Incidence and Genetic Diversity of Grapevine Virus G in Croatian Vineyards
Abstract
:1. Introduction
2. Results
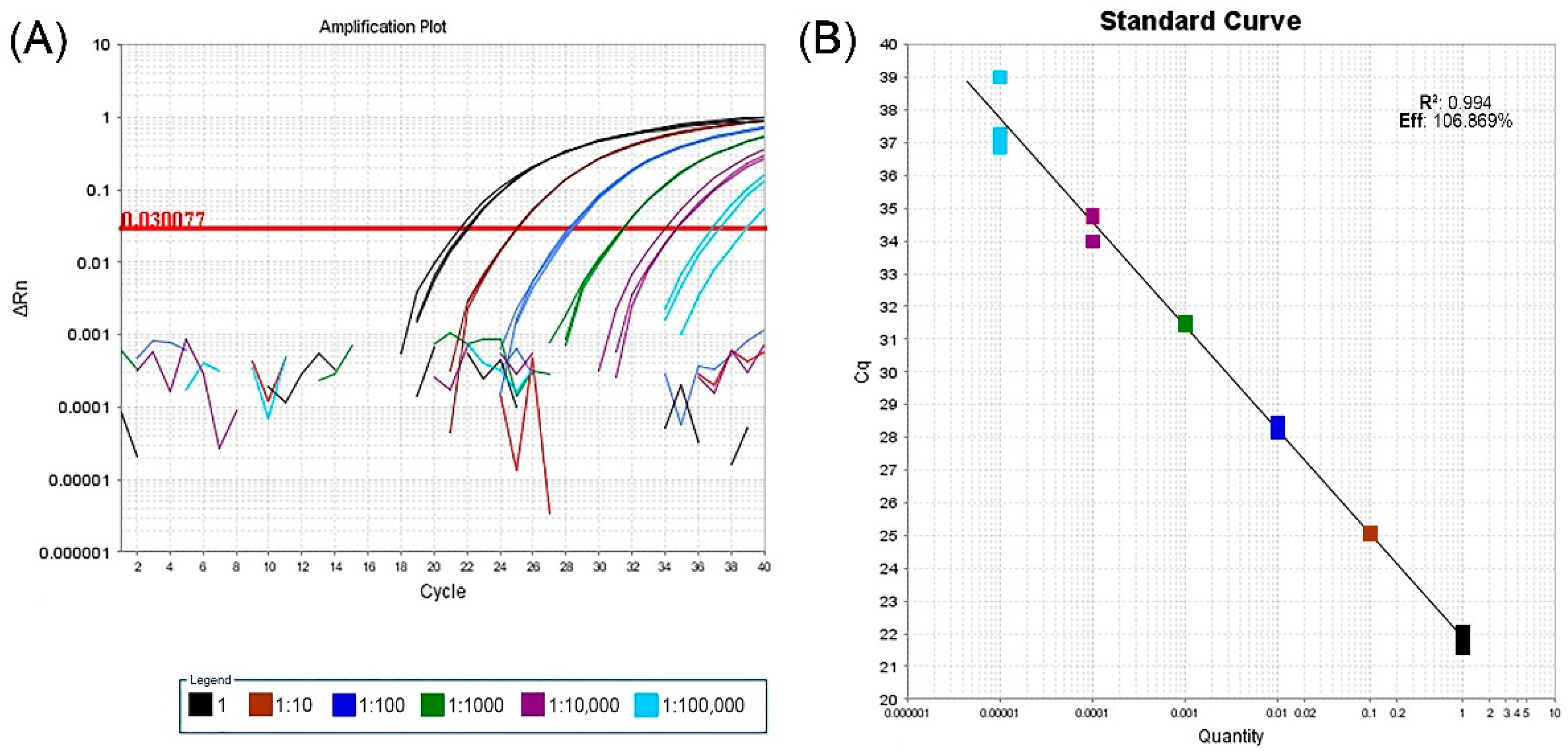
2.1. Real-Time RT-PCR Assay, HTS and Bioinformatic Analysis
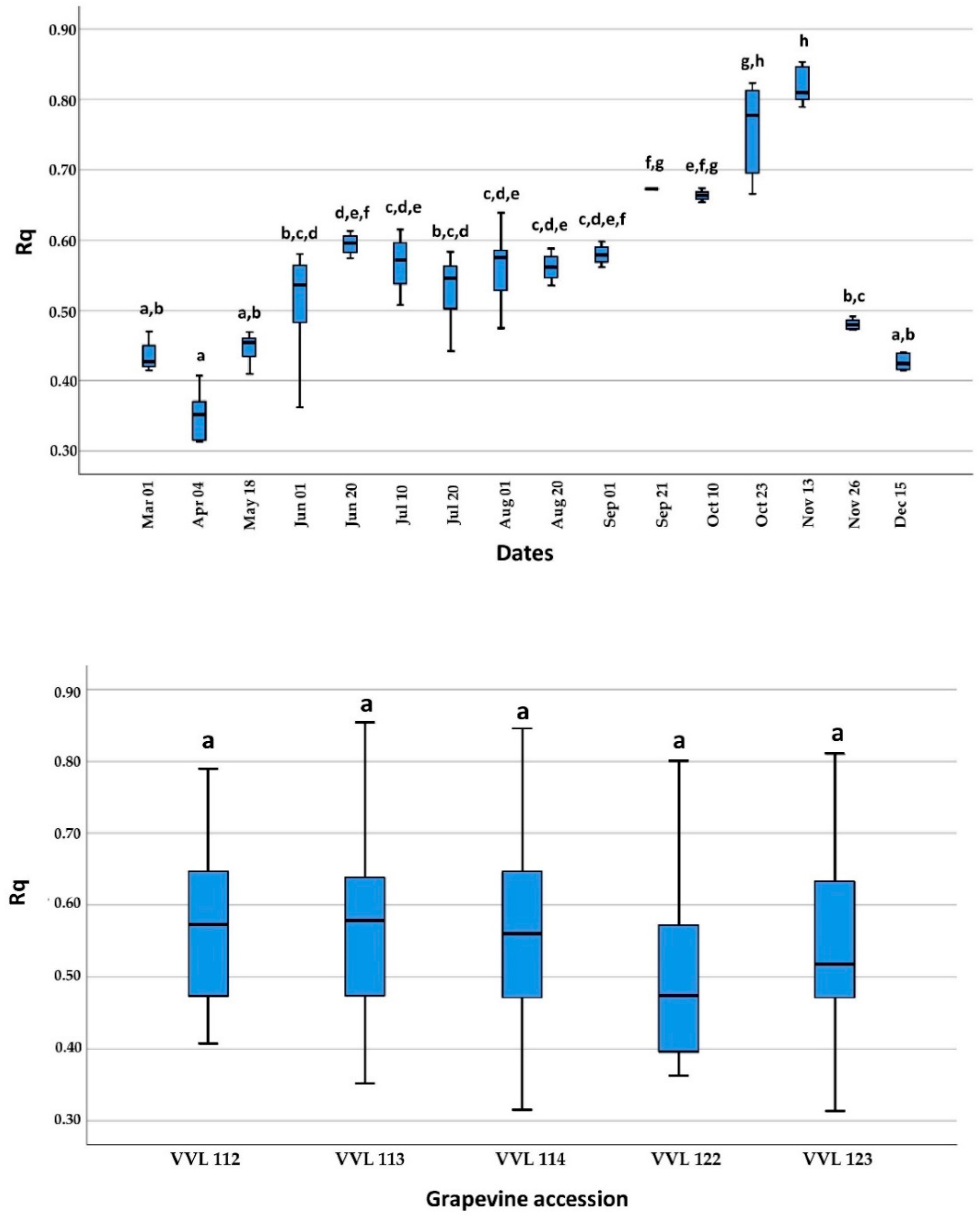
2.2. Detection during Dormancy and Growing Season
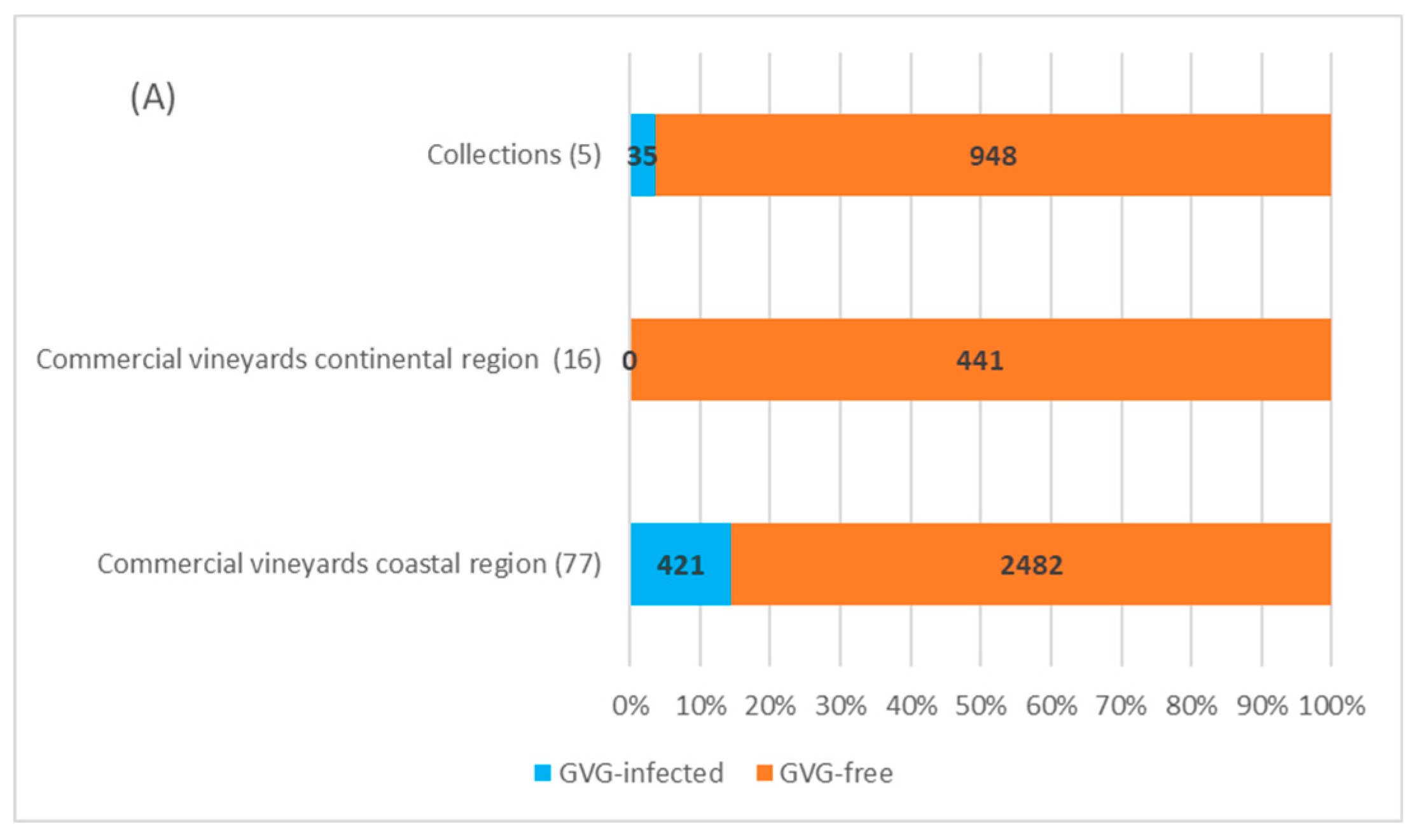
2.3. Field Survey
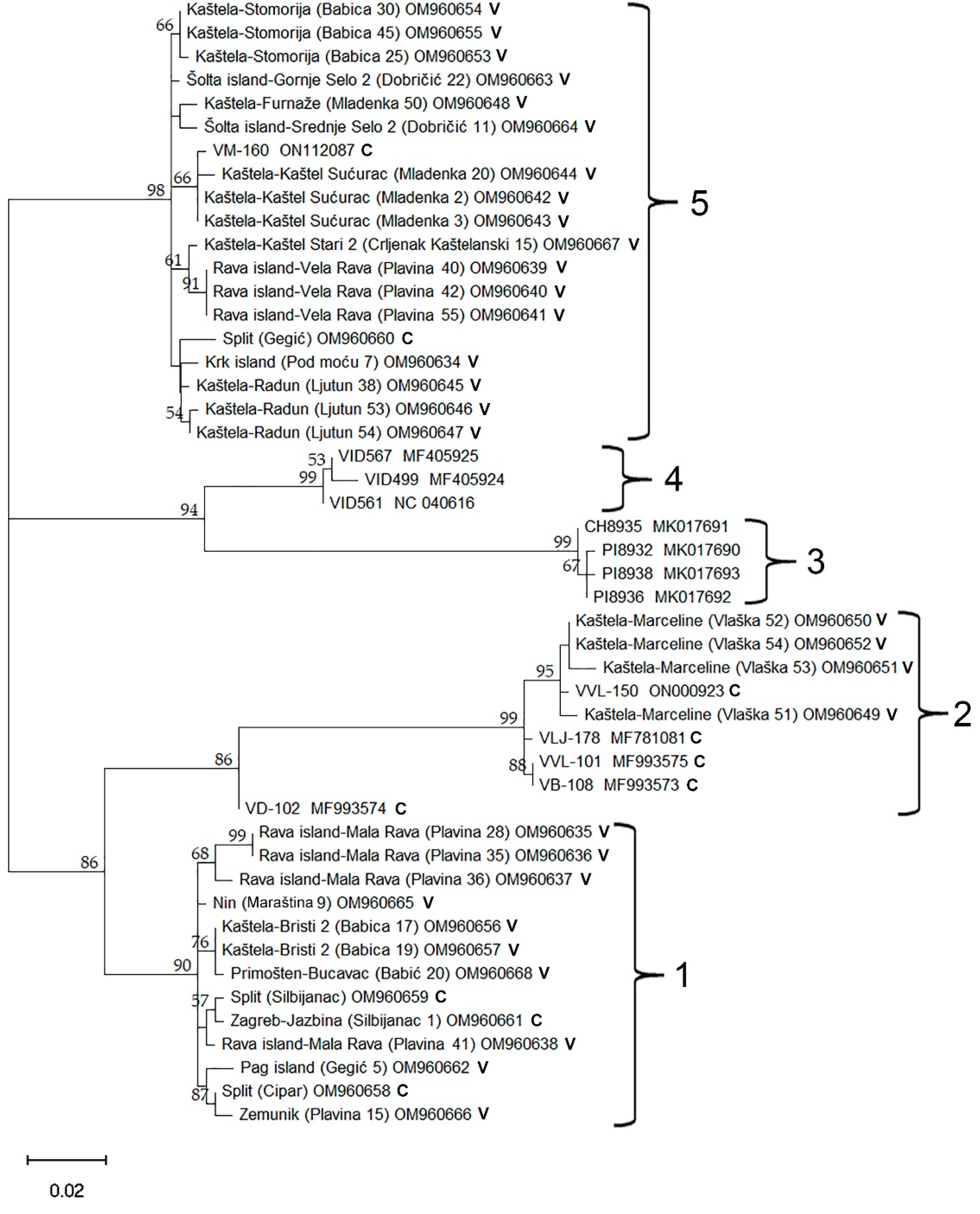
2.4. Phylogenetic Analysis Using Coat Protein Gene Sequence
3. Discussion
4. Materials and Methods
4.1. Real-Time RT-PCR Assay, HTS and Bioinformatic Analysis
4.2. Detection during Dormancy and Growing Season
4.3. Field Survey
4.4. Phylogenetic Analysis Using Coat Protein Gene Sequence
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conti, M.; Milne, R.G.; Luisoni, E.; Boccardo, G. A closterovirus from a stem-pitting-diseased grapevine. Phytopathology 1980, 70, 394–399. [Google Scholar] [CrossRef]
- Boscia, D.; Savino, V.; Minafra, A.; Namba, S.; Elicio, V.; Castellano, M.A.; Martelli, G.P. Properties of a filamentous virus isolated from grapevines affected by corky bark. Arch. Virol. 1993, 130, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Abou-Ghanem, N.; Saldarelli, P.; Minafra, A.; Buzkan, N.; Castellano, M.A.; Martelli, G.P. Properties of grapevine virus D, a novel putative trichovirus. J. Plant Pathol. 1997, 79, 15–25. [Google Scholar]
- Nakaune, R.; Toda, S.; Mochizuki, M.; Nakano, M. Identification and characterization of a new vitivirus from grapevine. Arch. Virol. 2008, 153, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Al Rwahnih, M.; Sudarshana, M.R.; Uyemoto, J.K.; Rowhani, A. Complete genome sequence of a novel vitivirus isolated from grapevine. J. Virol. 2012, 86, 9545. [Google Scholar] [CrossRef]
- Blouin, A.G.; Keenan, S.; Napier, K.R.; Barrero, R.A.; MacDiarmid, R.M. Identification of a novel vitivirus from grapevines in New Zealand. Arch. Virol. 2017, 163, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Candresse, T.; Theil, S.; Faure, C.; Marais, A. Determination of the complete genomic sequence of grapevine virus H, a novel vitivirus infecting grapevine. Arch. Virol. 2018, 163, 277–280. [Google Scholar] [CrossRef]
- Blouin, A.G.; Chooi, K.M.; Warren, B.; Napier, K.R.; Barrero, R.A.; MacDiarmid, R.M. Grapevine virus I, a putative new vitivirus detected in co-infection with grapevine virus G in New Zealand. Arch. Virol. 2018, 163, 1371–1374. [Google Scholar] [CrossRef]
- Diaz-Lara, A.; Golino, D.; Al Rwahnih, M. Genomic characterization of grapevine virus J, a novel virus identified in grapevine. Arch. Virol. 2018, 163, 1965–1967. [Google Scholar] [CrossRef]
- Jo, Y.; Song, M.K.; Choi, H.; Park, J.S.; Lee, J.W.; Lian, S.; Lee, B.C.; Cho, W.K. Genome sequence of grapevine virus K, a novel vitivirus infecting grapevine. Genome Announc. 2017, 5, 37. [Google Scholar] [CrossRef]
- Debat, H.; Zavallo, D.; Brisbane, R.S.; Vončina, D.; Almeida, R.P.P.; Blouin, A.G.; Al Rwahnih, M.; Gomez-Talquenca, S.; Asurmendi, S. Grapevine virus L: A novel vitivirus in grapevine. Eur. J. Plant Pathol. 2019, 155, 319–328. [Google Scholar] [CrossRef]
- Alabi, O.J.; McBride, S.; Appel, D.N.; Al Rwahnih, M.; Pontasch, F.M. Grapevine virus M, a novel vitivirus discovered in the American hybrid bunch grape cultivar Blanc du Bois in Texas. Arch. Virol. 2019, 164, 1739–1741. [Google Scholar] [CrossRef] [PubMed]
- Read, D.A.; Thompson, G.D.; Cordeur, N.L.; Swanevelder, D.; Pietersen, G. Genomic characterization of grapevine viruses N and O: Novel vitiviruses from South Africa. Arch. Virol. 2022, 167, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Maree, H.J.; Blouin, A.G.; Diaz-Lara, A.; Mostert, I.; Al Rwahnih, M.; Candresse, T. Status of the current vitivirus taxonomy. Arch. Virol. 2020, 165, 451–458. [Google Scholar] [CrossRef]
- Martelli, G.P.; Minafra, A.; Saldarelli, P. Vitivirus, a new genus of plant viruses. Arch. Virol. 1997, 142, 1929. [Google Scholar]
- Martelli, G.P. (Ed.) Rugose wood complex. In Graft-Transmissible Diseases of Grapevines: Handbook for Detection and Diagnosis, 1st ed.; FAO Publication Division: Rome, Italy, 1993; pp. 45–53. [Google Scholar]
- Martelli, G.P. Rugose wood complex. J. Plant Pathol. 2014, 96, 73–88. [Google Scholar]
- Minafra, A.; Mawassi, M.; Goszczynski, D.; Saldarelli, P. Grapevine vitiviruses. In Grapevine Viruses: Molecular Biology, Diagnostics and Management, 1st ed.; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 31–46. [Google Scholar] [CrossRef]
- Vončina, D.; Almeida, R.P.P. Screening of some Croatian autochthonous grapevine varieties reveals multitude of viruses including novel ones. Arch. Virol. 2018, 163, 2239–2243. [Google Scholar] [CrossRef]
- Diaz-Lara, A.; Brisbane, R.S.; Aram, K.; Golino, D.; Al Rwahnih, M. Detection of new vitiviruses infecting grapevine in California. Arch. Virol. 2019, 164, 2573–2580. [Google Scholar] [CrossRef]
- Rowhani, A.; Osman, F.; Daubert, S.D.; Al Rwahnih, M.; Saldarelli, P. Polymerase chain reaction methods for the detection of grapevine viruses and viroids. In Grapevine Viruses: Molecular Biology, Diagnostics and Management, 1st ed.; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 431–450. [Google Scholar] [CrossRef]
- Villamor, D.E.V.; Ho, T.; Al Rwahnih, M.; Martin, R.R.; Tzanetakis, I. High throughput sequencing in plant virus detection and discovery. Phytopathology 2019, 109, 716–725. [Google Scholar] [CrossRef]
- Saldarelli, P.; Giampetruzzi, A.; Maree, H.J.; Rwahnih, A. High throughput sequencing: Advantages beyong virus identification. In Grapevine Viruses: Molecular Biology, Diagnostics and Management, 1st ed.; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 625–642. [Google Scholar]
- Tsai, C.W.; Daugherty, M.P.; Almeida, R.P.P. Seasonal dynamics and virus translocation of grapevine leafroll-associated virus 3 in grapevine cultivars. Plant Pathol. 2011, 61, 977–985. [Google Scholar] [CrossRef]
- Chooi, K.M.; Cohen, D.; Pearson, M.N. Differential distribution and titre of selected grapevine leafroll-associated virus 3 genetic variants within grapevine rootstocks. Arch. Virol. 2016, 161, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Kahl, D.; Lowery, D.T.; Hart, M.; Úrbez-Torres, J.R. Seasonal dynamics and optimal diagnostics of grapevine red blotch virus in a British Columbian vineyard. Can. J. Plant Pathol. 2022, 44, 453–464. [Google Scholar] [CrossRef]
- Shabanian, M.; Xiao, H.; Meng, B. Seasonal dynamics and tissue distribution of two major viruses associated with grapevine Leafroll under cool climate condition. Eur. J. Plant Pathol. 2020, 158, 1017–1031. [Google Scholar] [CrossRef]
- Vončina, D.; Preiner, D.; Šimon, S.; Cvjetković, B.; Maletić, E.; Pejić, I.; Karoglan Kontić, J. Distribution of nine viruses in Croatian autochthonous grapevine (Vitis vinifera L.) cultivars from Dalmatian region included in clonal selection. J. Cent. Eur. Agric. 2019, 20, 262–273. [Google Scholar] [CrossRef]
- Karoglan Kontić, J.; Preiner, D.; Šimon, S.; Zdunić, G.; Poljuha, D.; Maletić, E. Sanitary status of Croatian native grapevine varieties. Agric. Conspec. Sci. 2009, 74, 99–103. [Google Scholar]
- Poljuha, D.; Sladonja, B.; Bubola, M. Incidence of viruses infecting grapevine varieties in Istria (Croatia). J. Food Agric. Environ. 2010, 8, 166–169. [Google Scholar]
- Čarija, M.; Radić, T.; Černi, S.; Mucalo, A.; Zdunić, G.; Vončina, D.; Jagunić, M.; Hančević, K. Prevalence of virus infections and GLRaV-3 genetic diversity in selected clones of Croatian indigenous grapevine cultivar Plavac Mali. Pathogens 2022, 11, 176. [Google Scholar] [CrossRef]
- Jagunić, M.; Lazarević, B.; Nikolić, K.; Stupić, D.; Preiner, D.; Vončina, D. Detection, transmission, and characterization of grapevine virus H in Croatia. Pathogens 2021, 10, 1578. [Google Scholar] [CrossRef]
- Sabaghian, S.; Rakhshandehroo, F.; Zamanizadeh, H.R.; Elbeaino, T. Biological, epidemiological and population structure analyses of vitiviruses in Iran. Eur. J. Plant Pathol. 2021, 159, 117–129. [Google Scholar] [CrossRef]
- Herrbach, E.; Alliaume, A.; Prator, C.A.; Daane, K.M.; Cooper, M.L.; Almeida, R.P.P. Vector transmission of grapevine leafroll-associated viruses. In Grapevine Viruses: Molecular Biology, Diagnostics and Management, 1st ed.; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 483–503. [Google Scholar] [CrossRef]
- Al Rwahnih, M.; Diaz-Lara, A.; Arnold, K.; Cooper, M.L.; Smith, R.J.; Zhuang, G.; Battany, M.C.; Bettiga, L.J.; Rowhani, A.; Golino, D. Incidence and genetic diversity of grapevine Pinot gris virus in California. Am. J. Enol. Vitic. 2021, 72, 64–169. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its fapplications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Osman, F.; Rowhani, A. Real-time RT-PCR (TaqMan®) assays for the detection of viruses associated with rugose wood complex of grapevine. J. Virol. Methods 2008, 154, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Rowhani, A.; Biardi, L.; Johnson, R.; Saldarelli, P.; Zhang, Y.P.; Chin, J.; Green, M. Simplified sample preparation method and one-tube RT-PCR for grapevine viruses. In Proceedings of the 13th Meeting of the ICVG, Adelaide, Australia, 12–18 March 2000; p. 148. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 25.0, Released 2017; IBM Corp: Armonk, NY, USA, 2017. Available online: https://www-01.ibm.com/support/docview.wss?uid=swg21476197(accessed on 24 March 2022).
- Berger, V.W.; Zhou, Y. Kolmogorov-Smirnov Test: Overview. In Wiley StatsRef: Statistics Reference Online, 1st ed.; Balakrishnan, N., Colton, T., Everit, B., Piegorsch, W., Ruggeri, F., Teugels, J., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Nei, M.; Gojobori, T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 1986, 3, 418–426. [Google Scholar]
- Hudson, R.R.; Slatkin, M.; Maddison, W.P. Estimation of levels of gene flow from DNA sequence data. Genetics 1992, 132, 583–589. [Google Scholar] [CrossRef]

| Primer/Probe Name | Orientation | Target Gene | Primer Sequence (5′–3′) |
|---|---|---|---|
| GVG F1 | Forward | Coat Protein (CP) | AAGGATGGGAGAGGAATCAAAGA |
| GVG F2 | Forward | AAGGATGGGAGAGGAATCCAAG | |
| GVG R1 | Reverse | TTTAGAGCCGGGTGCTTCAC | |
| GVG R2 | Reverse | TAGAGCCGGATGCTTCACG | |
| GVG R3 | Reverse | TTTAGGGCCGGGTGCTTC | |
| GVG P | TaqMan probe | NED-AGCAGTACTGACGCTGCT-MGB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagunić, M.; Diaz-Lara, A.; Szőke, L.; Rwahnih, M.A.; Stevens, K.; Zdunić, G.; Vončina, D. Incidence and Genetic Diversity of Grapevine Virus G in Croatian Vineyards. Plants 2022, 11, 2341. https://doi.org/10.3390/plants11182341
Jagunić M, Diaz-Lara A, Szőke L, Rwahnih MA, Stevens K, Zdunić G, Vončina D. Incidence and Genetic Diversity of Grapevine Virus G in Croatian Vineyards. Plants. 2022; 11(18):2341. https://doi.org/10.3390/plants11182341
Chicago/Turabian StyleJagunić, Martin, Alfredo Diaz-Lara, Lóránt Szőke, Maher Al Rwahnih, Kristian Stevens, Goran Zdunić, and Darko Vončina. 2022. "Incidence and Genetic Diversity of Grapevine Virus G in Croatian Vineyards" Plants 11, no. 18: 2341. https://doi.org/10.3390/plants11182341
APA StyleJagunić, M., Diaz-Lara, A., Szőke, L., Rwahnih, M. A., Stevens, K., Zdunić, G., & Vončina, D. (2022). Incidence and Genetic Diversity of Grapevine Virus G in Croatian Vineyards. Plants, 11(18), 2341. https://doi.org/10.3390/plants11182341








