. [Google Scholar] [CrossRef]
- Duan, K.; Yuan, Z.; Guo, W.; Meng, Y.; Cui, Y.; Kong, D.; Zhang, L.; Wang, N. LC–MS/MS Determination and Pharmacokinetic Study of Five Flavone Components after Solvent Extraction/Acid Hydrolysis in Rat Plasma after Oral Administration of Verbena officinalis L. Extract. J. Ethnopharmacol. 2011, 135, 201–208. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, Isolation and Characterization of Bioactive Compounds from Plants’ Extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Hafsi, C.; Mohsni, I.; Ksouri, R. Évaluation de Différents Procédés d’extraction Des Composés Phénoliques d’une Plante Médicinale: Verbena officinalis. Biol. Aujourd’hui 2021, 215, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Dziurka, M.; Kubica, P.; Kwiecień, I.; Biesaga-Kościelniak, J.; Ekiert, H.; Abdelmohsen, S.A.M.; Al-Harbi, F.F.; El-Ansary, D.O.; Elansary, H.O.; Szopa, A. In Vitro Cultures of Some Medicinal Plant Species (Cistus × Incanus, Verbena officinalis, Scutellaria Lateriflora, and Scutellaria Baicalensis) as a Rich Potential Source of Antioxidants—Evaluation by CUPRAC and QUENCHER-CUPRAC Assays. Plants 2021, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Fateh, A.H.; Mohamed, Z.; Chik, Z.; Alsalahi, A.; Zain, S.R.; Alshawsh, M.A. Mutagenicity and Genotoxicity Effects of Verbena officinalis Leaves Extract in Sprague-Dawley Rats. J. Ethnopharmacol. 2019, 235, 88–99. [Google Scholar] [CrossRef]
- Higgins, M.R.; Izadi, A.; Kaviani, M. Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation. Int. J. Environ. Res. Public Health 2020, 17, 8452. [Google Scholar] [CrossRef]
- Mason, S.A.; Trewin, A.J.; Parker, L.; Wadley, G.D. Antioxidant Supplements and Endurance Exercise: Current Evidence and Mechanistic Insights. Redox Biol. 2020, 35, 101471. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.-C.; Borrás, C.; Pallardó, F.V.; Sastre, J.; Ji, L.L.; Viña, J. Decreasing Xanthine Oxidase-Mediated Oxidative Stress Prevents Useful Cellular Adaptations to Exercise in Rats: Oxidants as Signals Modulating Muscle Adaptations to Exercise. J. Physiol. 2005, 567, 113–120. [Google Scholar] [CrossRef]
- La Gerche, A.; Heidbuchel, H. Can Intensive Exercise Harm the Heart? You Can Get Too Much of a Good Thing. Circulation 2014, 130, 992–1002. [Google Scholar] [CrossRef]
- Sellami, M.; Slimeni, O.; Pokrywka, A.; Kuvačić, G.; D Hayes, L.; Milic, M.; Padulo, J. Herbal Medicine for Sports: A Review. J. Int. Soc. Sport. Nutr. 2018, 15, 14. [Google Scholar] [CrossRef]
- Chen, C.K.; Muhamad, A.S.; Ooi, F.K. Herbs in Exercise and Sports. J. Physiol. Anthr. 2012, 31, 4. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen Consumption and Usage During Physical Exercise: The Balance Between Oxidative Stress and ROS-Dependent Adaptive Signaling. Antioxid. Redox Signal. 2013, 18, 1208–1246. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Tripaldi, R.; Davì, G.; Santilli, F. Oxidative Stress Modulation Through Habitual Physical Activity. Curr. Pharm. Des. 2016, 22, 3648–3680. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, S.; Valaei, K.; Arazi, H.; Suzuki, K. An Overview of Physical Exercise and Antioxidant Supplementation Influences on Skeletal Muscle Oxidative Stress. Antioxidants 2021, 10, 1528. [Google Scholar] [CrossRef] [PubMed]
- Verica, D.-U.; Bursać Kovačević, D.; Levaj, B.; Sandra, P.; Mirela, M.; Ana, T. Polyphenols and Antioxidant Capacity in Fruits and Vegetables Common in the Croatian Diet. Agric. Conspec. (ACS) 2009, 74, 175–179. [Google Scholar]
- Tauler, P.; Aguiló, A.; Fuentespina, E.; Tur, J.; Pons, A. Diet Supplementation with Vitamin E, Vitamin C and β-Carotene Cocktail Enhances Basal Neutrophil Antioxidant Enzymes in Athletes. Pflugers Arch. Eur. J. Physiol. 2002, 443, 791–797. [Google Scholar] [CrossRef]
- Tauler, P.; Aguiló, A.; Gimeno, I.; Fuentespina, E.; Tur, J.A.; Pons, A. Influence of Vitamin C Diet Supplementation on Endogenous Antioxidant Defences during Exhaustive Exercise. Pflugers Arch. Eur. J. Physiol. 2003, 446, 658–664. [Google Scholar] [CrossRef]
- Tauler, P.; Ferrer, M.D.; Sureda, A.; Pujol, P.; Drobnic, F.; Tur, J.A.; Pons, A. Supplementation with an Antioxidant Cocktail Containing Coenzyme Q Prevents Plasma Oxidative Damage Induced by Soccer. Eur. J. Appl. Physiol. 2008, 104, 777–785. [Google Scholar] [CrossRef]
- Bogdanska, J.J.; Korneti, P.; Todorova, B. Erythrocyte Superoxide Dismutase, Glutathione Peroxidase and Catalase Activities in Healthy Male Subjects in Republic of Macedonia. Bratisl Lek Listy 2003, 104, 108–114. [Google Scholar]
- Funes, L.; Laporta, O.; Cerdán-Calero, M.; Micol, V. Effects of Verbascoside, a Phenylpropanoid Glycoside from Lemon Verbena, on Phospholipid Model Membranes. Chem. Phys. Lipids 2010, 163, 190–199. [Google Scholar] [CrossRef]
- Buchwald-Werner, S.; Naka, I.; Wilhelm, M.; Schütz, E.; Schoen, C.; Reule, C. Effects of Lemon Verbena Extract (Recoverben®) Supplementation on Muscle Strength and Recovery after Exhaustive Exercise: A Randomized, Placebo-Controlled Trial. J. Int. Soc. Sport. Nutr. 2018, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Tobyn, G.; Denham, A.; Whitelegg, M. Verbena officinalis, Vervain. In Medical Herbs; Elsevier: Amsterdam, The Netherlands, 2011; pp. 327–336. ISBN 978-0-443-10344-5. [Google Scholar]
- Dwyer, D.; Browning, J. Endurance Training in Wistar Rats Decreases Receptor Sensitivity to a Serotonin Agonist. Acta Physiol. Scand. 2000, 170, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Benito, B.; Gay-Jordi, G.; Serrano-Mollar, A.; Guasch, E.; Shi, Y.; Tardif, J.-C.; Brugada, J.; Nattel, S.; Mont, L. Cardiac Arrhythmogenic Remodeling in a Rat Model of Long-Term Intensive Exercise Training. Circulation 2011, 123, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Abreu, P.; Vitzel, K.F.; Monteiro, I.C.C.R.; Lima, T.I.; Queiroz, A.N.; Leal-Cardoso, J.H.; Hirabara, S.M.; Ceccatto, V.M. Effects of Endurance Training on Reduction of Plasma Glucose during High Intensity Constant and Incremental Speed Tests in Wistar Rats. Braz. J. Med. Biol. Res. 2016, 49, e5226. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Copp, S.W.; Colburn, T.D.; Craig, J.C.; Allen, D.L.; Sturek, M.; O’Leary, D.S.; Zucker, I.H.; Musch, T.I. Guidelines for Animal Exercise and Training Protocols for Cardiovascular Studies. Am. J. Physiol. -Heart Circ. Physiol. 2020, 318, H1100–H1138. [Google Scholar] [CrossRef]
- Malgoyre, A.; Prola, A.; Meunier, A.; Chapot, R.; Serrurier, B.; Koulmann, N.; Bigard, X.; Sanchez, H. Endurance Is Improved in Female Rats After Living High-Training High Despite Alterations in Skeletal Muscle. Front. Sport. Act. Living 2021, 3, 663857. [Google Scholar] [CrossRef]
- Nagant, C.; Rozen, L.; Demulder, A. HIL Interferences on Three Hemostasis Analyzers and Contribution of a Preanalytical Module for Routine Coagulation Assays. Clin. Lab. 2016, 62, 1979–1987. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Warburton, D.E.R. Health Benefits of Physical Activity: The Evidence. Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Lu, Y.; Wiltshire, H.D.; Baker, J.S.; Wang, Q. Effects of High Intensity Exercise on Oxidative Stress and Antioxidant Status in Untrained Humans: A Systematic Review. Biology 2021, 10, 1272. [Google Scholar] [CrossRef]
- Deaton, C.M.; Marlin, D.J. Exercise-Associated Oxidative Stress. Clin. Tech. Equine Pract. 2003, 2, 278–291. [Google Scholar] [CrossRef]
- Ilhan, N.; Kamanli, A.; Ozmerdivenli, R.; Ilhan, N. Variable Effects of Exercise Intensity on Reduced Glutathione, Thiobarbituric Acid Reactive Substance Levels, and Glucose Concentration. Arch. Med. Res. 2004, 35, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Pannall, P.R. The Role of the Clinical Biochemist in Health Care. Pathology 1979, 11, 318. [Google Scholar] [CrossRef]
- Shepherd, J.R.A.; Dominelli, P.B.; Roy, T.K.; Secomb, T.W.; Hoyer, J.D.; Oliveira, J.L.; Joyner, M.J. Modelling the Relationships between Haemoglobin Oxygen Affinity and the Oxygen Cascade in Humans. J. Physiol. 2019, 597, 4193–4202. [Google Scholar] [CrossRef] [PubMed]
- Korniluk, A.; Koper-Lenkiewicz, O.M.; Kamińska, J.; Kemona, H.; Dymicka-Piekarska, V. Mean Platelet Volume (MPV): New Perspectives for an Old Marker in the Course and Prognosis of Inflammatory Conditions. Mediat. Inflamm. 2019, 2019, 9213074. [Google Scholar] [CrossRef]
- Garai, B.; Chatterjee, S.; Mondal, S.; Mondal, T. Effect of Exercise on Platelet Variables: An Overview. IJPESH 2017, 4, 506–510. [Google Scholar]
- Zhao, F.; Yan, Z.; Meng, Z.; Li, X.; Liu, M.; Ren, X.; Zhu, M.; He, Q.; Zhang, Q.; Song, K.; et al. Relationship between Mean Platelet Volume and Metabolic Syndrome in Chinese Patients. Sci. Rep. 2018, 8, 14574. [Google Scholar] [CrossRef]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The Compelling Link between Physical Activity and the Body’s Defense System. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can Exercise Affect Immune Function to Increase Susceptibility to Infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar]
- El-Wakil, E.S.; El-Shazly, M.A.M.; El-Ashkar, A.M.; Aboushousha, T.; Ghareeb, M.A. Chemical Profiling of Verbena officinalis and Assessment of Its Anti-Cryptosporidial Activity in Experimentally Infected Immunocompromised Mice. Arab. J. Chem. 2022, 15, 103945. [Google Scholar] [CrossRef]
- Biochemistry and Functions of the Liver. In Hepatology Textbook and Atlas; Springer: Berlin/Heidelberg, Germany, 2008; pp. 35–76. ISBN 978-3-540-76838-8.
- Eijsvogels, T.M.H.; Thompson, P.D.; Franklin, B.A. The “Extreme Exercise Hypothesis”: Recent Findings and Cardiovascular Health Implications. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Younus, H. Therapeutic Potentials of Superoxide Dismutase. Int. J. Health Sci. (Qassim) 2018, 12, 88–93. [Google Scholar]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Souissi, W.; Bouzid, M.A.; Farjallah, M.A.; Ben Mahmoud, L.; Boudaya, M.; Engel, F.A.; Sahnoun, Z. Effect of Different Running Exercise Modalities on Post-Exercise Oxidative Stress Markers in Trained Athletes. Int. J. Environ. Res. Public Health 2020, 17, 3729. [Google Scholar] [CrossRef] [PubMed]
- Marín-García, J. Oxidative Stress and Cell Death in Cardiovascular Disease. In Post-Genomic Cardiology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 471–498. ISBN 978-0-12-404599-6. [Google Scholar]
- Immunity and Inflammation in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-805417-8.
- Magherini, F.; Fiaschi, T.; Marzocchini, R.; Mannelli, M.; Gamberi, T.; Modesti, P.A.; Modesti, A. Oxidative Stress in Exercise Training: The Involvement of Inflammation and Peripheral Signals. Free. Radic. Res. 2019, 53, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, J.; Nijs, J.; Ickmans, K.; Godderis, L.; Ghosh, M.; Polli, A. The Interplay between Oxidative Stress, Exercise, and Pain in Health and Disease: Potential Role of Autonomic Regulation and Epigenetic Mechanisms. Antioxidants 2020, 9, 1166. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alsaqufi, A.S.; Omar, E.A.; El-Beltagi, H.S.; Srour, T.M.; Yousef, M.I. Ginseng, Tribulus Extracts and Pollen Grains Supplementation Improves Sexual State, Testes Redox Status, and Testicular Histology in Nile Tilapia Males. Antioxidants 2022, 11, 875. [Google Scholar] [CrossRef]
- Banan Khojasteh, S.M.; Javanmard Khameneh, R. Sophora Pachycarpa Root Extract Improves Testicular Damage in Carbon-Tetrachloride Intoxicated Rats. Zahedan J. Res. Med. Sci. 2018; in press. [Google Scholar] [CrossRef]
- Aramjoo, H.; Mohammadparast-Tabas, P.; Farkhondeh, T.; Zardast, M.; Makhdoumi, M.; Samarghandian, S.; Kiani, Z. Protective Effect of Sophora Pachycarpa Seed Extract on Carbon Tetrachloride-Induced Toxicity in Rats. BMC Complement. Med. Ther. 2022, 22, 76. [Google Scholar] [CrossRef]
- Khorsandi, L.; Oroojan, A.A. Toxic Effect of Tropaeolum majus L. Leaves on Spermatogenesis in Mice. JBRA Assist. Reprod. 2018, 22, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Rehecho, S.; Hidalgo, O.; García-Iñiguez de Cirano, M.; Navarro, I.; Astiasarán, I.; Ansorena, D.; Cavero, R.Y.; Calvo, M.I. Chemical Composition, Mineral Content and Antioxidant Activity of Verbena officinalis L. LWT Food Sci. Technol. 2011, 44, 875–882. [Google Scholar] [CrossRef]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research Advances in Their Phytochemistry, Biological Activities, and Pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Touaibia, M. Improvement of Testicular Steroidogenesis Using Flavonoids and Isoflavonoids for Prevention of Late-Onset Male Hypogonadism. Antioxidants 2020, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Olayinka, E.T.; Ore, A.; Adeyemo, O.A.; Ola, O.S. The Role of Flavonoid Antioxidant, Morin in Improving Procarbazine-Induced Oxidative Stress on Testicular Function in Rat. Porto Biomed. J. 2019, 4, e28. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, D.J.; Basu, A. Phenolic Compounds Potential Health Benefits and Toxicity. In Utilisation of Bioactive Compounds from Agricultural and Food Waste; Vuong, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; p. 33. ISBN 978-1-4987-4131-6. [Google Scholar]
- Ofosu, F.K.; Daliri, E.B.-M.; Elahi, F.; Chelliah, R.; Lee, B.-H.; Oh, D.-H. New Insights on the Use of Polyphenols as Natural Preservatives and Their Emerging Safety Concerns. Front. Sustain. Food Syst. 2020, 4, 525810. [Google Scholar] [CrossRef]
- Ly, C.; Yockell-Lelièvre, J.; Ferraro, Z.M.; Arnason, J.T.; Ferrier, J.; Gruslin, A. The Effects of Dietary Polyphenols on Reproductive Health and Early Development†. Hum. Reprod. Update 2015, 21, 228–248. [Google Scholar] [CrossRef]
- Kyselova, Z. Toxicological Aspects of the Use of Phenolic Compounds in Disease Prevention. Interdiscip. Toxicol. 2011, 4, 173–183. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Bešlo, D.; Došlić, G.; Agić, D.; Rastija, V.; Šperanda, M.; Gantner, V.; Lučić, B. Polyphenols in Ruminant Nutrition and Their Effects on Reproduction. Antioxidants 2022, 11, 970. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues Oliveira, S.M.; Dias, E.; Girol, A.P.; Silva, H.; Pereira, M.d.L. Exercise Training and Verbena officinalis L. Affect Pre-Clinical and Histological Parameters. Plants 2022, 11, 3115. https://doi.org/10.3390/plants11223115
Rodrigues Oliveira SM, Dias E, Girol AP, Silva H, Pereira MdL. Exercise Training and Verbena officinalis L. Affect Pre-Clinical and Histological Parameters. Plants. 2022; 11(22):3115. https://doi.org/10.3390/plants11223115
Chicago/Turabian StyleRodrigues Oliveira, Sonia M., Elsa Dias, Ana Paula Girol, Helena Silva, and Maria de Lourdes Pereira. 2022. "Exercise Training and Verbena officinalis L. Affect Pre-Clinical and Histological Parameters" Plants 11, no. 22: 3115. https://doi.org/10.3390/plants11223115
APA StyleRodrigues Oliveira, S. M., Dias, E., Girol, A. P., Silva, H., & Pereira, M. d. L. (2022). Exercise Training and Verbena officinalis L. Affect Pre-Clinical and Histological Parameters. Plants, 11(22), 3115. https://doi.org/10.3390/plants11223115









