Comparative Evaluation of the Potential Antitumor of Helleborus purpurascens in Skin and Breast Cancer
Abstract
:1. Introduction
2. Results
2.1. Liquid Chromatography–Mass Spectrometry (LC-MS) Assessment of Extract
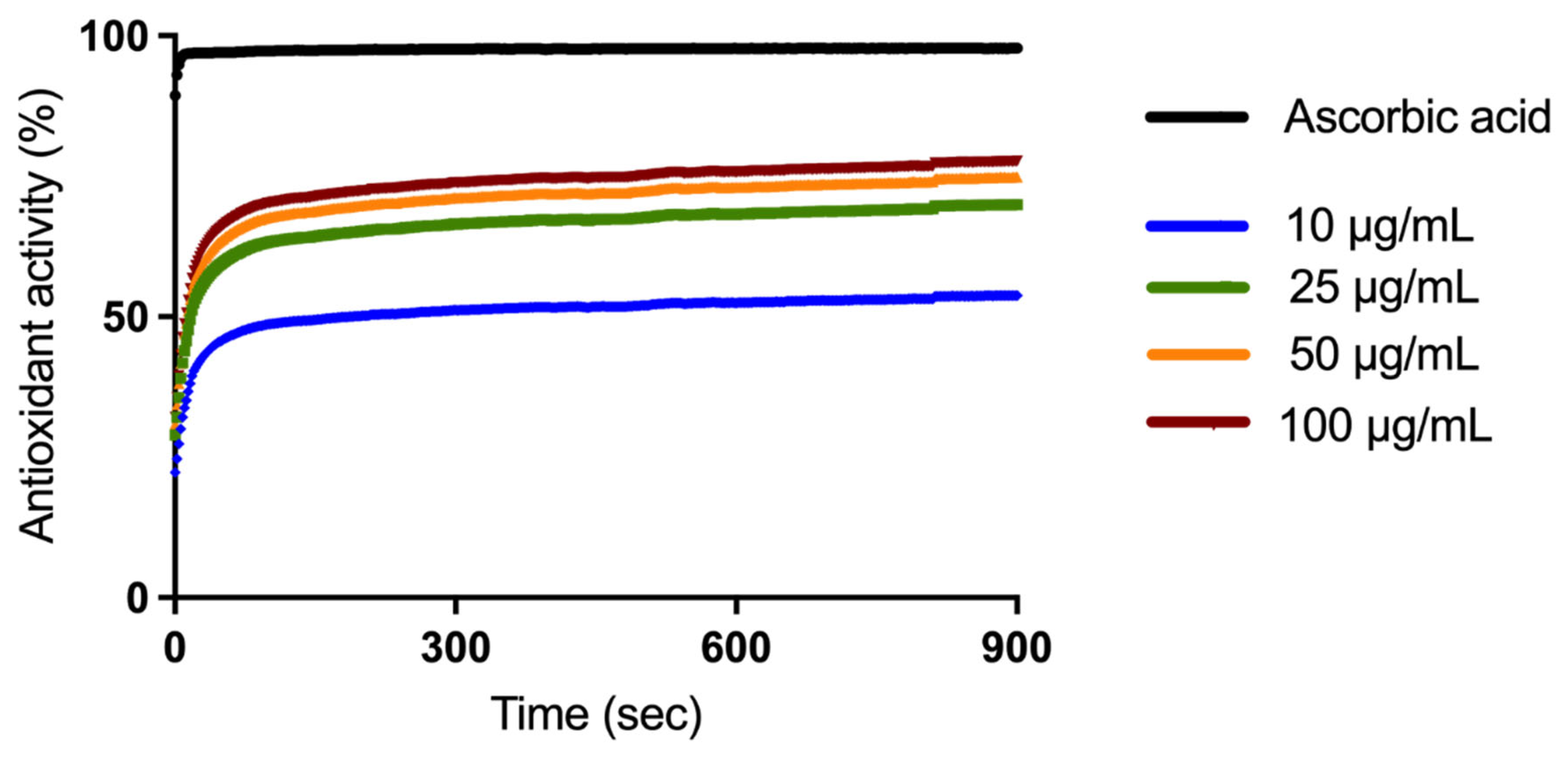
2.2. Antioxidant Activity Evaluation
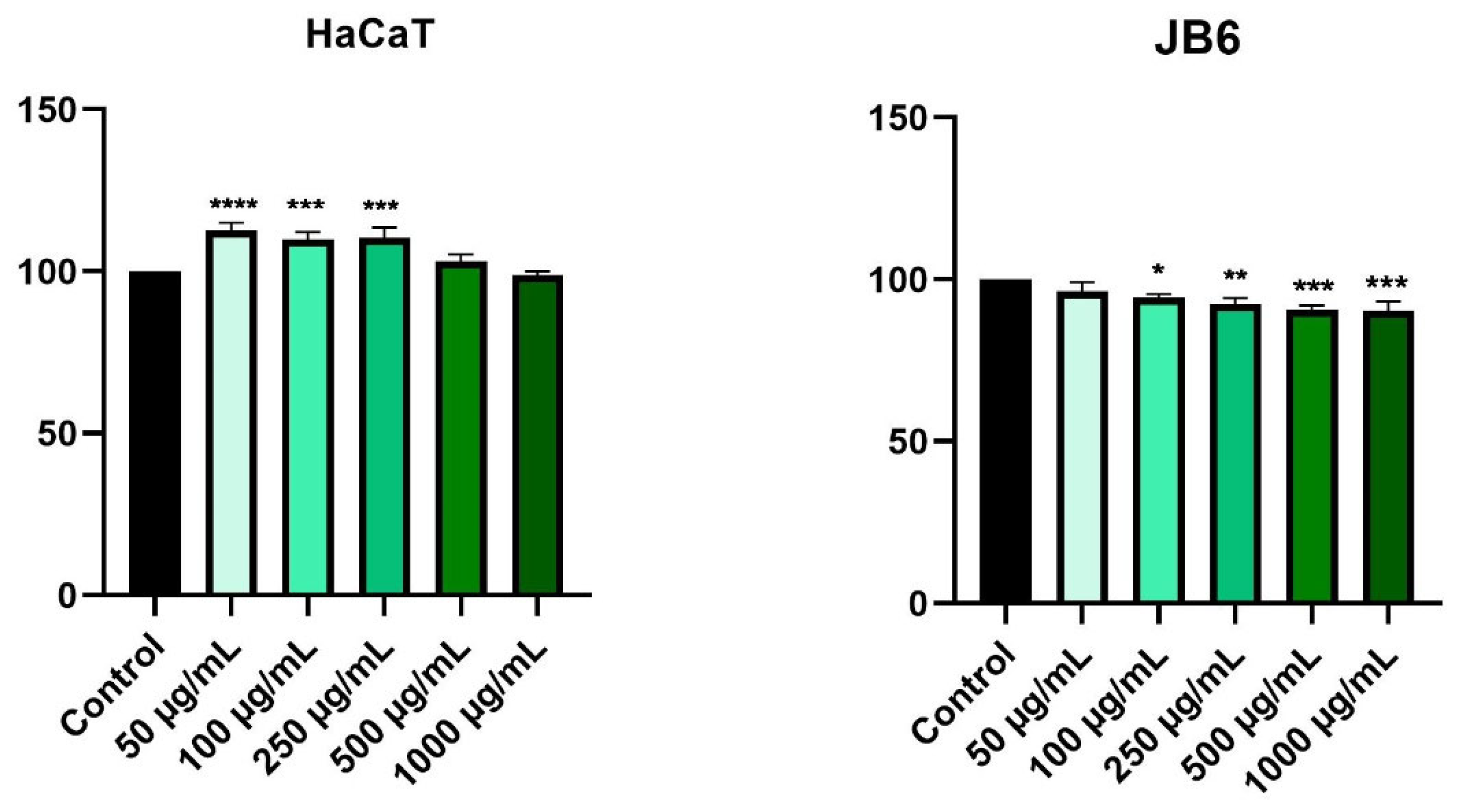
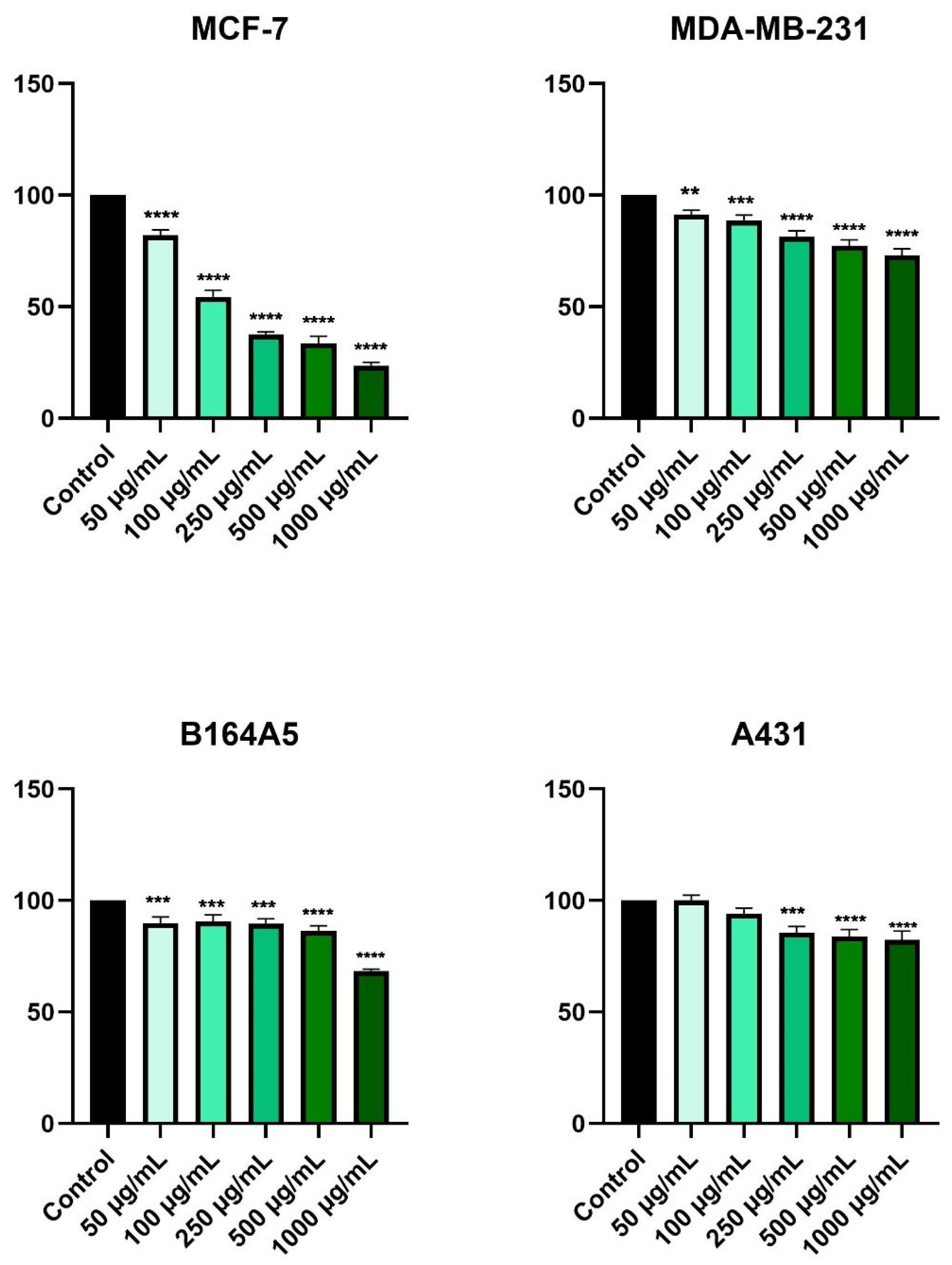
2.3. HPex Exerts a Selective Cytotoxic Effect
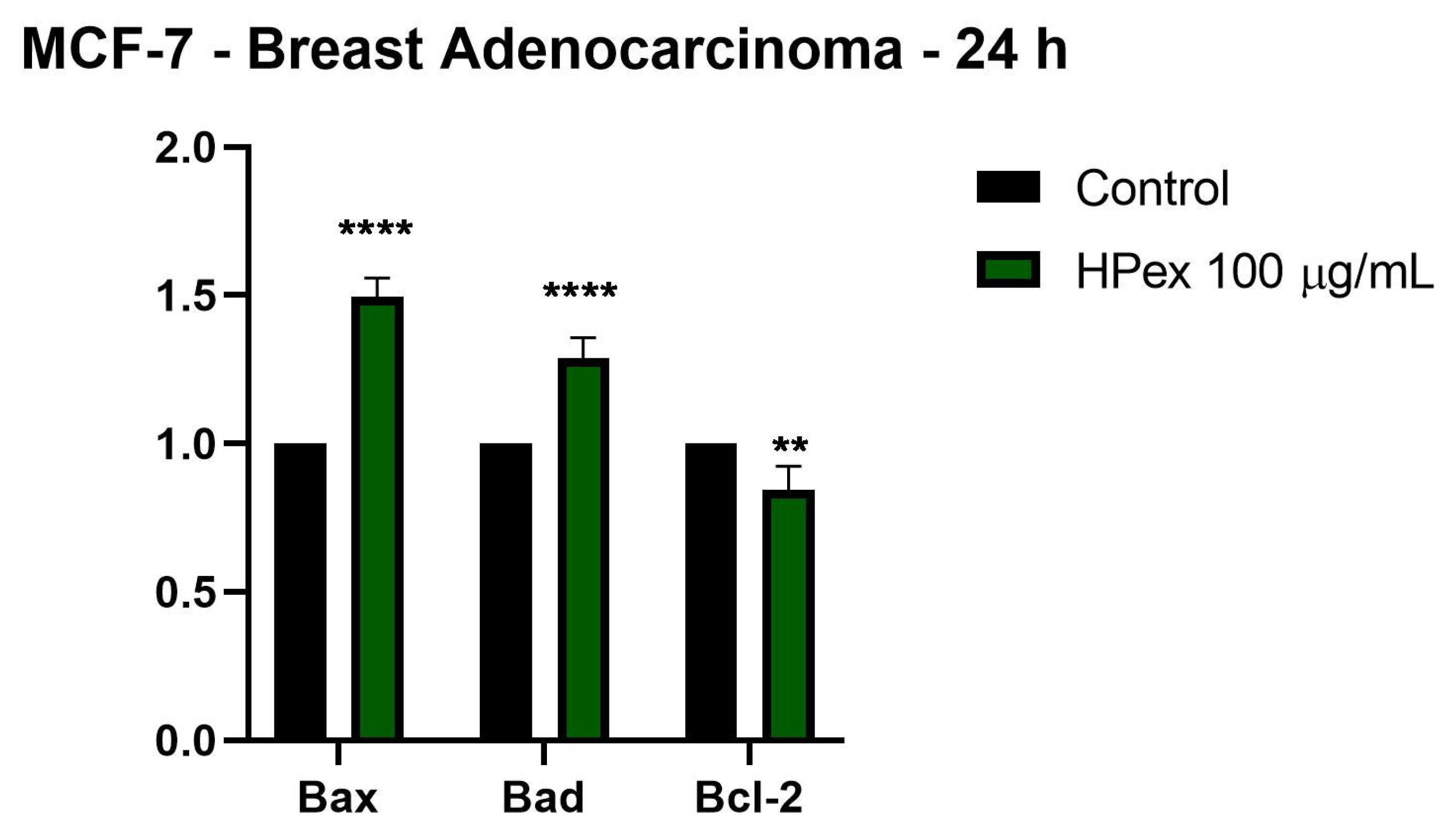
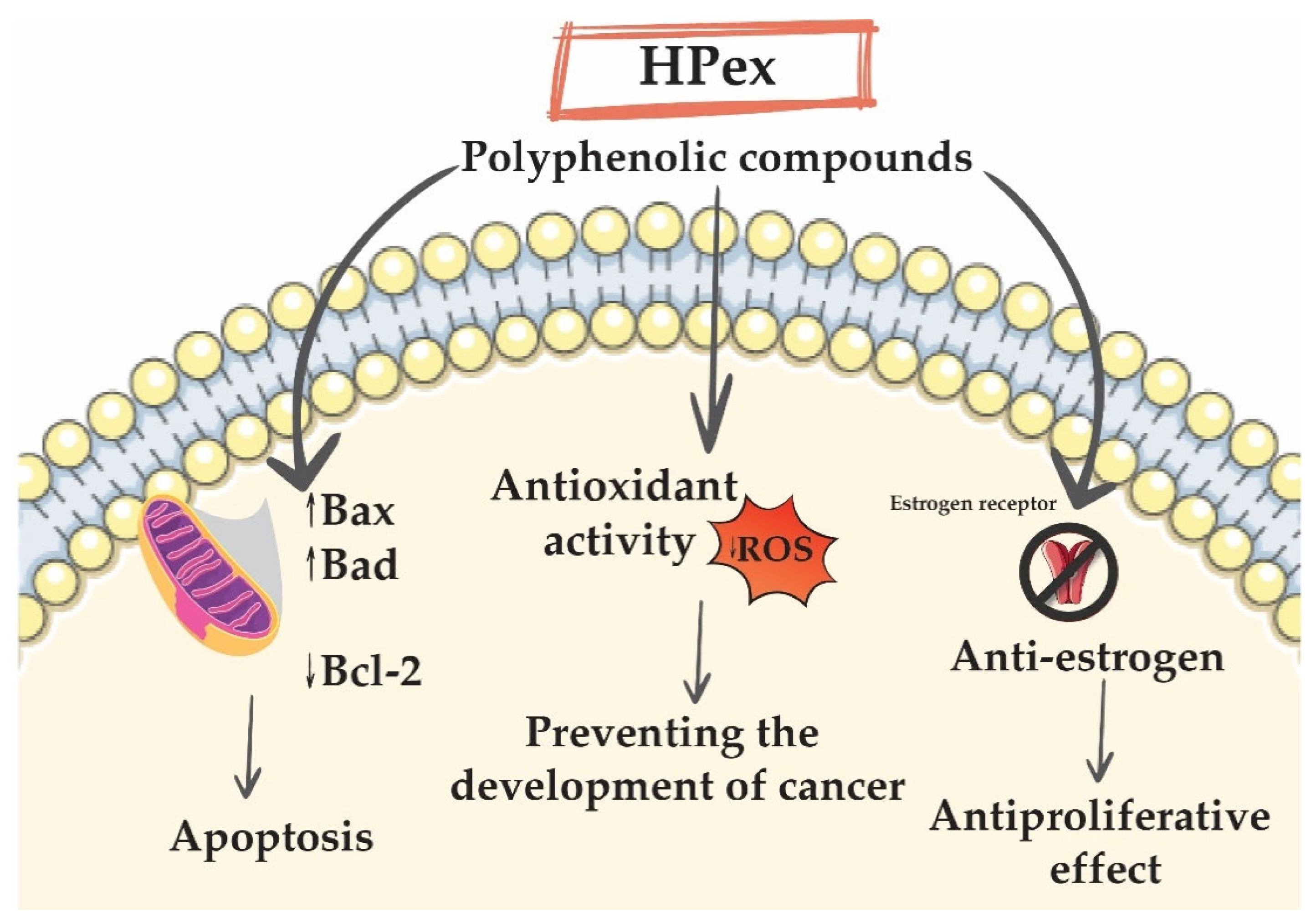
2.4. HPex Induces Changes in the Expression of Apoptotic Markers
3. Discussion
4. Materials and Methods
4.1. Preparation of Extract
4.2. Liquid Chromatography–Mass Spectrometry Analysis
4.3. DPPH Assay
4.4. Cell Culture
4.5. Cell Viability Assessment
4.6. Gene Expression
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Bcl-2 | B-cell CLL/lymphoma 2 |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EtOH | ethanol |
| FBS | fetal bovine serum |
| HPex | hydroalcoholic extract of H. purpurascens |
| LC-MS | Liquid chromatography–mass spectrometry |
| mRNA | messenger ribonucleic acid |
| Pen/Strep | penicillin/streptomycin solution |
| Ph. Eur. | European Pharmacopoeia |
| RT-PCR | reverse transcription–polymerase chain reaction |
| TAOxA | Total Antioxidant Activity |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Leiter, U.; Eigentler, T.; Garbe, C. Epidemiology of Skin Cancer. Adv. Exp. Med. Biol. 2014, 810, 120–140. [Google Scholar] [CrossRef] [PubMed]
- Bidram, E.; Esmaeili, Y.; Ranji-Burachaloo, H.; Al-Zaubai, N.; Zarrabi, A.; Stewart, A.; Dunstan, D.E. A Concise Review on Cancer Treatment Methods and Delivery Systems. J. Drug Deliv. Sci. Technol. 2019, 54, 101350. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Gabryszewska, E. Propagation In Vitro of Hellebores (Helleborus L.) Review. Acta Sci. Pol. Hortorum Cultus 2016, 16, 61–72. [Google Scholar]
- Lindholm, P.; Gullbo, J.; Claeson, P.; Göransson, U.; Johansson, S.; Backlund, A.; Larsson, R.; Bohlin, L. Selective Cytotoxicity Evaluation in Anticancer Drug Screening of Fractionated Plant Extracts. J. Biomol. Screen. 2002, 7, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Segneanu, A.E.; Grozescu, I.; Cziple, F.; Berki, D.; Damian, D.; Niculite, C.M.; Florea, A.; Leabu, M.; Ferreira, I.C.F.R. Helleborus purpurascens—Amino Acid and Peptide Analysis Linked to the Chemical and Antiproliferative Properties of the Extracted Compounds. Molecules 2015, 20, 22170–22187. [Google Scholar] [CrossRef] [Green Version]
- Grigore, A.; Bubueanu, C.; Pirvu, L.; Neagu, G.; Bejanaru, I.; Vulturescu, V.; Panteli, M.; Rasit, I. Immunomodulatory Effect of Helleborus purpurascens Waldst. & Kit. Plants 2021, 10, 1990. [Google Scholar]
- Roman, G.P.; Neagu, E.; Radu, G.L. Membrane Processes for the Purification and Concentration of Helleborus purpurascens Extracts and Evaluation of Antioxidant Activity. Assessment 2010, 13, 15. [Google Scholar]
- Gherghel, D.; Iurea, D.; Roman, G.; Radu, G.L.; Rotinberg, P. New Potential Antitumoral Agents of Polyphenolic Nature Obtained from Helleborus purpurascens by Membranary Micro- and Ultrafiltration Techniques. SAAIC 2011, 12, 41–51. [Google Scholar]
- Balázs, V.L.; Filep, R.; Ambrus, T.; Kocsis, M.; Farkas, Á.; Stranczinger, S.; Papp, N. Ethnobotanical, Historical and Histological Evaluation of Helleborus L. Genetic Resources Used in Veterinary and Human Ethnomedicine. Genet. Resour. Crop. Evol. 2020, 67, 781–797. [Google Scholar] [CrossRef] [Green Version]
- Milbradt, A.G.; Kerek, F.; Moroder, L.; Renner, C. Structural Characterization of Hellethionins from Helleborus purpurascens. Biochemistry 2003, 42, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting Apoptosis in Cancer Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [Green Version]
- Lopez, J.; Tait, S.W.G. Mitochondrial Apoptosis: Killing Cancer Using the Enemy Within. Br. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef] [Green Version]
- Franz, M.H.; Birzoi, R.; Vasile, C.; Maftei, E.; Kelter, G.; Herbert, H.; Ion, F. Studies on the Constituents of Helleborus purpurascens: Analysis and Biological Activity of the Aqueous and Organic Extracts. Amino Acids 2018, 50, 163–188. [Google Scholar] [CrossRef]
- Maior, M.C.; Dobrota, C. Natural Compounds with Important Medical Potential Found in Helleborus sp. Open Life Sci. 2013, 8, 272–285. [Google Scholar] [CrossRef] [Green Version]
- Hao, D.-C.; He, C.-N.; Shen, J.; Xiao, P.-G. Anticancer Chemodiversity of Ranunculaceae Medicinal Plants. In Current Genomics; Néri, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 223–259. ISBN 978-0-12-814232-5. [Google Scholar]
- Muzashvili, T.; Skhirtladze, A.; Sulakvelidze, T.; Benidze, M.; Mshvildadze, V.; Legault, J.; Pichette, A.; Kemertelidze, E. Cytotoxic Activity of Helleborus Caucasicus A. Georg. Chem. J. 2006, 6, 684–685. [Google Scholar]
- Felenda, J.E.; Turek, C.; Mörbt, N.; Herrick, A.; Müller, M.B.; Stintzing, F.C. Preclinical Evaluation of Safety and Potential of Black Hellebore Extracts for Cancer Treatment. BMC Complement. Altern. Med. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Schink, M.; Garcia-Käufer, M.; Bertrams, J.; Duckstein, S.; Müller, M.; Huber, R.; Stintzing, F.; Gründemann, C. Differential Cytotoxic Properties of Helleborus niger L. on Tumour and Immunocompetent Cells. J. Ethnopharmacol. 2014, 159, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Cakar, J.; Parić, A.; Vidic, D.; Haverić, A.; Haverić, S.; Maksimović, M.; Bajrović, K. Antioxidant and Antiproliferative Activities of Helleborus odorus Waldst. & Kit, H. multifidus Vis. and H. hercegovinus Martinis. Nat. Prod. Res. 2011, 25, 1969–1974. [Google Scholar] [CrossRef]
- Oh, S.; Xiaofei, E.; Ni, D.; Pirooz, S.D.; Lee, J.; Lee, D.; Zhao, Z.; Lee, S.; Lee, H.; Ku, B.; et al. Downregulation of Autophagy by Bcl-2 Promotes MCF7 Breast Cancer Cell Growth Independent of Its Inhibition of Apoptosis. Cell Death Differ. 2011, 18, 452–464. [Google Scholar] [CrossRef]
- Levine, B.; Sinha, S.C.; Kroemer, G. Bcl-2 Family Members: Dual Regulators of Apoptosis and Autophagy. Autophagy 2008, 4, 600–606. [Google Scholar] [CrossRef] [Green Version]
- Abcdefg, J.R.; Bcdf, S.M.; Defg, M.F. Bcl-2 Expression in Breast Carcinoma Cells In Vitro. Oncol. Radiother. 2018, 2, 23–26. [Google Scholar]
- Jesse, P.; Mottke, G.; Eberle, J.; Seifert, G.; Henze, G.; Prokop, A. Apoptosis-Inducing Activity of Helleborus Niger in ALL and AML. Pediatr. Blood Cancer 2009, 52, 464–469. [Google Scholar] [CrossRef]
- Yfanti, P.; Karkabounas, A.; Batistatou, A.; Tsapinou, A.; Leneti, E.; Manos, G.; Lekka, M.E. Study of Potent Cytotoxic Activity of Helleborus cyclophyllus Boiss against a Human Adenocarcinoma Cell Line. Cytotechnology 2020, 72, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, Inflammation, and Cardiovascular Disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial Effects of Polyphenols on Cardiovascular Disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Speer, H.; D’Cunha, N.M.; Botek, M.; McKune, A.J.; Sergi, D.; Georgousopoulou, E.; Mellor, D.D.; Naumovski, N. The Effects of Dietary Polyphenols on Circulating Cardiovascular Disease Biomarkers and Iron Status: A Systematic Review. Nutr. Metab. Insights 2019, 12, 1178638819882739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in Cancer Prevention: New Insights (Review). Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Kampa, M.; Nifli, A.-P.; Notas, G.; Castanas, E. Polyphenols and Cancer Cell Growth. Rev. Physiol. Biochem. Pharmacol. 2007, 159, 79–113. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Russo, G.L.; Tedesco, I.; Spagnuolo, C.; Russo, M. Antioxidant Polyphenols in Cancer Treatment: Friend, Foe or Foil? Semin. Cancer Biol. 2017, 46, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- D’Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-Oxidant and pro-Apoptotic Activity of Polyphenol Extract from Annurca Apple and Its Underlying Mechanisms in Human Breast Cancer Cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef]
- Huang, D.; Jiang, Y.; Chen, W.; Yao, F.; Sun, L. Polyphenols with Anti-Proliferative Activities from Penthorum Chinense Pursh. Molecules 2014, 19, 11045–11055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, D.; Soysa, P.; Wijeratne, S. Polyphenols Contribute to the Antioxidant and Antiproliferative Activity of Phyllanthus Debilis Plant In-Vitro. BMC Complement. Altern. Med. 2016, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kaur, M.; Katnoria, J.K.; Nagpal, A.K. Polyphenols in Food: Cancer Prevention and Apoptosis Induction. Curr. Med. Chem. 2017, 25, 4740–4757. [Google Scholar] [CrossRef]
- Dayem, A.A.; Choi, H.Y.; Yang, G.M.; Kim, K.; Saha, S.K.; Cho, S.G. The Anti-Cancer Effect of Polyphenols against Breast Cancer and Cancer Stem Cells: Molecular Mechanisms. Nutrients 2016, 8, 581. [Google Scholar] [CrossRef]
- Souto, E.B.; Sampaio, A.C.; Campos, J.R.; Martins-Gomes, C.; Aires, A.; Silva, A.M. Chapter 2—Polyphenols for skin cancer: Chemical properties, structure-related mechanisms of action and new delivery systems. In Studies in Natural Products Chemistry; ur-Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 63, pp. 21–42. ISBN 1572-5995. [Google Scholar]
- Almaguer, G.; Ortiz-Vilchis, P.; Cordero, P.; Martinez-Vega, R.; Perez-Durán, J.; Meaney, E.; Villarreal, F.; Ceballos, G.; Nájera, N. Anticancer Potential of (−)-Epicatechin in a Triple-Negative Mammary Gland Model. J. Pharm. Pharmacol. 2021, 73, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Li, Y.; Liu, Z.; Lin, Y.; Huang, J. Anti-Melanogenic Effects of Epigallocatechin-3-Gallate (EGCG), Epicatechin-3-Gallate (ECG) and Gallocatechin-3-Gallate (GCG) via down-Regulation of CAMP/CREB /MITF Signaling Pathway in B16F10 Melanoma Cells. Fitoterapia 2020, 145, 104634. [Google Scholar] [CrossRef]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F.A. Rosmarinic Acid-Induced Apoptosis and Cell Cycle Arrest in Triple-Negative Breast Cancer Cells. Eur. J. Pharmacol. 2020, 885, 173419. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Quan, J.; Xiang, D. Rosmarinic Acid Inhibits Proliferation and Migration, Promotes Apoptosis and Enhances Cisplatin Sensitivity of Melanoma Cells through Inhibiting ADAM17/EGFR/AKT/GSK3β Axis. Bioengineered 2021, 12, 3065–3076. [Google Scholar] [CrossRef]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A Review on Anti-Cancer Properties of Quercetin in Breast Cancer. Life Sci. 2020, 248, 117463. [Google Scholar] [CrossRef]
- Harris, Z.; Donovan, M.G.; Branco, G.M.; Limesand, K.H.; Burd, R. Quercetin as an Emerging Anti-Melanoma Agent: A Four-Focus Area Therapeutic Development Strategy. Front. Nutr. 2016, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.K.; Lalitha, K.G. Pharmacognostical and Phytochemical Studies of Helleborus niger L Root. Anc. Sci. Life 2017, 36, 151–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, Y.; Banuls, L.; Katz, A.; Miklos, W.; Cimmino, A.; Tal, D.M.; Ainbinder, E.; Zehl, M.; Urban, E.; Evidente, A.; et al. Hellebrin and Its Aglycone Form Hellebrigenin Display Similar in Vitro Growth Inhibitory Effects in Cancer Cells and Binding Profiles to the Alpha Subunits of the Na+/K+-ATPase. Mol. Cancer 2013, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; He, J.; Gao, B.; Han, L.; Mao, Y.; Zhao, H.; Si, N.; Wang, H.; Yang, J.; Bian, B. Hellebrigenin Anti-Pancreatic Cancer Effects Based on Apoptosis and Autophage. PeerJ 2020, 8, e9011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, B.; Bian, B.; Zhao, H.; Kiyomi, A.; Hayashi, H.; Iwatani, Y.; Sugiura, M.; Takagi, N. Cytotoxic Effects of Hellebrigenin and Arenobufagin Against Human Breast Cancer Cells. Front. Oncol. 2021, 11, 3320. [Google Scholar] [CrossRef]
- Liu, C.; Kong, Q.; Pan, F.; Jiang, S.; Meng, L.; Huang, G.; Lu, L.; Li, S.; Liu, Y. Hellebrigenin Induces Apoptosis in Colorectal Cancer Cells through Induction of Excessive Reactive Oxygen Species. Biocell 2021, 45, 943–951. [Google Scholar] [CrossRef]
- Martucciello, S.; Paolella, G.; Muzashvili, T.; Skhirtladze, A.; Pizza, C.; Caputo, I.; Piacente, S. Steroids from Helleborus caucasicus Reduce Cancer Cell Viability Inducing Apoptosis and GRP78 Down-Regulation. Chem. Biol. Interact. 2018, 279, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, A.; Inomata, M.; Yoshizawa, Y.; Iguchi, T.; Mimaki, Y. Bufadienolides and ecdysteroids from the whole plants of Helleborus niger and their cytotoxicity. J. Nat. Med. 2021, 75, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Theodossiou, T.A.; Ali, M.; Grigalavicius, M.; Grallert, B.; Dillard, P.; Schink, K.O.; Olsen, C.E.; Wälchli, S.; Inderberg, E.M.; Kubin, A.; et al. Simultaneous Defeat of MCF7 and MDA-MB-231 Resistances by a Hypericin PDT–Tamoxifen Hybrid Therapy. NPJ Breast Cancer 2019, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Aiyer, H.S.; Bouker, K.B.; Cook, K.L.; Facey, C.O.B.; Hu, R.; Schwartz, J.L.; Shajahan, A.N.; Hilakivi-Clarke, L.; Clarke, R. Interaction of Dietary Polyphenols with Molecular Signaling Pathways of Antiestrogen Resistance: Possible Role in Breast Cancer Recurrence. Horm. Mol. Biol. Clin. Investig. 2012, 9, 127–141. [Google Scholar] [CrossRef]
- Seung, M.O.; Yeon, P.K.; Kyu, H.C. Biphasic Effects of Kaempferol on the Estrogenicity in Human Breast Cancer Cells. Arch. Pharm. Res. 2006, 29, 354–362. [Google Scholar] [CrossRef]
- Shahzad, H.; Giribabu, N.; Muniandy, S.; Salleh, N. Quercetin Induces Morphological and Proliferative Changes of Rat’s Uteri under Estrogen and Progesterone Influences. Int. J. Clin. Exp. Pathol. 2014, 7, 5484–5494. [Google Scholar]
- Van Meeuwen, J.A.; Korthagen, N.; de Jong, P.C.; Piersma, A.H.; van den Berg, M. (Anti)Estrogenic Effects of Phytochemicals on Human Primary Mammary Fibroblasts, MCF-7 Cells and Their Co-Culture. Toxicol. Appl. Pharmacol. 2007, 221, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are Flavonoids Effective Antioxidants in Plants? Twenty Years of Our Investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Buleu, F.N.; Luca, C.T.; Tudor, A.; Badalica-petrescu, M.; Caraba, A.; Pah, A.; Georgescu, D.; Christodorescu, R.; Dragan, S. Correlations between Vascular Stiffness Indicators, OPG, and 25-OH Vitamin D3 Status in Heart Failure Patients. Medicina 2019, 55, 309. [Google Scholar] [CrossRef] [Green Version]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneto, H.; Katakami, N.; Matsuhisa, M.; Matsuoka, T. Role of Reactive Oxygen Species in the Progression of Type 2 Diabetes and Atherosclerosis. Mediat. Inflamm. 2010, 2010, 453892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrica, L.; Pusztai, A.-M.; Vlad, M.; Vlad, A.; Gadalean, F.; Dumitrascu, V.; Vlad, D.; Velciov, S.; Gluhovschi, C.; Bob, F.; et al. MiRNA Expression Is Associated with Clinical Variables Related to Vascular Remodeling in the Kidney and the Brain in Type 2 Diabetes Mellitus Patients. Endocr. Res. 2020, 45, 119–130. [Google Scholar] [CrossRef]
- Munteanu Ioan, A.; Manea, A.-M.; Jinca Marius, C.; Boia, M. Basic Biochemical and Hematological Parameters in Perinatal Asphyxia and Their Correlation with Hypoxic Ischemic Encephalopathy. Exp. Ther. Med. 2021, 21, 259. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Chlopicki, S. Revisiting Pharmacology of Oxidative Stress and Endothelial Dysfunction in Cardiovascular Disease: Evidence for Redox-Based Therapies. Free Radic. Biol. Med. 2020, 157, 15–37. [Google Scholar] [CrossRef]
- Doandes, F.M.; Manea, A.-M.; Lungu, N.; Cioboata, D.; Brandibur, T.; Costescu, O.; Hudisteanu, A.; Boia, E.R.; Boia, M. Clinical, Biological and Electroencephalographic Monitoring of Newborns with Neurological Risk in the Neonatal Intensive Care Unit. Exp. Ther. Med. 2021, 22, 760. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, G.; Liu, B.; Wang, Q. Flavonoids and Antioxidant Activity of Rare and Endangered Fern: Isoetes Sinensis. PLoS ONE 2020, 15, e0232185. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.C.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A.M. Flavonoids: A Review of Probable Mechanisms of Action and Potential Applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Öztürk, M.; Bulduk, İ.; Korcan, S.E.; Liman, R.; Çoban, F.K.; Kargıoğlu, M.; Konuk, M. Total Phenolics, Flavonoids Contents, Antioxidant Activity and DNA Protective Effect of Lenten Rose (Helleborus orientalis). Asian J. Biochem. Genet. Mol. Biol. 2019, 1, 1–12. [Google Scholar] [CrossRef]
- Mohammed, F.; Çınar, G.; Şahin Yiğit, S.; Akgül, H.; Dogan, M. Antioxidant and Oxidant Status of Endemic Helleborus Vesicarius. Turk. J. Agric. Food Sci. Technol. 2020, 8, 2008–2010. [Google Scholar] [CrossRef]
- Coricovac, D.-E.; Moacă, E.-A.; Pinzaru, I.; Cîtu, C.; Soica, C.; Mihali, C.-V.; Păcurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.-A. Biocompatible Colloidal Suspensions Based on Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Toxicological Profile. Front. Pharmacol. 2017, 8, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guran, K.; Buzatu, R.; Pinzaru, I.; Boruga, M.; Marcovici, I.; Coricovac, D.; Avram, S.; Poenaru, M.; Susan, M.; Susan, R.; et al. In Vitro Pharmaco-Toxicological Characterization of Melissa Officinalis Total Extract Using Oral, Pharynx and Colorectal Carcinoma Cell Lines. Processes 2021, 9, 850. [Google Scholar] [CrossRef]
- Maghiari, A.L.; Coricovac, D.; Pinzaru, I.A.; Macașoi, I.G.; Marcovici, I.; Simu, S.; Navolan, D.; Dehelean, C. High Concentrations of Aspartame Induce Pro-Angiogenic Effects in Ovo and Cytotoxic Effects in HT-29 Human Colorectal Carcinoma Cells. Nutrients 2020, 12, 3600. [Google Scholar] [CrossRef] [PubMed]
- Iftode, A.; Drăghici Andrei, G.; Macașoi, I.; Marcovici, I.; Coricovac, E.D.; Dragoi, R.; Tischer, A.; Kovatsi, L.; Tsatsakis, M.A.; Cretu, O.; et al. Exposure to Cadmium and Copper Triggers Cytotoxic Effects and Epigenetic Changes in Human Colorectal Carcinoma HT-29 Cells. Exp. Ther. Med. 2021, 21, 100. [Google Scholar] [CrossRef] [PubMed]
| Standard Phenolic Compound | Rt (min) | Monoisotopic Mass (Da) | m/z | Conc (µg/g d.m.) |
|---|---|---|---|---|
| Gallic acid | 4.74 | 170.02152329 | 169 | 3.075 |
| Proto catechuic acid | 11.104 | 154.02660867 | 153 | 0.496 |
| Caffeic acid | 20.896 | 180.04225873 | 179 | 3.168 |
| Epicatechin | 23.265 | 290.07903816 | 289 | 33.557 |
| p-Coumaric acid | 24.310 | 164.047344113 | 163 | 0.879 |
| Ferulic acid | 23.457 | 194.05790880 | 193 | 1.893 |
| Rutin | 25.925 | 610.15338487 | 609 | 5.546 |
| Rosmarinic acid | 29.001 | 360.08451746 | 359 | 21.301 |
| Resveratrol | 30.238 | 228.078644241 | 227 | 13.223 |
| Quercetin | 31.488 | 302.04265265 | 301 | 46.710 |
| Kaempferol | 34.870 | 286.04773803 | 285 | 67.761 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilut, C.N.; Manea, A.; Macasoi, I.; Dobrescu, A.; Georgescu, D.; Buzatu, R.; Faur, A.; Dinu, S.; Chioran, D.; Pinzaru, I.; et al. Comparative Evaluation of the Potential Antitumor of Helleborus purpurascens in Skin and Breast Cancer. Plants 2022, 11, 194. https://doi.org/10.3390/plants11020194
Pilut CN, Manea A, Macasoi I, Dobrescu A, Georgescu D, Buzatu R, Faur A, Dinu S, Chioran D, Pinzaru I, et al. Comparative Evaluation of the Potential Antitumor of Helleborus purpurascens in Skin and Breast Cancer. Plants. 2022; 11(2):194. https://doi.org/10.3390/plants11020194
Chicago/Turabian StylePilut, Ciprian Nicolae, Aniko Manea, Ioana Macasoi, Amadeus Dobrescu, Doina Georgescu, Roxana Buzatu, Alin Faur, Stefania Dinu, Doina Chioran, Iulia Pinzaru, and et al. 2022. "Comparative Evaluation of the Potential Antitumor of Helleborus purpurascens in Skin and Breast Cancer" Plants 11, no. 2: 194. https://doi.org/10.3390/plants11020194
APA StylePilut, C. N., Manea, A., Macasoi, I., Dobrescu, A., Georgescu, D., Buzatu, R., Faur, A., Dinu, S., Chioran, D., Pinzaru, I., Hancianu, M., Dehelean, C., & Malița, D. (2022). Comparative Evaluation of the Potential Antitumor of Helleborus purpurascens in Skin and Breast Cancer. Plants, 11(2), 194. https://doi.org/10.3390/plants11020194










