Role of Antioxidant Enzymes and Glutathione S-Transferase in Bromoxynil Herbicide Stress Tolerance in Wheat Plants
Abstract
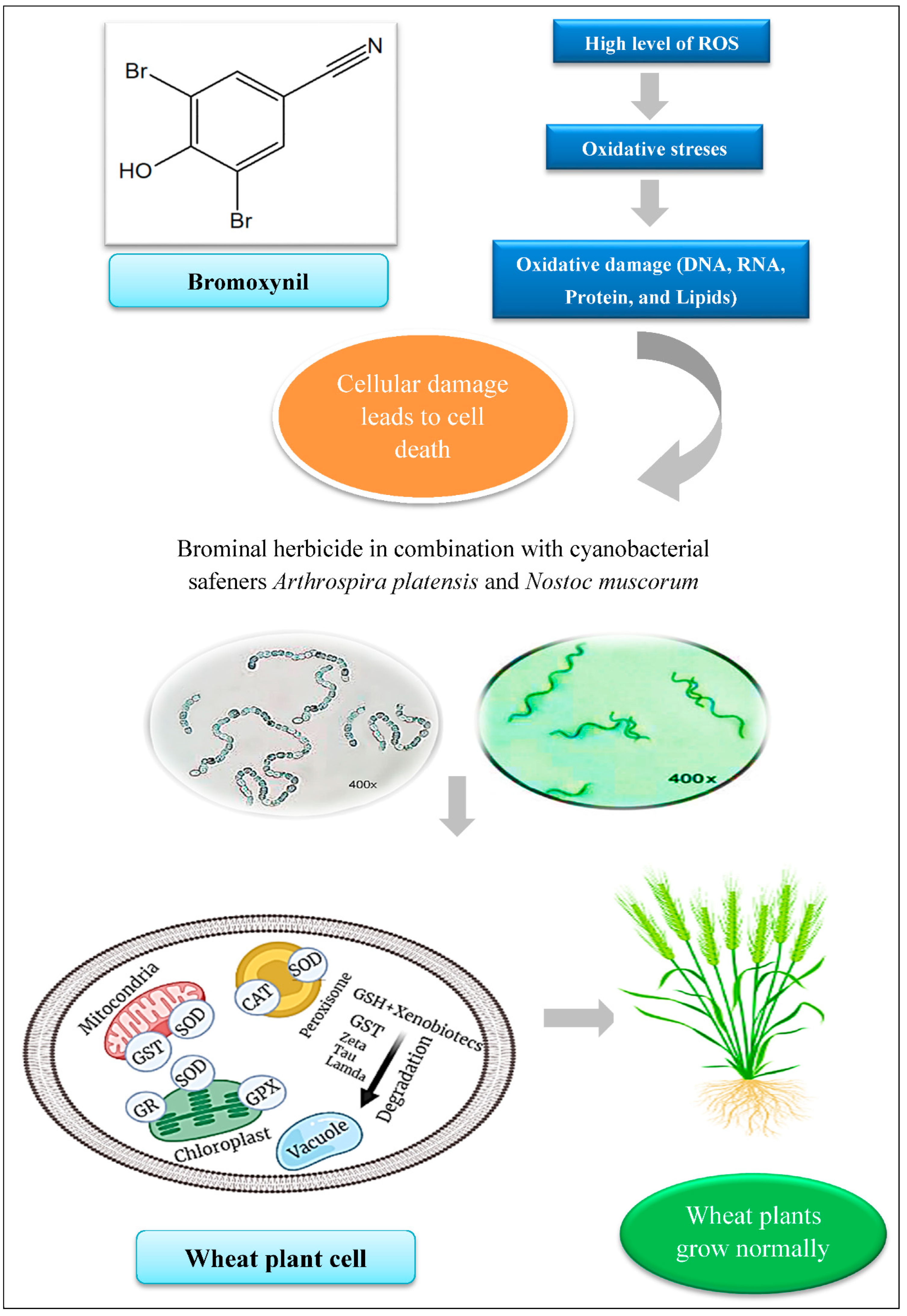
1. Introduction
2. Results
2.1. Growth Parameters
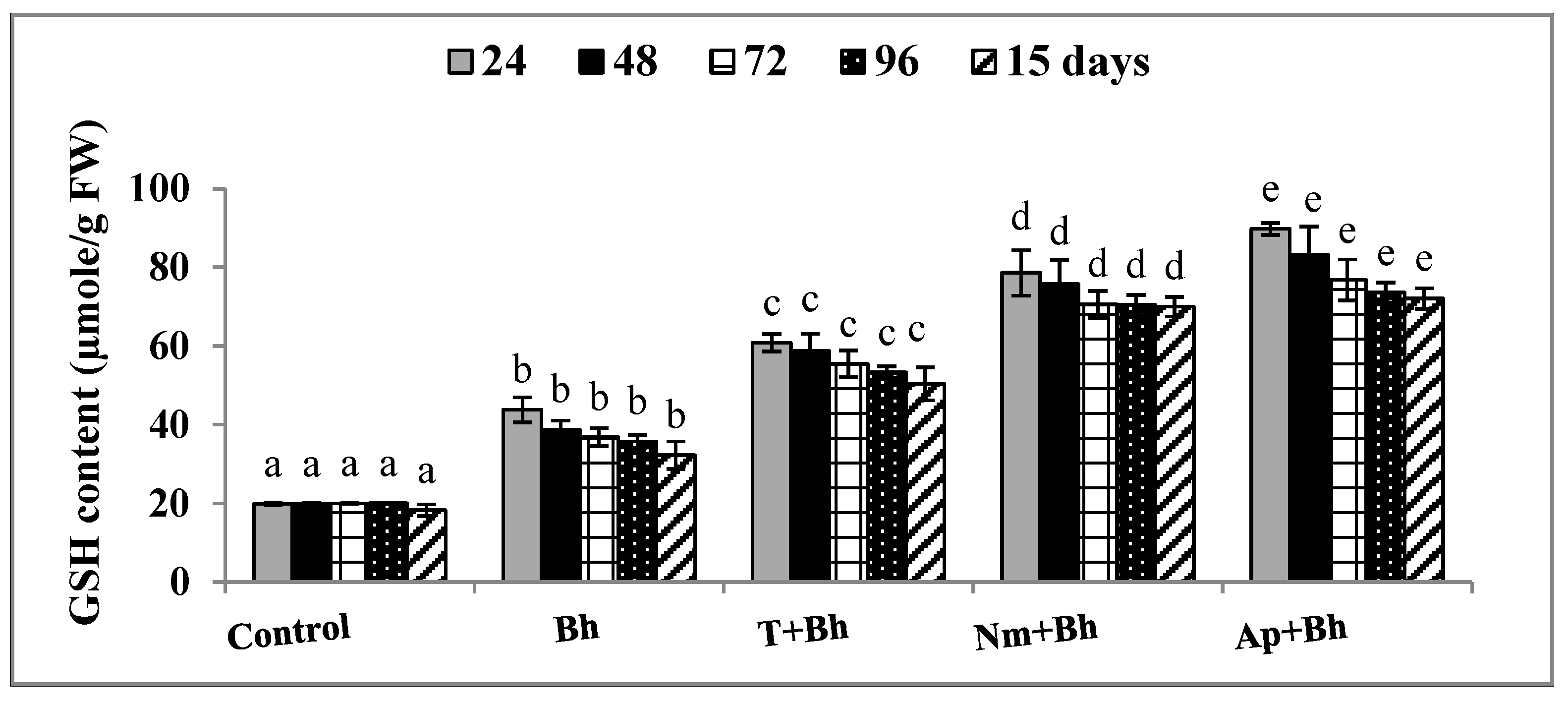
2.2. Biochemical Analysis
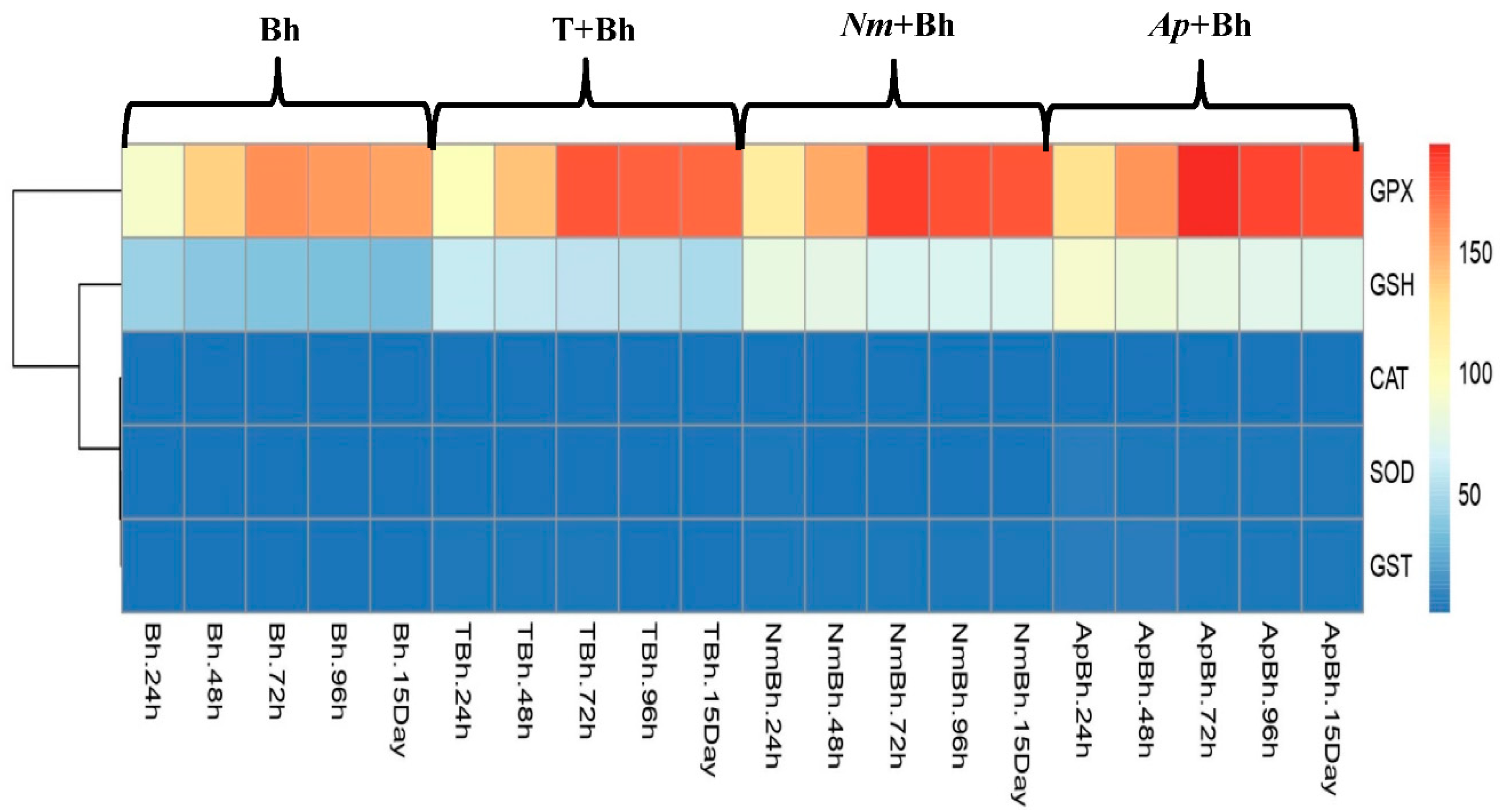
Antioxidant Activity
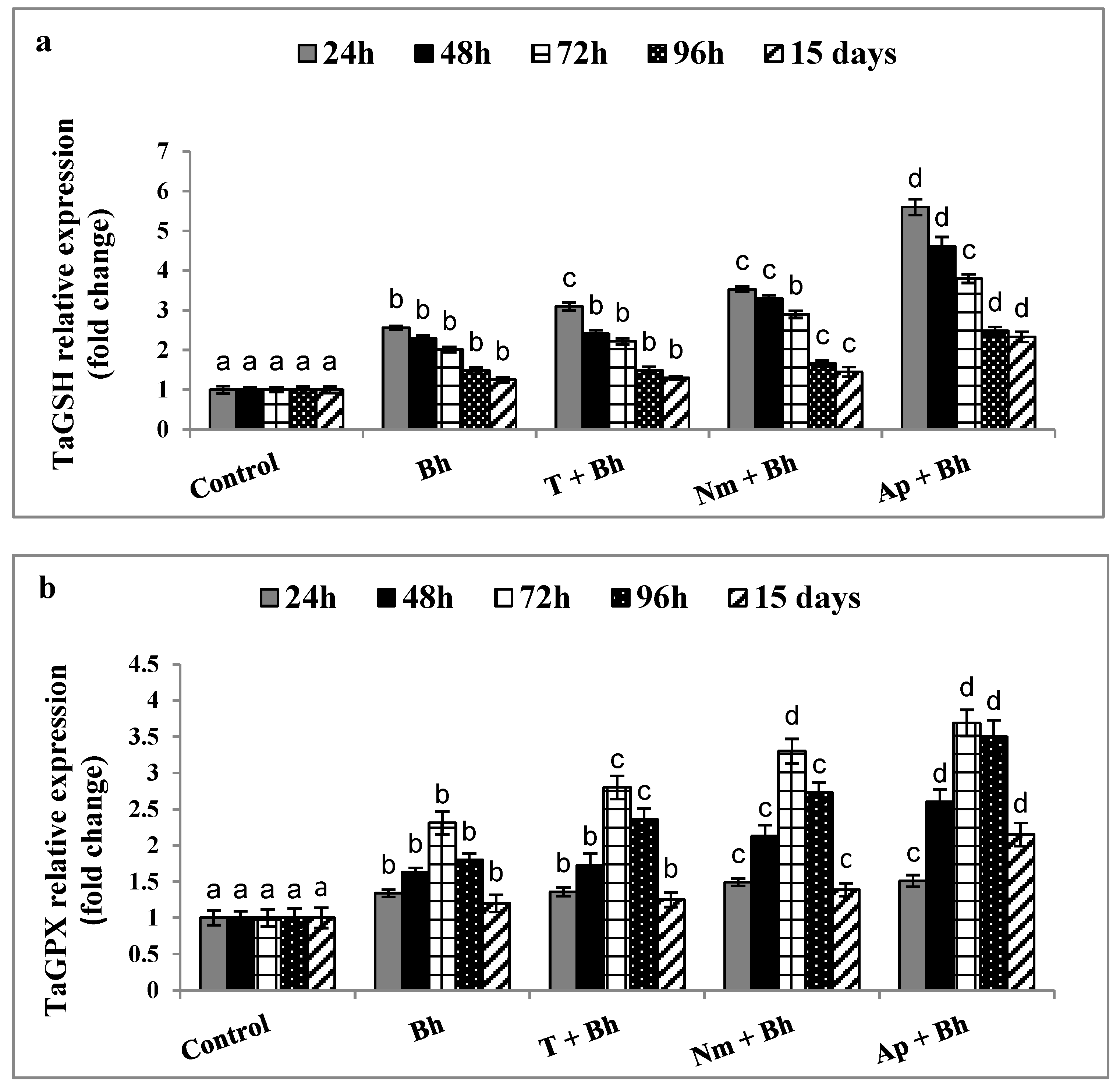
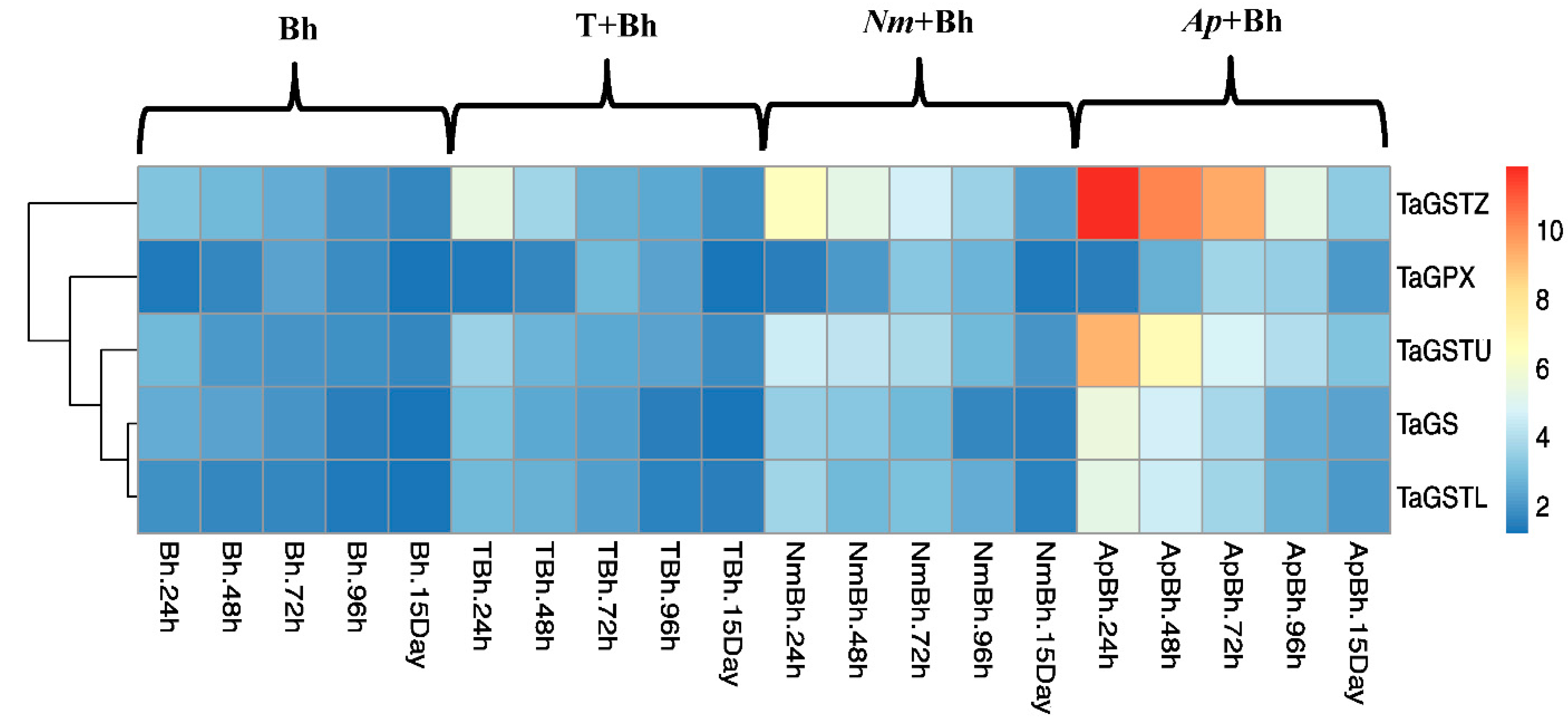
2.3. Genes Expression Analysis
Quantitative Real-Time PCR (qRT-PCR) of Antioxidant Enzyme-Related Genes
3. Discussion
4. Materials and Methods
4.1. Cyanobacteria Isolation, Identification, and Growth Culturing
4.2. Herbicide
4.3. Plant Growth and Treatments
4.4. Plant Samples Collection
4.4.1. Growth Parameters
4.4.2. Biochemical Analyses
Antioxidant Activity
4.4.3. Gene Expression Analysis Using qRT-PCR
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hossain, M.; Begum, M. Soil Weed Seed Bank: Importance and Management for Sustainable Crop Production—A Review. J. Bangladesh Agric. Univ. 2016, 13, 221–228. [Google Scholar] [CrossRef]
- Nishimoto, R.K. Purple Nutsedge Tuber Sprouting. Weed Biol. Manag. 2001, 1, 203–208. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Iza, S.C.; Rodríguez, A.I. Oxidative Stress and Pesticide Disease: A Challenge for Toxicology. Rev. Fac. Med. 2018, 66, 261–267. [Google Scholar] [CrossRef]
- Caverzan, A.; Piasecki, C.; Chavarria, G.; Stewart, C.N., Jr.; Vargas, L. Defenses against ROS in crops and weeds: The effects of interference and herbicides. Int. J. Mol. Sci. 2019, 20, 1086. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.; Doganlar, Z.B. Exogenous Jasmonic Acid Induces Stress Tolerance in Tobacco (Nicotiana tabacum) Exposed to Imazapic. Ecotoxicol. Environ. Saf. 2016, 124, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Riechers, D.E.; Kreuz, K.; Zhang, Q. Detoxification without intoxication: Herbicide safeners activate plant defense gene expression. Plant Physiol. 2010, 153, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Rosinger, C. Herbicide Safeners: An overview. Julius-Kühn-Archiv 2014, 443, 516–525. [Google Scholar] [CrossRef]
- Taylor, V.L.; Cummins, I.; Brazier-Hicks, M.; Edwards, R. Protective responses induced by herbicide safeners in wheat. Environ. Exp. Bot. 2013, 88, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.V.D.; Martins, C.C.; Silva, J.A.C.D.; Martins, D. Fluxofenim is used as a safener on sorghum seed for S-metolachlor herbicide TT—Fluxofenim em sementes de sorgo como protector ao herbicide S-metolachlor. Biosci. J. 2014, 30, 158–167. [Google Scholar]
- Hicks, L.C.; Rousk, K.; Rinnan, R.; Rousk, J. Soilmicrobial responses to 28 years of nutrient fertilization in a subarctic heath. Ecosystems 2019, 23, 1107–1119. [Google Scholar] [CrossRef]
- Cinelli, R.; Barbieri, A.S.; Polito, R.A.; Heck, T.; Bispo, N.B.; Nunes, A.L. The Use of Herbicide Safener Increases the Selectivity of Nicosulfuron to Maize Crops. J. Agric. Stud. 2020, 8, 460. [Google Scholar] [CrossRef]
- Hatzios, K.K. Metabolism-based herbicide resistance: Regulation by safeners. Weed Sci. 2004, 52, 454–467. [Google Scholar] [CrossRef]
- Schröder, P.; Scheer, C.E.; Diekmann, F.; Stampfl, A. How Plants Cope with Foreign Compounds. Translocation of xenobiotic glutathione conjugates in roots of barley (Hordeum vulgare). Environ. Sci. Pollut. Res. 2007, 14, 114–122. [Google Scholar] [CrossRef]
- Pan, S.; Jeevanandam, J.; Danquah, M. Benefits of Algal Extracts in Sustainable Agriculture. In Grand Challenges in Algae Biotechnology; Springer: Cham, Switzerland, 2020; pp. 501–553. [Google Scholar]
- Pathak, J.; Rajneesh; Maurya, P.K.; Singh, S.P.; Häder, D.P.; Sinha, R.P. Cyanobacterial farming for environment-friendly sustainable agriculture practices: Innovations and perspectives. Fron. Environ. Sci. 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Górka, B.; Korzeniowska, K.; Lipok, J.; Wieczorek, P.P. The Biomass of Algae and Algal Extracts in Agricultural Production. In Algae Biomass: Characteristics and Applications; Springer: Cham, Switzerland, 2018; pp. 103–114. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and bio-fertilizers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Han, X.; Zeng, H.; Bartocci, P.; Fantozzi, F.; Yan, Y. Phytohormones and effects on growth and metabolites of microalgae. Fermentation 2018, 4, 25. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture, and medicine: Current status and future prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- EL Arroussi, H.; Benhima, R.; Elbaouchi, A.; Sijilmassi, B.; EL Mernissi, N.; Aafsar, A.; Meftah-Kadmiri, I.; Bendaou, N.; Smouni, A. Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J. Appl. Phycol. 2018, 30, 2929–2941. [Google Scholar] [CrossRef]
- Sivey, J.D.; Lehmler, H.-J.; Salice, C.J.; Ricko, A.N.; Cwiertny, D.M. Environmental Fate and Effects of Dichloroacetamide Herbicide Safeners: “Inert” yet Biologically Active Agrochemical Ingredients. Environ. Sci. Technol. Lett. 2015, 2, 260–269. [Google Scholar] [CrossRef]
- Kraehmer, H.; Laber, B.; Rosinger, C.; Schulz, A. Herbicides as Weed Control Agents: State of the Art: I. Weed Control Research and Safener Technology: The Path to Modern Agriculture. Plant Physiol. 2014, 166, 1119–1131. [Google Scholar] [CrossRef]
- Priya, H.; Prasanna, R.; Ramakrishnan, B.; Bidyarani, N.; Babu, S.; Thapa, S.; Renuka, N. Influence of cyanobacterial inoculation on the culturable microbiome and growth of rice. Microbiol. Res. 2015, 171, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Gheda, S.F.; Ahmed, D.A. Improved Soil Characteristics and Wheat Germination as Influenced by Inoculation of Nostoc kihlmani and Anabaena cylindrica. Rend. Lincei 2015, 26, 121–131. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Abo-Shady, A.M.; El-Nagar, M.M.F. Cyanobacterial Arthrospira (Spirulina platensis) as Safener against Harmful Effects of Fusillade Herbicide on Faba Bean Plant. Rend. Lincei. 2016, 27, 455–462. [Google Scholar] [CrossRef]
- Godlewska, K.; Michalak, I.; Pacyga, P.; Baśladyńska, S.; Chojnacka, K. Potential applications of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J. Microbiol. Biotechnol. 2019, 35, 80. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, S.; Riahi, H.; Shariatmadari, Z.; Sonboli, A. Effects of cyanobacterial suspensions as bio-fertilizers on growth factors and the essential oil composition of chamomile, Matricaria chamomilla L. J. Appl.Phycol. 2020, 32, 1231–1241. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Abo-Shady, A.M.; Gaafar, R.M.; El-Nagar, M.M.F.; Ismail, G.A. Promoting Wheat Growth by Priming Grains with Water Extracts of Nostoc muscorum and Arthrospira platensis. Egypt. J. Bot. 2021, 61, 809–821. [Google Scholar] [CrossRef]
- Behrouzi, M.V.; Valizadeh, M.; Bandehagh, A. Primary Antioxidant Enzymes, and Their Important Role in Oxidative Stress in Plants and Mammalian. Biol. Forum Int. J. 2015, 7, 148–154. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First line defense antioxidants-superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defense grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Shumayla, S.T.; Singh, S.P.; Upadhyay, S.K. Role of Superoxide Dismutases (SODs) in Stress Tolerance in Plants. In Molecular Approaches in Plant Biology and Environmental Challenges; Springer: Singapore, 2019; pp. 51–77. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Glutathione S-Transferases: Role in Combating Abiotic Stresses Including Arsenic Detoxification in Plants. Front. Plant Sci. 2018, 9, 751. [Google Scholar] [CrossRef]
- Gullner, G.; Komives, T.; Király, L.; Schröder, P. Glutathione S-transferase enzymes in plant-pathogen interactions. Front. Plant Sci. 2018, 871, 1836. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.A.; Kaur, A.; Tyagi, S.; Upadhyay, S.K. Glutathione Peroxidases in Plants: Innumerable Role in Abiotic Stress Tolerance and Plant Development. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Kaur1, M.A.; Tyagi, S.; Singh, S.K.; Upadhyay, S.K. Exploration of glutathione reductase for abiotic stress response in bread wheat (Triticum aestivum L.). Plant Cell Rep. 2022, 41, 639–654. [Google Scholar] [CrossRef]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-induced oxidative stress and toxicity. J. Toxic. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, E.; Georgakis, N.; Nianiou-Obeidat, I.; Madesis, P.; Perperopoulou, F.; Pouliou, F.; Vasilopoulou, E.; Ioannou, E.; Ataya, F.S.; Labrou, N.E. Plant glutathione transferases in abiotic stress response and herbicide resistance. In Glutathione in Plant Growth, Development, and Stress Tolerance; Springer Science and Business Media LLC: Berlin, Germany, 2017; pp. 215–233. [Google Scholar] [CrossRef]
- Skopelitou, K.; Muleta, A.W.; Papageorgiou, A.C.; Chronopoulou, E.G.; Pavli, O.; Flemetakis, E.; Skaracis, G.N.; Labrou, N.E. Characterization and functional analysis of a recombinant tau class glutathione transferase GmGSTU2-2 from Glycine max. Int. J. Biol. Macromol. 2017, 94, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Pandey, T.; Chhetri, G.; Chinta, R.; Kumar, B.; Singh, D.B.; Tripathi, T.; Singh, A.K. Functional classification and biochemical characterization of a novel rho class glutathione S-transferase in Synechocystis PCC 6803. FEBS Open. Bio. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Moons, A. Regulatory and Functional Interactions of Plant Growth Regulators and Plant Glutathione S-Transferases (GSTs). Vitam. Horm. 2005, 72, 155–202. [Google Scholar] [CrossRef] [PubMed]
- Lo Cicero, L.; Madesis, P.; Tsaftaris, A.; Lo Piero, A.R. Tobacco plants over-expressing the sweet orange tau glutathione transferases (CsGSTUs) acquire tolerance to the diphenyl ether herbicide fluorodifen and salt and drought stresses. Phytochemistry 2015, 116, 69–77. [Google Scholar] [CrossRef]
- Baek, Y.S.; Goodrich, L.V.; Brown, P.J.; James, B.T.; Moose, S.P.; Lambert, K.N.; Riechers, D.E. Transcriptome profiling and genome-wide association studies reveal GSTs and other defense genes involved in multiple signaling pathways induced by herbicide safener in grain sorghum. Front. Plant Sci. 2019, 10, 192. [Google Scholar] [CrossRef]
- Brazier-Hicks, M.; Howell, A.; Cohn, J.; Hawkes, T.; Hall, G.; McIndoe, E.; Edwards, R. Chemically Induced Herbicide Tolerance in Rice by the Safener Metcamifen Is Associated with a Phased Stress Response. J. Exp. Bot. 2020, 71, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.I.; Prescott, G.W. How to Know the Fresh-Water Algae. Bull. Torrey Bot. Club. 1956, 83, 311. [Google Scholar] [CrossRef]
- Rogers, S.L.; Burns, R.G. Changes in aggregate stability, nutrient status, indigenous microbial populations, and seedling emergence, following inoculation of soil with Nostoc muscorum. Biol. Fertil. Soils 1994, 18, 209–215. [Google Scholar] [CrossRef]
- Nishikimi, M.; Appaji Rao, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Pabst, M.J.; Habig, W.H.; Jakoby, W.B. Glutathione-S-Transferase. J. Biol. Chem. 1974, 249, 7140–7148. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Whitworth, R.J.; Stuart, J.J.; Chen, M.S. Unbalanced Activation of Glutathione Metabolic Pathways Suggests Potential Involvement in Plant Defense against the Gall Midge Mayetiola destructor in Wheat. Sci. Rep. 2015, 5, srep 08092. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Treatments | Plant Height (cm) | Plant FW (g) | Plant DW (g) |
|---|---|---|---|
| Control | 98.07 ± 13.4 a | 10.47 ± 0.75 a | 3.09 ± 0.22 a |
| Bh | 74.94 ± 11.2 e | 6.89 ± 0.48 d | 1.78 ± 0.2 d |
| T + Bh | 80.30 ± 11.5 d | 7.8 ± 0.25 c | 1.89 ± 0.1 c |
| Nm + Bh | 83.20 ± 12.1 c | 8.56 ± 0.5 b | 1.98 ± 0.35 b |
| Ap + Bh | 89.2 ± 11.8 b | 9.01 ± 0.6 b | 2.10 ± 0.15 b |
| Treatment | Time | SOD (U/g FW) | CAT (U/g FW) | GPX (µmole/g FW) | GST (U/g FW) |
|---|---|---|---|---|---|
| Control | 24 h | 1.43 ± 0.02 a | 0.89 ± 0.04 a | 83.90 ± 5.6 a | 1.36 ± 0.2 a |
| 48 h | 1.44 ± 0.01 a | 0.90 ± 0.05 a | 84.05 ± 3.2 a | 1.38 ± 0.1 a | |
| 72 h | 1.44 ± 0.04 a | 0.92 ± 0.03 a | 84.40 ± 4.2 a | 1.40 ± 0.08 a | |
| 96 h | 1.46 ± 0.05 a | 0.93 ± 0.04 a | 85.03 ± 2.5 a | 1.41 ± 0.09 a | |
| 15 days | 1.39 ± 0.3 a | 0.86 ± 0.10 a | 81.23 ± 6.5 a | 1.29 ± 0.1 a | |
| Bh | 24 h | 1.68 ± 0.1 b | 0.95 ± 0.01 b | 90.38 ± 6.5 b | 2.70 ± 0.1 b |
| 48 h | 1.54 ± 0.03 b | 1.11 ± 0.03 a | 136.20 ± 4.9 b | 1.82 ± 0.03 b | |
| 72 h | 1.47 ± 0.01 a | 1.24 ± 0.02 b | 160.60 ± 7.6 b | 1.70 ± 0.01 b | |
| 96 h | 1.48 ± 0.03 a | 1.14 ± 0.02 b | 156.10 ± 8.6 b | 1.65 ± 0.02 b | |
| 15 days | 1.46 ± 0.2 b | 1.14 ± 0.20 b | 153.40 ± 17.3 b | 1.80 ± 0.15 b | |
| T + Bh | 24 h | 2.16 ± 0.3 c | 1.11 ± 0.03 c | 98.04 ± 2.2 c | 0.3 c ± 3.50 |
| 48 h | 1.90 ± 0.2 c | 1.26 ± 0.03 b | 141.80 ± 6.3 c | 3.23 ± 0.2 c | |
| 72 h | 1.89 ± 0.06 b | 1.47 ± 0.02 c | 180.40 ± 5.3 c | 2.96 ± 0.15 c | |
| 96 h | 1.59 ± 0.03 b | 1.21 ± 0.02 c | 00.176 ± 4.7 c | 2.88 ± 0.06 c | |
| 15 days | 1.72 ± 0.3 c | 1.19 ± 0.12 b | 174.00 ± 12.4 c | 2.50 ± 0.3 c | |
| Nm + Bh | 24 h | 3.76 ± 0.2 d | 1.15 ± 0.02 c | 116.80 ± 9.5 d | 4.81 ± 0.5 d |
| 48 h | 2.84 ± 0.1 d | 1.28 ± 0.3 b | 150.80 ± 2.8 d | 4.43 ± 0.2 d | |
| 72 h | 2.62 ± 0.3 c | 1.52 ± 0.1 c | 188.4 ± 10.4 d | 4.17 ± 0.5 d | |
| 96 h | 2.08 ± 0.4 c | 1.23 ± 0.02 c | 182.80 ± 12.6 d | 3.80 ± 0.15 d | |
| 15 days | 1.96 ± 0.2 d | 1.22 ± 0.15 b | 10.6 d ± 180.40 | 3.40 ± 0.4 d | |
| Ap + Bh | 24 h | 5.64 ± 0.6 e | 1.34 ± 0.03 d | 125.70 ± 8.7 e | 5.93 ± 0.8 e |
| 48 h | 4.23 ± 0.4 e | 1.46 ± 0.04 c | 158.90 ± 4.03 e | 4.94 ± 0.2 e | |
| 72 h | 3.73 ± 0.2 d | 1.66 ± 0.2 d | 194.80 ± 11.2 e | 4.78 ± 0.25 d | |
| 96 h | 3.66 ± 0.5 d | 1.28 ± 0.02 c | 185.80 ± 13.8 e | 4.62 ± 0.3 d | |
| 15 days | 3.13 ± 0.4 e | 1.26 ± 0.01 c | 182.70 ± 15.4 e | 4.28 ± 0.25 e |
| Gene Name | Primer Sequences (5′-3′) | |
|---|---|---|
| Wheat zeta GST (TaGSTZ) | For. 5′-CCTGGATCAGCTCTTGCTCT-3′ | [9] |
| Rev. 5′-AATCTGGAAGCGATTGATGG-3′ | ||
| Wheat tau GST (TaGSTU) | For. 5′-CAA CGA GTC CCT CAT CC-3′ | [9] |
| Rev. 5′-GAG GGT CTT GAG GAT GTC CA-3′ | ||
| Wheat lambda GST (TaGSTL) | For. 5′-GCA CTG CTT CCT CAA GAT CC-3′ | [9] |
| Rev. 5′-GTC ACG TAC GCA ATG TCC AC-3′ | ||
| Wheat glutathione peroxidase (TaGPX) | For. 5′-CTA GAC TTC AGG AAC TTG-3′ | [53] |
| Rev. 5′-CCT AAC TAA CTC CAA CTA C-3′ | ||
| Wheat glutathione synthetase (TaGS) | For. 5′-GAA CCA GCA TTG ACC TAC-3′ | [53] |
| Rev. 5′-TAG TGA TCC ACG AAA CAA G-3′ | ||
| TaGAPDH (housekeeping gene) | For. 5′-GGA GGA GTC TGA GGG AAA CC-3′ | [9] |
| Rev. 5′-GCT GTA TCC CCA CTC GTT GT -3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaafar, R.M.; Osman, M.E.-A.H.; Abo-Shady, A.M.; Almohisen, I.A.A.; Badawy, G.A.; El-Nagar, M.M.F.; Ismail, G.A. Role of Antioxidant Enzymes and Glutathione S-Transferase in Bromoxynil Herbicide Stress Tolerance in Wheat Plants. Plants 2022, 11, 2679. https://doi.org/10.3390/plants11202679
Gaafar RM, Osman ME-AH, Abo-Shady AM, Almohisen IAA, Badawy GA, El-Nagar MMF, Ismail GA. Role of Antioxidant Enzymes and Glutathione S-Transferase in Bromoxynil Herbicide Stress Tolerance in Wheat Plants. Plants. 2022; 11(20):2679. https://doi.org/10.3390/plants11202679
Chicago/Turabian StyleGaafar, Reda M., Mohamed El-Anwar H. Osman, Atef M. Abo-Shady, Ibrahim A. A. Almohisen, Ghada Ahmed Badawy, Maysa M. F. El-Nagar, and Gehan A. Ismail. 2022. "Role of Antioxidant Enzymes and Glutathione S-Transferase in Bromoxynil Herbicide Stress Tolerance in Wheat Plants" Plants 11, no. 20: 2679. https://doi.org/10.3390/plants11202679
APA StyleGaafar, R. M., Osman, M. E.-A. H., Abo-Shady, A. M., Almohisen, I. A. A., Badawy, G. A., El-Nagar, M. M. F., & Ismail, G. A. (2022). Role of Antioxidant Enzymes and Glutathione S-Transferase in Bromoxynil Herbicide Stress Tolerance in Wheat Plants. Plants, 11(20), 2679. https://doi.org/10.3390/plants11202679






