Membrane Localized GbTMEM214s Participate in Modulating Cotton Resistance to Verticillium Wilt
Abstract
:1. Introduction
2. Results
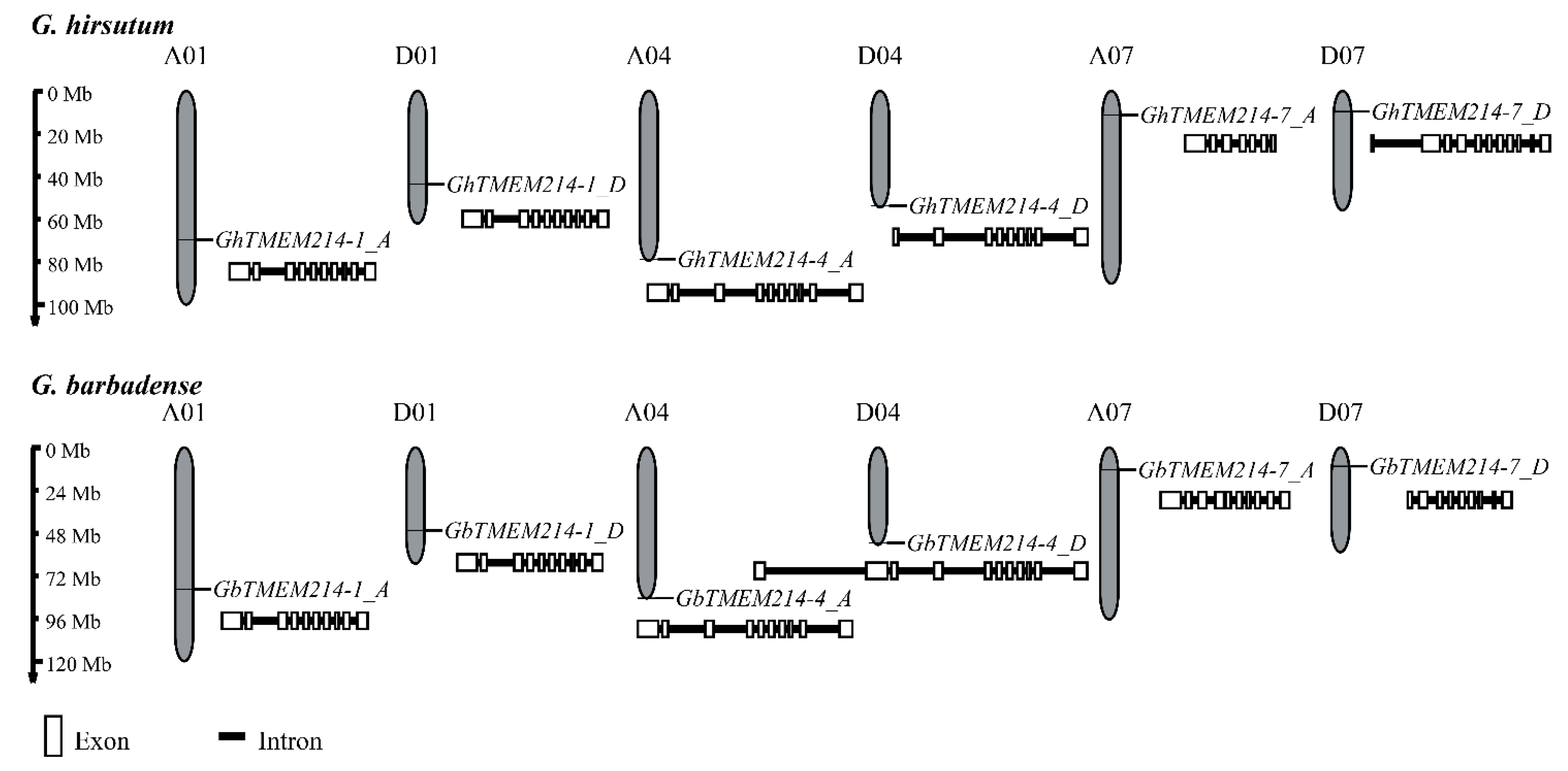
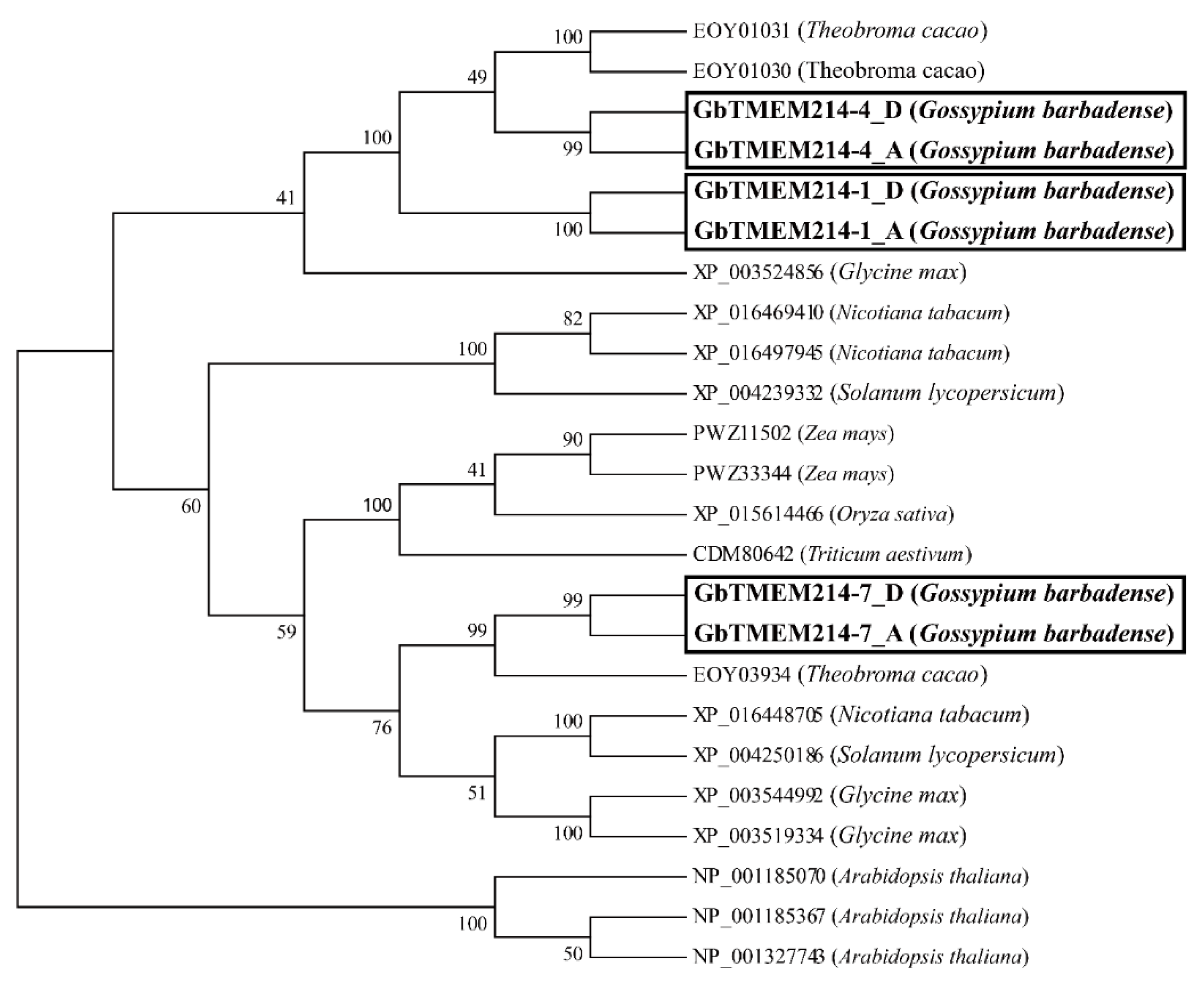
2.1. Identification and Phylogenetic Analysis of GbTMEM214s
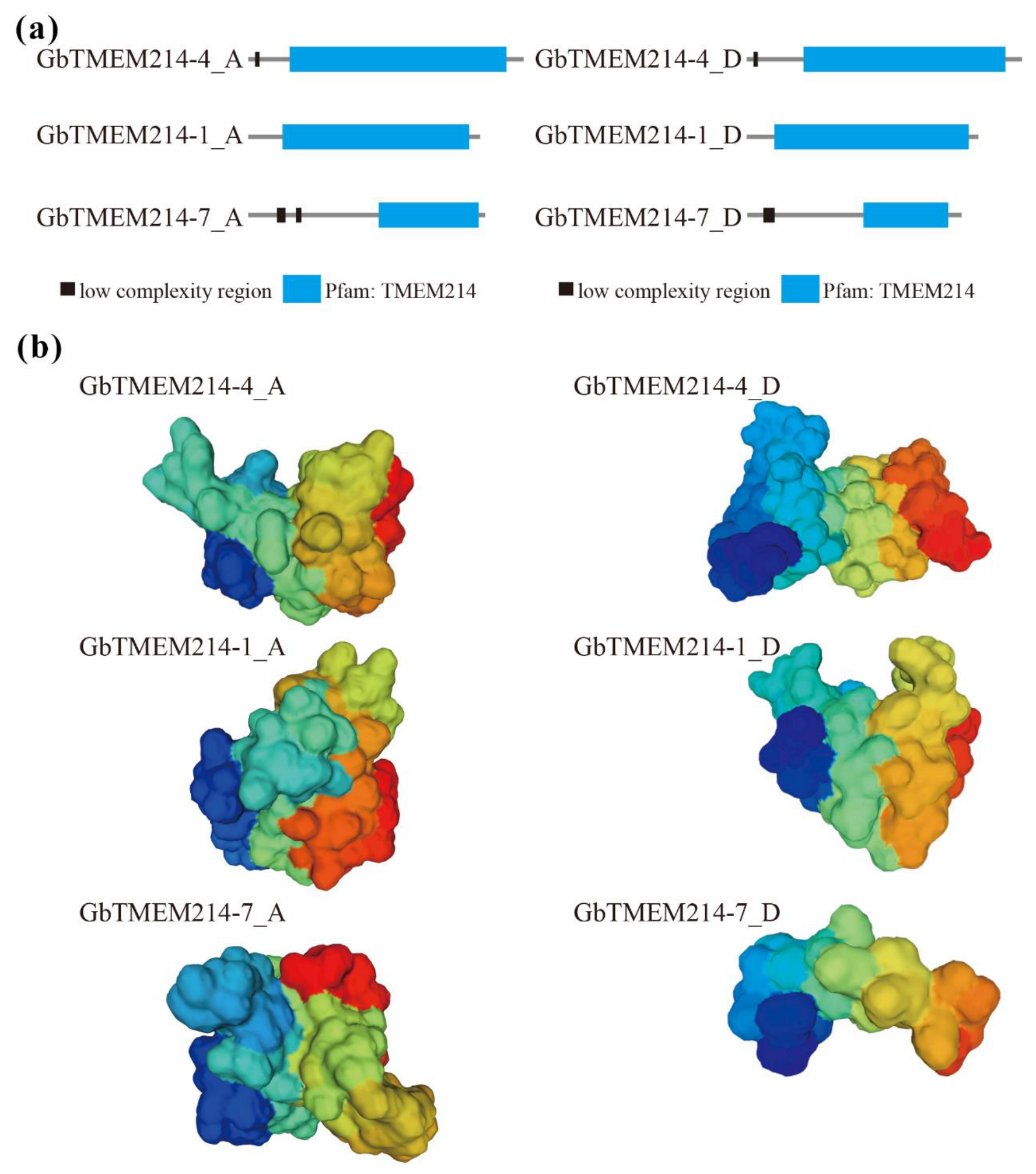
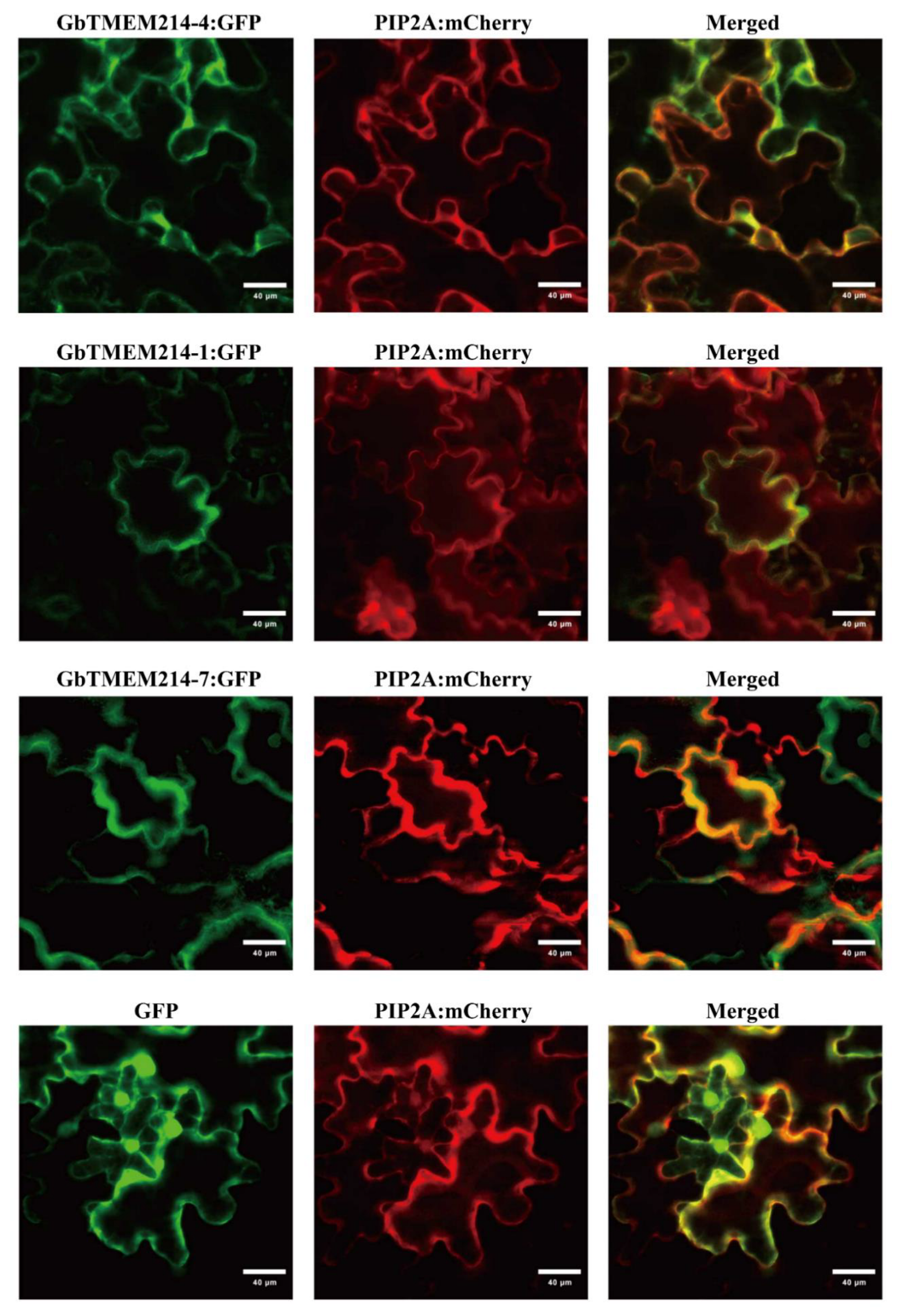
2.2. Protein Structure and Subcellular Localization of GbTMEM214s
2.3. Verticillium Dahliae Induced Expression Analysis of TMEM214s in Cotton
2.4. Phytohormone Induced Expression Analysis of GbTMEM214s
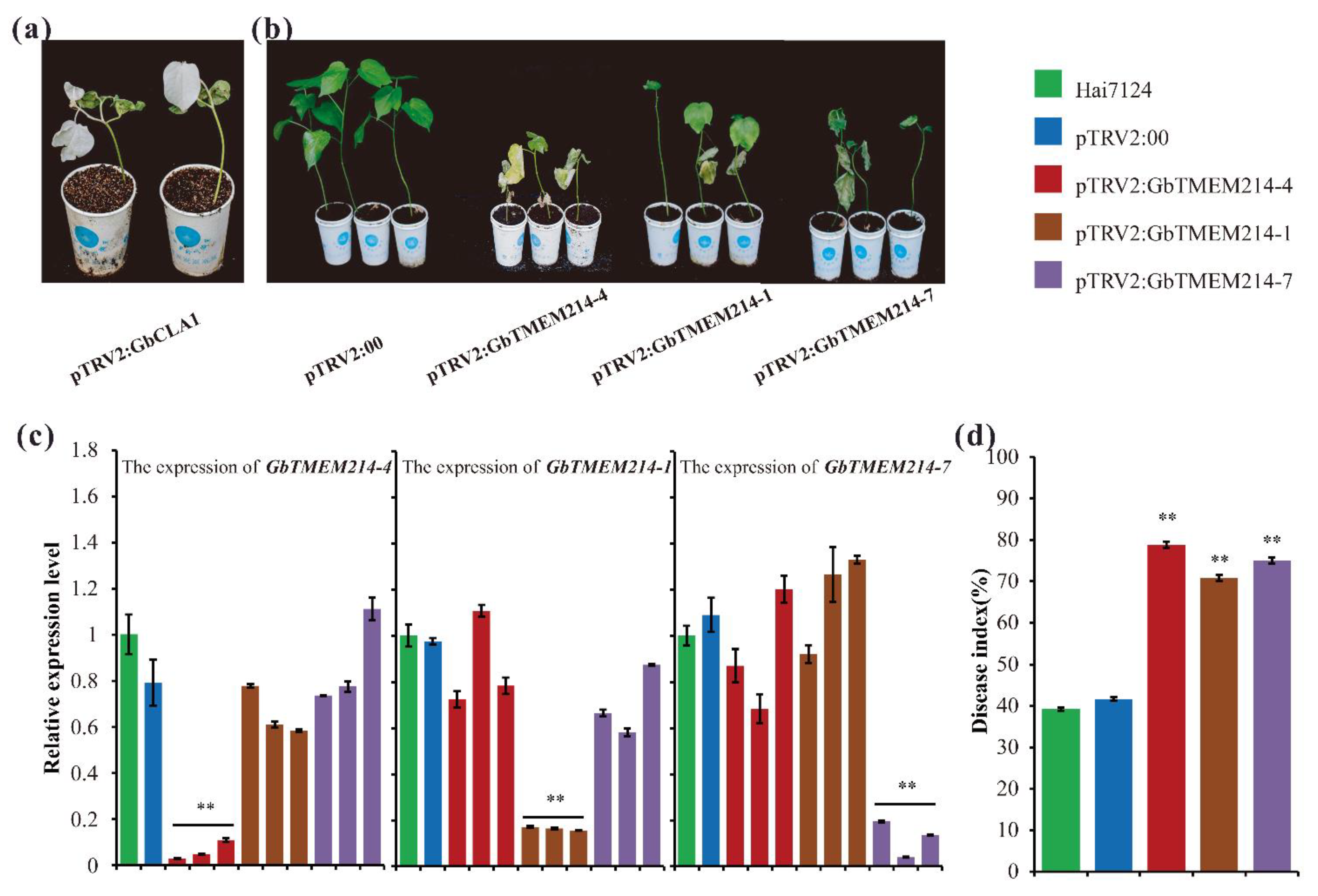
2.5. Resistance Function Analysis of GbTMEM214s
3. Discussion
4. Materials and Methods
4.1. The Bioinformatics Analysis of TMEM214 Superfamily
4.2. Protein Structure Analysis of TMEM214s
4.3. Plant Materials and Treatments
4.4. RNA Isolation and Expression Pattern Analysis
4.5. Cloning of the GbTMEM214s in Hai7124
4.6. VIGS Experiments
4.7. Subcellular Localization of GbTMEMs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, J.; Liu, J.; Xu, J.; Zhao, L.; Wu, Q.; Xiao, S. Quantitative trait locus mapping and candidate gene analysis for Verticillium Wilt resistance using Gossypium barbadense chromosomal segment introgressed line. Front. Plant Sci. 2018, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Li, Y.; Han, X.; Shen, F. Genome-wide profiling of miRNAs and other small non-coding RNAs in the Verticillium dahliae-inoculated cotton roots. PLoS ONE 2012, 7, e35765. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Jiang, H.; Zhu, X.; Wang, W.; He, X.; Shi, Y.; Yuan, Y.; Du, X.; Cai, Y. Analysis of sea-island cotton and upland cotton in response to Verticillium dahliae infection by RNA sequencing. BMC Genom. 2013, 14, 852. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, F.; Li, M.; Kianinejad, A.S.; Dever, J.K.; Wheeler, T.A.; Li, Z.; He, P.; Shan, L. Cotton GhBAK1 mediates Verticillium wilt resistance and cell death. J. Integr. Plant Biol. 2013, 55, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wheeler, T.; Li, Z.; Kenerley, C.M.; He, P.; Shan, L. Silencing GhNDR1 and GhMKK2 compromises cotton resistance to Verticillium wilt. Plant J. 2011, 66, 293–305. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Wang, S.; Wang, J.; Chen, X. Characterization of GaWRKY1, a Cotton Transcription Factor That Regulates the Sesquiterpene Synthase Gene (+)-δ-Cadinene Synthase. Plant Physiol. 2004, 135, 507–515. [Google Scholar] [CrossRef]
- Naoumkina, M.A.; Zhao, Q.; Gallego-Giraldo, L.; Dai, X.; Zhao, P.X.; Dixon, R.A. Genome-wide analysis of phenylpropanoid defence pathways. Mol. Plant Pathol. 2010, 11, 829–846. [Google Scholar] [CrossRef]
- Gayoso, C.; Pomar, F.; Novo-Uzal, E.; Merino, F.; de Ilárduya, Ó.M. The Ve-mediated resistance response of the tomato to Verticillium dahliae involves H2O2, peroxidase and lignins and drives PAL gene expression. BMC Plant Biol. 2010, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Staal, J.; Dixelius, C. Early responses in the Arabidopsis-Verticillium longisporum pathosystem are dependent on NDR1, JA- and ET-associated signals via cytosolic NPR1 and RFO1. Mol. Plant-Microbe Interact. 2006, 19, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.J.; Sun, Y.X.; Zhu, X.L.; Wang, X.F.; Zhang, Y.; Yang, J.; Yan, G.J.; Ma, Z.Y. Cotton S-adenosylmethionine decarboxylase-mediated spermine biosynthesis is required for salicylic acid- and leucine-correlated signaling in the defense response to Verticillium dahliae. Planta 2016, 243, 1023–1039. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, L.; Wang, Y.; Valkenburg, D.J.; Zhang, X.; Zhu, L.; Thomma, B. Transfer of tomato immune receptor Ve1 confers Ave1-dependent Verticillium resistance in tobacco and cotton. Plant Biotechnol. J. 2018, 16, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, Y.; Chen, T.; Yu, W.; Liu, T.; Li, H.; Fan, X.; Ren, Y.; Shen, D.; Liu, L. Island Cotton Gbve1 Gene Encoding A Receptor-Like Protein Confers Resistance to Both Defoliating and Non-Defoliating Isolates of Verticillium dahliae. PLoS ONE 2012, 7, e51091. [Google Scholar]
- Zhang, Y.; Wang, X.; Yang, S.; Chi, J.; Zhang, G.; Ma, Z. Cloning and characterization of a Verticillium wilt resistance gene from Gossypium barbadense and functional analysis in Arabidopsis thaliana. Plant Cell Rep. 2011, 30, 2085–2096. [Google Scholar] [CrossRef]
- Li, C.; He, X.; Luo, X.; Xu, L.; Liu, L.; Min, L.; Jin, L.; Zhu, L.; Zhang, X. Cotton WRKY1 mediates the plant defense-to-development transition during infection of cotton by Verticillium dahliae by activating JASMONATE ZIM-DOMAIN1 expression. Plant Physiol. 2014, 166, 2179–2194. [Google Scholar] [CrossRef] [PubMed]
- Jun, Z.; Zhang, Z.; Gao, Y.; Zhou, L.; Fang, L.; Chen, X.; Ning, Z.; Chen, T.; Guo, W.; Zhang, T. Overexpression of GbRLK, a putative receptor-like kinase gene, improved cotton tolerance to Verticillium wilt. Sci. Rep. 2015, 5, 15048. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ling, X.; Chen, T.; Cai, L.; Liu, T.; Wang, J.; Fan, X.; Ren, Y.; Yuan, H.; Zhu, W.; et al. A cotton Gbvdr5 gene encoding a leucine-rich-repeat receptor-Like protein confers resistance to Verticillium dahliae in transgenic Arabidopsis and Upland cotton. Plant Mol. Biol. Rep. 2015, 33, 987–1001. [Google Scholar] [CrossRef]
- Guo, W.; Jin, L.; Miao, Y.; He, X.; Hu, Q.; Guo, K.; Zhu, L.; Zhang, X. An ethylene response-related factor, GbERF1-like, from Gossypium barbadense improves resistance to Verticillium dahliae via activating lignin synthesis. Plant Mol. Biol. 2016, 91, 305–318. [Google Scholar] [CrossRef]
- Li, Y.B.; Han, L.B.; Wang, H.Y.; Zhang, J.; Sun, S.T.; Feng, D.Q.; Yang, C.L.; Sun, Y.D.; Zhong, N.Q.; Xia, G.X. The Thioredoxin GbNRX1 Plays a Crucial Role in Homeostasis of Apoplastic Reactive Oxygen Species in Response to Verticillium dahliae Infection in Cotton. Plant Physiol. 2016, 170, 2392–2406. [Google Scholar] [CrossRef]
- Chai, Q.; Shang, X.; Wu, S.; Zhu, G.; Cheng, C.; Cai, C.; Wang, X.; Guo, W. 5-Aminolevulinic Acid Dehydratase gene dosage affects programmed cell death and immunity. Plant Physiol. 2017, 175, 511–528. [Google Scholar] [CrossRef]
- Miao, W.; Wang, X.; Li, M.; Song, C.; Wang, Y.; Hu, D.; Wang, J. Genetic transformation of cotton with a harpin-encoding gene hpaXoo confers an enhanced defense response against different pathogens through a priming mechanism. BMC Plant Biol. 2010, 10, 67. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, X.; Liang, B.; Li, S.; Wu, Z.; Wang, Q.; Leng, C.; Dong, J.; Wang, T. Expression of baculovirus anti-apoptotic genes p35 and op-iap in cotton (Gossypium hirsutum L.) enhances tolerance to verticillium wilt. PLoS ONE 2010, 5, e14218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaspar, Y.M.; McKenna, J.A.; McGinness, B.S.; Hinch, J.; Poon, S.; Connelly, A.A.; Anderson, M.A.; Heath, R.L. Field resistance to Fusarium oxysporum and Verticillium dahliae in transgenic cotton expressing the plant defensin NaD1. J. Exp. Bot. 2014, 65, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, J.; Ding, L.; Zou, L.; Li, Y.; Chen, G.; Zhang, T. Constitutive expression of a novel antimicrobial protein, Hcm1, confers resistance to both Verticillium and Fusarium wilts in cotton. Sci. Rep. 2016, 6, 20773. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, G.; Chen, L.; Kim, H.S.; Pi, L.; Holsten, T.; Gardner, J.; Wang, B.; Zhai, W.; Zhu, L.; et al. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 1995, 270, 1804–1806. [Google Scholar] [CrossRef]
- Chen, X.; Shang, J.; Chen, D.; Lei, C.; Zou, Y.; Zhai, W.; Liu, G.; Xu, J.; Ling, Z.; Cao, G.; et al. A B-lectin receptor kinase gene conferring rice blast resistance. Plant J. 2006, 46, 794–804. [Google Scholar] [CrossRef]
- Robatzek, S.; Chinchilla, D.; Boller, T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 2006, 20, 537–542. [Google Scholar] [CrossRef]
- Deng, Y.; Zhai, K.; Zhen, X.; Yang, D.; Zhu, X.; Liu, J.; Wang, X.; Qin, P.; Yang, Y.; Zhang, G.; et al. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 2017, 355, 962–965. [Google Scholar] [CrossRef]
- Katoh, M.; Katoh, M. Characterization of human TMEM16G gene in silico. Int. J. Mol. Med. 2004, 14, 759–764. [Google Scholar] [CrossRef]
- Galietta, L.J.V. TMEM16A (ANO1) as a therapeutic target in cystic fibrosis. Curr. Opin. Pharm. 2022, 64, 102206. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Wang, W.; Feng, B.; Zhou, J.M.; Tang, D. Plant immune signaling: Advancing on two frontiers. J. Integr. Plant Biol. 2020, 62, 2–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanyuka, K.; Rudd, J.J. Cell surface immune receptors: The guardians of the plant’s extracellular spaces. Curr. Opin. Plant Biol. 2019, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chai, J. Structural insights into the plant immune receptors PRRs and NLRs. Plant Physiol. 2020, 182, 1566–1581. [Google Scholar] [CrossRef]
- Jamieson, P.A.; Shan, L.; He, P. Plant cell surface molecular cypher: Receptor-like proteins and their roles in immunity and development. Plant Sci. 2018, 274, 242–251. [Google Scholar] [CrossRef]
- Burkart, R.C.; Stahl, Y. Dynamic complexity: Plant receptor complexes at the plasma membrane. Curr. Opin. Plant Biol. 2017, 40, 15–21. [Google Scholar] [CrossRef]
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef]
- Saijo, Y.; Loo, E.P.; Yasuda, S. Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 2018, 93, 592–613. [Google Scholar] [CrossRef]
- Bohm, H.; Albert, I.; Fan, L.; Reinhard, A.; Nurnberger, T. Immune receptor complexes at the plant cell surface. Curr. Opin. Plant Biol. 2014, 20, 47–54. [Google Scholar] [CrossRef]
- Macho, A.P.; Zipfel, C. Plant PRRs and the activation of innate immune signaling. Mol. Cell 2014, 54, 263–272. [Google Scholar] [CrossRef]
- Wrzesinski, T.; Szelag, M.; Cieslikowski, W.A.; Ida, A.; Giles, R.; Zodro, E.; Szumska, J.; Pozniak, J.; Kwias, Z.; Bluyssen, H.A.; et al. Expression of pre-selected TMEMs with predicted ER localization as potential classifiers of ccRCC tumors. BMC Cancer 2015, 15, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentham, A.; Burdett, H.; Anderson, P.A.; Williams, S.J.; Kobe, B. Animal NLRs provide structural insights into plant NLR function. Ann. Bot. 2017, 119, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Calzado, M.A.; Bacher, S.; Schmitz, M.L. NF-kappaB inhibitors for the treatment of inflammatory diseases and cancer. Curr. Med. Chem. 2007, 14, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Dodeller, F.; Gottar, M.; Huesken, D.; Iourgenko, V.; Cenni, B. The lysosomal transmembrane protein 9B regulates the activity of inflammatory signaling pathways. J. Biol. Chem. 2008, 283, 21487–21494. [Google Scholar] [CrossRef] [PubMed]
- Condamine, T.; Le Texier, L.; Howie, D.; Lavault, A.; Hill, M.; Halary, F.; Cobbold, S.; Waldmann, H.; Cuturi, M.C.; Chiffoleau, E. Tmem176B and Tmem176A are associated with the immature state of dendritic cells. J. Leukocyte Biol. 2010, 88, 507–515. [Google Scholar] [CrossRef]
- Li, C.; Wei, J.; Li, Y.; He, X.; Zhou, Q.; Yan, J.; Zhang, J.; Liu, Y.; Liu, Y.; Shu, H.B. Transmembrane Protein 214 (TMEM214) mediates endoplasmic reticulum stress-induced caspase 4 enzyme activation and apoptosis. J. Biol. Chem. 2013, 288, 17908–17917. [Google Scholar] [CrossRef]
- Yang, K.; Rong, W.; Qi, L.; Li, J.; Wei, X.; Zhang, Z. Isolation and characterization of a novel wheat cysteine-rich receptor-like kinase gene induced by Rhizoctonia cerealis. Sci. Rep. 2013, 3, 3021. [Google Scholar] [CrossRef]
- Waadt, R.; Kudla, J. In Planta Visualization of Protein Interactions Using Bimolecular Fluorescence Complementation (BiFC). CSH Protoc. 2008, 5, prot4995. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Xu, J.; Wang, Y.; Liu, J.; Dong, C.; Zhao, L.; Ai, N.; Xu, Z.; Guo, Q.; Feng, G.; et al. Membrane Localized GbTMEM214s Participate in Modulating Cotton Resistance to Verticillium Wilt. Plants 2022, 11, 2342. https://doi.org/10.3390/plants11182342
Zhao J, Xu J, Wang Y, Liu J, Dong C, Zhao L, Ai N, Xu Z, Guo Q, Feng G, et al. Membrane Localized GbTMEM214s Participate in Modulating Cotton Resistance to Verticillium Wilt. Plants. 2022; 11(18):2342. https://doi.org/10.3390/plants11182342
Chicago/Turabian StyleZhao, Jun, Jianwen Xu, Yueping Wang, Jianguang Liu, Chengguang Dong, Liang Zhao, Nijiang Ai, Zhenzhen Xu, Qi Guo, Guoli Feng, and et al. 2022. "Membrane Localized GbTMEM214s Participate in Modulating Cotton Resistance to Verticillium Wilt" Plants 11, no. 18: 2342. https://doi.org/10.3390/plants11182342
APA StyleZhao, J., Xu, J., Wang, Y., Liu, J., Dong, C., Zhao, L., Ai, N., Xu, Z., Guo, Q., Feng, G., Xu, P., Cheng, J., Wang, X., Wang, J., & Xiao, S. (2022). Membrane Localized GbTMEM214s Participate in Modulating Cotton Resistance to Verticillium Wilt. Plants, 11(18), 2342. https://doi.org/10.3390/plants11182342