Current Status and Future Prospective for Nitrogen Use Efficiency in Wheat (Triticum aestivum L.)
Abstract
1. Introduction
2. Nitrogen Use Efficiency Indices
3. Key Traits for Wheat NUE
3.1. N Uptake, Assimilation and Use Effiency in Wheat
3.2. NUE in Relation to G × E × M
4. Future Prospective for Improving NUE in Wheat Crops
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat. Available online: https://ec.europa.eu/eurostat/databrowser/view/apro_cpsh1/default/table?lang=en (accessed on 11 July 2021).
- Barraclough, P.B.; Howarth, J.R.; Jones, J.; Lopez-Bellido, R.; Parmar, S.; Shepherd, C.E.; Hawkesford, M.J. Nitrogen efficiency of wheat: Genotypic and environmental variation and prospects for improvement. Eur. J. Agron. 2010, 33, 1–11. [Google Scholar] [CrossRef]
- Our World in Data. Greenhouse Gas Emissions. Available online: https://ourworldindata.org/greenhouse-gas-emissions#:~:text=Greenhouse%20gases%20are%20measured%20in,were%20around%2035%20billion%20tonnes (accessed on 2 March 2021).
- European Commission. Cap Specific Objectives. Brief No 4. Agriculture and Climate Mitigation. Available online: https://ec.europa.eu/info/sites/default/files/food-farming-fisheries/key_policies/documents/cap-specific-objectives-brief-4-agriculture-and-climate-mitigation_en.pdf (accessed on 2 March 2021).
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. J. Hum. Environ. 2002, 31, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, G.; Ciampitti, I. Crop Mass and N Status as Prerequisite Covariables for unraveling Nitrogen Use Efficiency across genotype-by-environment-by-management scenarios: A Review. Plants 2020, 9, 1309. [Google Scholar] [CrossRef]
- European Commission. The New Common Agricultural Policy: 2023-27. Available online: https://ec.europa.eu/info/food-farming-fisheries/key-policies/common-agricultural-policy/new-cap-2023-27_en (accessed on 12 June 2021).
- Vidican, R.; Mălinaș, A.; Rotar, I.; Kadar, R.; Deac, V.; Mălinaș, C. Assessing wheat response to N fertilization in a wheat–maize–soybean long-term rotation through NUE measurements. Agronomy 2020, 10, 941. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Griffiths, S. Exploiting genetic variation in nitrogen use efficiency for cereal crop improvement. Curr. Opin. Plant Biol. 2019, 49, 35–42. [Google Scholar] [CrossRef]
- Research Program on Wheat. Available online: https://wheat.org/wheat-in-the-world (accessed on 21 April 2021).
- Reynolds, M.P.; Foulkes, J.; Furbank, R.; Griffiths, S.; King, J.; Murchie, E.; Parry, M.; Slafer, G.A. Achieving yield gains in wheat. Plant Cell Environ. 2012, 35, 1799–1823. [Google Scholar] [CrossRef]
- Snape, J.W. Golden calves or white elephants? Biotechnologies for wheat improvement. Euphytica 1998, 100, 207–217. [Google Scholar] [CrossRef]
- Foulkes, M.J.; Hawkesford, M.J.; Barraclough, P.B.; Holdsworth, M.J.; Kerr, S.; Kightley, S.; Shewry, P.R. Identifying traits to improve the nitrogen economy of wheat: Recent advances and future prospects. Field Crops Res. 2009, 114, 329–342. [Google Scholar] [CrossRef]
- Hoang, V.N.; Alauddin, M. Assessing the eco-environmental performance of agricultural production in OECD countries: The use of nitrogen flows and balance. Nutr. Cycl. Agroecosystems 2010, 87, 353–368. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Available online: https://www.fao.org/3/a0800e/a0800e00.htm (accessed on 22 April 2021).
- Baresel, J.P.; Zimmerman, G.; Reents, H.J. Effect on genotype and environment on N uptake and N partition in organically grown winter wheat (Triticum aestivum L.) in Germany. Euphytica 2008, 163, 347–354. [Google Scholar] [CrossRef]
- Hirel, B.; Tétu, T.; Peter, J.L.; Dubois, F. Improving Nitrogen Use Efficiency in crops for sustainable agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Christie, K.M.; Smith, A.P.; Rawnsley, R.P.; Harrison, M.T.; Eckard, R.J. Simulated seasonal pasture responses of grazed dairy pastures to nitrogen fertiliser in SE Australia: Pasture production. Agric. Syst. 2018, 166, 36–47. [Google Scholar] [CrossRef]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and interpretation of factors which contribute to efficiency of nitrogen-utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Novoa, R.; Loomis, R.S. Nitrogen and plant production. Plant Soil 1981, 58, 177–204. [Google Scholar] [CrossRef]
- Congreves, K.A.; Otchere, O.; Ferland, D.; Farzadfar, S.; Williams, S.; Arcand, M.M. Nitrogen Use Efficiency Definitions of Today and Tomorrow. Front. Plant Sci. 2021, 12, 637108. [Google Scholar] [CrossRef]
- Fixen, P.; Brentrup, F.; Bruulsema, T.; Garcia, F.; Norton, R.; Zingore, S. Nutrient/fertilizer use efficiency: Measurement, current situation and trends. In Managing Water and fertilizer for Sustainable Agricultural Intensification, 1st ed.; Drechsel, P., Heffer, P., Magen, H., Mikkelsen, R., Wichelns, D., Eds.; International Fertilizer Industry Association (IFA); International Water Management Institute (IWMI); International Plant Nutrition Institute (IPNI); International Potash Institute (IPI): Paris, France, 2015; Volume 1, pp. 1–30. [Google Scholar]
- Dobermann, A. Nitrogen use efficiency—State of the art. Agron. Hortic. Fac. Publ. 2005, 316, 1–17. [Google Scholar]
- Che, S.; Zhao, B.; Li, Y.; Yuan, L.; Li, W.; Lin, Z.; Hu, S.; Shen, B. Review grain yield and nitrogen use efficiency in rice production regions in China. J. Integr. Agric. 2015, 14, 2456–2466. [Google Scholar] [CrossRef]
- Guttieri, M.J.; Frels, K.; Regassa, T.; Waters, B.M.; Baenziger, P.S. Variation for nitrogen use efficiency traits in current and historical great plains hard winter wheat. Euphytica 2017, 213, 87. [Google Scholar] [CrossRef]
- Sylvester-Bradley, R.; Kindred, D.R. Analysing nitrogen responses of cereals to prioritize routes to the improvement of nitrogen use efficiency. J. Exp. Bot. 2009, 60, 1939–1951. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Reducing the reliance on nitrogen fertilizer for wheat production. J. Cereal Sci. 2014, 59, 276–283. [Google Scholar] [CrossRef]
- Harper, L.A.; Sharpe, R.R.; Langdale, G.W.; Giddens, J.E. Nitrogen cycling in a wheat crop: Soil, plant, and aerial nitrogen transport. Agron. J. 1987, 79, 965–973. [Google Scholar] [CrossRef]
- Knowles, F.; Watkin, J.E. The assimilation and translocation of plant nutrients in wheat during growth. J. Agric. Sci. 1931, 21, 612–637. [Google Scholar] [CrossRef]
- Daigger, L.A.; Schnider, D.H.; Peterson, G.A. Nitrogen content of winter wheat during growth and maturation. Agron. J. 1976, 68, 815–818. [Google Scholar] [CrossRef]
- Parton, W.J.; Morgan, J.A.; Altenhofen, J.M.; Harper, L.A. Ammonia volatilization from Olaf spring wheat plants. Agron. J. 1988, 80, 419–425. [Google Scholar] [CrossRef]
- Denmead, O.T.; Freney, J.R.; Simpson, J.R. A closed ammonia cycle within a plant canopy. Soil Biol. Biochem. 1976, 8, 161–164. [Google Scholar] [CrossRef]
- Vallis, I.; Harper, L.A.; Catchpoole, V.R.; Weier, K.L. Volatilization of ammonia from urine patches in a subtropical pasture. Aust. J. Agric. Res. 1982, 33, 97–107. [Google Scholar] [CrossRef]
- Zhang, L.; Du, Y.-L.; Li, X.G. Modern wheat cultivars have greater root nitrogen uptake efficiency than old cultivars. J. Plant Nutr. Soil Sci. 2020, 183, 192–199. [Google Scholar] [CrossRef]
- William Whitehead DC. Grassland Nitrogen, 1st ed.; CAB International: Oxford, UK, 1998; pp. 108–120. [Google Scholar]
- de Vries, F.T.; Wallenstein, M.D. Below-ground connections underlying aboveground food production: A framework for optimising ecological connections in the rhizosphere. J. Ecol. 2017, 105, 913–920. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Hämmerle, I.; Fuchslueger, L.; Hofhansl, F.; Knoltsch, A.; Schnecker, J.; Takriti, M.; Watzka, M.; Wild, B.; et al. Adjustment of microbial nitrogen use efficiency to carbon: Nitrogen imbalances regulates soil nitrogen cycling. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, S.; Li, C.; Zhao, L.; Feng, H.; Yue, G.; Ren, Z.; Cheng, G. The soil carbon/nitrogen ratio and moisture affect microbial community structures in alkaline permafrost-affected soils with different vegetation types on the Tibetan plateau. Res. Microbiol. 2014, 165, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, B.; Olk, D.C.; Jia, Z.; Mao, J.; Cai, Y.; Zhang, J. Contributions of residue-C and -N to plant growth and soil organic matter pools under planted and unplanted conditions. Soil Biol. Biochem. 2018, 120, 91–104. [Google Scholar] [CrossRef]
- Sofo, A.; Ricciuti, P.; Fausto, C.; Mininni, A.N.; Crecchio, C.; Scagliola, M.; Malerba, A.D.; Xiloyannis, C.; Dichio, B. The metabolic and genetic diversity of soil bacterial communities depends on the soil management system and C/N dynamics: The case of sustainable and conventional olive groves. Appl. Soil Ecol. 2019, 137, 21–28. [Google Scholar] [CrossRef]
- Kitur, B.K.; Smith, S.S.; Blevins, R.L.; Frye, W.W. Fate of N-depleted ammonium nitrate applied to no-tillage and conventional tillage corn. Agron. J. 1984, 76, 240–242. [Google Scholar] [CrossRef]
- Davis, J. Nitrogen efficiency and management. Nutr. Manag. Tech. Note 2007, 6, 1–6. [Google Scholar]
- Peoples, M.B.; Boyer, E.W.; Goulding, K.W.T.; Heffer, P.; Ochwoh, V.A.; Vanlauwe, B.; Wood, S.; Yagi, K.; Van Cleemput, O. Pathways of nitrogen loss and their impact on human health and the environment. In Agriculture and the Nitrogen Cycle: Assessing the Impacts of Fertilizer Use on Food Production and the Environment; Mosier, A.R., Syers, J.K., Freney, J.R., Eds.; Island Press: Washington, WA, USA, 2004; pp. 53–69. [Google Scholar]
- Goulding, K.; Jarvis, S.; Whitmore, A. Optimizing nutrient management for farm systems. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Samborski, S.M.; Tremblay, N.; Fallon, E. Strategies to make use of plant sensors-based diagnostic information for nitrogen recommendations. Agron. J. 2009, 101, 800–816. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Haegele, J.W.; Cook, K.A.; Nichols, D.M.; Below, F.E. Changes in nitrogen use traits associated with genetic improvement for grain yield of maize hybrids released in different decades. Crop Sci. 2013, 53, 1256–1268. [Google Scholar] [CrossRef]
- Bogard, M.; Allard, V.; Brancourt-Hulmel, M.; Heumez, E.; Machet, J.M.; Jeuffroy, M.H.; Gate, P.; Martre, P.; Le Gouis, J. Deviation from the grain protein concentration–grain yield negative relationship is highly correlated to post-anthesis N uptake in winter wheat. J. Exp. Bot. 2010, 61, 4303–4312. [Google Scholar] [CrossRef]
- Takahashi, S.; Muhuddin, R.A. Wheat grain yield, phosphorus uptake and soil phosphorus fraction after 23 years of annual fertilizer application to an andosol. Field Crops Res. 2007, 101, 160–171. [Google Scholar] [CrossRef]
- Marschner, P.; Rengel, Z. Chapter 12-Nutrient Availability in Soils. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 315–330. [Google Scholar]
- Brundrett, M.C.; Ferguson, B.J.; Gressshoff, P.M.; Mathesius, U.; Munns, R.; Rasmussen, A.; Ryan, M.H.; Schmidt, S. Nutrient uptake from soils. In Plants in Action, 2nd ed.; Munns, R., Schmidt, S., Eds.; Australian Society of Plant Scientists: Adelaide, Australia; New Zealand Society of Plant Biologists: Christchurch, New Zealand, 2018; pp. 5–6. [Google Scholar]
- Gastal, F.; Lemaire, G. N uptake and distribution in crops: An agronomical and ecophysiological perspective. J. Exp. Bot. 2002, 53, 789–799. [Google Scholar] [CrossRef] [PubMed]
- King, J.; Gay, A.; Sylvester-Bradley, R.; Bingham, I.; Foulkes, J.; Gregory, P.; Robinson, D. Modelling cereal root systems for water and nitrogen capture: Towards an economic optimum. Ann. Bot. 2003, 91, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Allard, V.; Martre, P.; Le Gouis, J. Genetic variability in biomass allocation to roots in wheat is mainly related to crop tillering dynamics and nitrogen status. Eur. J. Agron. 2013, 46, 68–76. [Google Scholar] [CrossRef]
- Robinson, D. The responses of plants to non-uniform supplies of nutrients. New Phytol. 1994, 127, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D. Resource capture by localized root proliferation: Why do plants bother? Ann. Bot. 1996, 77, 179–186. [Google Scholar] [CrossRef]
- Robinson, D.; Hodge, A.; Griffiths, B.S.; Fitter, A.H. Plant root proliferation in nitrogen-rich patches confers competitive advantage. Proc. R. Soc. 1999, 266, 431–435. [Google Scholar] [CrossRef]
- Kristensen, H.L.; Thorup-Kristensen, K. Uptake of 15N labeled nitrate by root systems of sweet corn, carrot and white cabbage from 0.2–2.5 meters depth. Plant Soil 2004, 265, 93–100. [Google Scholar] [CrossRef]
- Haberle, J.; Svoboda, P.; Krejcová, J. Uptake of mineral nitrogen from subsoil by winter wheat. Plant Soil Environ. 2006, 52, 377–384. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Cortasa, M.S.; Loges, R. Winter wheat roots grow twice as deep as spring wheat roots, is this important for N uptake and N leaching losses? Plant Soil 2009, 322, 101–114. [Google Scholar] [CrossRef]
- Rasmussen, I.S.; Dresbøll, D.B.; Thorup-Kristensen, K. Winter wheat cultivars and nitrogen (N) fertilization—Effects on root growth, N uptake efficiency and N use efficiency. Europ. J. Agron. 2015, 68, 38–49. [Google Scholar] [CrossRef]
- Gregory, P.J.; McGowan, M.; Biscoe, P.V.; Hunter, B. Water relations of winter wheat. Growth of the root system. J. Agric. Sci 1978, 91, 91–102. [Google Scholar] [CrossRef]
- Kuhlmann, H.; Barraclough, P.B.; Weir, A.H. Utilization of mineral nitrogen in the subsoil by winter wheat. J. Plant Nutr. Soil Sci. 1989, 152, 291–295. [Google Scholar] [CrossRef]
- Anderson, G.C.; Fillery, I.R.P.; Dunin, F.X.; Dolling, P.J.; Asseng, S. Nitrogen andnwater flows under pasture–wheat and lupin–wheat rotations in deep sands in Western Australia. Drainage and nitrate leaching. Aust. J. Agric. Res. 1998, 49, 345–361. [Google Scholar] [CrossRef]
- Sauer, S.; Haussmann, W.; Harrach, T. Effective rooting depth, percolation water, and nitrate leaching in deeply developed loess soils of a water-shortage area. J. Plant Nutr. Soil Sci. 2002, 165, 269–273. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Lilley, J.M. Root penetration rate—A benchmark to identify soil and plant limitations to rooting depth in wheat. Aust. J. Exp. Agric. 2007, 47, 590–602. [Google Scholar] [CrossRef]
- Gao, S.; Pan, W.L.; Koenig, R.T. Wheat root growth responses to enhanced ammonium supply. Soil Sci. Soc. Am. J. 1998, 62, 1736–1740. [Google Scholar] [CrossRef]
- Barraclough, P.B.; Kuhlmann, H.; Weir, A.H. The effects of prolonged drought and nitrogen fertilizer on root and shoot growth and water uptake by winter wheat. J. Agron. Crop Sci. 1989, 163, 352–360. [Google Scholar] [CrossRef]
- Barber, S.A. Nutrient balance and nitrogen use. In Nitrogen in Crop Production; Hauck, R.D., Ed.; American Society of Agronomy: Madison, WI, USA, 1984; pp. 87–96. [Google Scholar]
- Haynes, R.J. Uptake and assimilation of mineral nitrogen by plants. In Mineral Nitrogen in the Plant-Soil System; Haynes, R.J., Ed.; Academic Press: London, UK, 1986; pp. 303–378. [Google Scholar]
- Buchner, P.; Hawkesford, M.J. Complex phylogeny and gene expression patterns of members of the NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family (NPF) in wheat. J. Exp. Bot. 2014, 64, 5697–5710. [Google Scholar] [CrossRef]
- Cormier, F.; Le Gouis, J.; Dubreuil, P.; Lafarge, S.; Praud, S. A genome-wide identification of chromosomal regions determining nitrogen use efficiency components in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2014, 127, 2679–2693. [Google Scholar] [CrossRef]
- Wittgenstein, V.; Neil, J.B.; Hawkins, B.J.; Ehlting, J. Evolutionary classification of ammonium, nitrate, and peptide transporters in land plants. BMC Evol. Biol. 2014, 14, 11. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Cheng, Y.H.; Chen, K.E.; Tsay, Y.F. Nitrate transport, signaling, and use efficiency. Annu. Rev. Plant Biol. 2018, 69, 85–122. [Google Scholar] [CrossRef] [PubMed]
- Lupini, A.; Preiti, G.; Badagliacca, G.; Abenavoli, M.R.; Sunseri, F.; Monti, M.; Bacchi, M. Nitrogen Use Efficiency in durum wheat under different nitrogen and water regimes in the Mediterranean Basin. Front. Plant Sci. 2021, 11, 607226. [Google Scholar] [CrossRef]
- Yuan, L.; Loque, D.; Kojima, S.; Rauch, S.; Ishiyama, K.; Inoue, E.; Takahashi, H.; von Wiren, N. The organization of high affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. Plant Cell 2007, 19, 2636–2652. [Google Scholar] [CrossRef]
- van der Eerden, L.J.M. Toxicity of ammonia to plants. Agric. Environ. 1982, 7, 223–235. [Google Scholar] [CrossRef]
- Russell, B.; Marshall, G.; Jarvis, P. Plant Canopies: Their Growth, form and Function; Cambridge University Press: Cambridge, UK, 1989; pp. 7–10. [Google Scholar]
- Jenner, F.C.; Ugalde, T.D.; Aspinall, D. The physiology of starch and protein deposition in the endosperm of wheat. Aust. J. Plant Physiol. 1991, 18, 211–226. [Google Scholar] [CrossRef]
- Barraclough, P.B.; Lopez-Bellido, R.; Hawkesford, M.J. Genotypic variation in the uptake, partitioning and remobilization of nitrogen during grain-filling in wheat. Field Crops Res. 2014, 156, 242–248. [Google Scholar] [CrossRef]
- Bai, C.H.; Liang, Y.L.; Hawkesford, M.J. Identification of QTLs associated with seedling root traits and their correlation with plant height in wheat. J. Exp. Bot. 2013, 64, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, T.R.; Horie, T. Leaf nitrogen, photosynthesis and crop radiation use efficiency: A review. Crop Sci. 1989, 29, 90–98. [Google Scholar] [CrossRef]
- Lemaire, G.; Jeuffroy, M.H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage. Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Jeuffroy, M.H.; Bouchard, C. Intensity and duration of nitrogen deficiency on wheat grain number. Crop Sci. 1999, 39, 1385–1393. [Google Scholar] [CrossRef]
- Lemaire, G.; Gastal, F. N uptake and distribution in plant canopies. In Diagnosis of the Nitrogen Status in Crops, 1st ed.; Lemaire, G., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 3–43. [Google Scholar]
- Millard, P. The accumulation and storage of nitrogen by herbaceous plants. Plant Cell Environ. 1988, 11, 1–8. [Google Scholar] [CrossRef]
- Spratt, E.D.; Gasser, J.K.R. Effects of fertiliser, nitrogen and water supply on distribution of dry matter and nitrogen between the different parts of wheat. Can. J. Plant Sci. 1970, 50, 613–625. [Google Scholar] [CrossRef][Green Version]
- Austin, R.B.; Ford, M.A.; Edrich, J.A.; Blackwell, R.D. The nitrogen economy of winter wheat. J. Agric. Sci. 1977, 88, 159–167. [Google Scholar] [CrossRef]
- Gregory, P.J.; Crawford, D.V.; McGowan, M. Nutrient relations of winter wheat. Accumulation and distribution of Na, K, Ca, Mg, P, S and N. J. Agric. Sci. 1979, 93, 485–494. [Google Scholar] [CrossRef]
- Cox, M.C.; Qualset, C.O.; Rains, D.W. Genetic variation for nitrogen assimilation and translocation in wheat III. Nitrogen translocation in relation to grain yield and protein. Crop Sci. 1986, 26, 737–740. [Google Scholar] [CrossRef]
- Barbottin, A.; Lecomte, C.; Bouchard, C.; Jeuffroy, M.-H. Nitrogen remobilization during grain filling in wheat: Genotypic and environmental effects. Crop Sci. 2005, 45, 1141–1150. [Google Scholar] [CrossRef]
- Gaju, O.; Allard, V.; Martre, P.; Snape, J.; Heumez, E.; Le Gouis, J.; Moreau, D.; Bogard, M.; Griffiths, S.; Orford, S.; et al. Identification of traits to improve nitrogen-use efficiency of wheat genotypes. Field Crops Res. 2011, 123, 139–152. [Google Scholar] [CrossRef]
- Kichey, T.; Hirel, B.; Heumez, E.; Dubois, F.; Le Gouis, J. In winter wheat (Triticum aestivum L.), post-anthesis nitrogen uptake and remobilisation to the grain correlatesmwith agronomic traits and nitrogen physiological markers. F. Crop. Res. 2007, 102, 22–32. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Riche, A.B. Impacts of G × E × M on Nitrogen Use Efficiency in Wheat and Future Prospects. Front. Plant Sci. 2020, 11, 1157. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, M.S.; Malhi, S.S. Interactions of nitrogen with other nutrients and water: Effect on crop yield and quality, nutrient use efficiency, carbon sequestration, and environmental pollution. Adv. Agron. 2005, 86, 341–409. [Google Scholar]
- McAllister, C.H.; Beatty, P.H.; Good, A.G. Engineering nitrogen use efficient crop plants: The current status. Plant Biotech. J. 2012, 10, 1011–1025. [Google Scholar] [CrossRef]
- Laperche, A.; Devienne-Barret, F.; Maury, O.; Le Gouis, J.; Ney, B. A simplified conceptual model of carbon/nitrogen functioning for QTL analysis of winter wheat adaptation to nitrogen deficiency. Theor. Appl. Genet. 2006, 113, 1131–1146. [Google Scholar] [CrossRef]
- Cormier, F.; Foulkes, J.; Hirel, B.; Gouache, D.; Moenne-Loccoz, Y.; Le Gouis, J. Breeding for increased nitrogen-use efficiency: A review for wheat (T. aestivum L.). Plant Breed. 2016, 135, 255–278. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Sørensen, J.N. Soil nitrogen depletion by vegetable crops with variable root growth. Acta Agric. Scand. Sect. B Soil Plant Sci. 1999, 49, 92–97. [Google Scholar] [CrossRef]
- Wuest, S.B.; Cassman, K.G. Fertilizer-Nitrogen Use Efficiency of irrigated wheat: I. uptake efficiency of preplant versus late-season application. Agron. J. 1992, 84, 682–688. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, F.; Chen, X.; Li, F.; Tong, Y. Using In-Season Nitrogen management and wheat cultivars to improve Nitrogen Use Efficiency. Agron. J. Soil Fertil. Plant Nutr. 2011, 75, 976–983. [Google Scholar] [CrossRef]
- Guo, H.; Tian, Z.; Sun, S.; Li, Y.; Jiang, D.; Cao, W.; Dai, T. Preanthesis root growth and nitrogen uptake improved wheat grain yield and Nitrogen Use Efficiency. Agron. J. Crop Ecol. Physiol. 2019, 111, 3048–3056. [Google Scholar] [CrossRef]
- Campbell, C.A.; Zentner, R.P.; Selles, F.; McConkey, B.G.; Dyck, F.B. Nitrogen management for spring wheat grown annually on zero-tillage: Yields and Nitrogen Use Efficiency. Agron. J. 1993, 85, 107–114. [Google Scholar] [CrossRef]
- Sowers, K.E.; Pan, W.L.; Miller, B.C.; Smith, J.L. Nitrogen Use Efficiency of split nitrogen applications in soft white winter wheat. Agron. J. 1994, 86, 942–948. [Google Scholar] [CrossRef]
- Sowers, K.E.; Miller, B.C.; Pan, W.L. Optimizing Yield and Grain Protein in Soft White Winter Wheat with Split Nitrogen Applications. Agron. J. 1994, 86, 1020–1025. [Google Scholar] [CrossRef]
- Woolfolk, C.W.; Raun, W.R.; Johnson, G.V.; Thomason, W.E.; Mullen, R.W.; Wynn, K.J.; Freeman, K.W. Influence of late-season foliar nitrogen applications on yield and grain nitrogen in winter wheat. Agron. J. 2002, 94, 429–434. [Google Scholar]
- Bly, A.G.; Woodard, H.J. Foliar nitrogen application timing influence on grain yield and protein concentration of hard red winter and spring wheat. Agron. J. 2003, 95, 335–338. [Google Scholar] [CrossRef]
- Whalley, W.R.; Binley, A.; Watts, C.; Shanahan, P.; Dodd, I.C.; Ober, E.S.; Ashton, R.W.; Webster, C.P.; White, R.P.; Hawkesford, M.J. Methods to estimate changes in soil water for phenotyping root activity in the field. Plant Soil 2017, 415, 407–422. [Google Scholar] [CrossRef]
- EU Nitrogen Expert Panel. Nitrogen Use Efficiency (NUE): An Indicator for the Utilization of Nitrogen in Agriculture and Food Systems; Wageningen University & Research: Wageningen, The Netherlands, 2015; p. 6. [Google Scholar]
- Syswerda, S.P.; Basso, B.; Hamilton, S.K.; Tausig, J.B.; Robertson, G.P. Long-term nitrate loss along an agricultural intensity gradient in the Upper Midwest USA. Agric. Ecosyst. Environ. 2012, 149, 10–19. [Google Scholar] [CrossRef]
- Meena, S.K.; Rakshit, A.; Meena, V.S. Effect of seed bio-priming and N doses under varied soil type on nitrogen use efficiency (NUE) of wheat (Triticum aestivum L.) under greenhouse conditions. Biocatal. Agric. Biotechnol. 2016, 6, 68–75. [Google Scholar] [CrossRef]
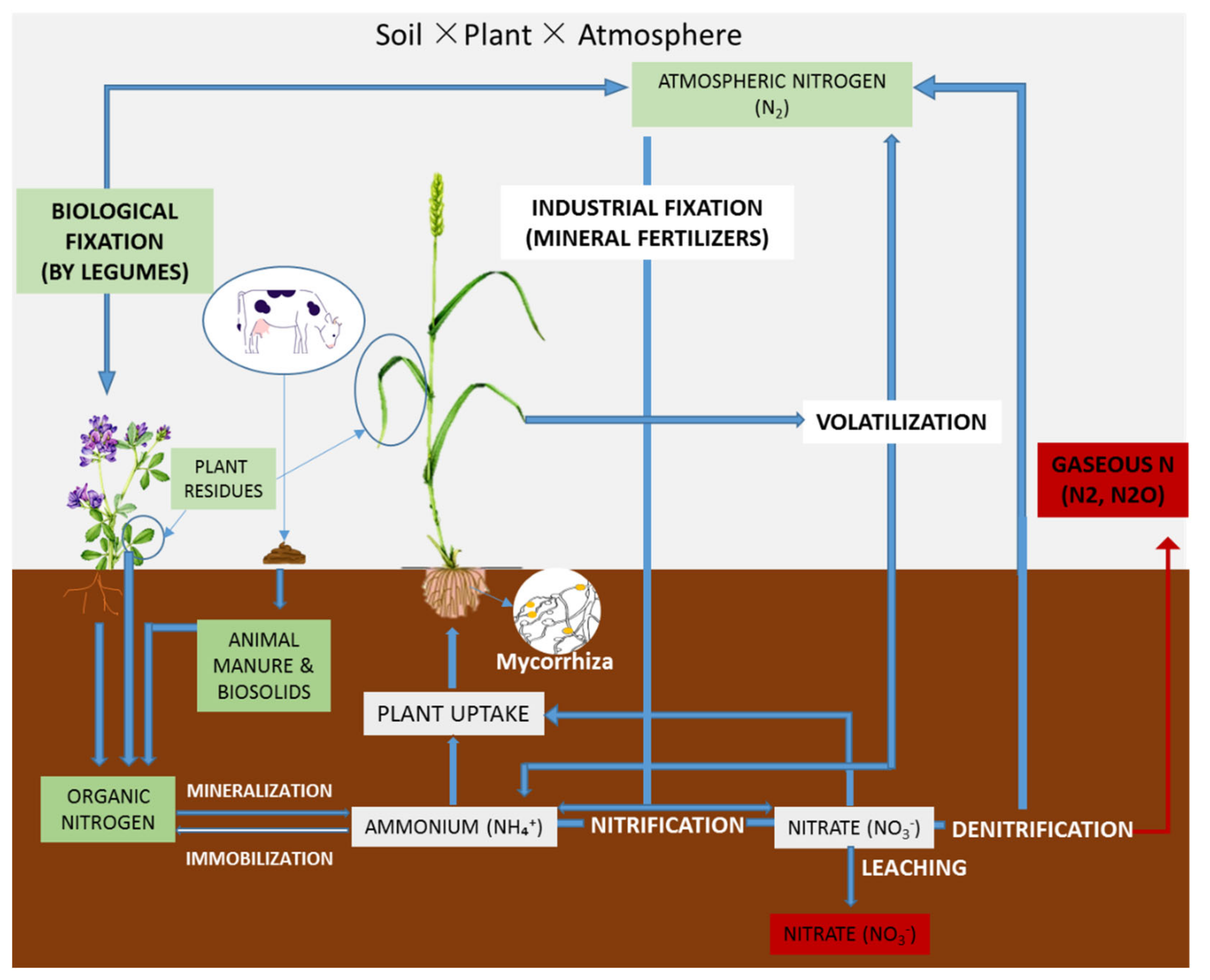
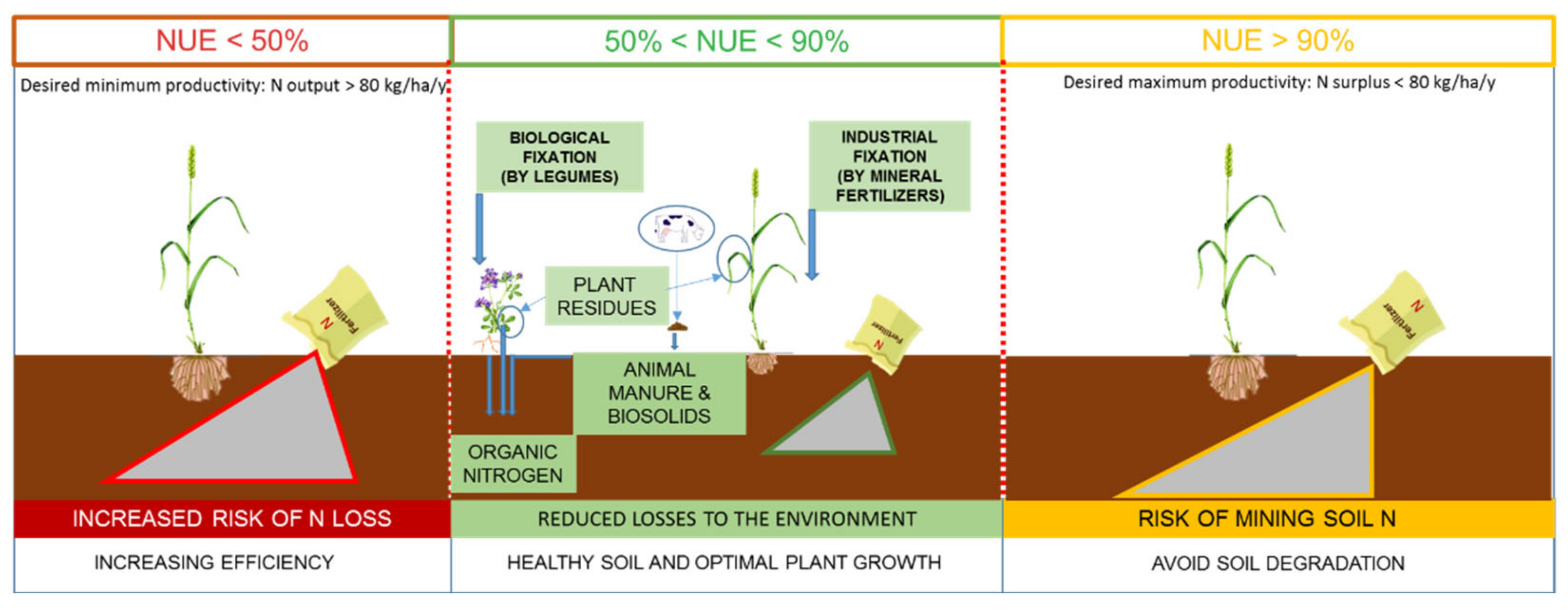

| Approach | NUE Index | Calculation | Definition |
|---|---|---|---|
| Fertilizer-based | Agronomic efficiency [kg yield increase kg nutrient applied−1] | AE = (Y − Y0)/F | The productivity recorded as a result of N input [22,23] |
| Partial factor productivity [kg harvested product kg nutrient applied−1] | PFP = Y/F | The productivity of the cropping system in relation to N inputs [22] | |
| Partial nutrient balance [kg harvested yield kg N applied−1] | PNB = U/F | The ratio between the amount of nutrients taken out of the system and the N applied [22] | |
| Apparent recovery efficiency [%] | RE = (U − U0)/F | The amount of nutrients applied assimilated by the plant [22,23] | |
| Plant-based | Physiological efficiency [kg yield increase/kg increase in N uptake from the fertilizer] | PE = (Y − Y0)/(U − U0) | The ability of the plant to transform nutrients acquired from the source applied to economic yield [22] |
| N Utilization efficiency [kg yield increase/kg increase in N uptake from the fertilizer] | NutE = Y/U | Similar to PE, but does not account for background N [21] | |
| Internal utilization efficiency [kg yield kg nutrient uptake−1] | IE = YN/U | The fraction of plant tissue N that is contained in the yield component [21] | |
| N Harvest index [%] | NHI = (Y/U) × 100 | The same as IE, but expressed as a percentage [21] | |
| Soil-based | N Uptake efficiency [%] | NUpE = (U/F + S) × 100 | The percentage of available soil N that is utilized by the plant; also conceptualized as the apparent recovery efficiency of the N supply [21] |
| NUEyield | NUEyield = NUpE × NUtE | The contribution of N supplied from the soil that is allocated to the yield N [21] | |
| Ecology-based | Nitrogen productivity | NP = Relative growth rate/U | The ratio of the relative growth rate to the concentration of N in plant tissues [21] |
| NUEecology | NUEecology = NP × MRT | The product of N productivity and the mean residency time of plant N [21] | |
| System-based | N Balance index of a system | Snbi = F − U − S | The accumulation or reduction in soil N over a set time |
| NUE of a system (sNUE) | sNUE = YN/YN + Nloss | The fraction of system N outputs that is captured as the N yield rather than lost to the environment [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mălinaş, A.; Vidican, R.; Rotar, I.; Mălinaş, C.; Moldovan, C.M.; Proorocu, M. Current Status and Future Prospective for Nitrogen Use Efficiency in Wheat (Triticum aestivum L.). Plants 2022, 11, 217. https://doi.org/10.3390/plants11020217
Mălinaş A, Vidican R, Rotar I, Mălinaş C, Moldovan CM, Proorocu M. Current Status and Future Prospective for Nitrogen Use Efficiency in Wheat (Triticum aestivum L.). Plants. 2022; 11(2):217. https://doi.org/10.3390/plants11020217
Chicago/Turabian StyleMălinaş, Anamaria, Roxana Vidican, Ioan Rotar, Cristian Mălinaş, Cristina Maria Moldovan, and Marian Proorocu. 2022. "Current Status and Future Prospective for Nitrogen Use Efficiency in Wheat (Triticum aestivum L.)" Plants 11, no. 2: 217. https://doi.org/10.3390/plants11020217
APA StyleMălinaş, A., Vidican, R., Rotar, I., Mălinaş, C., Moldovan, C. M., & Proorocu, M. (2022). Current Status and Future Prospective for Nitrogen Use Efficiency in Wheat (Triticum aestivum L.). Plants, 11(2), 217. https://doi.org/10.3390/plants11020217







