Ultrasound-Assisted Natural Deep Eutectic Solvents Extraction of Bilberry Anthocyanins: Optimization, Bioactivities, and Storage Stability
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening Analysis of NaDES
2.2. Modelling and Optimization
2.2.1. Model Fitting Assessment
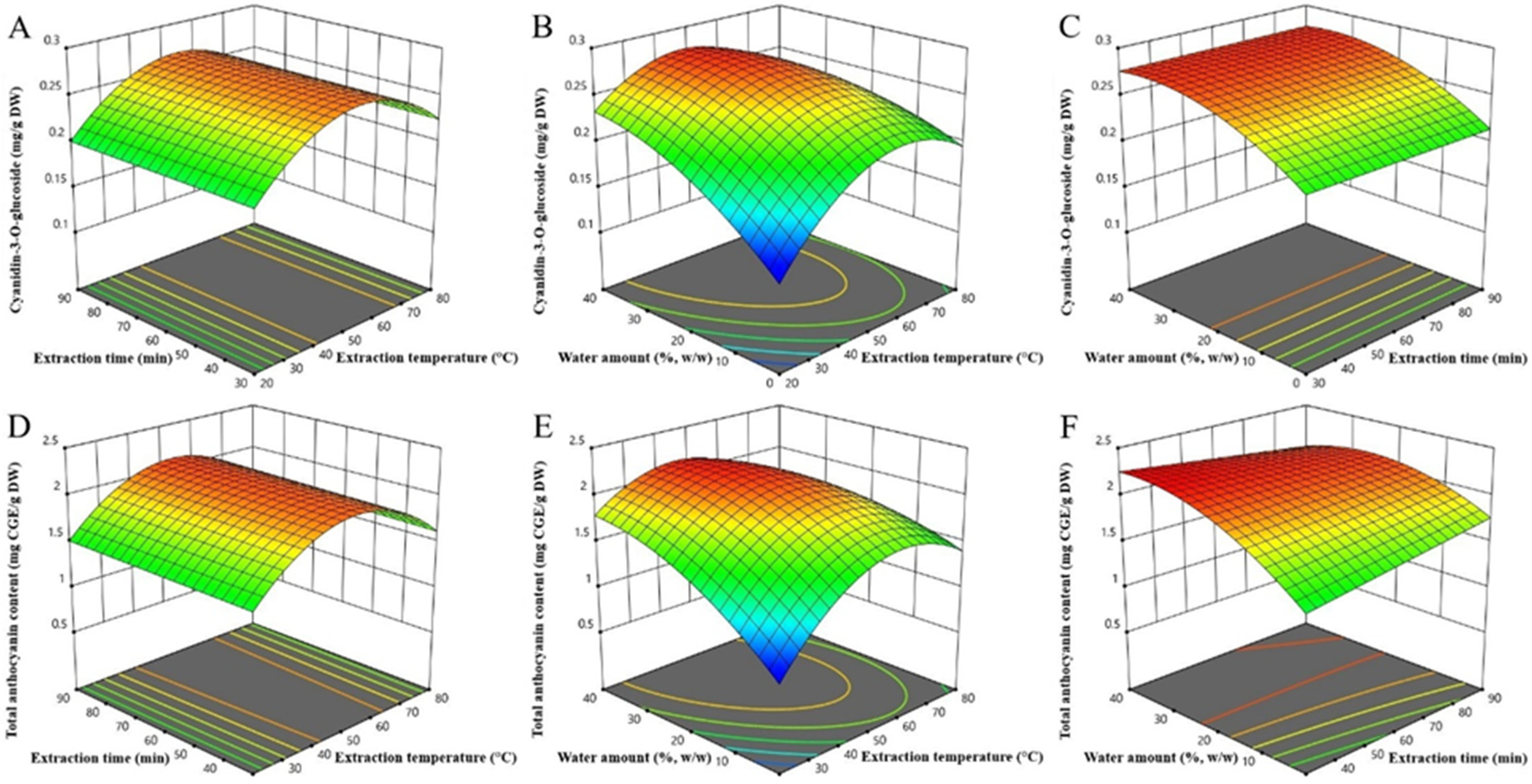
2.2.2. Influence of Extraction Parameters on Cyanidin-3-O-glucoside Extraction
| Source | p-Values | |
|---|---|---|
| Cyanidin-3-O-glucoside | Total Anthocyanin Content | |
| Linear terms | ||
| X1—Extraction temperature | 0.0433 * | 0.0797 |
| X2—Extraction time | / | 0.6098 |
| X3—Water amount | <0.0001 *** | <0.0001 *** |
| Quadratic terms | ||
| X21 | <0.0001 *** | <0.0001 *** |
| X22 | / | / |
| X23 | 0.0118 * | 0.0013 ** |
| Interaction terms | ||
| X1X2 | / | / |
| X1X3 | 0.0118 * | 0.0008 *** |
| X2X3 | / | 0.0288 * |
| Model fitting assessment | ||
| Model | <0.0001 *** | <0.0001 *** |
| Lack of fit | 0.0755 | 0.1690 |
| R2 | 0.9059 | 0.9577 |
| Adjusted R2 | 0.8666 | 0.9281 |
| Predicted R2 | 0.7477 | 0.8338 |
| CV (%) | 6.49 | 5.94 |
2.2.3. Influence of Extraction Parameters on TAC Extraction
2.2.4. Optimization and Model Validation
2.3. Biological Activities
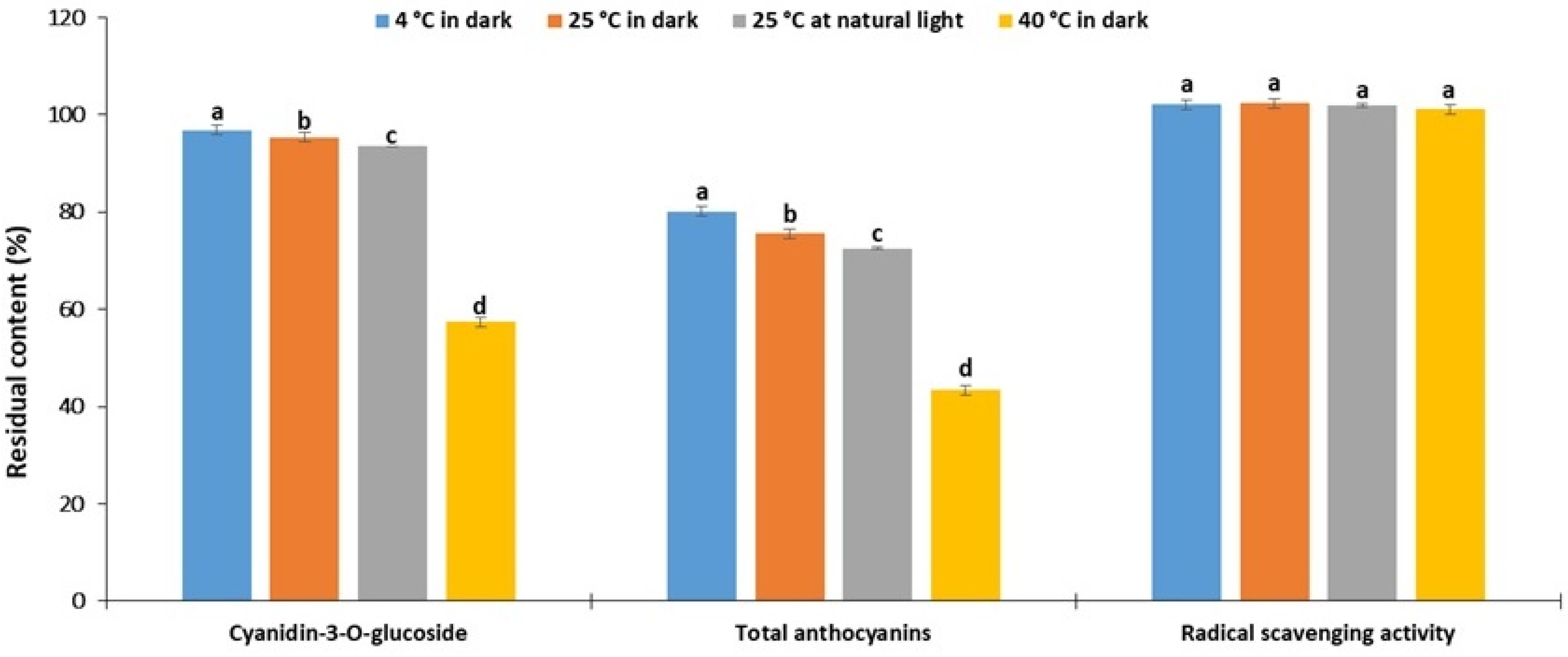
2.4. Storage Stability
3. Materials and Methods
3.1. Plant Material
3.2. Screening Analysis of NaDES
3.2.1. Preparation of NaDES
3.2.2. Extraction Procedure
3.3. Optimization by RSM
3.4. Chemical Analysis
3.4.1. HPLC Analysis
3.4.2. Quantification of TAC
3.5. Biological Activities
3.5.1. Antimicrobial Activity
3.5.2. Radical Scavenging Activity
3.6. Storage Stability
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dare, A.P.; Günther, C.S.; Grey, A.C.; Guo, G.; Demarais, N.J.; Cordiner, S.; McGhie, T.K.; Boldingh, H.; Hunt, M.; Deng, C.; et al. Resolving the developmental distribution patterns of polyphenols and related primary metabolites in bilberry (Vaccinium myrtillus) fruit. Food Chem. 2022, 374, 131703. [Google Scholar] [CrossRef] [PubMed]
- ESCOP Monographs: The Scientific Foundation for Herbal Medicinal Products; European Scientific Cooperative on Phytotherapy (ESCOP): Exeter, UK, 2014.
- Primetta, A.K.; Jaakola, L.; Ayaz, F.A.; Inceer, H.; Riihinen, K.R. Anthocyanin fingerprinting for authenticity studies of bilberry (Vaccinium myrtillus L.). Food Control 2013, 30, 662–667. [Google Scholar] [CrossRef]
- Dinkova, R.; Heffels, P.; Shikov, V.; Weber, F.; Schieber, A.; Mihalev, K. Effect of enzyme-assisted extraction on the chilled storage stability of bilberry (Vaccinium myrtillus L.) anthocyanins in skin extracts and freshly pressed juices. Food Res. Int. 2014, 65, 35–41. [Google Scholar] [CrossRef]
- Da Silva, D.T.; Pauletto, R.; Da Silva Cavalheiro, S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Compost. Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- Klimaviciute, R.; Navikaite, V.; Jakstas, V.; Ivanauskas, L. Complexes of dextran sulfate and anthocyanins from Vaccinium myrtillus: Formation and stability. Carbohydr. Polym. 2015, 129, 70–78. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Minuzzi, N.M.; Rodrigues, R.F.; Pauletto, R.; Rodrigues, E.; Emanuelli, T.; Bochi, V.C. Citric acid water-based solution for blueberry bagasse anthocyanins recovery: Optimization and comparisons with microwave-assisted extraction (MAE). LWT 2020, 133, 110064. [Google Scholar] [CrossRef]
- Mohd Fuad, F.; Mohd Nadzir, M.; Kamaruddin, H.A. Hydrophilic natural deep eutectic solvent: A review on physicochemical properties and extractability of bioactive compounds. J. Mol. Liq. 2021, 339, 116923. [Google Scholar] [CrossRef]
- Xing, C.; Cui, W.Q.; Zhang, Y.; Zou, X.S.; Hao, J.Y.; Zheng, S.D.; Wang, T.T.; Wang, X.Z.; Wu, T.; Liu, Y.Y.; et al. Ultrasound-assisted deep eutectic solvents extraction of glabridin and isoliquiritigenin from Glycyrrhiza glabra: Optimization, extraction mechanism and in vitro bioactivities. Ultrason. Sonochem. 2022, 83, 105946. [Google Scholar] [CrossRef]
- Santana, A.P.R.; Mora-Vargas, J.A.; Guimarães, T.G.S.; Amaral, C.D.B.; Oliveira, A.; Gonzalez, M.H. Sustainable synthesis of natural deep eutectic solvents (NADES) by different methods. J. Mol. Liq. 2019, 293, 111452. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, D.; Belwal, T.; Xie, J.; Xu, Y.; Li, L.; Luo, Z. Natural deep eutectic solvent enhanced pulse-ultrasonication assisted extraction as a multi-stability protective and efficient green strategy to extract anthocyanin from blueberry pomace. LWT 2021, 144, 111220. [Google Scholar] [CrossRef]
- Türker, D.A.; Doğan, M. Ultrasound-assisted natural deep eutectic solvent extraction of anthocyanin from black carrots: Optimization, cytotoxicity, in-vitro bioavailability and stability. Food Bioprod. Process. 2022, 132, 99–113. [Google Scholar] [CrossRef]
- Da Silva, D.T.; Smaniotto, F.A.; Costa, I.F.; Baranzelli, J.; Muller, A.; Somacal, S.; Emanuelli, T. Natural deep eutectic solvent (NADES): A strategy to improve the bioavailability of blueberry phenolic compounds in a ready-to-use extract. Food Chem. 2021, 364, 130370. [Google Scholar] [CrossRef]
- Jovanović, M.; Mudrić, J.; Drinić, Z.; Matejić, J.; Kitić, D.; Bigović, D.; Šavikin, K. Optimization of ultrasound-assisted extraction of bitter compounds and polyphenols from willow gentian underground parts. Sep. Purif. Technol. 2022, 281, 119868. [Google Scholar] [CrossRef]
- Rae, J.; Ashokkumar, M.; Eulaerts, O.; Von Sonntag, C.; Reisse, J.; Grieser, F. Estimation of ultrasound induced cavitation bubble temperatures in aqueous solutions. Ultrason. Sonochem. 2005, 12, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing By-Products: A Review. Ultrason. Sonochem. 2020, 70, 105325. [Google Scholar] [CrossRef]
- Dai, Y.; Varypataki, E.M.; Golovina, E.A.; Jiskoot, W.; Witkamp, G.J.; Choi, Y.H.; Verpoorte, R. Natural deep eutectic solvents in plants and plant cells: In vitro evidence for their possible functions. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2020; Volume 97, pp. 159–184. [Google Scholar]
- Gómez, A.V.; Tadini, C.C.; Biswas, A.; Buttrum, M.; Kim, S.; Boddu, V.M.; Cheng, H.N. Microwave-assisted extraction of soluble sugars from banana puree with natural deep eutectic solvents (NADES). LWT 2019, 107, 79–88. [Google Scholar] [CrossRef]
- Quadrelli, E.A. 25 years of energy and green chemistry: Saving, storing, distributing and using energy responsibly. Green Chem. 2016, 18, 328–330. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural deep eutectic solvent mediated pretreatment of rice straw: Bioanalytical characterization of lignin extract and enzymatic hydrolysis of pretreated biomass residue. Environ. Sci. Pollut. Res. Int. 2016, 23, 9265–9275. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Alakomi, H.L.; Oksman-Caldentey, K.M. The action of berry phenolics against human intestinal pathogens. BioFactors 2005, 23, 243–251. [Google Scholar] [CrossRef]
- Klavins, L.; Mezulis, M.; Nikolajeva, V.; Klavins, M. Composition, sun protective and antimicrobial activity of lipophilic bilberry (Vaccinium myrtillus L.) and lingonberry (Vaccinium vitis-idaea L.) extract fractions. LWT 2021, 138, 110784. [Google Scholar] [CrossRef]
- Satoh, Y.; Ishihara, K. Investigation of the antimicrobial activity of Bilberry (Vaccinium myrtillus L.) extract against periodontopathic bacteria. J. Oral Biosci. 2020, 62, 169–174. [Google Scholar] [CrossRef]
- Thornthwaite, J.T.; Thibado, S.P.; Thornthwaite, K.A. Bilberry anthocyanins as agents to address oxidative stress. In Pathology, Oxidative Stress and Dietary Antioxidants, 1st ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2020; pp. 179–187. [Google Scholar]
- Zimmer, R.; Blum-Silva, H.; Kulkamp Souza, L.; WulffSchuch, M.; Reginatto, H.; Pereira, P.; Lencina, L. The antibiofilm effect of blueberry fruit cultivars against Staphylococcus epidermidis and Pseudomonas aeruginosa. J. Med. Food 2014, 17, 324–331. [Google Scholar] [CrossRef]
- Silva, S.; Costa, M.; Mendes, M.; Morais, M.; Calhau, C.; Pintado, P. Antimicrobial, antiadhesive and antibiofilm activity of an ethanolic, anthocyanin-rich blueberry extract purified by solid phase extraction. J. Appl. Microbiol. 2016, 121, 693–703. [Google Scholar] [CrossRef]
- Cisowska, A.; Wojnicz, D.; Hendrich, A. Anthocyanins as Antimicrobial Agents of Natural Plant Origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef]
- Tavares, D.; Antunes, C.; Padrão, J.; Ribeiro, I.; Zille, A.; Amorim, P.; Ferreira, F.; Felgueiras, P. Activity of Specialized Biomolecules against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, S.; Fei, Y.; Liu, G.; Jang, H.; Fang, J. Antimicrobial activity of anthocyanins and catechins against foodborne pathogens Escherichia coli and Salmonella. Food Control 2019, 106, 106712. [Google Scholar] [CrossRef]
- Sinela, A.; Rawat, N.; Mertz, C.; Achir, N.; Fulcrand, H.; Dornier, M. Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chem. 2017, 214, 234–241. [Google Scholar] [CrossRef]
- Yugoslavian Pharmacopeia (Pharmacopoea Jugoslavica), 5th ed.; National Institute for Health Protection, Contemporaneous Administration: Belgrade, Serbia, 2000; Section 2.1.4; p. 18.
- European Pharmacopoeia 10.0; Council of Europe: Strasbourg CEDEX, France, 2019.
- National Committee for Clinical Laboratory Standards (CLSI) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved standard. NCCLS Document M38-A; NCCLS: Wayne, PA, USA, 2002.
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
| Solvent * | Composition | Molar Ratio | Cyanidin-3-O-glucoside (mg/g DW) | Total Anthocyanin Content (mg CGE/g DW) |
|---|---|---|---|---|
| NaDES 1 | Choline chloride:Lactic acid | 1:2 | 0.19 ± 0.01 d | 2.48 ± 0.02 b |
| NaDES 2 | Choline chloride:Citric acid:Water | 1:1:2 | 0.28 ± 0.00 a | 1.78 ± 0.04 f |
| NaDES 3 | Choline chloride:Malic acid:Water | 1:1:2 | 0.28 ± 0.01 a | 2.24 ± 0.06 c |
| NaDES 4 | Choline chloride:Tartaric acid | 1:2 | 0.20 ± 0.00 cd | 1.25 ± 0.04 h |
| NaDES 5 | Choline chloride:Glycerol | 1:2 | 0.28 ± 0.01 a | 1.90 ± 0.04 e |
| NaDES 6 | Choline chloride:1,2-propanediol | 1:3 | 0.21 ± 0.00 c | 2.00 ± 0.02 de |
| NaDES 7 | Choline chloride:Sorbitol | 1:1 | 0.29 ± 0.00 a | 2.03 ± 0.01 d |
| NaDES 8 | Choline chloride:Glucose:Water | 2:1:1 | 0.29 ± 0.01 a | 1.74 ± 0.06 fg |
| NaDES 9 | Choline chloride:Fructose:Water | 2:1:1 | 0.25 ± 0.00 b | 1.64 ± 0.06 g |
| NaDES 10 | Choline chloride:Urea | 1:2 | 0.20 ± 0.01 cd | 0.92 ± 0.01 i |
| MeOH | Methanol | 0.20 ± 0.00 cd | 2.93 ± 0.01 a |
| Experiment | Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 |
|---|---|---|---|---|---|
| X1: Extraction Temperature (°C) | X2: Extraction Time (min) | X3: Water Amount (%, w/w) | Cyanidin-3-O-glucoside (mg/g DW) | Total Anthocyanin Content (mg CGE/g DW) | |
| 1 | 20 (−1) | 30 (−1) | 20 (0) | 0.19 | 1.33 |
| 2 | 80 (+1) | 30 (−1) | 20 (0) | 0.24 | 1.78 |
| 3 | 20 (−1) | 90 (+1) | 20 (0) | 0.19 | 1.50 |
| 4 | 80 (+1) | 90 (+1) | 20 (0) | 0.21 | 1.57 |
| 5 | 20 (−1) | 60 (0) | 0 (−1) | 0.14 | 0.85 |
| 6 | 80 (+1) | 60 (0) | 0 (−1) | 0.20 | 1.38 |
| 7 | 20 (−1) | 60 (0) | 40 (+1) | 0.24 | 1.85 |
| 8 | 80 (+1) | 60 (0) | 40 (+1) | 0.21 | 1.38 |
| 9 | 50 (0) | 30 (−1) | 0 (−1) | 0.18 | 1.39 |
| 10 | 50 (0) | 90 (+1) | 0 (−1) | 0.23 | 1.76 |
| 11 | 50 (0) | 30 (−1) | 40 (+1) | 0.28 | 2.22 |
| 12 | 50 (0) | 90 (+1) | 40 (+1) | 0.27 | 2.05 |
| 13 | 50 (0) | 60 (0) | 20 (0) | 0.27 | 2.06 |
| 14 | 50 (0) | 60 (0) | 20 (0) | 0.28 | 2.22 |
| 15 | 50 (0) | 60 (0) | 20 (0) | 0.28 | 2.19 |
| 16 | 50 (0) | 60 (0) | 20 (0) | 0.26 | 2.10 |
| 17 | 50 (0) | 60 (0) | 20 (0) | 0.26 | 2.07 |
| 18 | 50 (0) | 60 (0) | 20 (0) | 0.26 | 2.02 |
| Optimized Extraction Conditions | Responses under Optimized Extraction Conditions | ||
|---|---|---|---|
| Extraction temperature: 48.38 °C Extraction time: 37.63 min Water amount: 34.79% (w/w) (Desirability: 0.98) | Target compounds | Predicted mean (95% confidence interval) | Validated values (mean ± standard deviation) |
| Cyanidin-3-O-glucoside (mg/g DW) | 0.28 (0.26–0.29) | 0.27 ± 0.00 | |
| Total anthocyanin content (mg CGE/g DW) | 2.23 (2.10–2.36) | 2.12 ± 0.02 | |
| Microorganism | MIC (mg/mL) | MBC/MFC (mg/mL) | |
|---|---|---|---|
| Foodborne pathogens | Escherichia coli O157:H7 | 20 | 25 |
| Salmonella typhimurium ATCC 14028 | 35 | >35 | |
| Shigella flexneri ATCC 12022 | 25 | 30 | |
| Listeria monocytogenes ATCC 19114 | 15 | 20 | |
| Enterococcus faecalis ATCC 29212 | 25 | 25 | |
| Causatives of skin infections and cosmetic products spoilage | Escherichia coli ATCC 8739 | 25 | 30 |
| Pseudomonas aeruginosa ATCC 27853 | >35 | >35 | |
| Staphylococcus aureus ATCC 25923 | 15 | 20 | |
| Staphylococcus epidermidis ATCC 12228 | 15 | 15 | |
| Candida albicans ATCC 10231 | 15 | 20 | |
| Aspergillus brasiliensis ATCC 16404 | 25 | 30 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, M.S.; Krgović, N.; Živković, J.; Stević, T.; Zdunić, G.; Bigović, D.; Šavikin, K. Ultrasound-Assisted Natural Deep Eutectic Solvents Extraction of Bilberry Anthocyanins: Optimization, Bioactivities, and Storage Stability. Plants 2022, 11, 2680. https://doi.org/10.3390/plants11202680
Jovanović MS, Krgović N, Živković J, Stević T, Zdunić G, Bigović D, Šavikin K. Ultrasound-Assisted Natural Deep Eutectic Solvents Extraction of Bilberry Anthocyanins: Optimization, Bioactivities, and Storage Stability. Plants. 2022; 11(20):2680. https://doi.org/10.3390/plants11202680
Chicago/Turabian StyleJovanović, Miloš S., Nemanja Krgović, Jelena Živković, Tatjana Stević, Gordana Zdunić, Dubravka Bigović, and Katarina Šavikin. 2022. "Ultrasound-Assisted Natural Deep Eutectic Solvents Extraction of Bilberry Anthocyanins: Optimization, Bioactivities, and Storage Stability" Plants 11, no. 20: 2680. https://doi.org/10.3390/plants11202680
APA StyleJovanović, M. S., Krgović, N., Živković, J., Stević, T., Zdunić, G., Bigović, D., & Šavikin, K. (2022). Ultrasound-Assisted Natural Deep Eutectic Solvents Extraction of Bilberry Anthocyanins: Optimization, Bioactivities, and Storage Stability. Plants, 11(20), 2680. https://doi.org/10.3390/plants11202680








