Abstract
The sessile plant has developed mechanisms to survive the “rough and tumble” of its natural surroundings, aided by its evolved innate immune system. Precise perception and rapid response to stress stimuli confer a fitness edge to the plant against its competitors, guaranteeing greater chances of survival and productivity. Plants can “eavesdrop” on volatile chemical cues from their stressed neighbours and have adapted to use these airborne signals to prepare for impending danger without having to experience the actual stress themselves. The role of volatile organic compounds (VOCs) in plant–plant communication has gained significant attention over the past decade, particularly with regard to the potential of VOCs to prime non-stressed plants for more robust defence responses to future stress challenges. The ecological relevance of such interactions under various environmental stresses has been much debated, and there is a nascent understanding of the mechanisms involved. This review discusses the significance of VOC-mediated inter-plant interactions under both biotic and abiotic stresses and highlights the potential to manipulate outcomes in agricultural systems for sustainable crop protection via enhanced defence. The need to integrate physiological, biochemical, and molecular approaches in understanding the underlying mechanisms and signalling pathways involved in volatile signalling is emphasised.
1. Introduction
Climate change has exacerbated the multifarious effects of environmental stresses on crop growth and development, thereby compromising sustainable agricultural productivity worldwide. Biotic stresses in the form of insects, bacteria, viruses, fungi, nematodes, arachnids, and weeds account for over 30% of losses from the annual global food production capacity, or approximately US$500 billion [1]. Abiotic stresses including drought, extreme temperatures, and salinity are major yield-limiting factors of economically important food crops globally [2]. Of these stresses, drought is one of the most significant, given that its frequency and severity has been forecasted to increase due to climate change [3]. Greater effort has therefore been directed towards the implementation of sustainable agricultural management and drought mitigation strategies in major crop-growing regions worldwide.
To compensate for their immobile nature, plants acclimatise to various environmental stresses with an array of complex molecular, physiological, and biochemical adaptations, which ultimately allow them to survive and even maintain potential rates of growth [4]. These adaptations may be expressed constitutively, or in many cases, are activated only in the presence of stressors. The evolved innate immunity or basal resistance of a plant is regulated by an intricate network of endogenous signalling molecules, receptor proteins, and transcriptional regulators. In response to environmental stress, plants exhibit an upregulation of the expression of stress-related genes encoding for defence proteins, such as trypsin protease inhibitors (PI) and pathogenesis-related (PR) proteins, which act against herbivory and pathogen attack, respectively. There is also an elevated production of secondary metabolites including osmoprotectants and toxins with deterrent/antifeedant activity [5,6,7,8,9,10,11,12,13]. Timely defence responses to biotic and abiotic stresses offer a fitness benefit to plants that largely depends on the capacity to quickly and accurately recognise external stress stimuli [14,15]. Upon stress perception, the plant will activate various defence-signalling pathways, leading to subsequent gene expression [15,16].
Among the secondary metabolites, phytochemicals in the form of volatile organic compounds (VOCs) have been identified as chemical-signalling molecules involved in both intra- and inter-plant communication, providing a fitness benefit to both the emitter and neighbouring receiver plants [17]. Plants respond to various biotic and abiotic stresses by emitting VOCs that fall into various compound classes, namely: terpenoids, benzenoids/phenylpropanoids, and fatty acid derivatives [17,18,19,20,21,22,23,24,25]. Recent studies in various crop species have explored an intriguing possibility of developing stress tolerance in plants through their exposure to VOCs from stressed neighbours, a process referred to as “priming” [5,6,7,8,9,10,11,12,13,26,27,28,29,30]. In the classical study by Karban et al. [27], volatile-mediated airborne signalling between native tobacco (Nicotiana attenuata) and sagebrush (Artemisia tridentata) was demonstrated. The sagebrush plants were clipped experimentally to mimic insect damage, and the emitted VOCs induced herbivore resistance in the neighbouring tobacco plants.
A follow-up study by Kessler et al. [31] reported an upregulation of herbivore-regulated genes in the receiver tobacco plants, but with no evidence of direct elicitation of defensive secondary metabolites. Interestingly, following post-challenge with Manduca sexta caterpillars, the receiver tobacco plants had an accelerated production of PI proteins, which was not evident in plants not previously exposed to the clipped sagebrush volatiles [31]. This study demonstrated how volatile-mediated plant–plant interaction primes defence responses in non-stressed receiver plants, inducing a faster and stronger response to a real stress. The production of defensive metabolites such as (Z)-3-hexenyl-vicianoside [12] and genes of defence-related enzymes including PI, threonine deaminase, and α-dioxygenase [31] have been shown to increase as a result of VOC exposure, ultimately conferring tolerance or resistance in non-stressed plants. Overall, however, the signalling pathways and mechanisms in volatile-mediated plant–plant interactions are vaguely understood and have been sparsely investigated in stress physiology studies.
This review offers a comprehensive synopsis of volatile-mediated inter-plant communication, referencing studies published from 2000–2021 and using Google Scholar and Web of Science as the main academic search engines. It explores the range of VOCs that elicit various stress responses in non-stressed, neighbouring receiver plants in the face of both biotic and abiotic stresses. Understanding how plants can prepare for impending stresses by recognising VOC signals from stressed plants without themselves having to experience the actual stress is a noble endeavour towards the development of crops that are more tolerant against environmental stresses. Furthermore, the identification of specific receptors, transcription factors (TFs), and other regulatory proteins involved in volatile-mediated signalling, as well as the characterisation of the various defence-signalling pathways induced after VOC perception, is necessary for a full appreciation of airborne signalling between plants under stress. The integration of physiological, metabolome, and transcriptome analyses in volatile-mediated signalling and defence priming will be discussed.
2. Plant Volatile Organic Compounds
Plants emit and respond to a wide range of VOCs, which are generally lipophilic and of adequate volatility to be released into the atmosphere from the liquid phase [22]. Up to 10% of the carbon fixed during photosynthesis can be lost through complex volatile plumes [32]. Beyond terpenoids, which constitute the most complex group, other major classes of VOCs include fatty acid catabolites, aromatic compounds, and amino acid derivatives as products of the shikimic acid pathway [17,18,19,20,21,22,23,24]. Volatiles can either be emitted constitutively [33] or induced in response to factors associated with abiotic stress [21,22] or biotic stress [34]. VOCs have relatively high vapour pressure, low molecular weight, and low polarity properties, accounting for their high volatility [35]. The rate of emission from plant tissue into the atmosphere has been suggested to depend on the volatility, solubility, and diffusivity of the particular volatile compound rather than its rate of synthesis or other physiological mechanisms [36]. Nonetheless, the ratio of compounds in a constitutively emitted VOC bouquet is considered to be dependent on species taxonomy and varies greatly within species [37].
VOC Biosynthesis
About 1700 VOCs have been characterised in different plant volatile blends, where they convey information about the plant identity and physiological condition [38]. The biosynthesis of VOCs occurs in the leaf mesophyll tissues, particularly in the palisade mesophyll cells, prior to their release either via inflicted wounds or the stomata on leaves and stems, which regulate emission rates by the opening and closing of their apertures [33,36,39]. The secretory cells of glandular trichomes are also active sites of VOC production [40]. In non-chlorophyll-containing tissues such as flowers and roots, VOC biosynthesis has been reported to occur in specialised crenulated epidermal cells, whose close proximity to the atmosphere or rhizosphere ensures immediate release [32,40]. Roots produce volatiles in epidermal cells and release them into the rhizosphere to mediate below-ground plant–plant interactions, thereby influencing the diversity of soil microbial communities [32,41,42].
Damage to the plant tissues triggers hydrolytic cleavage of complex membrane lipids by lipases to produce free polyunsaturated fatty acids such as the C18 linoleic and linolenic acids [43,44]. Following peroxidation of the fatty acids by lipoxygenase (LOX) enzymes, subsequent cleavage of lipid hydroperoxide by hydroperoxide lyase (HPL) will give rise to a suite of C6 aldehydes, alcohols and esters—collectively known as green leaf volatiles (GLVs) (Figure 1, plastid) [7,8,34,45]. In the presence of allene oxide synthase (AOS), the lipid hydroperoxide is rechannelled to the jasmonic acid (JA) pathway for JA synthesis [44]. Terpenoids form the largest and most diverse group of organic compounds synthesised via two distinct metabolic pathways: the cystolic mevalonic acid (MVA) pathway and the plastidic 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway. All terpenoids are derived from C5 isoprene precursors, isopentenyl pyrophosphate (IPP), and dimethylallyl diphosphate (DMADP), with reactions catalysed by various terpene synthases [46] (Figure 1, plastid). Aromatic compounds (in terms of chemistry rather than any potential aroma) are derived from L-phenylalanine and include products from the chain-shortening of trans-cinnamic acid and structures derived from lignin biosynthesis that form benzenoids [19,47] (Figure 1, plastid). Additionally, amino acid methionine produces 1-aminocyclopropane-1-carboxylic acid (ACC), which is enzymatically oxidised to form ethylene [48] (Figure 1, cytosol). Methanol is also one of the most abundant VOCs found in plants and has been reported to serve as a signalling molecule in intra- and inter-plant communication [49,50]. It is produced through the methylation of the cell wall constituent pectin during plant growth and senescence. The high pH of herbivore salivary secretions is reported to also activate cell-wall pectin methylesterases, leading to copious amounts of methanol being released (Figure 1, cell wall) [40].
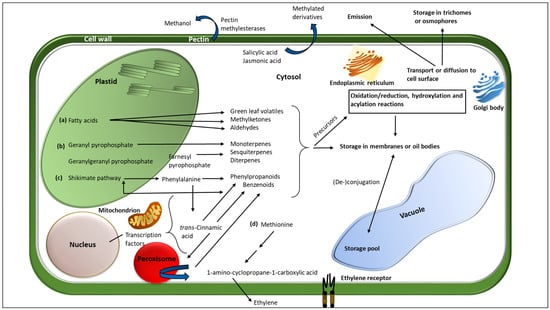
Figure 1.
Biosynthetic sites, storage, and transport of major plant volatile organic compounds. The four major biosynthetic classes, (a) fatty acids, (b) terpenoids, (c) benzenoids, and (d) amino acids, are represented in the figure. Modification reactions including redox reactions, hydroxylation, acylation, and degradative reactions occur mainly in the cell cytosol. Some reactions may also occur in the membrane-bound sub-cellular compartments such as plastids, mitochondria, and endoplasmic reticulum. Inactive forms of the VOCs such as glycosides are stored in the vacuole, ducts, and other extracellular compartments such as trichomes. Transport of the volatiles is by passive diffusion and possibly via vesicular trafficking by the endoplasmic reticulum, golgi apparatus, and the trans-golgi network. (Modified from Pichersky et al. [51]).
3. Plant Volatile Storage, Transport, and Emission
Considering that many of the VOCs produced by plants are likely to be lethal to the plant itself at high concentrations, several self-resistance mechanisms are employed by plants during VOC storage, transport, and emission to avoid self-toxicity (Figure 1). These include vacuolar sequestration, vesicle transport, extracellular excretion, extracellular biosynthesis, and storage of VOCs in cells as inactive non-volatile glycoside precursors [52,53]. Storage of VOCs occurs in various extracellular storage organs, including glandular trichomes, ducts, and laticifers, as well as the sub-cellular membrane-bound compartments such as vacuoles, plastids, mitochondria, and the endoplasmic reticulum (ER) [40,51,54].
Stored VOCs are released after deconjugation of their precursors upon mixing with lytic enzymes after the rupturing of storage organs through mechanical damage [40]. Studies have shown that specific components from herbivore salivary secretions such as β-glucosidases, glucose oxidases, and volicitin act as elicitors of HIPV emission during herbivory to release toxic aglycones of the glycoside conjugates, which have antibiotic and/or antixenotic effects on the herbivores [42,55,56,57,58,59]. When gut regurgitant of cabbage caterpillar Pieris brassicae was used in a study by Mattiacci et al. [60] to treat artificially damaged cabbages, a release of volatile blends similar to those observed from herbivore-damaged plants was noted. Parallel results were observed when β-glucosidase from bitter almonds was used to treat undamaged P. lunatus [61]. Interestingly, when lima bean plants were treated with JA solution, a similar VOC blend was emitted. It was postulated that the lytic enzymes in herbivore saliva hydrolyse cell structures into oligosaccharides and pectin, which in turn act as first signals leading to gene activation and de novo metabolite synthesis via internal signal transduction pathways such as the JA pathway [62,63].
Despite the presumption that VOC movement from the site of synthesis into the atmosphere occurs via passive diffusion, Widhalm et al. [32] questioned this supposition and highlighted the possibility of high barrier resistance in the movement of lipophilic VOCs across the cytosol, plasma membrane, aqueous cell wall, and cuticle. Movement via diffusion alone is likely to be too slow to account for the high emission rates observed during stress responses. Based on Fick’s first law of diffusion, it was determined that VOCs had to accumulate to toxic levels internally before they could be emitted at the observed emission rates, so it has been suggested that more active trafficking mechanisms may be involved [32]. Plausible mechanisms involve vesicular trafficking associated with the endoplasmic reticulum and Golgi apparatus [32,64], soluble carrier proteins [65], plasma-membrane localised transporters such as ATP-Binding Cassette (ABC) transporters [66,67], and small carrier proteins in the cell apoplast, such as lipid transfer proteins (LTPs) [68,69]. From these studies, it can be hypothesised that the same mechanisms that facilitate volatile emission from cells into the atmosphere may also be involved in VOC recognition and uptake [70].
Apart from VOC emissions from ruptured specialised storage structures such as glandular trichomes and ducts, emissions from plants are predominantly via stomata in leaves or directly from the epidermal cells in tissues lacking stomata, such as flower petals and roots. Emission of VOCs via the cuticle is an alternative pathway for some monoterpenoids. This has been calculated to contribute only 10–20% of the total monoterpenoid emissions, and therefore would not account for the high emission rates observed when stomatal conductance is low [71]. Stomata provide a low resistance pathway for volatile emissions, but they may decrease the efflux of VOCs from plants during stomatal closure resulting from various stressors [72,73].
The magnitude of stress-induced VOC emissions in a plant depends on its stress tolerance as well as the severity, timing, and duration of that particular stressor [21]. Unlike storage emissions, de novo emissions are generally more sensitive to stress and have been suggested to be the primary source of emitted isoprenoids [74,75]. Constitutive emissions can be altered through immediate stress responses and acclimation after stress recovery [76,77]. Volatiles including LOX-pathway products [78] and methanol [79] are induced during early stress (mild stress), reflecting signal activation at the membrane level and in cell walls [80]. Later stress responses include emissions of specialised isoprenoids [22]. Induced volatile phytohormones including MeJA, MeSA, and ET mediate stress signal perception, transduction, and propagation, leading to the activation of gene expression of defence-related genes, including enzymes involved in VOC synthesis [21]. The lifetime of these induced VOC emissions is finite following stress exposure. LOX products and MeSA have been shown to last for 15h after ozone exposure [79] and five to seven days under heat stress [81], and isoprenoid emissions continued for two days after herbivory [82].
Physicochemical and physiological factors limit VOC emission rates in plants, with the extent of control varying with the specific volatiles stored in the leaf [36]. Physicochemical constraints affect the emission of the synthesised VOCs to the ambient air by limiting their volatility and diffusion [36]. Leaf anatomy, specifically the presence of specialised VOC storage structures such as glands and resin ducts, has also been shown to influence emission rates [83]. Large diffusion resistances of various VOC storage pools exist between the intercellular spaces and the ambient air as a result of layers of epithelial and sclerenchyma cells lining the storage structures [74], unless rupturing of the pools occurs. Biochemical and molecular control over VOC synthesis in response to physiological factors such as light [84], temperature, drought [75,83], and high carbon dioxide concentration [83,85] has been shown to relate to the availability of immediate VOC precursors as well as on the activity rate of flux-controlling enzymes.
The biosynthesis of signalling VOCs depends on the plastidic pool of intermediate products of photosynthesis, including glyceraldehyde 3-phosphate (G3P) and erythrose 4-phosphate (Ery4P), as well as sufficient supply of phosphoenol pyruvate (PEP) from glycolysis [74] (Figure 2). During mild stress, the photosynthetic machinery is affected such that the carbon assimilation rate is significantly decreased [86]. Emission rates of most VOCs are expected to be reduced under such conditions [84]. However, an increase in emissions after stress exposure has been observed during drought [76,87], heat [88], salinity [89], and ozone stress [90], indicating the acclimation of VOC synthesis under stress conditions and the plant’s ability to maintain high emission rates after stress relief.
Mild water stress conditions, like most abiotic stressors, typically reduce stomatal conductance, thereby reducing intercellular CO2 concentration [86] and increasing leaf temperature because of constrained leaf transpiration [91]. The temperature response of VOC emissions is a function of increased enzyme activity and substrate availability [92], such that an increase in temperature reduces photosynthetic metabolites and energy, subsequently reducing de novo VOC synthesis and emission [93]. Although the reduction in stomatal conductance limits CO2 supply to the Calvin–Benson cycle of photosynthesis, photosynthetic electron transport is not inhibited, as the triggering of photorespiration may effectively supply CO2 and phosphoglycerate (G3P) needed to drive the cycle [21] (Figure 2). Therefore, ATP and NADPH remain available for the reduction in carbon reserves in the form of starch and sugar for isoprenoid synthesis [21]. Typically, sustained moderate or strong rapid drought stress will eventually lead to a significant reduction in VOC emissions, with augmented emissions after rewatering [76,87]. However, prolonged water stress can lead to accelerated leaf senescence and retardation in key terminal enzyme activity, leading to low emission rates during and after stress [84,94,95,96].
Mild heat stress enhances the activities of flux-controlling enzymes involved in the terpenoid synthesis pathway at the expense of carbon fixation enzymes. Subsequently, emission increases as the VOC synthesis pathway becomes more competitive for carbon and photosynthetic electrons than carbon fixation [88,94]. Increases in temperature also affect photorespiration, which can indirectly regulate PEP levels available to form intermediate substrates for VOC biosynthesis [97]. Light intensity affects the amount of the terpenoid precursor G3P available from photosynthesis, as well as the energetic co-factors ATP and NADPH required for its chemical reduction [98].
The diffusion of individual VOCs is influenced by the width of the stomatal opening, the leaf anatomy, and compound molar size [36]. The volatility of a specific VOC is determined by equilibrium partitioning between the intercellular airspaces and the ambient air, which then affect the diffusion gradient [36,74]. Changes in stomatal conductance can differentially affect the emission rates of various VOCs under stress conditions [39]. The emission of methanol [99], linalool [39], acetic acid [100], and acetaldehyde [101] were vulnerable to stomatal conductance, whereas several monoterpenoids such as limonene, trans-β-ocimene [39], isoprene [102], and α-pinene [103] were emitted independently of stomatal regulation. This independence from stomatal conductance has been associated with the large Henry’s Law constant (H; gas/liquid phase partition coefficient) of the monoterpenoids, which implies that they can maintain a high intercellular partial pressure for a given liquid phase concentration. An increase in the diffusion gradient from the intercellular space to the external atmosphere may compensate, at least partially, for the reduced stomatal conductance, in order to control VOC emission rates [39,102,104].
Following an earlier hypothesis that VOC emissions in plants may display hormetic responses to environmental stressors [105], studies involving herbivory [106,107], drought [108,109], CO2 concentration, light, temperature [92,110], and ozone [105,111,112] have also provided evidence that VOC emission is biphasic by time and dose in response to the stressors, thus indicating hormesis [113,114]. Hormesis is a biphasic stress dose response that depicts adaptive responses of organisms to low-dose stresses whereby they can improve their tolerance to severe stress challenges, whereas higher doses of the stresses will have negative effects on organisms [115] (Figure 2). The occurrence of hormesis of VOC emission in plants suggests an evolutionary adaptation that acts to maintain fitness in a changing environment in the context of enhancing intra- and interplant communication, defence priming, protection, and defence functions [114].
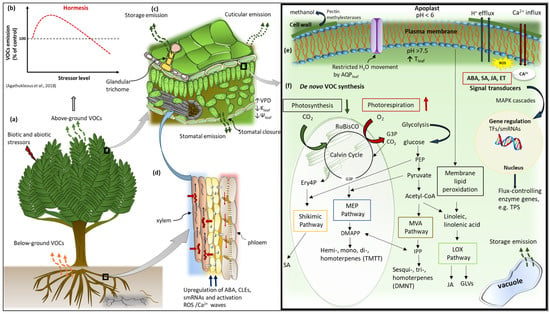
Figure 2.
A simplified schematic of the interactions among major VOC biosynthesis pathways in response to stress. (a) VOC emissions respond to biotic and abiotic stresses from both below and aboveground sources of a plant. (b) A generalised VOC emission response to stress is given, which depicts a hormetic-like biphasic pattern. (c) Increased stress results in decreased intercellular CO2 concentration favouring photorespiration over photosynthesis, which is the source of VOC precursors. (d) Various root-to-shoot signalling molecules that are involved in stress signalling. (e) Membrane depolarisation activities involving ROS accumulation and Ca2+ influx as well as stress-induced phytohormones, e.g., ABA, JA, SA, and ET, activate defence signal transduction pathways that trigger gene expression of flux-controlling enzymes, e.g., TPS genes. Stressors affecting leaf temperature (Tleaf) influence the activity of the enzymes. (f) De novo VOC synthesis in the chloroplast and cytosol involves VOC precursors such as G3P (for the MEP pathway) and Ery4P (for the shikimic pathway), PEP from glycolysis, as well as its downstream metabolites pyruvate and acetyl-CoA that are involved in the Shikimic, MVA, and LOX biosynthesis pathways. Note that the sizes of organelles are not drawn to scale. Abbreviations: ABA, abscisic acid; JA, jasmonic acid; SA—salicylic acid; ET, ethylene; TPS, terpene synthase; G3P, glyceraldehyde 3-phosphate; MEP, 2-C-methyl-d-erythritol 4-phosphate; Ery4P, erythrose 4-phosphate; PEP, phosphoenol pyruvate; MVA, mevalonic acid; LOX, lipoxygenase; CLE25, CLAVATA3/EMBRYO-SURROUNDING REGION-RELATED 25; smRNA, small RNAs [114].
4. Volatile Uptake, Perception, and Signalling
The adsorption of VOCs on the surface of the leaves as well as their solubilisation and enzymatic detoxification within the leaves are what drive the uptake, deposition, and storage of VOCs [116]. VOCs’ chemical reactivity, physicochemical properties, and involvement in leaf metabolism all play a role in how much they react in leaves and can potentially be absorbed by vegetation [116]. Though stomatal uptake is a significant sink for VOCs in leaves, VOCs can adsorb directly onto the cuticle as a result of gas-phase deposition [117,118,119,120]. The cuticle opens up as a route to and from the leaf tissues for organic compounds with substantial lipophilicity and low vapour pressure in addition to the stomatal route [118]. The degree of stomatal conductance along with the combination of VOC physicochemical properties will determine whether VOCs are released or absorbed through the cuticle, the stomatal pores, or both.
The direction of the concentration gradient between the leaf intercellular air space and the surrounding air determines whether a certain VOC is released or absorbed at any particular time. At very low internal concentrations, the uptake rate is controlled essentially by stomatal conductance as well as the ambient concentration of the VOC [116]. A more significant uptake of monoterpenes was observed when the ambient air concentration was higher versus when it was lower [121]. The thickness and conductivity of the leaf boundary layer also have a bearing on VOC foliar uptake and deposition, which itself is influenced by leaf morphology and wind speed [119]. Higher uptake rates have been noted in plants with thinner leaves and greater surface area per unit dry mass [121]. Overall, it is anticipated that temperature, VOC and cuticle physicochemical properties, and cuticular structure would all play major roles in regulating the deposition and release of compounds from leaf surfaces [116].
Once deposited on the plant’s surface, stress-induced VOCs may act as resistance elicitors perceivable by the plant’s evolved and highly sophisticated surveillance system. Subsequently, unique molecular mechanisms of several defence-signalling pathways are initiated [122,123], thereby conferring some degree of immunity in distal regions of the same plant or a neighbouring plant, thus acting as long-distance damage-associated molecular patterns (DAMPs) [124,125]. Elicitation by the volatile DAMPs ranges from early signalling cascades (including Ca2+ influxes, oxidative burst, and MAPK activation) to phenotypic defence responses [126]. Methanol emissions in plants, just like GLVs, are reliably associated with injury and have been suggested to act as wound signals in plants [49]. Studies have shown that methanol activated oxidative burst and MAPK cascades in Festuca arundinacea (tall fescue) grass and tomato [127]. Isoprene was shown in Arabidopsis to induce signal transduction networks associated with stress response and plant growth regulators, such as JA, SA, and ET, which promote defence [128,129].
Although the molecular basis for VOC perception and signalling is vaguely understood, it is likely that VOC signals are first perceived at the membranes, making the transmembrane pathway a more plausible route for signal generation and transduction. On the other hand, a plant’s response could be simply evoked by volatiles that might be deposited on the plasma membrane due to their lipophilic or amphiphilic nature, which could probably distort the membrane organisation [130]. Subsequent VOC metabolisation or VOC recognition in the cytoplasm of those VOCs that manage to pass through the membrane is also a possibility, as observed in studies related to the uptake of 13C- labelled GLVs in plants [131,132]. Unlike most VOCs, ethylene perception and signal transduction have been well-established through extensive studies using Arabidopsis [133,134,135,136,137,138,139,140]. First suggested by Burg and Burg [133], this gaseous phytohormone binds reversibly to its receptor via a protein-bound transition metal co-factor such as copper. Compelling evidence from biochemical data has shown that the ethylene signal is perceived at the membrane of the endoplasmic reticulum by a family of receptors, including ETHYLENE RESPONSE1 (ETR1), ETHYLENE RESPONSE SENSOR (ERS1), ETR2, ETHYLENE INSENSITIVE 4 (EIN4), and (ERS2), which repress ethylene through the negative regulator CONSTITUTIVE RESPONSE1 (CTR1). Downstream and secondary ethylene responsive gene expression are positively regulated by EIN2 and EIN3 [137,139,141,142,143].
Efforts to understand the molecular aspects of VOC perception and signalling have involved studies in exogenous applications of VOCs to plants. In these studies, VOC-binding proteins involved in gene transcriptional regulation have been identified [70]. A transcriptional co-repressor, TOPLESS-like protein (TPL), was shown in tobacco to bind to β-caryophyllene, a sesquiterpene, to initiate the expression of stress response gene NtOsmotin, a pathogenesis-related protein [70]. The study suggested significant specificity of the receptors for individual VOCs as well as selectivity in inducing gene expression in plants. Though the involvement of membrane receptors in the VOC-sensing mechanism cannot be ruled out, it is clear that some nuclear proteins may act as VOC receptors in plants.
Documented studies on mechanisms by which plants perceive various stimuli such as light [144,145], sound [146,147,148,149], and touch [150] have shown uncanny similarities with those exhibited in mammalian systems. It is also probable that the mechanisms involved in how plants “smell” odours follow the same paradigm as that observed in mammals—excluding the existence of a nervous system. In mammals, the olfactory system has been described as discriminatory, in that each volatile compound is perceived to have a distinct odour, and even subtle changes in odourant structure or concentration can potentially alter the VOC’s “code”, thereby shifting the perception of its odour quality [151]. A similar discriminatory behaviour has also been observed in the parasitic dodder plant (C. pentagona), which locates its host plants based on its perception of their unique volatile blends [152] and is repelled by specific VOC blends of its non-hosts. In mammals, a combinatorial receptor coding scheme is used by the olfactory system, where specific combinations of odourant receptors (ORs) recognise specific volatiles. Studies by Malnic et al. [151] have shown that even subtle alterations in the volatile compound or changes in its concentration may result in changes in its “code”, thereby affecting the perceived quality of the odour. Whether plants follow a similar model for their VOC receptors remains unclear, but it is worth considering in future endeavours to understand the mechanisms of volatile perception and signalling.
5. Volatile-Mediated Intra- and Inter-Plant Communication
Plants have evolved unique communication mechanisms between various organs for the enhancement of their development and providing resistance to stress. Upon perception of an environmental stress, a plant will activate several long-distance signals to trigger systemic stress responses with the aid of the plant’s vasculature (Figure 3). Environmental information can be conveyed from the roots to the aerial parts of the plant, and vice-versa, via well-orchestrated cell-to-cell and/or long-distance signals. This root-to-shoot/shoot-to-root signalling is crucial in the activation of defence responses in the target tissues, which allow for plant adaptation to both biotic and abiotic stresses at the whole-plant level. These possibly interconnected signals, which include electrical, hydraulic, and chemical signals, differ in their mode of action as well as propagation speed [153,154]. As a consequence of multiple stresses, these signals tend to overlap in their presence and speed, making it difficult to associate a particular response to a specific stress stimulus [154,155].
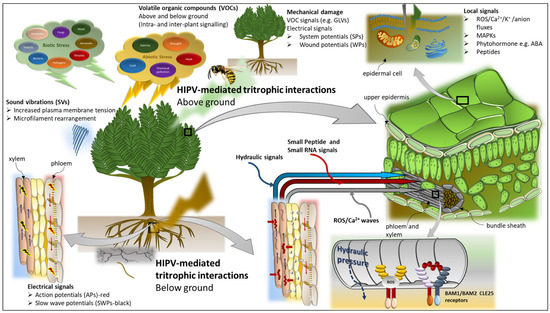
Figure 3.
Overview of long distance signals in plants. Long-distance signals include electrical, hydraulic, and chemical signals. Electrical signals found in plants include: slow wave potentials (SWPs) propagated in the functional xylem; action potentials (APs) initiated in the phloem; system potentials (SPs) propagated in the apoplast following mechanical perturbations or wounding; and wound potentials (WPs) through changes in cell turgor leading to plasma membrane depolarisation. Hydraulic signals involve changes in turgor pressure, mass flow, and pressure waves. SWPs are closely linked to hydraulic signals as a result of cavitation events or changes in turgor [154,155,156]. Chemical signals can be classified as: (i) secondary messengers including reactive oxygen species (ROS), inositol triphosphate (IP), Ca2+, K+, and anion fluxes; (ii) signalling cascade chemicals including mitogen-activated protein kinases (MAPKs); and (iii) chemical response signals including phytohormones and volatile organic compounds (VOCs). Herbivore-induced plant volatiles (HIPVs) have been well-documented involving both above- and below-ground biocontrol of herbivores by insect predators and parasitoids, such as the yellow jacket wasp and the parasitic wasp, respectively [157,158]. Recent advanced analyses have elucidated the role of various mobile molecules including small peptides [155,159,160] and small RNAs (small interfering RNA and micro RNAs) in long-distance systemic signalling [159,161,162,163]. Stomatal closure is one of the initial responses to osmotic stress to prevent hydraulic failure [164] and is regulated by abscisic acid (ABA) [165,166]. During water stress, the root-to-shoot communication is mediated by a small mobile root-derived peptide, CLAVATA3/EMBRYO-SURROUNDING REGION-RELATED 25 (CLE25), which then triggers ABA accumulation in the leaves via BARELY ANY MERISTEM1 (BAM1) and BAM2 receptors in the leaf vascular bundles [159]. Sound vibrations (SVs) or acoustic signals produced by both biotic and abiotic stresses act as stimuli capable of priming plants for future stress challenges and as long-range signals that activate plant-signalling pathways [148]. Leaf vibrations caused by herbivore chewing [148,149] and the “clicking” sound produced by the collapsing water column (cavitation) in the xylem [167] have been demonstrated to trigger systemic responses in distal regions of the plant. The perturbation of the plasma membrane by SVs is characterised by a sequence of molecular episodes including cell wall modification and microfilament rearrangement in plant cells [147,149,168].
Unlike most chemical signals, VOCs are long-distance signals that can induce systemic stress responses in distant plant organs that have little or no distinct vascular connection with parts of the stressed plant [169,170]. The role of volatiles in intra- and inter-specific communication, their chemical diversity, and mode of action is well-documented [20,171,172,173,174,175,176,177,178,179]. They also serve as signalling molecules that are involved in intra- and inter-plant communication, providing a fitness benefit to the “emitter” and modifying behaviour in neighbouring “receiver” plants [17].
Plants are reported to be able to distinguish between the different volatile blends [152]. As individual VOCs are not species-specific, plants formulate unique messages by adjusting the individual components of a volatile bouquet [180], which in turn induce specific responses in the receiver. For instance, the parasitic dodder plant (Cuscuta pentagonia) uses specific VOC blends as foraging cues to locate its host plants [152]. The presence of (Z)-3-hexenyl acetate in the constitutive VOC blend of wheat, a non-host to the dodder plant, was reported to be absent in tomato, suggesting its role as a repellent to the dodder [152].
Volatile blend composition has a bearing on the specificity of volatile-mediated plant–plant communication in congeneric species such that “eavesdropping” by other species can be avoided [27,180,181]. In such cases, competing plant heterospecies may not be able to “decode” the chemical stress signals, which may otherwise have served to prepare them for a potential threat [179,182].
The allelopathic effect of VOCs, particularly terpenoids, has also been employed as part of the plant’s defence against its competing neighbours by remotely suppressing the competitors’ growth [183]. The cellular mode of action that underlies the allelopathic effect of terpenoids, such as eucalyptol, β-myrcene, camphor, camphene, menthol, and α- and β-pinene, occurs via cytoskeletal disruption, with microtubules being the primary targets [184,185,186,187,188]. Essential oils containing these terpenoids have been used for weed control as well as biological control of pests [188,189], facilitated by the specific nature of allelopathic compounds [190,191,192].
Under biotic stress, plants respond to insect and pathogen attacks by emitting herbivore-induced plant volatiles (HIPVs) and pathogen-induced volatiles (PIPVs), respectively [30,193,194,195,196,197,198]. Such volatiles may be exploited by neighbouring hetero- or con-specifics to either benefit or compromise the fitness of the emitter plant. The HIPVs/PIPVs can either benefit the emitter plant by attracting pollinators and natural enemies of its herbivores, or they can negatively affect the plant by acting as foraging cues to parasitic plants and as host location cues by herbivores [40,152]. Interestingly, in some HIPV-mediated plant interactions, receiver plants have been shown to adsorb VOCs from their stressed neighbours and process them into their lethal derivatives, which are detrimental to herbivore growth and survival [12]. In some instances, the receiver plant will re-release VOCs that have been adsorbed from the source plant against insect pests, in what is known as associational resistance (AR) [199].
Abiotic factors such as high light intensity [200], nutrient availability [201], salinity [11], temperature [29,72,73,104,202], wind and UV radiation [203,204], ozone exposure [205], and mechanical damage [5] have also been shown to induce VOC emissions in plants. In response to abiotic stress, VOCs offer protection against high-temperature exposure in addition to improving the thermo-tolerance of photosynthetic tissues [22,206,207,208,209,210]. To alleviate the negative effects of oxidative stress induced in response to environmental stressors, plant VOCs can stabilise and protect cellular membranes by quenching ROS species or by altering ROS signalling [211,212]. The role and mechanism of action of stress-induced VOCs in curbing the detrimental effects of biotic and abiotic stresses is summarised in Figure 4.
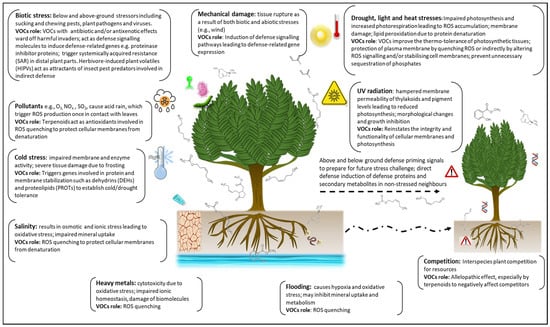
Figure 4.
Role and mechanism of action of stress-induced volatile organic compounds (VOCs). In response to both biotic and abiotic stresses, VOCs induce various defence-signalling pathways and are involved in intra- and inter-plant priming against future stress challenges. The antixenotic and antibiotic effects of VOCs facilitate direct and indirect defence responses against biotic stressors. Both abiotic and biotic stress induce the production of reactive oxygen species (ROS) whose negative oxidative effects are curbed by VOCs through ROS quenching and cell membrane stabilisation. Thermo-tolerance of photosynthetic tissue is also facilitated by some VOCs under high temperature conditions. (Modified from Vickers et al. [208]).
The potential role of stress-induced VOCs in priming neighbouring plants for enhanced stress tolerance has been explored [5,6,7,8,9,10,11,12,13,27,29,30,213], with only a few studies focusing on abiotic stress. Priming is an adaptive strategy characterised by an enhanced responsiveness of defence mechanisms to stress as a result of a previous stress challenges. This involves subtle physiological, molecular, and epigenetic alterations in the plant leading to increased stress resistance and/or tolerance [214]. GLVs comprise an important chemical group in the HIPV/PIPV plume emitted in response to mechanical damage, herbivory, or pathogen attack. The priming effect of GLVs has been characterised by an increase in defence-related gene expression and an augmented production of secondary metabolites with antixenotic or antibiotic effects on the biotic stressors [20,193,215,216]. In studies that involved priming for salinity stress, a significant increase in salt tolerance was observed in Arabidopsis and lima beans (Phaseolus lunatus) plants, independent of ABA and salinity stress-signalling pathways) [11,13]. An increase in photosynthetic rate and relative growth rate was observed in the plants previously exposed to VOCs from salt-stressed plants. In a similar study by Landi et al. [28], enhanced photosynthetic activity and reproductive success was also observed in Ocimum basilicum. In this particular study, the observed VOC profile differences of the emitter and the receiver plants were thought to suggest the ability of the receiver plants to propagate the volatile signal to their own neighbours [28]. Beyond these examples, Table 1 gives a brief account of a selection of studies on VOC-mediated priming in plant–plant communication under biotic and abiotic stresses.

Table 1.
Volatile-mediated priming in plant–plant communication under biotic and abiotic stress conditions.
Apart from the GLVs that are released in response to herbivory and mechanical damage, terpenoids including monoterpenes, sesquiterpenes, and hemiterpenes have also been identified and associated with defence priming in receiver plants. Typical VOCs include monoterpenes β-ocimene, 1,8-cineole, linalool, α- and β-pinene; sesquiterpenes β-caryophyllene, (E)-α-bergamotene and (E)-β-farnesene; hemiterpenes (E)-4,8-dimethylnona-1,3,7-triene (DMNT), and 4,8,12-trimethyltrideca-1,3,7,11-tetraene (TMTT). Phenylpropenes including eugenol and methyl eugenol were detected in salt-stressed plant emissions.
6. Priming: The Cost of Defence
The concept of priming, an indication of hormesis, has gained interest in plant stress physiology due to its feasibility and efficiency, as well as being a cost- and resource-effective tool [8,214]. Priming has been associated with induced resistance (IR) whereby a plant is sensitised for a more robust basal defence response to subsequent attacks [217]. This immunological memory can last throughout the life cycle of a plant and is possibly heritable [214]. IR thus puts the plant in an alert state for future stress challenges and confers broad-spectrum protection against various environmental stresses [214].
To ascertain that effective defence priming has been achieved, more robust defence responses with a low fitness cost must be observed. In addition, a superior response in the presence of the actual stress challenge must be evident to imply that the plant has a memory of the stress stimulus [218]. In the context of priming using VOCs, a receiver plant must be able to associate a particular volatile plume to a specific stress in order to affect corresponding and specific defence responses. The priming of defence responses in receiver plants due to volatile signals may depend on the plant’s receptiveness to different concentrations of specific volatile blends or individual compounds, the duration of exposure, and the effective distance from the source plant before the VOC bouquet is diluted in the air to inactive concentrations [42,180,219,220]. In the latter case, as the VOCs are released into the atmosphere, eddy currents dilute them to concentrations that are adequate to prime neighbouring plants for impending stresses [217,221,222,223]. Since the concentrations of airborne VOC signals required to induce priming are generally lower than those in which a full defence response is elicited [223], low fitness costs are likely to be incurred by the emitter plants. Although it has been presumed that priming does not alter plant metabolic pathways or initiate defence-related gene expression until actual exposure to the stress [11,218,221,224,225], a study by Balmer et al. [226] has shown that direct changes for enhanced tolerance can in fact be triggered with negligible fitness costs in the receiver plants. Priming may, therefore, offer an ecological fitness benefit to plants that are able to launch faster and stronger defence responses when the impending stress arrives [31,227].
Besides the ability to prime or induce defence-related responses in receiver plants, VOC exposure may have the potential to affect nutrient uptake and photosynthesis, thereby modulating primary and secondary metabolisms. This would subsequently lead to a change in the overall growth and development of the plants [228], as observed in VOC-exposed broad bean plants that reduced their photosynthetic rates in response to VOCs whilst they achieved increased salt resilience [13]. A reduction in photosynthesis was also shown in sweet basil plants only after subsequent exposure to salt stress, and they were observed to have early flowering, a greater seed set, and early senescence [28]. A downside of priming has also been observed in studies reporting the downregulation of specific resistance pathways, potentially leading to a reduced defence ability to unrelated stressors that the plant may not have been primed for [214].
This has been observed in transgenerational priming, in which inherited defence hormone crosstalk seemed to compromise the primed progeny’s defence response against stresses the parents had not been primed for [214]. Using the study by Luna et al. [229] as an example, it was observed that JA-dependent defence responses were compromised in salicylic acid (SA)-primed Arabidopsis progeny, leading to significant susceptibility to necrotrophic fungi [229]. This was accounted for by the probable existence of crosstalk between the JA and SA pathways.
Continued metabolic investment in the primed state in the absence of stress has a low fitness cost to a plant, but it may compromise growth and yield [214,230]. In a study carried out by Crisp et al. [230], the growth and yield of Arabidopsis plants previously primed by exposure to drought or high light intensity was significantly reduced in one group of plants, whereas another was observed to recover to their pre-stressed state after rewatering. The latter group was deemed to have “forgotten” about the primed state in contrast to the former, which maintained the hardened/primed state whereby defence responses remained activated. Considering that stresses are usually transitory and often repetitive in a given environment, plants that are able to balance the formation of stress memories for protection against future stress challenges with resetting for normal growth and yield may have developed an evolutionary adaptation strategy that allows them to compete effectively against their con- and hetero-specifics [230]. Where priming is observed to be maladaptive in transgenerational inheritance, plants would be better off resetting their memory and “forgetting” previous stresses to avoid compromising their growth and survival [230].
Overall, priming offers an opportunity for the development and implementation of sustainable crop protection strategies for Smart Agriculture [231]. Whereas studies on volatile-mediated priming of defence responses against biotic stresses in plants have been well-documented [5,6,8,27,31,169,172,222,232], they exist only sparsely for abiotic stresses [11,13,233]. Therefore, the need to explore opportunities that VOC-mediated priming could offer against the various abiotic stressors is greatly emphasised.
7. Exploiting VOC-Mediated Signalling for Future Sustainable Agriculture
7.1. The Ecological Aspects: A Community Perspective
In nature, a plant may respond to a myriad of signals from its neighbours—above and below ground—and/or under different environmental conditions [234]. Despite their sessile nature, plants are able to modulate their phenotype in response to a plethora of environmental stresses and to interactions with various members of their associated community. In these communities, plants are unlikely to experience single stress factors sequentially but rather multiple stresses simultaneously [157,234] while also interacting with both con- and hetero-species [235]. The co-occurrence of multiple stresses may have some additive effects, although in some cases, only one stress takes precedence. This means that plant response due to several stress combinations cannot be accurately extrapolated based on responses to individual stress factors [236], suggesting an interaction effect of the combined stresses on plant response. This too will result in a diverse mix of VOCs being emitted by the plant. In the context of VOC-mediated interactions, the cocktail of stress-induced VOCs emitted by the individual plants contributes significantly towards the complex transcriptional responses observed in the neighbouring plants [237]. It is plausible that receiver plants acquire a stronger basal resistance resulting from the stress-specific VOC blend, rendering them more resilient and fit in the face of diverse future stressors as they remain exposed to the different volatile blends over time.
Studies on volatile-mediated plant–plant communication have indicated its ecological and functional relevance, although most work has understandably been carried out in controlled environments such as glasshouses and growth chambers due to the complexities of building a robust experimental design as well as incorporating adequate controls [238]. Evidence from studies on the volatile-mediated priming of non-stressed plants under various environmental conditions inspires the exploration of further opportunities to induce tolerance in crops against a broad spectrum of stresses. However, this should include moving on from studies carried out under controlled environmental conditions to those that better represent that which occurs in the natural environment. Normal agricultural management activities such as pruning, hedging, and crop harvesting, as well as key phenological events such as flowering and fruit set, are also potential factors that induce VOC emission in plants. The questions of how the neighbouring con- and hetero-specifics respond to the resultant volatile bouquet released by the multi-stressed plant and whether they will be primed for broader tolerance require exploration. Additional considerations include the examination of the effect of wind eddies on the airborne signals, because these may dilute the volatile bouquet to concentrations that may not effectively prime the intended receiver plants as mentioned earlier; the effective distance between the source and receiver plants in different agricultural systems; and diurnal changes in humidity and temperature, which will impact the volatility of the VOCs. These considerations will be relevant in ascertaining the feasibility of plant–plant VOC priming for enhanced stress resilience.
7.2. Technologies for Exploring Plant VOC Signalling Interactions
Attempting to address both the mechanistic and functional-level questions concerning inter-plant VOC signalling, several advances in the fields of metabolomics, volatilomics, transcriptomics, and bioinformatics offer an opportunity for a more holistic understanding of VOC-induced defence responses in plants. Significant progress in the development of VOC collection and analysis technologies that are sensitive and relatively inexpensive has been observed over the past decade [239,240,241]. These advances include gas chromatography-mass spectrometry (GC-MS) and static and dynamic headspace (SHS, DHS) techniques.
The headspace VOC sampling methods involve non-solvent sorptive extraction techniques, which include headspace solid phase microextraction (HS-SPME) and purge-and-trap headspace (P&T-HS) for static and dynamic extraction, respectively [239,242,243], using collection chambers (refer to Figure 4 in the review by Tholl et al. [239]). Live plants can be analysed with such techniques to provide a more representative volatile profile than with traditional methods such as solvent extraction or steam distillation. It is important, however, to consider changes that may occur in the plant’s micro-environment while in the headspace collection chambers. Increases in humidity [39] and temperature, as well as reduced light intensity in the chambers [244,245] are likely to affect transpiration rates and, subsequently, VOC composition and emission rates. Additionally, given the potential for the scalping of VOCs by hydrophobic materials, their adsorption by the lining of collection chambers or sampling bags may require the use of non-reactive materials such as fluorinated ethylene propylene (FEP) or polytetrafluoroethylene (PTFE), which have been shown to be effective in plant VOC sampling [210,246,247].
Innovations that allow for VOC sampling under natural environments in which plants grow have emerged in the past decade. Trends include direct analysis in real time (DART), which is an ionisation technology for rapid non-contact analyte detection on solid or liquid surfaces [248]. Portable devices suitable for both laboratory and field sampling include a portable gas chromatograph (GC) with photoionisation detector (GC-PID) such as the FROG-4000 VOC analyser. Volatile analysis using these devices can be undertaken in water, soil, and air [249]. Micro-versions of these detectors, μGC–PID, are currently being developed and have been demonstrated to facilitate rapid vapour analysis in the field [250]. The FlavourSpec is another portable analytical device consisting of GC coupled to an ion mobility spectrometer (IMS), which can analyse headspace volatiles of solid and liquid samples without prior treatment [243,251]. Additionally, another very cost-effective field sampling technique involving minimum headspace and organism manipulation involves the use of polydimethylsiloxane (PDMS) in the form of silicone tubing. This technique involves using small pieces of the tubing for headspace sampling, combined with thermal desorption (TD)-GC-MS analysis [252]. GC-independent methods such as proton-transfer reaction-mass spectrometry (PTR-MS) have also been relevant in both field and laboratory real-time VOC monitoring [253]. A high-resolution version of the PTR-MS uses a time-of-flight mass spectrometer (PTR-TOF-MS) that is capable of rapid measurement of VOCs at ultra-low concentrations with a resolution of isobaric ions [254]. A brief highlight of the various practical and novel technologies being employed in the metabolite profiling of VOCs is given in this review (Table 2); however, more detailed and extensive reviews have been documented.

Table 2.
Practical technologies used in plant volatile metabolite profiling.
With the advent of high-performance computing and statistical analysis packages such as R, there is an opportunity to mine and analyse the data collected from one or a combination of the above-mentioned methodologies to unveil relationships between VOC signals and plant physiology that would allow for a systems-based understanding of plant communication. Furthermore, data fusion approaches [255] that combine real-time VOC signalling measurements, e.g., PTR-MS, with high temporal resolution phenotyping data such as plant growth, spectral responses, and water and nutrient status are available. Modern “omics” methods such as metabolomics, volatilomics, proteomics, and transcriptomics methods all generate vast quantities of data requiring advanced analytics such as chemometrics, machine learning, and deep learning techniques for statistical analyses and prediction of plant responses to biotic and abiotic stresses. For example, Principal Component Analysis (PCA) is a multivariate statistical method to group variables and highlight their relative contributions to other variables based on variance. In contrast, machine learning algorithms based on supervised learning are available, which provide extensive and reliable datasets and can offer significant advantages over traditional chemometrics methods with regards to the prediction of VOC responses,; Random Forest is one example [256,257].
Though the use of physiological data in the evaluation of VOC signalling is relevant under both biotic and abiotic stresses, the impact of the signalling may be overlooked or assumed to be absent if the parameters selected in the study are influenced by several other environmental factors. For instance, in trying to investigate VOC signalling under abiotic stresses such as drought, it may be reasonable to consider observing changes in transpiration rate in the receiver plant. However, transpiration rate may not give a clear indication of signalling due to factors such as glasshouse lighting and stomatal oscillations [258], which depend on diurnal variations. Whether or not the receiver plant is responding to VOCs or to its inherent circadian rhythm may not be well-defined. Other physiological parameters such as ROS scavenging, stimulated root growth, and xylem hydraulic changes may be more difficult to measure directly. A more reliable option may be to investigate the subtle changes that could occur at the transcript level, which would offer an opportunity to understand the immediate responses of the receiver plants to VOC perception. Targeted gene expression using quantitative PCR (qPCR) is relevant in this regard and has been employed in several VOC-signalling studies under biotic [31,259] and abiotic stresses [11]. However, only a few putative genes can be investigated at a time, and those may not represent all the genes involved in VOC signalling. Investigating the gene expression of rate-limiting genes involved in GLV and terpenoid biosynthesis, as well as the various phytohormone signalling pathways, may be important for understanding VOC signalling in plants.
As opposed to qPCR analysis, which determines gene expression levels of a few pre-selected gene transcripts, next generation sequencing (NGS) technologies such as RNA-seq provide in-depth knowledge of a myriad of genes involved in various metabolic pathways and the complex interactions of plants during stress [260]. Gene expression profiling and transcriptome analyses are functional genomics approaches that have facilitated the identification of two broad classes of genes associated with stress responses. One category encompasses genes encoding the proteins involved with cellular osmotic homeostasis and stress protection, and the other consists of TFs and kinases involved in the regulation of stress transduction and gene expression in conjunction with various phytohormones [261]. NGS reveals transcript information, allowing for the identification of unique genes and single nucleotide variants, as well as allele-specific gene expression [261]. The platform offers an opportunity to investigate possible VOC protein receptors, TFs, and regulatory proteins involved in volatile signalling under various environmental stresses, which could prove invaluable in future studies.
To complement gene expression studies, the use of genetically modified plants possessing the ability to either emit or perceive specific components of the wild-type volatile bouquet will be useful in understanding plant–plant VOC signalling [222]. The use of mutant “deaf” receiver plants with functionally impaired VOC receptors for specific volatile substances [141] and “mute” emitter lines with silenced genes of enzymes needed for VOC synthesis [262,263,264,265,266,267] may be useful in investigating the possible receptors involved in VOC signalling. Confirmation studies involving the use of synthetic analogues of the VOCs could then be undertaken in conjunction with the “mute” emitter plant to ascertain whether the response by receiver is restored after a specific VOC has been made absent in the “mute” emitter.
Efforts directed towards understanding VOC signalling pathways and defence priming can greatly benefit from the integration of physiological, metabolome, proteome, and transcriptome analyses (Figure 5), aided by the technological advancements in those individual fields witnessed over the past decade.
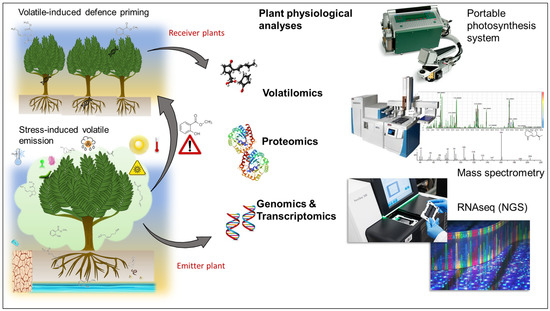
Figure 5.
Integrating physiological, metabolome, proteome, and transcriptome analyses in understanding plant volatile signalling.
8. Conclusions and Future Perspectives
The increasing burden of climate change has exacerbated the effects of both biotic and abiotic stresses, thus posing a threat to global agricultural production. The employment of VOCs to enhance plant resilience to stress offers an eco-sustainable strategy for Smart Agricultural practices. The wider application of both natural and synthetic VOCs in most agricultural systems has focused on controlling insect pests by the VOCs acting as herbivore repellents or as attractants of their natural enemies, or on combining volatiles and pheromones for tailored herbivore trapping. The role of plant volatiles in intra- and inter-specific communication, the chemical diversity of VOCs, and their mode of action have been relatively well-documented. Studies have indicated the ecological and functional relevance of VOC-mediated communication, including their priming effect in non-stressed neighbouring plants for augmented defence responses following future stress challenges. Interestingly, most studies to date have mainly focused on biotic stress, with only a minority addressing several abiotic stresses. Moreover, information on the relevant signalling pathways and mechanisms involved in volatile-mediated plant–plant interactions remains nascent and has been sparsely studied in stress physiology studies.
Efforts directed towards metabolite VOC profiling of plant volatile blends emitted under various environmental stresses, the identification of the specific VOC receptors, and TFs and other regulatory proteins involved in the signalling pathways are still required. Future endeavours will benefit greatly from technological advances in the fields of plant physiology, metabolomics, transcriptomics, and bioinformatics that have emerged over the past decade. Indeed, the integration of physiological, metabolomic, and transcriptomic analyses in volatile-mediated signalling and defence priming studies will contribute to achieving a more holistic understanding of VOC-induced defence responses in plants under both biotic and abiotic stresses. As most studies have been carried out under controlled environments such as glasshouses and growth chambers, it would be worthwhile to integrate such studies with those that consider more realistic environments in which plants grow. Plants in their natural environments are part of a community of constantly interacting hetero- and conspecifics and are prone to multiple stresses occurring simultaneously. Investigations into the responses by neighbouring plants to the resultant VOC emissions will provide more conclusive results regarding the feasibility of implementing VOC-mediated priming not only against biotic stresses, but also against various abiotic stresses in agricultural systems. If plants are able to manipulate responses in their neighbours against biotic stresses via VOCs, it is plausible that they could also “warn” them against impending abiotic stresses. The potential of integrating plant–plant VOC-mediated defence priming into existing plant protection strategies against a myriad of abiotic stresses appears convincing and would potentially assist in overcoming major yield-limiting factors that contribute to over 70% of crop yield losses worldwide. As indicated in this review, however, additional studies, are still required in the face of growing pressures on ecosystems and the need to drastically improve the sustainability of agricultural production.
Author Contributions
Author contributions are as follows: Conceptualisation, investigation, visualisation, writing—original draft preparation, J.M.; supervision, writing—review and editing, D.W.J., U.B., S.R., S.D.T. and V.P; Conceptualisation, project administration, funding acquisition, writing—review and editing, V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Australian Research Council Training Centre for Innovative Wine Production (www.ARCwinecentre.org.au; project number IC170100008), funded by the Australian Government with additional support from Wine Australia, Waite Research Institute and industry partners. The University of Adelaide is a member of the Wine Innovation Cluster. J.M. recognises the support of a Wine Australia supplementary PhD scholarship (WA Ph2104).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Mantri, N.; Patade, V.; Penna, S.; Ford, R.; Pang, E. Abiotic stress responses in plants—Present and future. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Springer: New York, NY, USA, 2012; pp. 1–19. [Google Scholar]
- IPCC. Climate change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; p. 1132.
- Bohnert, H.J.; Gong, Q.; Li, P.; Ma, S. Unraveling abiotic stress tolerance mechanisms--getting genomics going. Curr. Opin. Plant Biol. 2006, 9, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Baldwin, I.T.; Baxter, K.J.; Laue, G.; Felton, G.W. Communication between plants: Induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 2000, 125, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Thiessen, S.; Dolch, R.; Boland, W. Herbivory, induced resistance, and interplant signal transfer in Alnus glutinosa. Biochem. Syst. Ecol. 2001, 29, 1025–1047. [Google Scholar] [CrossRef]
- Arimura, G.-I.; Ozawa, R.; Nishioka, T.; Boland, W.; Koch, T.; Kühnemann, F.; Takabayashi, J. Herbivore-induced volatiles induce the emission of ethylene in neighboring lima bean plants. Plant J. 2002, 29, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne signals prime plants against insect herbivore attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781. [Google Scholar] [CrossRef]
- Ton, J.; D’Alessandro, M.; Jourdie, V.; Jakab, G.; Karlen, D.; Held, M.; Mauch-Mani, B.; Turlings, T.C.J. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 2007, 49, 16–26. [Google Scholar] [CrossRef]
- Kessler, A.; Halitschke, R. Specificity and complexity: The impact of herbivore-induced plant responses on arthropod community structure. Curr. Opin. Plant 2007, 10, 409–414. [Google Scholar] [CrossRef]
- Lee, K.; Seo, P.J. Airborne signals from salt-stressed Arabidopsis plants trigger salinity tolerance in neighboring plants. Plant Signal. Behav. 2014, 9, e28392. [Google Scholar] [CrossRef]
- Sugimoto, K.; Matsui, K.; Iijima, Y.; Akakabe, Y.; Muramoto, S.; Ozawa, R.; Uefune, M.; Sasaki, R.; Alamgir, K.M.; Akitake, S.; et al. Intake and transformation to a glycoside of (Z)-3-hexenol from infested neighbors reveals a mode of plant odor reception and defense. Proc. Natl. Acad. Sci. USA 2014, 111, 7144–7149. [Google Scholar] [CrossRef]
- Caparrotta, S.; Boni, S.; Taiti, C.; Palm, E.; Mancuso, S.; Pandolfi, C. Induction of priming by salt stress in neighbouring plants. Environ. Exp. Bot. 2018, 147, 261–270. [Google Scholar] [CrossRef]
- Zhang, J.; Nguyen, H.T.; Blum, A. Genetic analysis of osmotic adjustment in crop plants. J. Exp. Bot. 1999, 50, 291–302. [Google Scholar] [CrossRef]
- Saijo, Y.; Loo, E.P.-I. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Lata, C.; Prasad, M. Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 2011, 62, 4731–4748. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Silva Bueno, J.C. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl. Acad. Sci. USA 2007, 104, 5467. [Google Scholar] [CrossRef] [PubMed]
- Shute, C.C. Chemical transmitter systems in the brain. Mod. Trends. Neurol. 1975, 6, 183–203. [Google Scholar]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Arneth, A.; Kuhn, U.; Monson, R.K.; Peñuelas, J.; Staudt, M. The emission factor of volatile isoprenoids: Stress, acclimation, and developmental responses. Biogeosciences 2010, 7, 2203–2223. [Google Scholar] [CrossRef]
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Weingart, G.; Kluger, B.; Forneck, A.; Krska, R.; Schuhmacher, R. Establishment and application of a metabolomics workflow for identification and profiling of volatiles from leaves of Vitis vinifera by HS-SPME-GC-MS. Phytochem. Anal. 2012, 23, 345–358. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kännaste, A.; Copolovici, L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front. Plant Sci. 2013, 4, 262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Jander, G. Molecular ecology of plant volatiles in interactions with insect herbivores. J. Exp. Bot. 2021, 73, 449–462. [Google Scholar] [CrossRef]
- Farmer, E.E.; Ryan, C.A. Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl. Acad. Sci. USA 1990, 87, 7713. [Google Scholar] [CrossRef]
- Karban, R.; Huntzinger, M.; McCall, A.C. The specificity of eavesdropping on sagebrush by other plants. Ecology 2004, 85, 1846–1852. [Google Scholar] [CrossRef]
- Landi, M.; Araniti, F.; Flamini, G.; Piccolo, E.L.; Trivellini, A.; Abenavoli, M.R.; Guidi, L. “Help is in the air”: Volatiles from salt-stressed plants increase the reproductive success of receivers under salinity. Planta 2020, 251, 48. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, L.; Wang, J.; Jin, J.; Zhang, N.; Lei, L.; Gao, T.; Jing, T.; Zhang, S.; Wu, Y.; et al. Induction of priming by cold stress via inducible volatile cues in neighboring tea plants. J. Integr. Plant Biol. 2020, 62, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Lazazzara, V.; Avesani, S.; Robatscher, P.; Oberhuber, M.; Pertot, I.; Schuhmacher, R.; Perazzolli, M. Biogenic volatile organic compounds in the grapevine response to pathogens, beneficial microorganisms, resistance inducers and abiotic factors. J. Exp. Bot. 2021, 73, 529–554. [Google Scholar] [CrossRef]
- Kessler, A.; Halitschke, R.; Diezel, C.; Baldwin, I. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 2006, 148, 280–292. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Jaini, R.; Morgan, J.A.; Dudareva, N. Rethinking how volatiles are released from plant cells. Trends Plant Sci. 2015, 20, 545–550. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Staudt, M. Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Paré, P.W.; Tumlinson, J.H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999, 121, 325. [Google Scholar] [CrossRef] [PubMed]
- Piechulla, B.; Degenhardt, J. The emerging importance of microbial volatile organic compounds. Plant Cell Environ. 2014, 37, 811–812. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü.; Loreto, F.; Reichstein, M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 2004, 9, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Reichstein, M. Controls on the emission of plant volatiles through stomata: Differential sensitivity of emission rates to stomatal closure explained. J. Geophys. Res. Atmos. 2003, 108, 4211. [Google Scholar] [CrossRef]
- Baldwin, I.T. Plant volatiles. Curr. Biol. 2010, 20, R392–R397. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zamanizadehnajari, S.; Baldwin, I.T. Phytotoxic volatiles in the roots and shoots of Artemisia tridentata as detected by headspace solid-phase microextraction and gas chromatographic-mass spectrometry analysis. J. Chem. Ecol. 2010, 36, 1398–1407. [Google Scholar] [CrossRef]
- Heil, M.; Karban, R. Explaining evolution of plant communication by airborne signals. Trends Ecol. Evol. 2010, 25, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Gigot, C.; Ongena, M.; Fauconnier, M.-L.; Wathelet, J.-P.; Jardin, P.D.; Thonart, P. The lipoxygenase metabolic pathway in plants: Potential for industrial production of natural green leaf volatiles. Biotechnol. Agron. Soc. Environ. 2010, 14, 451–460. [Google Scholar]
- Nakashima, A.; von Reuss, S.H.; Tasaka, H.; Nomura, M.; Mochizuki, S.; Iijima, Y.; Aoki, K.; Shibata, D.; Boland, W.; Takabayashi, J.; et al. Traumatin- and dinortraumatin-containing galactolipids in Arabidopsis: Their formation in tissue-disrupted leaves as counterparts of green leaf volatiles. J. Biol. Chem. 2013, 288, 26078–26088. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Cheng, H.; Yang, H.; Yuan, H.; Zhang, J.; Chi, D. Effects of plant volatiles on the EAG response and behavior of the grey tiger longicorn, Xylotrechus rusticus (L.) Coleoptera: Cerambycidae. Kun Chong Xue Bao 2006, 49, 759–767. [Google Scholar]
- Wu, W.; Tran, W.; Taatjes, C.A.; Alonso-Gutierrez, J.; Lee, T.S.; Gladden, J.M. Rapid discovery and functional characterization of terpene synthases from four Endophytic xylariaceae. PLoS ONE 2016, 11, e0146983. [Google Scholar] [CrossRef] [PubMed]
- Boatright, J.; Negre, F.; Chen, X.; Kish, C.M.; Wood, B.; Peel, G.; Orlova, I.; Gang, D.; Rhodes, D.; Dudareva, N. Understanding in vivo benzenoid metabolism in petunia petal tissue. Plant Physiol. 2004, 135, 1993–2011. [Google Scholar] [CrossRef]
- Rudus, I.; Sasiak, M.; Kępczyński, J. Regulation of ethylene biosynthesis at the level of 1-aminocyclopropane-1-carboxylate oxidase (ACO) gene. Acta Physiol. Plant 2013, 35, 295–307. [Google Scholar] [CrossRef]
- Dorokhov, Y.L.; Komarova, T.V.; Petrunia, I.V.; Frolova, O.Y.; Pozdyshev, D.V.; Gleba, Y.Y. Airborne signals from a wounded leaf facilitate viral spreading and induce antibacterial resistance in neighboring plants. PLoS Pathog. 2012, 8, e1002640. [Google Scholar] [CrossRef]
- Komarova, T.; Sheshukova, E.; Dorokhov, Y. Cell wall methanol as a signal in plant immunity. Front. Plant Sci. 2014, 5, 101. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Jerkovic, I.; Mastelic, J. Composition of free and glycosidically bound volatiles of Mentha aquatica L. Croat. Chem. Acta 2001, 74, 431–439. [Google Scholar]
- Sirikantaramas, S.; Yamazaki, M.; Saito, K. Mechanisms of resistance to self-produced toxic secondary metabolites in plants. Phytochem. Rev. 2007, 7, 467. [Google Scholar] [CrossRef]
- Maffei, M.E. Sites of synthesis, biochemistry and functional role of plant volatiles. S. Afr. J. Bot. 2010, 76, 612–631. [Google Scholar] [CrossRef]
- Mithöfer, A.; Wanner, G.; Boland, W. Effects of feeding Spodoptera littoralis on lima bean leaves: Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiol. 2005, 137, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, G.; VanDoorn, A.; Baldwin, I.T. Herbivore-associated elicitors: FAC signaling and metabolism. Trends Plant Sci. 2011, 16, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Louis, J.; Luthe, D.S.; Felton, G.W. Salivary signals of European corn borer induce indirect defenses in tomato. Plant Signal. Behav. 2013, 8, e27318. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Erb, M. tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Arce, C.M.; Besomi, G.; Glauser, G.; Turlings, T.C.J. Caterpillar-induced volatile emissions in cotton: The relative importance of damage and insect-derived factors. Front. Plant Sci. 2021, 12, 709858. [Google Scholar]
- Mattiacci, L.; Dicke, M.; Posthumus, M.A. beta-Glucosidase: An elicitor of herbivore-induced plant odour that attracts host-searching parasitic wasps. Proc. Natl. Acad. Sci. USA 1995, 92, 2036–2040. [Google Scholar] [CrossRef]
- Hopke, J.; Donath, J.; Blechert, S.; Boland, W. Herbivore-induced volatiles: The emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a β-glucosidase and jasmonic acid. FEBS Lett. 1994, 352, 146–150. [Google Scholar] [CrossRef]
- Doares, S.H.; Syrovets, T.; Weiler, E.W.; Ryan, C.A. Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proc. Natl. Acad. Sci. USA 1995, 92, 4095–4098. [Google Scholar] [CrossRef]
- Bergey, D.R.; Orozco-Cardenas, M.; de Moura, D.S.; Ryan, C.A. A wound- and systemin-inducible polygalacturonase in tomato leaves. Proc. Natl. Acad. Sci. USA 1999, 96, 1756. [Google Scholar] [CrossRef]
- McFarlane, H.E.; Watanabe, Y.; Yang, W.; Huang, Y.; Ohlrogge, J.; Samuels, A.L. Golgi- and trans-Golgi network-mediated vesicle trafficking is required for wax secretion from epidermal cells. Plant Physiol. 2014, 164, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Samuels, L.; McFarlane, H.E. Plant cell wall secretion and lipid traffic at membrane contact sites of the cell cortex. Protoplasma 2012, 249 (Suppl. 1), S19–S23. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, H.E.; Shin, J.J.; Bird, D.A.; Samuels, A.L. Arabidopsis ABCG transporters, which are required for export of diverse cuticular lipids, dimerize in different combinations. Plant Cell 2010, 22, 3066–3075. [Google Scholar] [CrossRef] [PubMed]
- Adebesin, F.; Widhalm, J.R.; Boachon, B.; Lefèvre, F.; Pierman, B.; Lynch, J.H.; Alam, I.; Junqueira, B.; Benke, R.; Ray, S.; et al. Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter. Science 2017, 356, 1386–1388. [Google Scholar] [CrossRef]
- Debono, A.; Yeats, T.H.; Rose, J.K.; Bird, D.; Jetter, R.; Kunst, L.; Samuels, L. Arabidopsis LTPG is a glycosylphosphatidylinositol-anchored lipid transfer protein required for export of lipids to the plant surface. Plant Cell 2009, 21, 1230–1238. [Google Scholar] [CrossRef]
- Choi, Y.E.; Lim, S.; Kim, H.J.; Han, J.Y.; Lee, M.H.; Yang, Y.; Kim, J.A.; Kim, Y.S. Tobacco NtLTP1, a glandular-specific lipid transfer protein, is required for lipid secretion from glandular trichomes. Plant J. 2012, 70, 480–491. [Google Scholar] [CrossRef]
- Nagashima, A.; Higaki, T.; Koeduka, T.; Ishigami, K.; Hosokawa, S.; Watanabe, H.; Matsui, K.; Hasezawa, S.; Touhara, K. Transcriptional regulators involved in responses to volatile organic compounds in plants. J. Biol. Chem. 2019, 294, 2256–2266. [Google Scholar] [CrossRef]
- Schurmann, W. Emission of biosynthesized monoterpenes from needles of Norway spruce. Naturwissenschaften 1993, 80, 276–278. [Google Scholar] [CrossRef]
- Llusià, J.; Peñuelas, J. Changes in terpene content and emission in potted Mediterranean woody plants under severe drought. Can. J. Bot. 1998, 76, 1366–1373. [Google Scholar]
- Bourtsoukidis, E.; Schneider, H.; Radacki, D.; Schütz, S.; Hakola, H.; Hellén, H.; Noe, S.; Mölder, I.; Ammer, C.; Bonn, B. Impact of flooding and drought conditions on the emission of volatile organic compounds of Quercus robur and Prunus serotina. Trees 2013, 28, 193–204. [Google Scholar] [CrossRef]
- Niinemets, U.L.; Reichstein, M.; Staudt, M.; Seufert, G.N.; Tenhunen, J.D. Stomatal Constraints May Affect Emission of Oxygenated Monoterpenoids from the Foliage of Pinus pinea. Plant Physiol. 2002, 130, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, L.; Niinemets, Ü. Environmental Impacts on Plant Volatile Emission. In Deciphering Chemical Language of Plant Communication; Blande, J.D., Glinwood, R., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 35–59. [Google Scholar]
- Peñuelas, J.; Filella, I.; Seco, R.; Llusià, J. Increase in isoprene and monoterpene emissions after re-watering of droughted Quercus ilex seedlings. Biol. Plant. 2009, 53, 351–354. [Google Scholar] [CrossRef]
- Velikova, V.; Várkonyi, Z.; Szabó, M.; Maslenkova, L.; Nogues, I.; Kovács, L.; Peeva, V.; Busheva, M.; Garab, G.; Sharkey, T.D.; et al. Increased thermostability of thylakoid membranes in isoprene-emitting leaves probed with three biophysical techniques. Plant Physiol. 2011, 157, 905. [Google Scholar] [CrossRef]
- Brilli, F.; Hörtnagl, L.; Bamberger, I.; Schnitzhofer, R.; Ruuskanen, T.M.; Hansel, A.; Loreto, F.; Wohlfahrt, G. Qualitative and quantitative characterization of volatile organic compound emissions from cut grass. Environ. Sci. Technol. 2012, 46, 3859–3865. [Google Scholar] [CrossRef]
- Beauchamp, J.; Wisthaler, A.; Hansel, A.; Kleist, E.; Miebach, M.; Niinemets, Ü.; Schurr, U.L.I.; Wildt, J. Ozone induced emissions of biogenic VOC from tobacco: Relationships between ozone uptake and emission of LOX products. Plant Cell Environ. 2005, 28, 1334–1343. [Google Scholar] [CrossRef]
- Grote, R.; Samson, R.; Alonso, R.; Amorim, J.H.; Cariñanos, P.; Churkina, G.; Fares, S.; Thiec, D.L.; Niinemets, Ü.; Mikkelsen, T.N.; et al. Functional traits of urban trees: Air pollution mitigation potential. Front. Ecol. Environ. 2016, 14, 543–550. [Google Scholar] [CrossRef]
- Karl, T.; Guenther, A.; Turnipseed, A.; Patton, E.G.; Jardine, K. Chemical sensing of plant stress at the ecosystem scale. Biogeosciences 2008, 5, 1287–1294. [Google Scholar] [CrossRef]
- Staudt, M.; Lhoutellier, L. Volatile organic compound emission from holm oak infested by gypsy moth larvae: Evidence for distinct responses in damaged and undamaged leaves. Tree Physiol. 2007, 27, 1433–1440. [Google Scholar] [CrossRef]
- Possell, M.; Loreto, F. The Role of volatile organic compounds in plant resistance to abiotic stresses: Responses and mechanisms. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson, R.K., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 209–235. [Google Scholar]
- Niinemets, Ü.; Sun, Z. How light, temperature, and measurement and growth [CO2] interactively control isoprene emission in hybrid aspen. J. Exp. Bot. 2015, 66, 841–851. [Google Scholar] [CrossRef]
- Li, Z.; Sharkey, T.D. Molecular and pathway controls on biogenic volatile organic compound emissions. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson, R.K., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 119–151. [Google Scholar]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, E.; Rey, A.; Bobich, E.G.; Barron-Gafford, G.; Grieve, K.A.; Malhi, Y.; Murthy, R. Effect of elevated CO2 concentration and vapour pressure deficit on isoprene emission from leaves of Populus deltoides during drought. Funct. Plant Biol. 2004, 31, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Loreto, F. On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell Environ. 2005, 28, 318–327. [Google Scholar] [CrossRef]
- Loreto, F.; Delfine, S. Emission of isoprene from salt-stressed Eucalyptus globulus leaves. Plant Physiol. 2000, 123, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Pinelli, P.; Manes, F.; Kollist, H. Impact of ozone on monoterpene emissions and evidence for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiol. 2004, 24, 361–367. [Google Scholar] [CrossRef]
- Grote, R.; Monson, R.K.; Niinemets, Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson, R.K., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 315–355. [Google Scholar]
- Rasulov, B.; Hüve, K.; Bichele, I.; Laisk, A.; Niinemets, Ü. Temperature response of isoprene emission in vivo reflects a combined effect of substrate limitations and isoprene synthase activity: A Kinetic Analysis. Plant Physiol. 2010, 154, 1558–1570. [Google Scholar] [CrossRef]
- Singsaas, E.L.; Sharkey, T.D. The effects of high temperature on isoprene synthesis in oak leaves. Plant Cell Environ. 2000, 23, 751–757. [Google Scholar] [CrossRef]
- Loreto, F.; Barta, C.; Brilli, F.; Nogues, I. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ. 2006, 29, 1820–1828. [Google Scholar] [CrossRef]
- Brilli, F.; Barta, C.; Fortunati, A.; Lerdau, M.; Loreto, F.; Centritto, M. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytol. 2007, 175, 244–254. [Google Scholar] [CrossRef]
- Grote, R.; Lavoir, A.-V.; Rambal, S.; Staudt, M.; Zimmer, I.; Schnitzler, J.-P. Modelling the drought impact on monoterpene fluxes from an evergreen Mediterranean forest canopy. Oecologia 2009, 160, 213–223. [Google Scholar] [CrossRef]
- Rosenstiel, T.N.; Fisher, A.J.; Fall, R.; Monson, R.K. Differential accumulation of dimethylallyl diphosphate in leaves and needles of isoprene- and methylbutenol-emitting and nonemitting species. Plant Physiol. 2002, 129, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Yeh, S. Isoprene emission from plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 407–436. [Google Scholar] [CrossRef] [PubMed]
- Nemecek-Marshall, M.; MacDonald, R.C.; Franzen, J.J.; Wojciechowski, C.L.; Fall, R. Methanol emission from leaves (enzymatic detection of gas-phase methanol and relation of methanol fluxes to stomatal conductance and leaf development). Plant Physiol. 1995, 108, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, R.; Schäfer, L.; Gerlach, C.; Rausch, T.; Kesselmeier, J. Factors controlling the emissions of volatile organic acids from leaves of Quercus ilex L. (Holm oak). Atmos. Environ. 1999, 33, 1347–1355. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Harren, F.J.M.; Laarhoven, L.J.J.; Boamfa, I.; te Lintel-Hekkert, S.; Scheerer, U.; Hüglin, C.; Rennenberg, H. Acetaldehyde emission by the leaves of trees—Correlation with physiological and environmental parameters. Physiol. Plant 2001, 113, 41–49. [Google Scholar] [CrossRef]
- Fall, R.; Monson, R.K. Isoprene emission rate and intercellular isoprene concentration as influenced by stomatal distribution and conductance. Plant Physiol. 1992, 100, 987. [Google Scholar] [CrossRef]
- Loreto, F. Influence of environmental factors and air composition on the emission of alpha-pinene from Quercus ilex leaves. Plant Physiol. 1996, 110, 267–275. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Loreto, F. Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia 1993, 95, 328–333. [Google Scholar] [CrossRef]
- Calfapietra, C.; Fares, S.; Loreto, F. Volatile organic compounds from Italian vegetation and their interaction with ozone. Environ. Pollut. 2009, 157, 1478–1486. [Google Scholar] [CrossRef]
- Piesik, D.; Delaney, K.J.; Wenda-Piesik, A.; Sendel, S.; Tabaka, P.; Buszewski, B. Meligethes aeneus pollen-feeding suppresses, and oviposition induces, Brassica napus volatiles: Beetle attraction/repellence to lilac aldehydes and veratrole. Chemoecology 2013, 23, 241–250. [Google Scholar] [CrossRef]
- Harren, F.J.M.; Cristescu, S.M. Online, real-time detection of volatile emissions from plant tissue. AoB Plants 2013, 5, plt003. [Google Scholar] [CrossRef] [PubMed]
- Jubany-Marí, T.; Prinsen, E.; Munné-Bosch, S.; Alegre, L. The timing of methyl jasmonate, hydrogen peroxide and ascorbate accumulation during water deficit and subsequent recovery in the Mediterranean shrub Cistus albidus L. Environ. Exp. Bot. 2010, 69, 47–55. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Remmel, T.; Niinemets, Ü. Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environ. Exp. Bot. 2014, 100, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Rasulov, B.; Hüve, K.; Laisk, A.; Niinemets, Ü. Induction of a longer term component of isoprene release in darkened aspen leaves: Origin and regulation under different environmental conditions. Plant Physiol. 2011, 156, 816–831. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Calatayud, V.; Gao, F.; Fares, S.; Paoletti, E.; Tian, Y.; Feng, Z. Interaction of drought and ozone exposure on isoprene emission from extensively cultivated poplar. Plant Cell Environ. 2016, 39, 2276–2287. [Google Scholar] [CrossRef] [PubMed]
- Tani, A.; Ohno, T.; Saito, T.; Ito, S.; Yonekura, T.; Miwa, M. Effects of ozone on isoprene emission from two major Quercus species native to East Asia. J. Agric. Meteorol. 2017, 73, 195–202. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Emission of volatile organic compounds from plants shows a biphasic pattern within an hormetic context. Environ. Pollut. 2018, 239, 318–321. [Google Scholar] [CrossRef]
- Jiang, Y.; Ye, J.; Li, S.; Niinemets, Ü. Methyl jasmonate-induced emission of biogenic volatiles is biphasic in cucumber: A high-resolution analysis of dose dependence. J. Exp. Bot. 2017, 68, 4679–4694. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Fares, S.; Harley, P.; Jardine, K.J. Bidirectional exchange of biogenic volatiles with vegetation: Emission sources, reactions, breakdown and deposition. Plant Cell Environ. 2014, 37, 1790–1809. [Google Scholar] [CrossRef]
- Bakker, M.I. Atmospheric Deposition of Semivolatile Organic Compounds to Plants. Ph.D. Thesis, Utrecht University Repository, Utrecht, The Netherlands, 2000. [Google Scholar]
- Riederer, M.; Daiß, A.; Gilbert, N.; Köhle, H. Semi-volatile organic compounds at the leaf/atmosphere interface: Numerical simulation of dispersal and foliar uptake. J. Exp. Bot. 2002, 53, 1815–1823. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keyte, I.; Wild, E.; Dent, J.; Jones, K.C. Investigating the foliar uptake and within-leaf migration of phenanthrene by moss (Hypnum Cupressiforme) using two-photon excitation microscopy with autofluorescence. Environ. Sci. Technol. 2009, 43, 5755–5761. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, J.; Pariyar, S. Particulate pollutants are capable to ‘degrade’ epicuticular waxes and to decrease the drought tolerance of Scots pine (Pinus sylvestris L.). Environ. Pollut. 2014, 184, 659–667. [Google Scholar] [CrossRef]
- Noe, S.M.; Copolovici, L.; Niinemets, Ü.; Vaino, E. Foliar limonene uptake scales positively with leaf lipid content: “Non-emitting” species absorb and release monoterpenes. Plant Biol. 2007, 9, e79–e86. [Google Scholar] [CrossRef] [PubMed]
- Tör, M.; Lotze, M.T.; Holton, N. Receptor-mediated signalling in plants: Molecular patterns and programmes. J. Exp. Bot. 2009, 60, 3645–3654. [Google Scholar] [CrossRef]
- He, Y.; Zhou, J.; Shan, L.; Meng, X. Plant cell surface receptor-mediated signaling—A common theme amid diversity. J. Cell Sci. 2018, 131, jcs209353. [Google Scholar] [CrossRef]
- Choi, H.W.; Klessig, D.F. DAMPs, MAMPs, and NAMPs in plant innate immunity. BMC Plant Biol. 2016, 16, 232. [Google Scholar] [CrossRef]
- Quintana-Rodriguez, E.; Duran-Flores, D.; Heil, M.; Camacho-Coronel, X. Damage-associated molecular patterns (DAMPs) as future plant vaccines that protect crops from pests. Sci. Hortic. 2018, 237, 207–220. [Google Scholar] [CrossRef]
- Gust, A.A.; Pruitt, R.; Nürnberger, T. Sensing danger: Key to activating plant immunity. Trends Plant Sci. 2017, 22, 779–791. [Google Scholar] [CrossRef]
- Hann, C.T.; Bequette, C.J.; Dombrowski, J.E.; Stratmann, J.W. Methanol and ethanol modulate responses to danger- and microbe-associated molecular patterns. Front. Plant Sci. 2014, 5, 550. [Google Scholar] [CrossRef]
- Harvey, C.M.; Sharkey, T.D. Exogenous isoprene modulates gene expression in unstressed Arabidopsis thaliana plants. Plant Cell Environ. 2016, 39, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Weraduwage, S.M.; Lantz, A.T.; Sanchez, L.M.; Weise, S.E.; Wang, J.; Childs, K.L.; Sharkey, T.D. Isoprene acts as a signaling molecule in gene networks important for stress responses and plant growth. Plant Physiol. 2019, 180, 124–152. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Sopory, S.K. Chemical signaling under abiotic stress environment in plants. Plant Signal. Behav. 2008, 3, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.C.; Rott, A.S.; Zeder, M.; Jüttner, F.; Dorn, S. 13C-labelling patterns of green leaf volatiles indicating different dynamics of precursors in Brassica leaves. Phytochemistry 2008, 69, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Paré, P.W. C6-Green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry 2002, 61, 545–554. [Google Scholar] [CrossRef]
- Burg, S.P.; Burg, E.A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967, 42, 144–152. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Schaller, G.E. The Mechanism of ethylene perception. Plant Physiol. 1996, 111, 653–660. [Google Scholar] [CrossRef]
- Ecker, J.R. The ethylene signal transduction pathway in plants. Science 1995, 268, 667–675. [Google Scholar] [CrossRef]
- Chen, Q.G.; Bleecker, A.B. Analysis of ethylene signal-transduction kinetics associated with seedling-growth response and chitinase induction in wild-type and mutant Arabidopsis. Plant Physiol. 1995, 108, 597–607. [Google Scholar] [CrossRef]
- Chang, C.; Kwok, S.F.; Bleecker, A.B.; Meyerowitz, E.M. Arabidopsis ethylene-response gene ETR1: Similarity of product to two-component regulators. Science 1993, 262, 539–544. [Google Scholar] [CrossRef]
- Guzmán, P.; Ecker, J.R. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 1990, 2, 513–523. [Google Scholar] [PubMed]
- Ju, C.; Chang, C. Mechanistic insights in ethylene perception and signal transduction. Plant Physiol. 2015, 169, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cui, X.; Sun, Y.; Dong, C.H. Ethylene signaling and regulation in plant growth and stress responses. Plant Cell Rep. 2013, 32, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Chang, C.; Sun, Q.; Meyerowitz, E.M. Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 1995, 269, 1712. [Google Scholar] [CrossRef]
- Sakai, H.; Hua, J.; Chen, Q.G.; Chang, C.; Medrano, L.J.; Bleecker, A.B.; Meyerowitz, E.M. ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 1998, 95, 5812–5817. [Google Scholar] [CrossRef]
- Hall, B.P.; Shakeel, S.N.; Schaller, G.E. Ethylene Receptors: Ethylene perception and signal transduction. J. Plant Growth Regul. 2007, 26, 118–130. [Google Scholar] [CrossRef]
- Chory, J. Light signal transduction: An infinite spectrum of possibilities. Plant J. 2010, 61, 982–991. [Google Scholar] [CrossRef]
- Chamovitz, D. Rooted in sensation: Sight. New Sci. 2012, 215, 35. [Google Scholar] [CrossRef]
- Chamovitz, D. Rooted in sensation: Hearing. New Sci. 2012, 215, 37. [Google Scholar] [CrossRef]
- Gagliano, M. Green symphonies: A call for studies on acoustic communication in plants. Behav. Ecol. 2013, 24, 789–796. [Google Scholar] [CrossRef]
- Appel, H.M.; Cocroft, R.B. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 2014, 175, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.C.; Ghosh, R.; Bae, H. Plant acoustics: In the search of a sound mechanism for sound signaling in plants. J. Exp. Bot. 2016, 67, 4483–4494. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.G.; Adesina, T.; Jovanov, E. Closing of venus flytrap by electrical stimulation of motor cells. Plant Signal. Behav. 2007, 2, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Malnic, B.; Hirono, J.; Sato, T.; Buck, L.B. Combinatorial receptor codes for odours. Cell 1999, 96, 713–723. [Google Scholar] [CrossRef]
- Mescher, M.C.; Runyon, J.B.; De Moraes, C.M. Plant host finding by parasitic plants: A new perspective on plant to plant communication. Plant Signal. Behav. 2006, 1, 284–286. [Google Scholar] [CrossRef][Green Version]
- Tiwari, V.; Patel, M.K.; Chaturvedi, A.K.; Mishra, A.; Jha, B. Functional characterisation of the Tau Class Glutathione-S-Transferases Gene (SbGSTU) Promoter of Salicornia brachiata under salinity and osmotic stress. PLoS ONE 2016, 11, e0148494. [Google Scholar] [CrossRef]
- Huber, A.E.; Bauerle, T.L. Long-distance plant signaling pathways in response to multiple stressors: The gap in knowledge. J. Exp. Bot. 2016, 67, 2063–2079. [Google Scholar] [CrossRef]
- Johns, S.; Hagihara, T.; Toyota, M.; Gilroy, S. The fast and the furious: Rapid long-range signaling in plants. Plant Physiol. 2021, 185, 694–706. [Google Scholar] [CrossRef]
- Malone, M. Wound-induced hydraulic signals and stimulus transmission in Mimosa pudica L. New Phytol. 1994, 128, 49–56. [Google Scholar] [CrossRef]
- Dicke, M.; van Loon, J.J.; Soler, R. Chemical complexity of volatiles from plants induced by multiple attack. Nat. Chem. Biol. 2009, 5, 317–324. [Google Scholar] [CrossRef]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Shinozaki, K. Long-distance signaling in plant stress response. Curr. Opin. Plant 2019, 47, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Reinhart, B.J.; Bartel, D.P.; Zamore, P.D. A biochemical framework for RNA silencing in plants. Genes Dev 2003, 17, 49–63. [Google Scholar] [CrossRef]
- Yoo, B.-C.; Kragler, F.; Varkonyi-Gasic, E.; Haywood, V.; Archer-Evans, S.; Lee, Y.M.; Lough, T.J.; Lucas, W.J. A systemic small RNA signaling system in plants. Plant Cell 2004, 16, 1979–2000. [Google Scholar] [CrossRef]
- Kondhare, K.R.; Patil, N.S.; Banerjee, A.K. A historical overview of long-distance signalling in plants. J. Exp. Bot. 2021, 72, 4218–4236. [Google Scholar] [CrossRef]
- Charrier, G.; Delzon, S.; Domec, J.-C.; Zhang, L.; Delmas, C.E.L.; Merlin, I.; Corso, D.; King, A.; Ojeda, H.; Ollat, N.; et al. Drought will not leave your glass empty: Low risk of hydraulic failure revealed by long-term drought observations in world’s top wine regions. Sci. Adv. 2018, 4, eaao6969. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Goodger, J.Q. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008, 13, 281–287. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef]
- Zweifel, R.; Zeugin, F. Ultrasonic acoustic emissions in drought-stressed trees—More than signals from cavitation? New Phytol. 2008, 179, 1070–1079. [Google Scholar] [CrossRef]
- Gagliano, M.; Renton, M.; Duvdevani, N.; Timmins, M.; Mancuso, S. Acoustic and magnetic communication in plants: Is it possible? Plant Signal. Behav. 2012, 7, 1346–1348. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.J.; Appel, H.M.; Carlson, J.E.; De Moraes, C.M.; Mescher, M.C.; Schultz, J.C. Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol. Lett. 2007, 10, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Kessler, A.; Halitschke, R. Volatile signaling in plant–plant herbivore interactions: What is real? Curr. Opin. Plant 2002, 5, 351–354. [Google Scholar] [CrossRef]
- Howe, G.; Schaller, A. Direct Defenses in Plants and Their Induction by Wounding and Insect Herbivores. In Induced Plant Resistance to Herbivory; Schaller, A., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 7–29. [Google Scholar]
- Zimmermann, M.R.; Maischak, H.; Mithöfer, A.; Boland, W.; Felle, H.H. System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant Physiol. 2009, 149, 1593–1600. [Google Scholar] [CrossRef]
- Wortemann, R.; Herbette, S.; Barigah, T.S.; Fumanal, B.; Alia, R.; Ducousso, A.; Gomory, D.; Roeckel-Drevet, P.; Cochard, H. Genotypic variability and phenotypic plasticity of cavitation resistance in Fagus sylvatica L. across Europe. Tree Physiol. 2011, 31, 1175–1182. [Google Scholar] [CrossRef]
- Arimura, G.-I.; Ozawa, R.; Maffei, M.E. Recent advances in plant early signaling in response to herbivory. Int. J. Mol. Sci. 2011, 12, 3723–3739. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef]
- Ninkovic, V.; Rensing, M.; Dahlin, I.; Markovic, D. Who is my neighbor? Volatile cues in plant interactions. Plant Signal. Behav. 2019, 14, 1634993. [Google Scholar] [CrossRef]
- Ninkovic, V.; Markovic, D.; Rensing, M. Plant volatiles as cues and signals in plant communication. Plant Cell Environ. 2020, 44, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Kikuta, Y.; Matsuda, K. Plant communication: Mediated by individual or blended VOCs? Plant Signal. Behav. 2012, 7, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Yoneya, K.; Takabayashi, J. Plant—Plant communication mediated by airborne signals: Ecological and plant physiological perspectives. Plant Biotechnol. 2014, 31, 409–416. [Google Scholar]
- Karban, R.; Maron, J. The fitness consequences of interspecific eavesdropping between plants. Ecology 2002, 83, 1209–1213. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Ecological Biochemistry: Allelopathy and defense against herbivores. In Plant Physiological Ecology; Lambers, H., Chapin, F.S., Pons, T.L., Eds.; Springer: New York, NY, USA, 2008; pp. 445–477. [Google Scholar]
- Anthony, R.G.; Waldin, T.R.; Ray, J.A.; Bright, S.W.J.; Hussey, P.J. Herbicide resistance caused by spontaneous mutation of the cytoskeletal protein tubulin. Nature 1998, 393, 260–263. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of Cell Proliferation and DNA Synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Chaimovitsh, D.; Abu-Abied, M.; Belausov, E.; Rubin, B.; Dudai, N.; Sadot, E. Microtubules are an intracellular target of the plant terpene citral. Plant J. 2010, 61, 399–408. [Google Scholar] [CrossRef]
- Kriegs, B.; Jansen, M.; Hahn, K.; Peisker, H.; Samajová, O.; Beck, M.; Braun, S.; Ulbrich, A.; Baluška, F.; Schulz, M. Cyclic monoterpene mediated modulations of Arabidopsis thaliana phenotype: Effects on the cytoskeleton and on the expression of selected genes. Plant Signal. Behav. 2010, 5, 832–838. [Google Scholar] [CrossRef]
- Sarheed, M.M.; Rajabi, F.; Kunert, M.; Boland, W.; Wetters, S.; Miadowitz, K.; Kaźmierczak, A.; Sahi, V.P.; Nick, P. Cellular base of mint allelopathy: Menthone affects plant microtubules. Front. Plant Sci. 2020, 11, 1320. [Google Scholar] [CrossRef]
- Mofikoya, A.O.; Bui, T.N.T.; Kivimäenpää, M.; Holopainen, J.K.; Himanen, S.J.; Blande, J.D. Foliar behaviour of biogenic semi-volatiles: Potential applications in sustainable pest management. Arthropod-Plant Interact. 2019, 13, 193–212. [Google Scholar] [CrossRef]
- Bajalan, I.; Zand, M.; Rezaee, S. The study on allelopathic effects of Mentha longifolia on seed germination of velvet flower and two cultivars of wheat. Int. Res. J. Appl. Basic Sci. 2013, 4, 2539–2543. [Google Scholar]
- Cheng, F.; Cheng, Z. Research Progress on the use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Edyta, S.; Peter, R.; Alina, S.-S.; Beata, B.-K.; Katarzyna, M. Allelopathic effect of aqueous extracts from the leaves of peppermint (Mentha piperita L.) on selected physiological processes of common sunflower (Helianthus annuus L.). Not. Bot. Horti. Agrobot. Cluj Napoca 2015, 43, 335–342. [Google Scholar]
- Martini, X.; Pelz-Stelinski, K.; Stelinski, L. Plant pathogen-induced volatiles attract parasitoids to increase parasitism of an insect vector. Front. Ecol. Evol. 2014, 2, 8. [Google Scholar] [CrossRef]
- Chitarrini, G.; Soini, E.; Riccadonna, S.; Franceschi, P.; Zulini, L.; Masuero, D.; Vecchione, A.; Stefanini, M.; Di Gaspero, G.; Mattivi, F.; et al. Identification of biomarkers for defense response to Plasmopara viticola in a resistant grape variety. Front. Plant Sci. 2017, 8, 1524. [Google Scholar] [CrossRef]
- Camacho-Coronel, X.; Molina-Torres, J.; Heil, M. Sequestration of exogenous volatiles by plant cuticular waxes as a mechanism of passive associational resistance: A proof of concept. Front. Plant Sci. 2020, 11, 121. [Google Scholar] [CrossRef]
- Ciubotaru, R.M.; Franceschi, P.; Zulini, L.; Stefanini, M.; Škrab, D.; Rossarolla, M.D.; Robatscher, P.; Oberhuber, M.; Vrhovsek, U.; Chitarrini, G. Mono-Locus and pyramided resistant grapevine cultivars reveal early putative biomarkers upon artificial inoculation with Plasmopara viticola. Front. Plant Sci. 2021, 12, 693887. [Google Scholar] [CrossRef]
- Ricciardi, V.; Marcianò, D.; Sargolzaei, M.; Maddalena, G.; Maghradze, D.; Tirelli, A.; Casati, P.; Bianco, P.A.; Failla, O.; Fracassetti, D.; et al. From plant resistance response to the discovery of antimicrobial compounds: The role of volatile organic compounds (VOCs) in grapevine downy mildew infection. Plant Physiol. Biochem. 2021, 160, 294–305. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.-M. Social networking in crop plants: Wired and wireless cross-plant communications. Plant Cell Environ. 2021, 44, 1095–1110. [Google Scholar] [CrossRef]
- Himanen, S.J.; Blande, J.D.; Klemola, T.; Pulkkinen, J.; Heijari, J.; Holopainen, J.K. Birch (Betula spp.) leaves adsorb and re-release volatiles specific to neighbouring plants—A mechanism for associational herbivore resistance? New Phytol. 2010, 186, 722–732. [Google Scholar] [CrossRef]
- Hansen, U.; Seufert, G. Temperature and light dependence of β-caryophyllene emission rates. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Engelberth, J.; Alborn, H.T.; O’Donnell, P.; Sammons, M.; Toshima, H.; Tumlinson, J.H. 3rd, Simultaneous analysis of phytohormones, phytotoxins, and volatile organic compounds in plants. Proc. Natl. Acad. Sci. USA 2003, 100, 10552–10557. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.C.; Daniel, J.C.; Raymond, M.W.; Ara, M.; Robert, R.H. A System and methodology for measuring volatile organic compounds produced by hydroponic lettuce in a controlled environment. J. Am. Soc. Hortic. Sci. 1996, 121, 483–487. [Google Scholar]
- Zepp, R.G.; Callaghan, T.V.; Erickson, D.J. Effects of increased solar ultraviolet radiation on biogeochemical cycles. Ambio 1995, 24, 181–187. [Google Scholar]
- Harley, P.; Guenther, A.; Zimmerman, P. Environmental controls over isoprene emission in deciduous oak canopies. Tree Physiol. 1997, 17, 705–714. [Google Scholar] [CrossRef]
- Heiden, A.C.; Hoffmann, T.; Kahl, J.; Kley, D.; Klockow, D.; Langebartels, C.; Mehlhorn, H.; Sandermann Jr, H.; Schraudner, M.; Schuh, G.; et al. Emission of volatile organic compounds from ozone-exposed plants. Ecol. Appl. 1999, 9, 1160–1167. [Google Scholar] [CrossRef]
- Brüggemann, N.; Schnitzler, J.P. Comparison of isoprene emission, intercellular isoprene concentration and photosynthetic performance in water-limited oak (Quercus pubescens Willd. and Quercus robur L.) saplings. Plant Biol. 2002, 4, 456–463. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Wiberley, A.E.; Donohue, A.R. Isoprene emission from plants: Why and how. Ann. Bot. 2008, 101, 5–18. [Google Scholar] [CrossRef]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781. [Google Scholar] [CrossRef]
- Bertamini, M.; Faralli, M.; Varotto, C.; Grando, M.S.; Cappellin, L. Leaf monoterpene emission limits photosynthetic downregulation under heat stress in field-grown grapevine. Plants 2021, 10, 181. [Google Scholar] [CrossRef]
- Mahajan, P.; Singh, H.P.; Kaur, S.; Batish, D.R.; Kohli, R.K. β-pinene moderates Cr(VI) phytotoxicity by quenching reactive oxygen species and altering antioxidant machinery in maize. Environ. Sci. Pollut. Res. 2019, 26, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Pollastri, S.; Baccelli, I.; Loreto, F. Isoprene: An antioxidant itself or a molecule with multiple regulatory functions in plants? Antioxidants 2021, 10, 684. [Google Scholar] [CrossRef] [PubMed]
- Landi, M. Airborne signals and abiotic factors: The neglected side of the plant communication. Commun. Integr. Biol. 2020, 13, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef] [PubMed]
- Karban, R. Communication between sagebrush and wild tobacco in the field. Biochem. Syst. Ecol. 2001, 29, 995–1005. [Google Scholar] [CrossRef]
- Zakir, A.; Khallaf, M.A.; Hansson, B.S.; Witzgall, P.; Anderson, P. Herbivore-induced changes in cotton modulates reproductive behavior in the moth Spodoptera littoralis. Front. Ecol. Evol. 2017, 5, 49. [Google Scholar] [CrossRef]
- Conrath, U. Systemic acquired resistance. Plant Signal. Behav. 2006, 1, 179–184. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing plant defense priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef]
- Preston, C.A.; Laue, G.; Baldwin, I.T. Plant–plant Signaling: Application of trans- or cis-methyl jasmonate equivalent to sagebrush releases does not elicit direct defenses in native tobacco. J. Chem. Ecol. 2004, 30, 2193–2214. [Google Scholar] [CrossRef]
- Hagiwara, T.; Ishihara, M.I.; Takabayashi, J.; Hiura, T.; Shiojiri, K. Effective distance of volatile cues for plant–plant communication in beech. Ecol. Evol. 2021, 11, 12445–12452. [Google Scholar] [CrossRef] [PubMed]
- van Hulten, M.; Pelser, M.; van Loon, L.C.; Pieterse, C.M.; Ton, J. Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 5602–5607. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; von Dahl, C.C.; Preston, C.A. Volatile signaling in plant-plant interactions: ’Talking trees’ in the Genomics era. Science 2006, 311, 812. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Ton, J. Long-distance signalling in plant defence. Trends Plant Sci. 2008, 13, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Katz, V.A.; Thulke, O.U.; Conrath, U. A benzothiadiazole primes parsley cells for augmented elicitation of defense responses. Plant Physiol. 1998, 117, 1333. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, L.; Métraux, J.-P.; Mauch-Mani, B. β-Aminobutyric acid-induced protection of Arabidopsis against the necrotrophic fungus Botrytis cinerea. Plant Physiol. 2001, 126, 517. [Google Scholar] [CrossRef] [PubMed]
- Balmer, A.; Pastor, V.; Glauser, G.; Mauch-Mani, B. Tricarboxylates induce defense priming against bacteria in Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 1221. [Google Scholar] [CrossRef]
- Balmer, A.; Pastor, V.; Gamir, J.; Flors, V.; Mauch-Mani, B. The ’prime-ome’: Towards a holistic approach to priming. Trends Plant Sci. 2015, 20, 443–452. [Google Scholar] [CrossRef]
- Brosset, A.; Blande, J.D. Volatile-mediated plant-plant interactions: VOCs as modulators of receiver plant defence, growth and reproduction. J. Exp. Bot. 2021, 73, 511–528. [Google Scholar] [CrossRef]
- Luna, E.; Bruce, T.J.; Roberts, M.R.; Flors, V.; Ton, J. Next-generation systemic acquired resistance. Plant Physiol. 2012, 158, 844–853. [Google Scholar] [CrossRef]
- Crisp, P.A.; Ganguly, D.; Eichten, S.R.; Borevitz, J.O.; Pogson, B.J. Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Sci. Adv. 2016, 2, e1501340. [Google Scholar] [CrossRef] [PubMed]
- Brilli, F.; Loreto, F.; Baccelli, I. Exploiting plant volatile organic compounds (VOCs) in agriculture to improve sustainable defense strategies and productivity of crops. Front. Plant Sci. 2019, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Agrawal, A.A.; Bruin, J. Plants talk, but are they deaf? Trends Plant Sci. 2003, 8, 403–405. [Google Scholar] [CrossRef]
- Theocharis, A.; Bordiec, S.; Fernandez, O.; Paquis, S.; Dhondt-Cordelier, S.; Baillieul, F.; Clement, C.; Barka, E.A. Burkholderia phytofirmans PsJN primes Vitis vinifera L. and confers a better tolerance to low nonfreezing temperatures. Mol. Plant Microbe Interact. 2012, 25, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Pierik, R.; BallarÉ, C.L.; Dicke, M. Ecology of plant volatiles: Taking a plant community perspective. Plant Cell Environ. 2014, 37, 1845–1853. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Kessler, A. The information landscape of plant constitutive and induced secondary metabolite production. Curr. Opin. Insect Sci. 2015, 8, 47–53. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Blande, J.D. Molecular plant volatile communication. In Sensing in Nature; López-Larrea, C., Ed.; Springer: New York, NY, USA, 2012; pp. 17–31. [Google Scholar]
- Tholl, D.; Boland, W.; Hansel, A.; Loreto, F.; Röse, U.S.R.; Schnitzler, J.-P. Practical approaches to plant volatile analysis. Plant J. 2006, 45, 540–560. [Google Scholar] [CrossRef]
- Beck, J.; Smith, L.; Baig, N. An overview of plant volatile metabolomics, sample treatment and reporting considerations with emphasis on mechanical damage and biological control of weeds. Phytochem. Anal. 2014, 25, 331–341. [Google Scholar] [CrossRef]
- Kfoury, N.; Scott, E.; Orians, C.; Robbat, A., Jr. Direct contact sorptive extraction: A robust method for sampling plant volatiles in the field. J. Agric. Food Chem. 2017, 65, 8501–8509. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.G.; Yamaguchi, M.S.; McCartney, M.M.; Aksenov, A.A.; Pasamontes, A.; Davis, C.E. SPME-based mobile field device for active sampling of volatiles. Microchem. J. 2019, 146, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Louw, S. Recent trends in the chromatographic analysis of volatile flavor and fragrance compounds: Annual review 2020. Anal. Sci. Adv. 2021, 2, 157–170. [Google Scholar] [CrossRef]
- Stewart-Jones, A.; Poppy, G.M. Comparison of glass vessels and plastic bags for enclosing living plant parts for headspace analysis. J. Chem. Ecol. 2006, 32, 845–864. [Google Scholar] [CrossRef]
- Timkovsky, J.; Gankema, P.; Pierik, R.; Holzinger, R. A plant chamber system with downstream reaction chamber to study the effects of pollution on biogenic emissions. Environ. Sci. Process. Impacts 2014, 16, 2301–2312. [Google Scholar] [CrossRef]
- Beck, J.J.; Smith, L.; Merrill, G.B. In situ volatile collection, analysis, and comparison of three Centaurea species and their relationship to biocontrol with herbivorous insects. J. Agric. Food Chem. 2008, 56, 2759–2764. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Koziel, J.A.; Cai, L.; Wright, D.; Kuhrt, F. Testing odorants recovery from a novel metallized fluorinated ethylene propylene gas sampling bag. J. Air Waste Manag. Assoc. 2015, 65, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.H. Direct analysis in real time—A critical review on DART-MS. Anal. Bioanal. Chem. 2014, 406, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Soo, J.-C.; Lee, E.G.; LeBouf, R.F.; Kashon, M.L.; Chisholm, W.; Harper, M. Evaluation of a portable gas chromatograph with photoionization detector under variations of VOC concentration, temperature, and relative humidity. J. Occup. Environ. Hyg. 2018, 15, 351–360. [Google Scholar] [CrossRef]
- Wei-Hao Li, M.; Ghosh, A.; Venkatasubramanian, A.; Sharma, R.; Huang, X.; Fan, X. High-sensitivity micro-gas chromatograph–photoionization detector for trace vapor detection. ACS Sens. 2021, 6, 2348–2355. [Google Scholar] [CrossRef]
- Arce, L.; Gallegos, J.; Garrido, R.; Medina, L.; Sielemann, S.; Wortelmann, T. Ion mobility spectrometry a versatile analytical tool for metabolomics applications in food science. Curr. Metab. 2014, 2, 264–271. [Google Scholar] [CrossRef]
- Kallenbach, M.; Veit, D.; Eilers, E.J.; Schuman, M.C. Application of silicone tubing for robust, simple, high-throughput, and time-resolved analysis of plant volatiles in field experiments. Bio Protoc. 2015, 5, e1391. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.M.; Mayhew, C.A. Proton Transfer Reaction Mass Spectrometry: Principles and Applications; John Wiley & Sons: Chichester, UK, 2014. [Google Scholar]
- Jordan, A.; Haidacher, S.; Hanel, G.; Hartungen, E.; Märk, L.; Seehauser, H.; Schottkowsky, R.; Sulzer, P.; Märk, T.D. A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS). Int. J. Mass Spectrom. 2009, 286, 122–128. [Google Scholar] [CrossRef]
- Schwolow, S.; Gerhardt, N.; Rohn, S.; Weller, P. Data fusion of GC-IMS data and FT-MIR spectra for the authentication of olive oils and honeys—Is it worth to go the extra mile? Anal. Bioanal. Chem. 2019, 411, 6005–6019. [Google Scholar] [CrossRef]
- Ranganathan, Y.; Borges, R.M. To transform or not to transform. Plant Signal. Behav. 2011, 6, 113–116. [Google Scholar] [CrossRef]
- Houhou, R.; Bocklitz, T. Trends in artificial intelligence, machine learning, and chemometrics applied to chemical data. Anal. Sci. Adv. 2021, 2, 128–141. [Google Scholar] [CrossRef]
- Marenco, R.A.; Siebke, K.; Farquhar, G.D.; Ball, M.C. Hydraulically based stomatal oscillations and stomatal patchiness in Gossypium hirsutum. Funct. Plant Biol. 2006, 33, 1103–1113. [Google Scholar] [CrossRef]
- Peng, J.; van Loon, J.J.A.; Zheng, S.; Dicke, M. Herbivore-induced volatiles of cabbage (Brassica oleracea) prime defence responses in neighbouring intact plants. Plant Biol. 2011, 13, 276–284. [Google Scholar] [CrossRef]
- Silva, P.; Gerós, H. Regulation by salt of vacuolar H+-ATPase and H+-pyrophosphatase activities and Na+/H+ exchange. Plant Signal. Behav. 2009, 4, 718–726. [Google Scholar] [CrossRef]
- Haider, M.S.; Zhang, C.; Kurjogi, M.M.; Pervaiz, T.; Zheng, T.; Zhang, C.; Lide, C.; Shangguan, L.; Fang, J. Insights into grapevine defense response against drought as revealed by biochemical, physiological and RNA-Seq analysis. Sci. Rep. 2017, 7, 13134. [Google Scholar] [CrossRef]
- Hamilton, A.J.; Lycett, G.W.; Grierson, D. Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature 1990, 346, 284–287. [Google Scholar] [CrossRef]
- Oeller, P.W.; Lu, M.W.; Taylor, L.P.; Pike, D.A.; Theologis, A. Reversible inhibition of tomato fruit senescence by antisense RNA. Science 1991, 254, 437. [Google Scholar] [CrossRef] [PubMed]
- Vancanneyt, G.; Sanz, C.; Farmaki, T.; Paneque, M.; Ortego, F.; Castañera, P.; Sánchez-Serrano, J.J. Hydroperoxide lyase depletion in transgenic potato plants leads to an increase in aphid performance. Proc. Natl. Acad. Sci. USA 2001, 98, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Halitschke, R.; Baldwin, I.T. Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J. 2003, 36, 794–807. [Google Scholar] [CrossRef]
- Halitschke, R.; Ziegler, J.; Keinänen, M.; Baldwin, I.T. Silencing of hydroperoxide lyase and allene oxide synthase reveals substrate and defense signaling crosstalk in Nicotiana attenuata. Plant J. 2004, 40, 35–46. [Google Scholar] [CrossRef]
- Paschold, A.; Halitschke, R.; Baldwin, I.T. Using ‘mute’ plants to translate volatile signals. Plant J. 2006, 45, 275–291. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).