A Phytocomplex Obtained from Salvia officinalis by Cell Culture Technology Effectively Controls the Grapevine Downy Mildew Pathogen Plasmopara viticola
Abstract
1. Introduction
2. Results
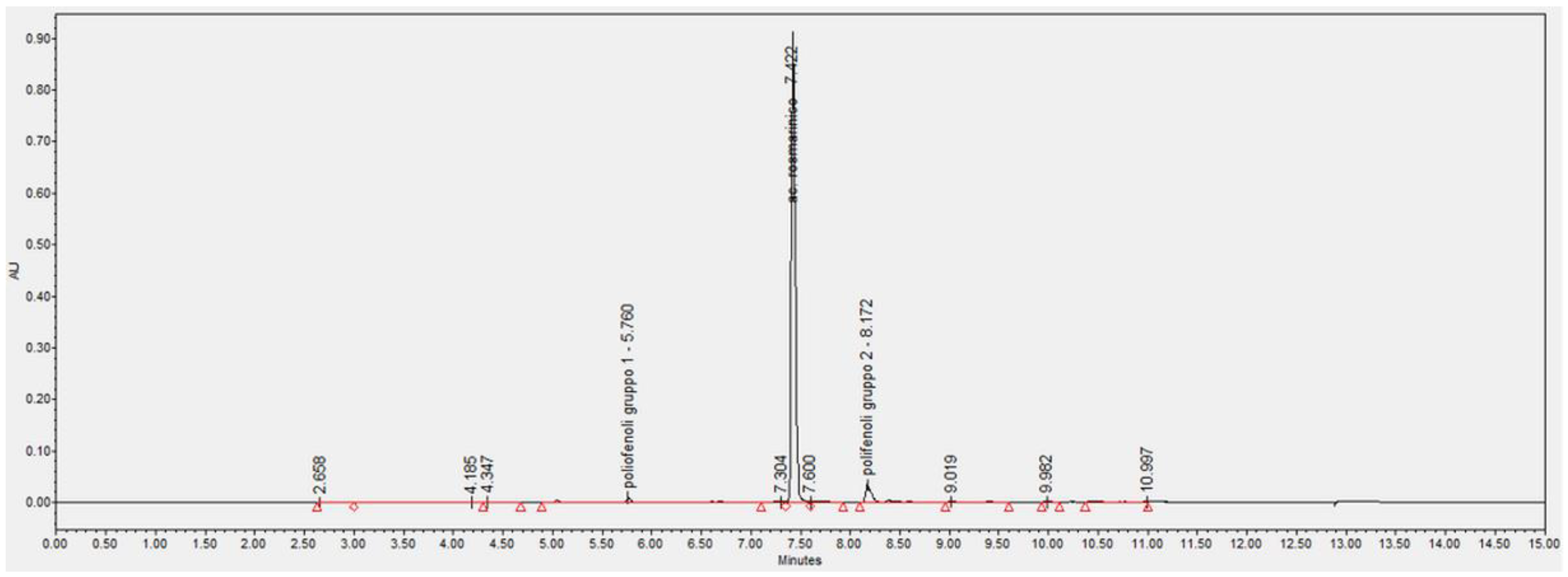
2.1. Salvia Officinalis Phytocomplex Was Obtained from a Stable and Selected Cell Line
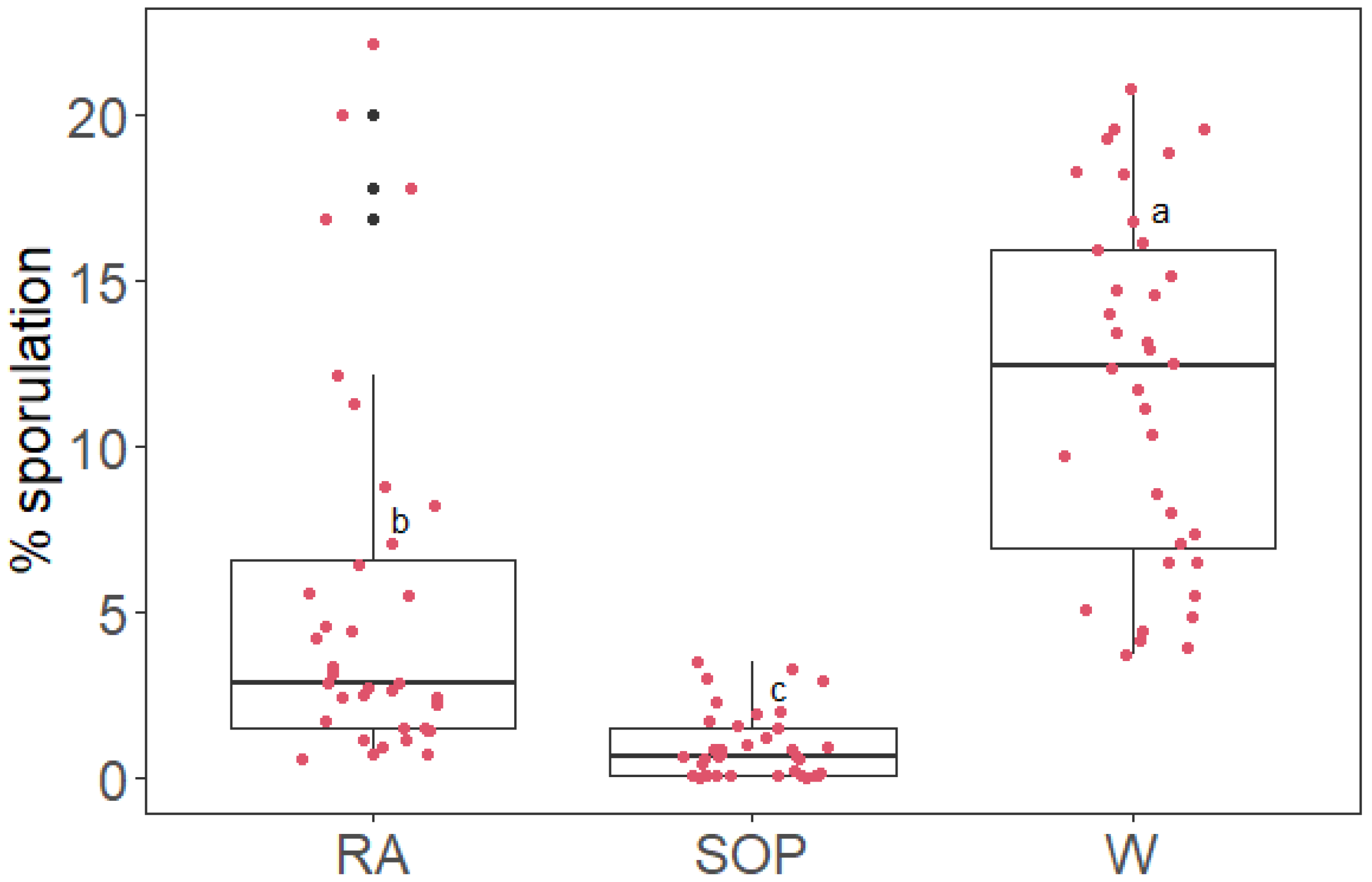
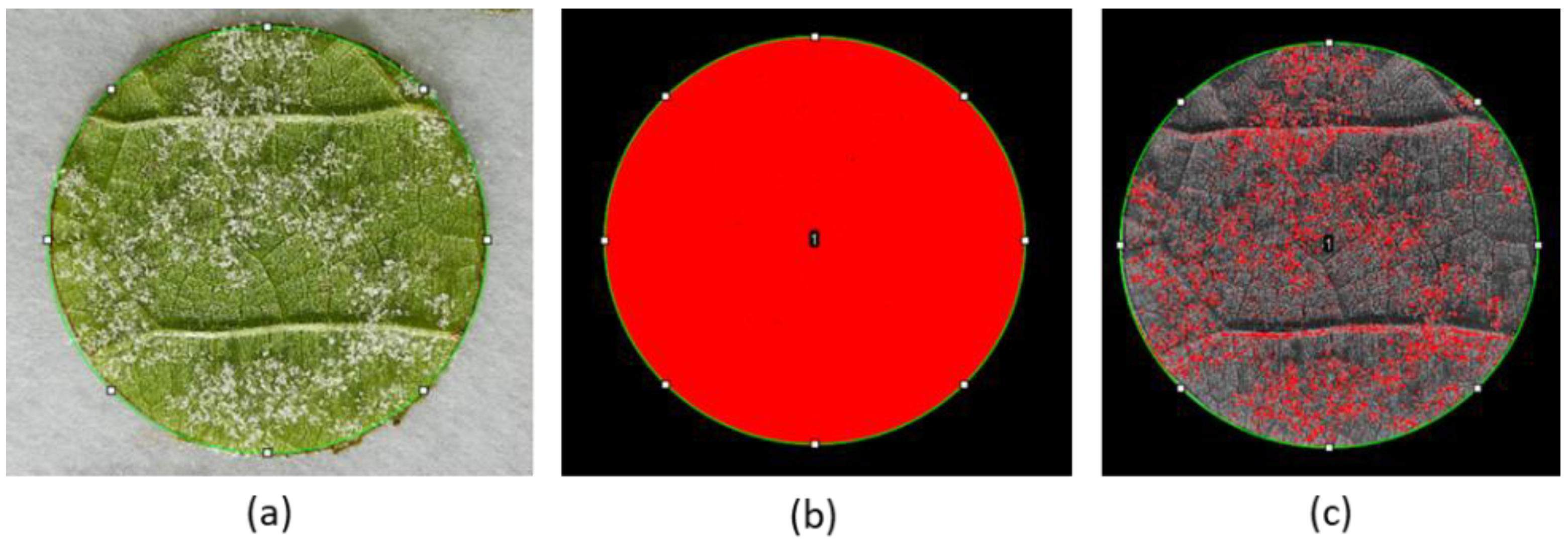
2.2. SOP Inhibits Plasmopara viticola Sporulation on Grapevine Leaf Discs
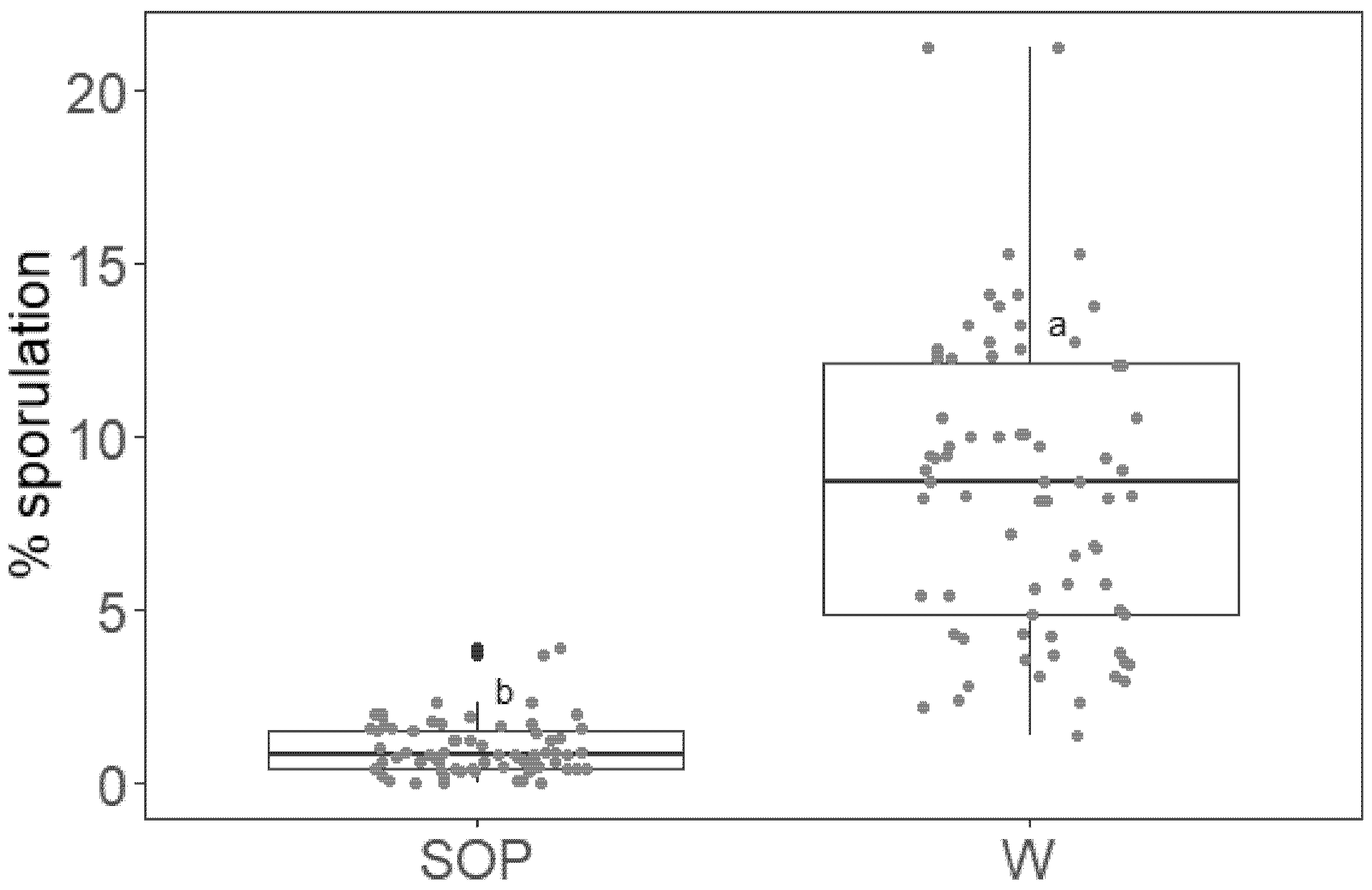
2.3. SOP Effect Persists on Grapevine Leaves
3. Discussion
4. Materials and Methods
4.1. Salvia Officinalis Cell Culture
4.2. Phytocomplex Preparation from Salvia officinalis Cell Culture
4.3. Quantification of Rosmarinic Acid in the S. officinalis Phytocomplex by UPLC-DAD Analysis
4.4. Plant Material
4.5. SOP and Rosmarinic Acid Preparations
4.6. Leaf Inoculation with Plasmopara viticola
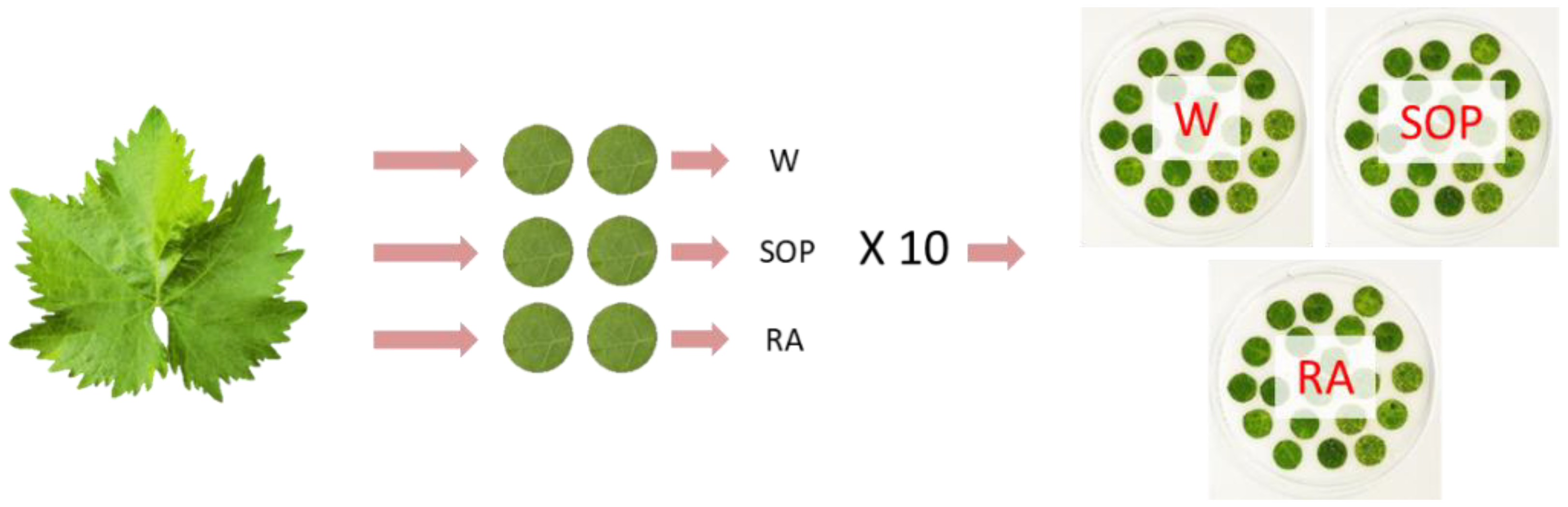
4.7. SOP Inhibitory Assay against Plasmopara viticola Infection
4.8. Persistence of SOP Treatment
4.9. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ogunnupebi, T.A.; Oluyori, A.P.; Dada, A.O.; Oladeji, O.S.; Inyinbor, A.A.; Egharevba, G.O. Promising Natural Products in Crop Protection and Food Preservation: Basis, Advances, and Future Prospects. Int. J. Agron. 2020, 2020, 8840046. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Counsil, the European Economic and Social Committee and the Committee of the Regions: A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Fiendly Food System; COM/2020/381 final; Publications Office of the European Union: Brussels, Belgium, 2020.
- Gessler, C.; Pertot, I.; Perazzolli, M. Plasmopara Viticola: A Review of Knowledge on Downy Mildew of Grapevine and Effective Disease Management. Phytopathol. Mediterr. 2011, 50, 3–44. [Google Scholar] [CrossRef]
- Fontaine, M.C.; Labbé, F.; Dussert, Y.; Delière, L.; Richart-Cervera, S.; Giraud, T.; Delmotte, F. Europe as a Bridgehead in the Worldwide Invasion History of Grapevine Downy Mildew, Plasmopara viticola. Curr. Biol. 2021, 31, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Smith, C.J.; Perfetti, T.A. A Comparison of the Persistence, Toxicity, and Exposure to High-Volume Natural Plant-Derived and Synthetic Pesticides. Toxicol. Res. Appl. 2020, 4, 2397847320940561. [Google Scholar] [CrossRef]
- Durán-Lara, E.F.; Valderrama, A.; Marican, A. Natural Organic Compounds for Application in Organic Farming. Agriculture 2020, 10, 41. [Google Scholar] [CrossRef]
- Aguilar-Veloz, L.M.; Calderón-Santoyo, M.; González, Y.V.; Ragazzo-Sánchez, J.A. Application of Essential Oils and Polyphenols as Natural Antimicrobial Agents in Postharvest Treatments: Advances and Challenges. Food Sci. Nutr. 2020, 8, 2555–2568. [Google Scholar] [CrossRef]
- Yoon, M.-Y.; Cha, B.; Kim, J.-C. Recent Trends in Studies on Botanical Fungicides in Agriculture. Plant Pathol. J. 2013, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V. Mass Propagation of Plant Cells—An Emerging Technology Platform for Sustainable Production of Biopharmaceuticals. Biochem. Pharmacol. Los Angel. 2015, 4, e180. [Google Scholar] [CrossRef]
- Hellwig, S.; Drossard, J.; Twyman, R.M.; Fischer, R. Plant Cell Cultures for the Production of Recombinant Proteins. Nat. Biotechnol. 2004, 22, 1415–1422. [Google Scholar] [CrossRef]
- Yue, W.; Ming, Q.; Lin, B.; Rahman, K.; Zheng, C.-J.; Han, T.; Qin, L. Medicinal Plant Cell Suspension Cultures: Pharmaceutical Applications and High-Yielding Strategies for the Desired Secondary Metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.A.; Roberts, S.C. Recent Advances towards Development and Commercialization of Plant Cell Culture Processes for the Synthesis of Biomolecules. Plant Biotechnol. J. 2012, 10, 249–268. [Google Scholar] [CrossRef]
- Imseng, N.; Schillberg, S.; Schürch, C.; Schmid, D.; Schütte, K.; Gorr, G.; Eibl, D.; Eibl, R. Suspension Culture of Plant Cells Under Heterotrophic Conditions. In Industrial Scale Suspension Culture of Living Cells; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 224–258. ISBN 978 3 527 68332 1. [Google Scholar]
- Marchev, A.; Haas, C.; Schulz, S.; Georgiev, V.; Steingroewer, J.; Bley, T.; Pavlov, A. Sage in Vitro Cultures: A Promising Tool for the Production of Bioactive Terpenes and Phenolic Substances. Biotechnol. Lett. 2014, 36, 211–221. [Google Scholar] [CrossRef]
- Pinto, E.; Salgueiro, L.R.; Cavaleiro, C.; Palmeira, A.; Gonçalves, M.J. In Vitro Susceptibility of Some Species of Yeasts and Filamentous Fungi to Essential Oils of Salvia officinalis. Ind. Crops Prod. 2007, 26, 135–141. [Google Scholar] [CrossRef]
- Dagostin, S.; Formolo, T.; Giovannini, O.; Pertot, I.; Schmitt, A. Salvia Officinalis Extract Can Protect Grapevine Against Plasmopara viticola. Plant Dis. 2010, 94, 575–580. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic Acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Ivanov, M.; Kostić, M.; Stojković, D.; Soković, M. Rosmarinic Acid–Modes of Antimicrobial and Antibiofilm Activities of a Common Plant Polyphenol. South Afr. J. Bot. 2022, 146, 521–527. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Wysokińska, H. The Effect of Methyl Jasmonate on Production of Antioxidant Compounds in Shoot Cultures of Salvia officinalis L. Herba Pol. 2009, 55, 238–243. [Google Scholar]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.O.; Jin, Y.-W.; Lee, E.-K.; Loake, G.J. Plant Cell Culture Strategies for the Production of Natural Products. BMB Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef]
- Shekarchi, M.; Hajimehdipoor, H.; Saeidnia, S.; Gohari, A.R.; Hamedani, M.P. Comparative Study of Rosmarinic Acid Content in Some Plants of Labiatae Family. Pharmacogn. Mag. 2012, 8, 37–41. [Google Scholar] [CrossRef]
- Hakkim, F.; Idrees, M.; Bakshi, H.A.; Mombasawala, L.; Rashan, L. Enhancement of Rosmarinic Acid Content by Biotechnological Approaches and Metabolic Engineering. In Natural Bio-Active Compounds; Akhtar, M.S., Swamy, M.K., Eds.; Springer: Singapore; Volume 3, pp. 317–330. ISBN 978 981 13 7437 1.
- Grzegorczyk, I.; Matkowski, A.; Wysokińska, H. Antioxidant Activity of Extracts from in vitro Cultures of Salvia officinalis L. Food Chem. 2007, 104, 536–541. [Google Scholar] [CrossRef]
- Bais, P.H.; Walker, T.S.; Schweizer, H.P.; Vivanco, J.M. Root Specific Elicitation and Antimicrobial Activity of Rosmarinic Acid in Hairy Root Cultures of Ocimum basilicum. Plant Physiol. Biochem. 2002, 40, 983–995. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological Properties of Salvia officinalis and Its Components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of Phenolics and Essential Oil from Dried Sage (Salvia officinalis) Using Ethanol-Water Mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Mulder-Krieger, T.H.; Verpoorte, R.; Svendsen, A.B.; Scheffer, J.J.C. Production of Essential Oils and Flavours in Plant Cell and Tissue Cultures. A Review. Plant Cell Tissue Organ Cult. 1988, 13, 85–154. [Google Scholar] [CrossRef]
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of Essential Oil Production in Plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C.F.R. Exploring Plant Tissue Culture to Improve the Production of Phenolic Compounds: A Review. Ind. Crops Prod. 2016, 82, 9–22. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and Antagonism in Natural Product Extracts: When 1 + 1 Does Not Equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Nybom, H.; Weising, K.; Rotter, B. DNA Fingerprinting in Botany: Past, Present, Future. Investig. Genet. 2014, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient Requirements of Suspension Cultures of Soybean Root Cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Md Rahman, M.; Md Islam, B.; Biswas, M.; Khurshid Alam, A.H.M. In vitro Antioxidant and Free Radical Scavenging Activity of Different Parts of Tabebuia pallida Growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Peressotti, E.; Duchêne, E.; Merdinoglu, D.; Mestre, P. A Semi-Automatic Non-Destructive Method to Quantify Grapevine Downy Mildew Sporulation. J. Microbiol. Methods 2011, 84, 265–271. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busato, I.; Bertaiola, O.; Tundo, S.; Guarnerio, C.; Lucchetta, M.; Sella, L.; Pressi, G.; Favaron, F. A Phytocomplex Obtained from Salvia officinalis by Cell Culture Technology Effectively Controls the Grapevine Downy Mildew Pathogen Plasmopara viticola. Plants 2022, 11, 2675. https://doi.org/10.3390/plants11202675
Busato I, Bertaiola O, Tundo S, Guarnerio C, Lucchetta M, Sella L, Pressi G, Favaron F. A Phytocomplex Obtained from Salvia officinalis by Cell Culture Technology Effectively Controls the Grapevine Downy Mildew Pathogen Plasmopara viticola. Plants. 2022; 11(20):2675. https://doi.org/10.3390/plants11202675
Chicago/Turabian StyleBusato, Isabella, Oriana Bertaiola, Silvio Tundo, Chiara Guarnerio, Marco Lucchetta, Luca Sella, Giovanna Pressi, and Francesco Favaron. 2022. "A Phytocomplex Obtained from Salvia officinalis by Cell Culture Technology Effectively Controls the Grapevine Downy Mildew Pathogen Plasmopara viticola" Plants 11, no. 20: 2675. https://doi.org/10.3390/plants11202675
APA StyleBusato, I., Bertaiola, O., Tundo, S., Guarnerio, C., Lucchetta, M., Sella, L., Pressi, G., & Favaron, F. (2022). A Phytocomplex Obtained from Salvia officinalis by Cell Culture Technology Effectively Controls the Grapevine Downy Mildew Pathogen Plasmopara viticola. Plants, 11(20), 2675. https://doi.org/10.3390/plants11202675





