Molecular and Morphological Investigations of Two Giant Diatom Cymbella Species from the Transbaikal Area (Russia, Siberia) with Comments on Their Distributions
Abstract
:1. Introduction
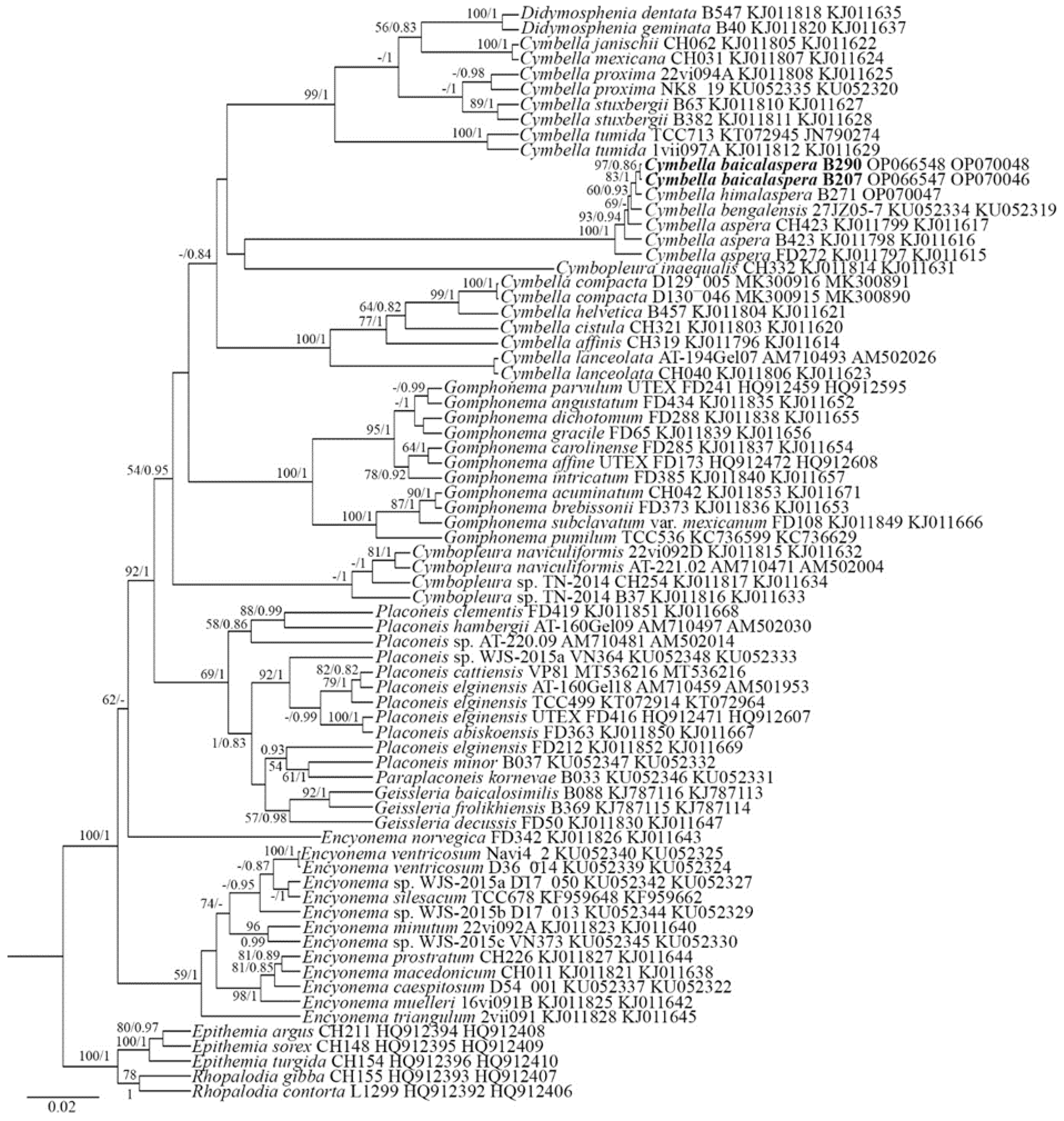
2. Results
2.1. Molecular Investigation
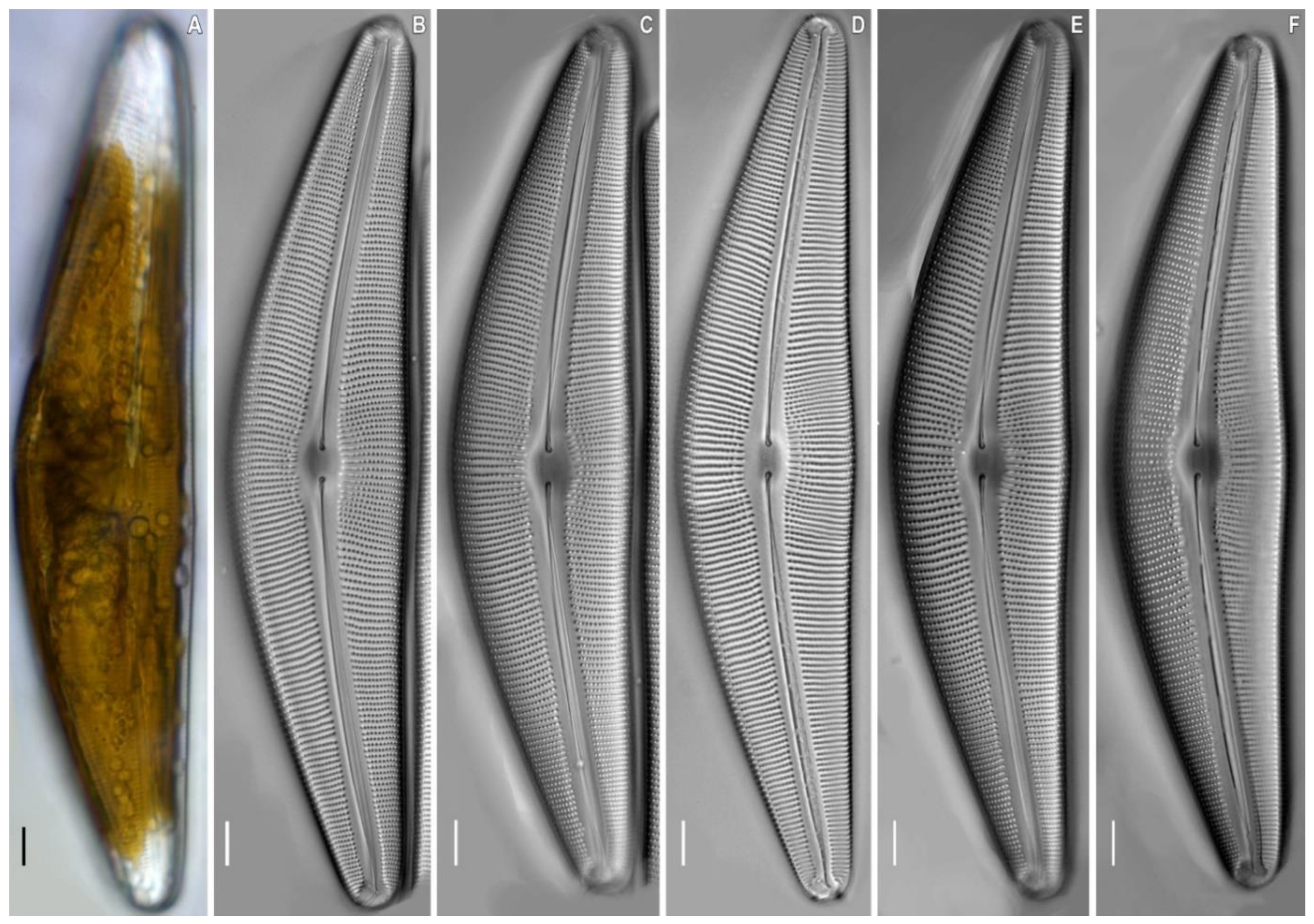
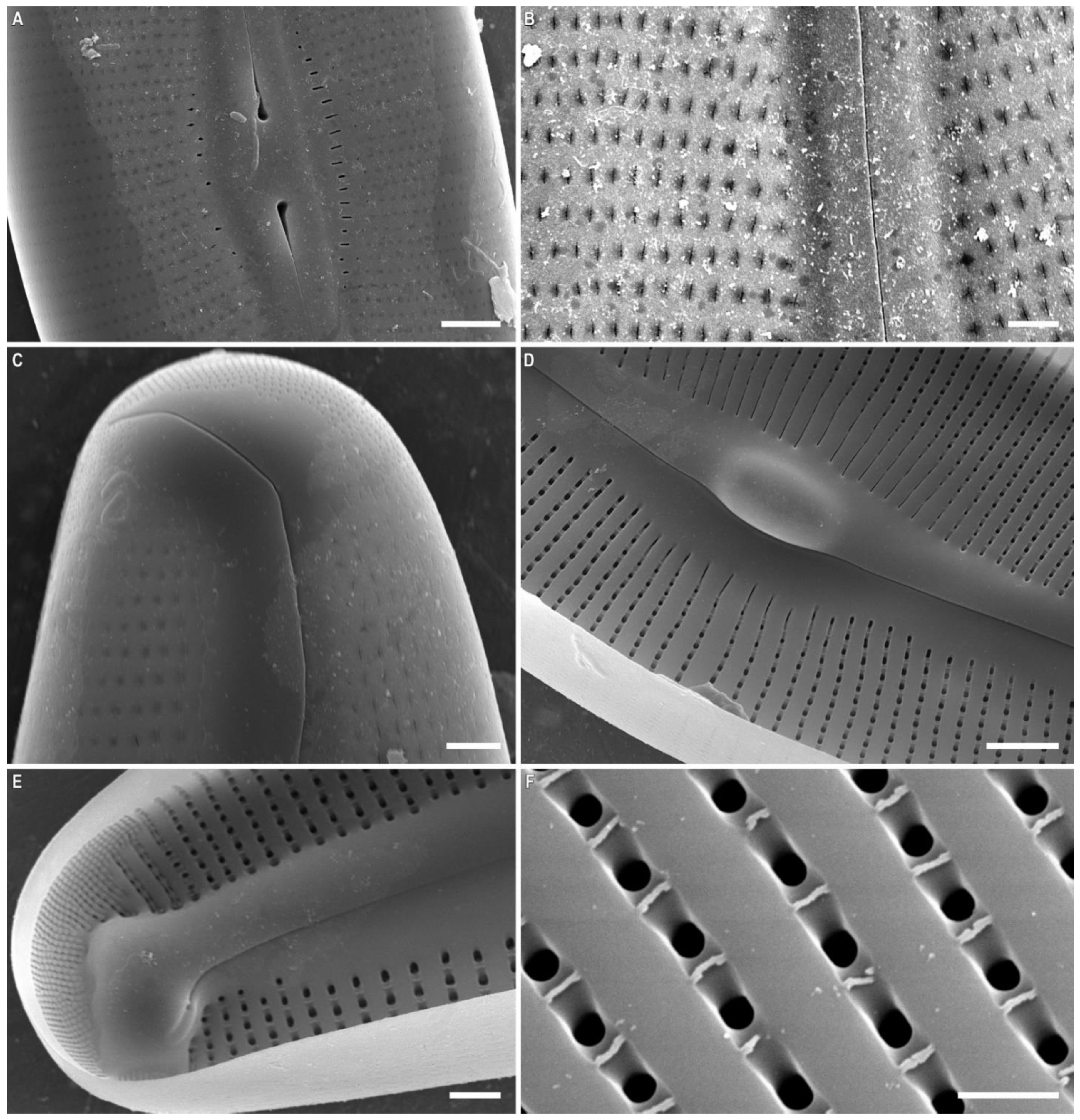
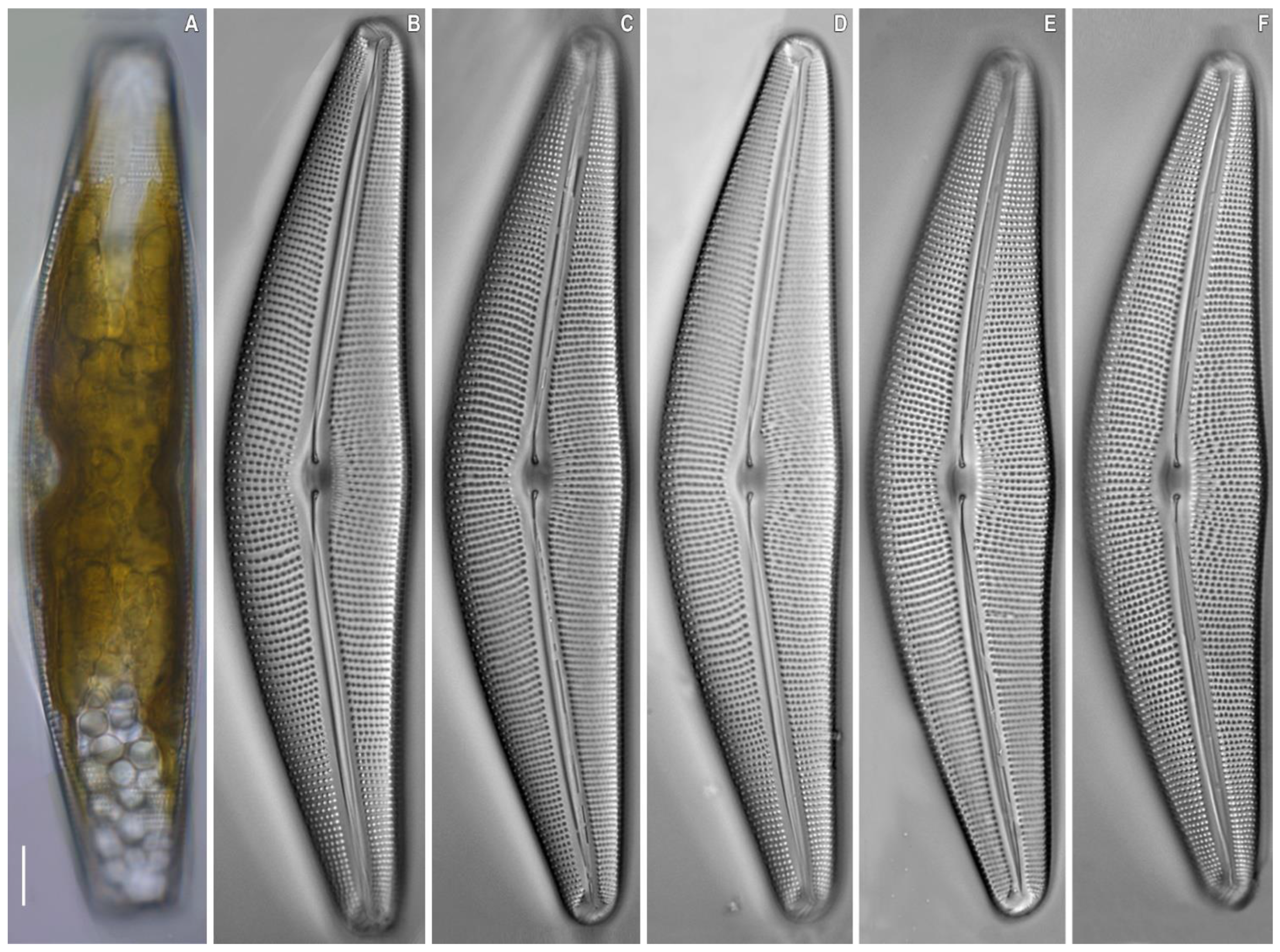
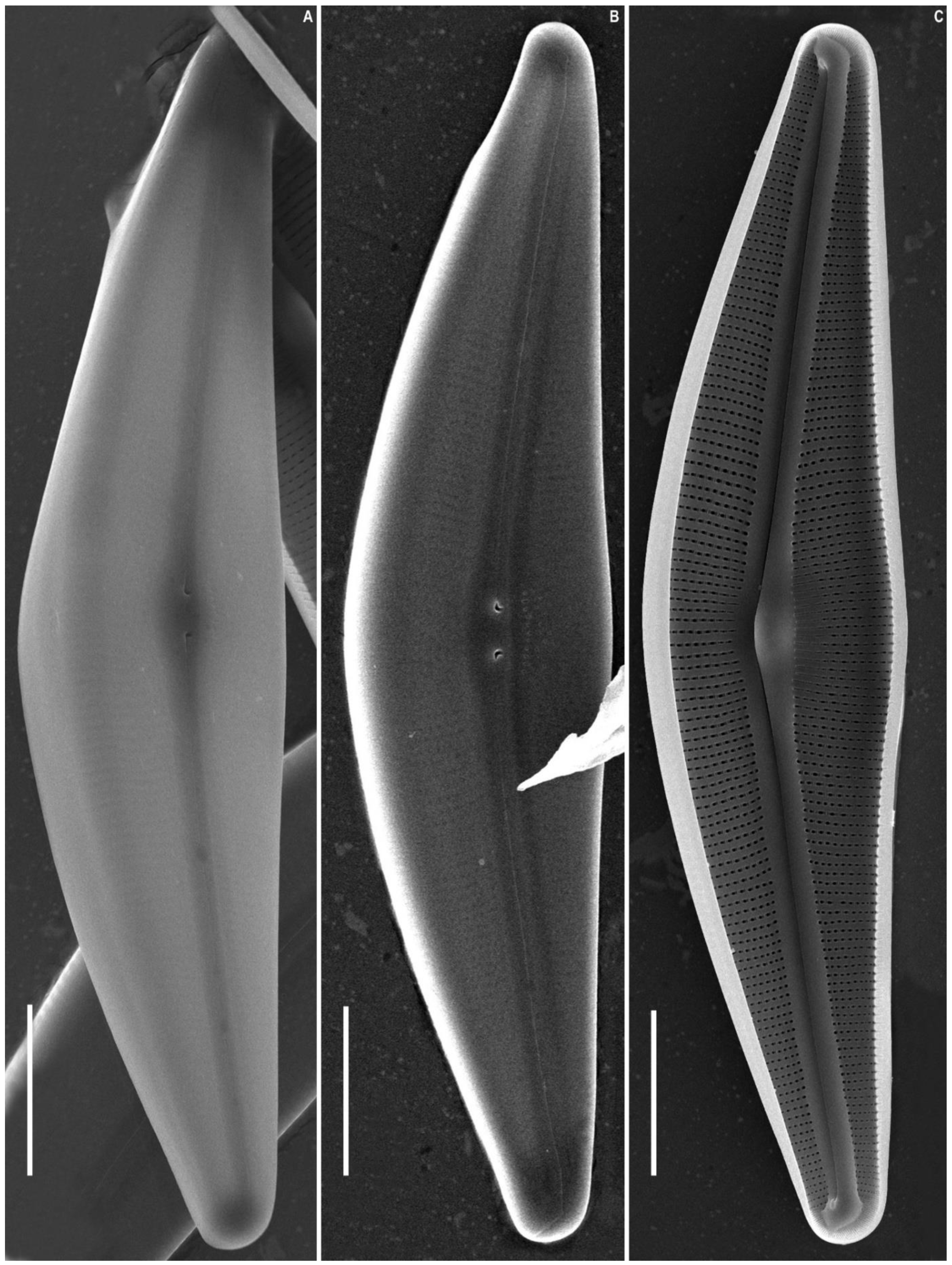
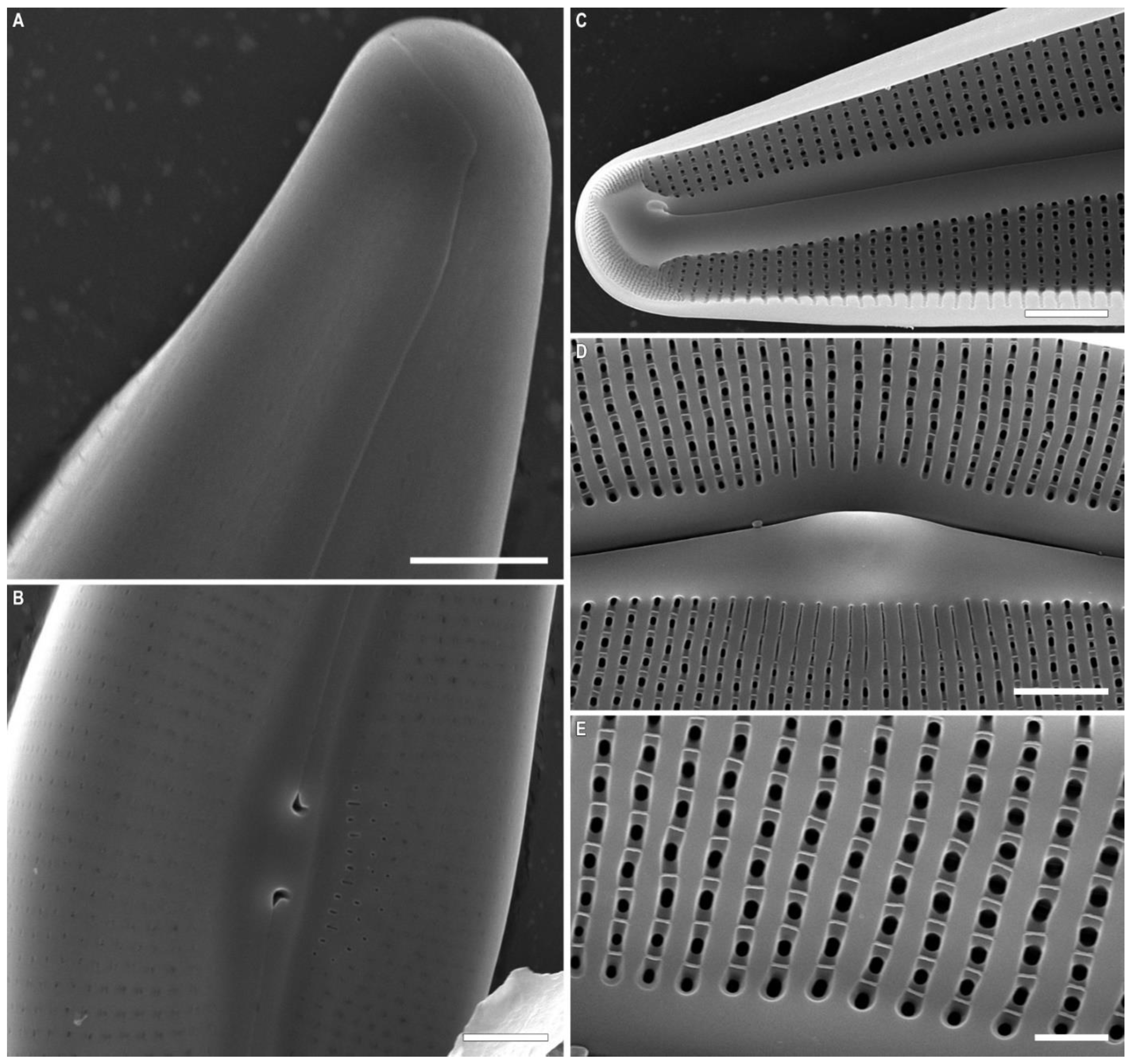
2.2. Morphological Investigation
3. Discussion
| Species/Feature | C. baicalaspera sp. nov. | C. himalaspera B271 | C. himalaspera | C. aspera | C. peraspera | C. subhimalaspera | C. ameliana |
| Central Raphe Endings | weakly deflected ventrally, tipped with inflated drop-shaped pores | weakly deflected ventrally, tipped with inflated drop-shaped pores | large drop-like, ventrally deflected | moderately large, round, almost straight or slightly ventrally deflected | moderately large, round, almost straight or slightly ventrally deflected | large drop-like, straight or slightly ventrally deflected | are typically crook-shaped and almost entirely curved backwards |
| Length, μm | 143.4–153.0 | 190.0–201.5 | 150–280 | 110–200 | (130) 154–320 | 104–133 | 160–270 |
| Breadth, μm | 30.6–32.2 | 40.0–42.4 | 35–49 | 26–35 | 44–52 | 21–25 | 40–46 |
| Length/Breadth Ratio | 4.6–4.8 | 4.6–4.8 | 4.1–5.6 | max. 5.7 | max. 6 | 4.9–5.8 | 5.5–6.0 |
| Striae in 10 μm | 8–9 (11–12 near the ends) | 7–8 (9.5–10.5 near the ends) | 7–9 | 6.5–8.0 (10 near the ends) | 5–8 (10 near the ends) | 8–11 (10–12 near the ends) | 7–8 (11–12 near the ends) |
| Areolae, Shape | X-, Y-, star- or tree-shaped closer to central part of valve, becoming longitudinally-slits to the valve ends | X-, Y-, star- or tree-shaped closer to central part of valve, becoming longitudinally-slits to the valve ends | X-, Y-, star-, tree-shaped | I-shaped | I-shaped | X-, I-shaped, oval, curved | X-, I-shaped, slit-like weakly curved |
| Areolae in 10 μm | 10–11 | 11–12 | 10–12 | 8–10 (11) | 7–10 (11) | 10–12 (15 near the ends) | 10–11 |
| Stigmata | 8–13, round and slit-like | 13–18, round and slit-like | large number, round to slightly elongate | 7–10 | large number, round | 8–11, elongate | large number, small round |
| Reference | This study | This study | [11] | [2,11] | [2,11] | [2,11] | [10] |
4. Materials and Methods
Molecular Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agardh, C.A. Conspectus Criticus Diatomacearum, Part 1; Lundae: Lund, Sweden, 1830; pp. 1–16. [Google Scholar]
- Krammer, K. Cymbella. Diatoms Eur. 2002, 3, 1–584. [Google Scholar]
- Krammer, K. Cymbopleura, Delicata, Navicymbula, Gomphocymbellopsis, Afrocymbella. Diatoms Eur. 2003, 4, 1–529. [Google Scholar]
- Jüttner, I.; Krammer, K.; Van de Vijver, B.; Tuji, A.; Simkhada, B.; Gurung, S.; Sharma, S.; Sharma, C.; Cox, E.J. Oricymba (Cymbellales, Bacillariophyceae), a new cymbelloid genus and three new species from the Nepalese Himalaya. Phycologia 2010, 49, 407–423. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Glushchenko, A.M.; Genkal, S.I.; Kuznetsova, I.V. Identification Book of Diatoms from Russia; Filigran: Yaroslavl, Russia, 2016; 804p. [Google Scholar]
- Kulikovskiy, M.; Kociolek, J.P.; Liu, Y.; Kuznetsova, I.; Glushchenko, A. Vladinikolaevia, gen. nov.—A new enigmatic freshwater diatom genus (Cymbellaceae; Bacillariophyceae) from Mongolia. Fottea 2022, 22, 1–7. [Google Scholar] [CrossRef]
- Glushchenko, A.; Kuznetsova, I.; Kociolek, J.P.; Kulikovskiy, M. Karthickia verestigmata gen. et sp. nov.—An interesting diatom with frustular morphology similar to several different cymbelloid diatom genera. Phycologia 2019, 58, 605–613. [Google Scholar] [CrossRef]
- Liu, Y.; Kociolek, J.P.; Kulikovskiy, M.S.; Glushchenko, A.M.; Yu, P.; Wang, Q.; Lu, X.; Fan, Y. A new genus of freshwater cymbelloid diatoms, Qinia gen. nov. from Yunnan Province, China. J. Oceanol. Limnol. 2022, 5, 1359–1368. [Google Scholar] [CrossRef]
- Voigt, M. Notes sur quelques espèces chinoises du genre Cymbella. Musée Heude, Université l’Aurore, Changhai. Notes Bot. Chin. 1942, 5, 1–46. [Google Scholar]
- Van de Vijver, B.; Lange-Bertalot, H. Cymbella amelieana sp. nov., a new large Cymbella species from Swedish rivers. Diatom Res. 2008, 23, 511–518. [Google Scholar] [CrossRef]
- Jüttner, I.; Gurung, S.; Sharma, C.; Sharma, S.; De Hann, M.; Van de Vijver, B. Morphology of new taxa in the Cymbella aspera and Cymbella neocistula groups, Cymbella yakii sp. nov., and Cymbella cf. hantzschiana form Everest National Park, Nepal. Pol. Bot. J. 2010, 55, 73–92. [Google Scholar]
- Da Silva, W.J.; Souza, M.D.G.M.D.; Proença, C.E.B. Cymbella neolanceolata sp. nov., a species formerly known as Cymbella lanceolata. Diatom Res. 2013, 28, 131–138. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Lange-Bertalot, H.; Metzeltin, D.; Witkowski, A. Lake Baikal: Hotspot of endemic diatoms I. Iconogr. Diatomol. 2012, 23, 7–607. [Google Scholar]
- Andreeva, S.A.; Kociolek, J.P.; Maltsev, Y.I.; Dorofeyuk, N.I.; Kezlya, E.S.; Shkurina, N.A.; Kuznetsova, I.V.; Gusev, E.S.; Kulikovskiy, M.S. Sellaphora balashovae (Bacillariophyta), a new species from Siberian mountain Lake Frolikha (Baikal region), Russia. Phytotaxa 2018, 371, 73–83. [Google Scholar] [CrossRef]
- Rimet, F.; Gusev, E.S.; Kahlert, M.; Kelly, M.; Kulikovskiy, M.S.; Maltsev, Y.I.; Mann, D.; Pfannkuchen, M.; Trobajo, R.; Vasselon, V.; et al. Diat.barcode, an open-access barcode library for diatoms. Sci. Rep. 2019, 9, 15116. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, Y.I.; Maltseva, S.Y.; Kociolek, J.P.; Jahn, R.; Kulikovskiy, M.S. Biogeography of the cosmopolitan terrestrial diatom Hantzschia amphioxys sensu lato based on molecular and morphological data. Sci. Rep. 2021, 11, 4266. [Google Scholar] [CrossRef] [PubMed]
- Kulikovskiy, M.S.; Kuznetsova, I.V. Biogeography of Bacillariophyta. Main Concepts and Approaches. Int. J. Algae 2014, 16, 207–228. [Google Scholar] [CrossRef]
- Glushchenko, A.M.; Gusev, E.S.; Maltsev, Y.I.; Kociolek, J.P.; Kuznetsova, I.V.; Kulikovskiy, M.S. Cymbopleura natellia—a new species from Transbaikal area (Russia, Siberia) described on the basis of molecular and morphological investigation. PhytoKeys 2021, 183, 95–105. [Google Scholar] [CrossRef]
- Kezlya, E.M.; Glushchenko, A.M.; Maltsev, Y.I.; Gusev, E.S.; Genkal, S.I.; Kociolek, J.P.; Kulikovskiy, M.S. Three New Species of Placoneis Mereschkowsky (Bacillariophyceae: Cymbellales) with Comments on Cryptic Diversity in the P. elginensis—Group. Water 2021, 13, 3276. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Lange-Bertalot, H.; Witkowski, A. Gliwiczia gen. nov. a new monoraphid diatom genus from Lake Baikal with a description of four species new for science. Phytotaxa 2013, 109, 1–16. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Gusev, E.; Andreeva, S.; Annenkova, N. Phylogenetic position of the diatom genus Geissleria Lange-Bertalot & Metzeltin and description of two new species from Siberian mountain lakes. Phytotaxa 2014, 177, 249–260. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Lange-Bertalot, H.; Annenkova, N.V.; Gusev, E.S.; Kociolek, J.P. Morphological and molecular evidence support description of two new diatom species from the genus Ulnaria in Lake Baikal. Fottea 2016, 16, 34–42. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Glushchenko, A.M.; Kuznetsova, I.V.; Genkal, S.I.; Kociolek, J.P. Gololobovia gen. nov.—A new genus from Lake Baikal with comments on pore occlusion in monoraphid diatoms. Phycologia 2020, 59, 616–633. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Glushchenko, A.M.; Genkal, S.; Kuznetsova, I.V.; Kociolek, J.P. Platebaikalia—a new monoraphid diatom genus from ancient Lake Baikal with comments on the genus Platessa. Fottea 2020, 20, 58–67. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Lange-Bertalot, H.; Kuznetsova, I.V. Lake Baikal: Hotspot of endemic diatoms II. Iconogr. Diatomol. 2015, 26, 1–657. [Google Scholar]
- Guillard, R.R.L.; Lorenzen, C.J. Yellow-green algae with chlorophyllide c. J. Phycol. 1972, 8, 10–14. [Google Scholar] [CrossRef]
- Zimmermann, J.; Jahn, R.; Gemeinholzer, B. Barcoding diatoms: Evaluation of the V4 subregion on the 18S rRNA gene, including new primers and protocols. Org. Divers. Evol. 2011, 11, 173–192. [Google Scholar] [CrossRef]
- Ruck, E.C.; Theriot, E.C. Origin and Evolution of the Canal Raphe System in Diatoms. Protist 2011, 162, 723–737. [Google Scholar] [CrossRef]
- Alverson, A.J.; Jansen, R.K.; Theriot, E.C. Bridging the Rubicon: Phylogenetic analysis reveals repeated colonizations of marine and fresh waters by thalassiosiroid diatoms. Mol. Phylogenetics Evol. 2007, 45, 193–210. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Katoh, K.; Toh, H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 2010, 26, 1899–1900. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A Rapid Bootstrap Algorithm for the RAxML Web Servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef] [PubMed]


| Strains | Slide No | Sample Locality | Collection of Date | Coordinates | t (°C) | pH | Cond. (μS cm−1) | Substratum | GenBank Accession Number, SSU rDNA, Partial | GenBank Accession Number, rbcL, Partial |
|---|---|---|---|---|---|---|---|---|---|---|
| B207 | B207 | Sample no. 11.2, Eastern Siberia, Buryatia, Zagza River | 15 July 2011 | 52°31.656′ N 107°05.114′ E | 14 | 8.5 | 40 | periphyton | OP070046 | OP066547 |
| B271 | B271 | Sample no. 28.1, Eastern Siberia, Buryatia, unnamed river near the Zarechnyj Village | 17 July 2011 | 52°33.418′ N 107°08.564′ E | 15.4 | 6.1 | 63 | benthos | OP070047 | – |
| B290 | B290 | Sample no. 11.2, Eastern Siberia, Buryatia, Zagza River | 15 July 2011 | 52°31.656′ N 107°05.114′ E | 14 | 8.5 | 40 | periphyton | OP070048 | OP066548 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glushchenko, A.M.; Maltsev, Y.I.; Kociolek, J.P.; Kuznetsova, I.V.; Kulikovskiy, M.S. Molecular and Morphological Investigations of Two Giant Diatom Cymbella Species from the Transbaikal Area (Russia, Siberia) with Comments on Their Distributions. Plants 2022, 11, 2445. https://doi.org/10.3390/plants11182445
Glushchenko AM, Maltsev YI, Kociolek JP, Kuznetsova IV, Kulikovskiy MS. Molecular and Morphological Investigations of Two Giant Diatom Cymbella Species from the Transbaikal Area (Russia, Siberia) with Comments on Their Distributions. Plants. 2022; 11(18):2445. https://doi.org/10.3390/plants11182445
Chicago/Turabian StyleGlushchenko, Anton M., Yevhen I. Maltsev, John Patrick Kociolek, Irina V. Kuznetsova, and Maxim S. Kulikovskiy. 2022. "Molecular and Morphological Investigations of Two Giant Diatom Cymbella Species from the Transbaikal Area (Russia, Siberia) with Comments on Their Distributions" Plants 11, no. 18: 2445. https://doi.org/10.3390/plants11182445
APA StyleGlushchenko, A. M., Maltsev, Y. I., Kociolek, J. P., Kuznetsova, I. V., & Kulikovskiy, M. S. (2022). Molecular and Morphological Investigations of Two Giant Diatom Cymbella Species from the Transbaikal Area (Russia, Siberia) with Comments on Their Distributions. Plants, 11(18), 2445. https://doi.org/10.3390/plants11182445






