Physiological and Proteomic Responses of Cassava to Short-Term Extreme Cool and Hot Temperature
Abstract
:1. Introduction
2. Results
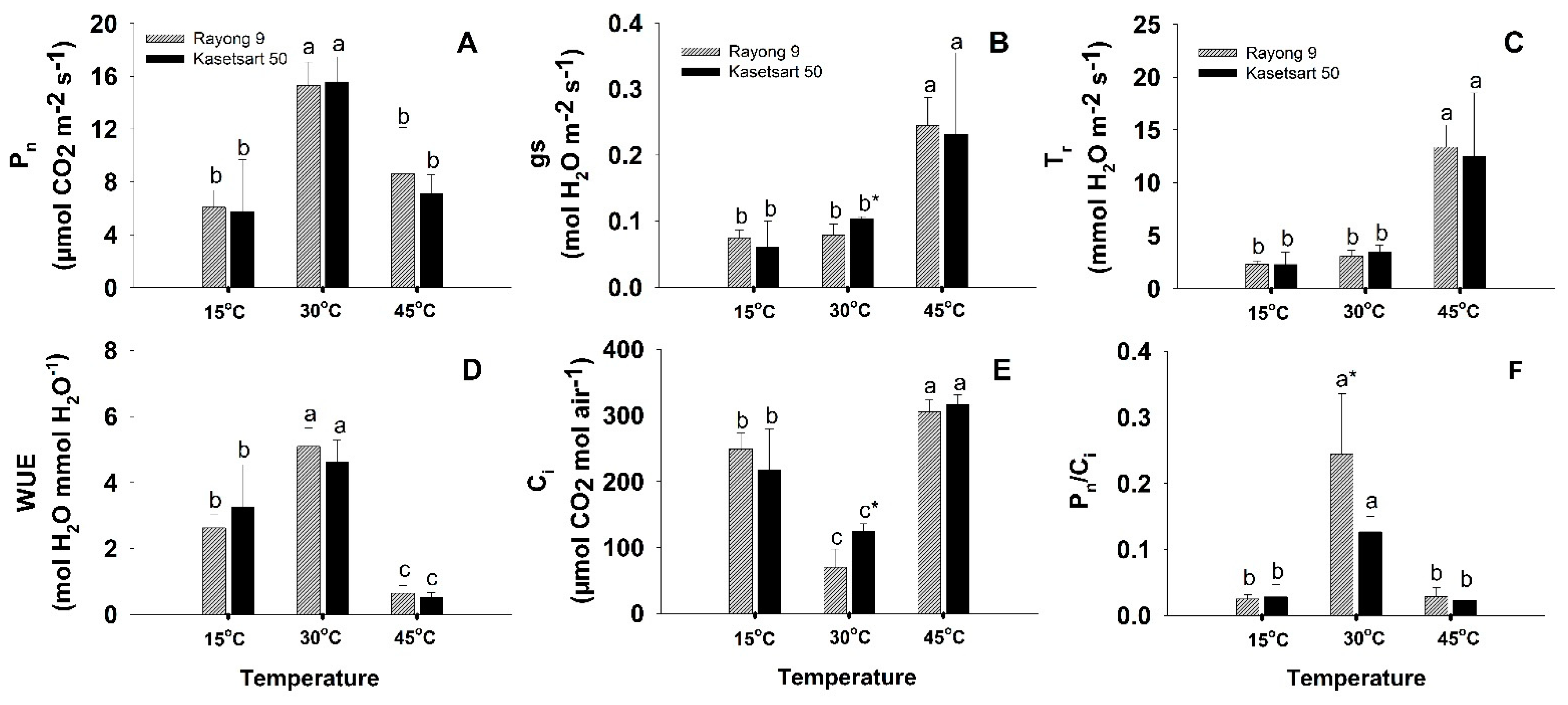
2.1. Photosynthetic Performance
2.2. Chlorophyll Fluorescence Parameters
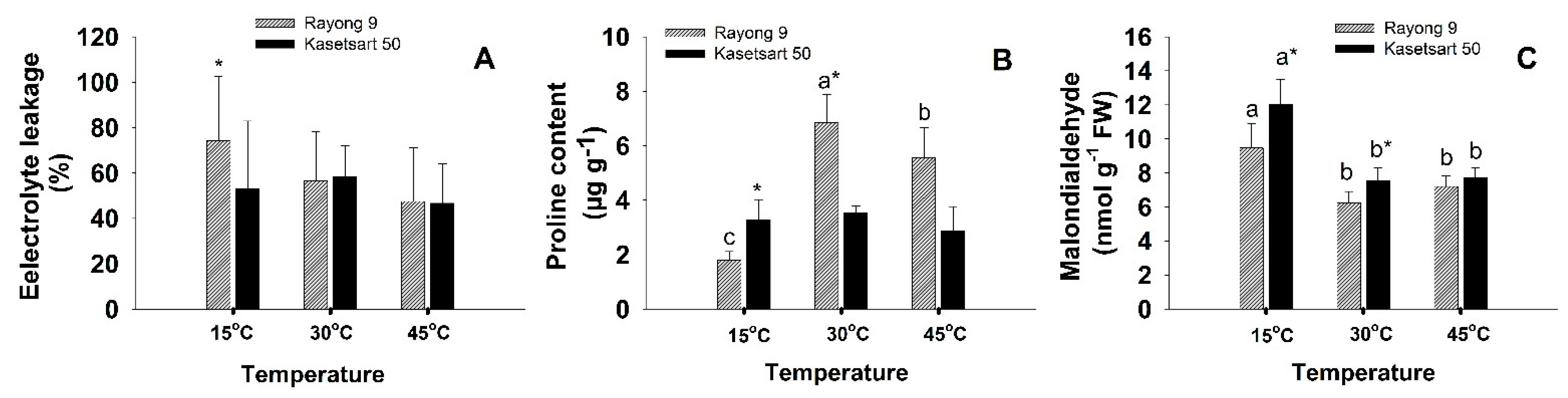
2.3. Electrolyte Leakage, Proline, and Malondialdehyde
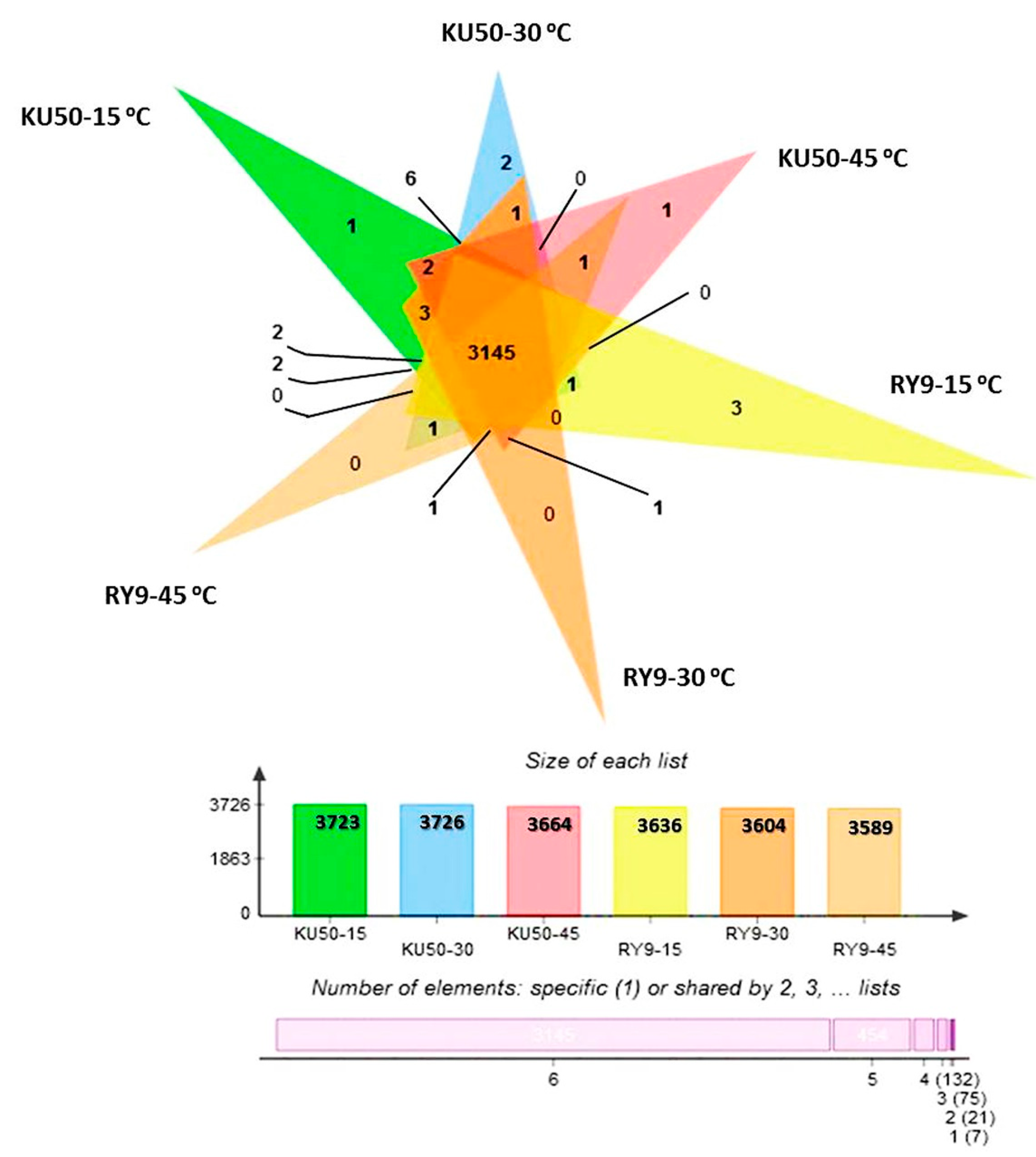
2.4. The Effects of Temperature on Proteomes of Cassava Leaves
3. Discussion
3.1. Short-Term Low- and High-Temperature Stress Induced Different Patterns of Changes in Photosynthesis and Related Physiology
3.2. Different Early Response Proteins Were Detected under Short-Term Low- and High- Temperature Stress
4. Materials and Methods
4.1. Plant Materials, Growing Conditions, and Temperature Treatment
4.2. Photosynthesis and Chlorophyll Fluorescence
4.3. Electrolyte Leakage (EL), Proline, and Malondialdehyde (MDA)
4.4. Proteomics Analysis
4.5. Data and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reyer, C.P.O.; Leuzinger, S.; Rammig, A.; Wolf, A.; Bartholomeus, R.P.; Bonfante, A.; de Lorenzi, F.; Dury, M.; Gloning, P.; AbouJaoudé, R.; et al. A plant’s perspective of extremes: Terrestrial plant responses to changing climatic variability. Glob. Chang. Biol. 2013, 19, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Sultan, B.; Gaetani, M. Agriculture in West Africa in the twenty-first century: Climate change and impacts scenarios, and potential for adaptation. Front. Plant Sci. 2016, 7, 1262. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.; Long, D.; Berger, P.; Russell, C.; Drewes, A. Expanding the foundation: Climate change and opportunities for educational research. Educ. Stud. 2017, 53, 412–425. [Google Scholar] [CrossRef]
- Sclenker, W.; Roberts, M.J. Nonlinear temperature effects indicate severe damages to U.S. crop yields under climate change. Proc. Natl. Acad. Sci. USA 2009, 106, 15594–15598. [Google Scholar] [CrossRef]
- Damatta, F.M.; Grandis, A.; Arenque, B.C.; Buckeridge, M.S. Impacts of climate changes on crop physiology and food quality. Int. Food Res. J. 2010, 43, 1814–1823. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Bray, E.A.; Bailey-Serres, J.; Weretilnyk, E. Responses to abiotic stress. Biochemistry & molecular biology of plants. In American Society of Plant Physiologists; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; Willey Blackwell: West Sussex, UK, 2000; pp. 1158–1203. [Google Scholar]
- Mabrouk, A.; El-Sharkawy, M.A.; Hernández, A.D.P.; Hershey, C. Yield stability of cassava during prolonged mid-season water stress. Exp. Agric. 1992, 28, 165–174. [Google Scholar]
- Keating, B.A.; Evenson, J.P. Effect of soil temperature on sprouting and sprout elongation of stem cuttings of cassava (Manihot esculenta crantz.). Field Crops Res. 1979, 2, 241–251. [Google Scholar] [CrossRef]
- Phoncharoen, P.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P.; Hoogenboom, G. Growth rates and yields of cassava at different planting dates in a tropical savanna climate. Sci. Agric. 2019, 76, 376–388. [Google Scholar] [CrossRef]
- Pannakkong, W.; Parthanadee, P.; Buddhakulsomsiri, J. Impacts of harvesting age and pricing schemes on economic sustainability of cassava farmers in Thailand under market uncertainty. Sustainability 2022, 14, 7768. [Google Scholar] [CrossRef]
- Vongcharoen, K.; Santanoo, S.; Banterng, P.; Jogloy, S.; Vorasoot, N.; Theerakulpisut, P. Diurnal and seasonal variations in the photosynthetic performance and chlorophyll fluorescence of cassava ‘Rayong 9’ under irrigated and rainfed conditions. Photosynthetica 2019, 57, 268–285. [Google Scholar] [CrossRef] [Green Version]
- Janket, A.; Vorasoot, N.; Toomsan, B.; Kaewpradit, W.; Jogloy, S.; Theerakulpisut, P.; Corley Holbrook, C.; Craig, K.; Kvien, C.K.; Banterng, P. Starch accumulation and granule size distribution of cassava cv. Rayong 9 grown under irrigated and rainfed conditions using different growing Seasons. Agronomy 2020, 10, 412. [Google Scholar] [CrossRef]
- Orsenigo, S.; Mondoni, A.; Rossi, G.; Abeli, T. Some like it hot and some like it cold, but not too much: Plant responses to climate extremes. Plant Ecol. 2014, 215, 677–688. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; Cock, J.H. Photosynthesis of cassava (Manihot esculenta). Exp. Agric. 1990, 26, 325–340. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; Cock, J.H.; Held, A.A. Photosynthetic responses of cassava cultivars (Manihot esculenta Crantz) from different habitats to temperature. Photosynth. Res. 1984, 5, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F.; Peixoto, M.M.; Sang, T.L. Photosynthesis in sugarcane. In Sugarcane: Physiology, Biochemistry, and Fictional Biology; Moore, P.H., Botha, F.C., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 121–152. [Google Scholar]
- Vongcharoen, K.; Santanoo, S.; Banterng, P.; Jogloy, S.; Vorasoot, N.; Theerakulpisut, P. Seasonal variation in photosynthesis performance of cassava at two different growth stages under irrigated and rain-fed conditions in a tropical savanna climate. Photosynthetica 2018, 56, 1398–1413. [Google Scholar] [CrossRef]
- Yabuta, S.; Fukuta, T.; Tamaru, S.; Goto, K.; Nakao, Y.; Khanthavong, P.; Ssenyonga, P.; Sakagami, J. The productivity of cassava (Manihot esculenta Crantz) in Kagoshima, Japan, which belongs to the temperate zone. Agronomy 2021, 11, 2021. [Google Scholar] [CrossRef]
- Santanoo, S.; Vongcharoen, K.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Roytrakul, S.; Theerakulpisut, P. Seasonal variation in diurnal photosynthesis and chlorophyll fluorescence of four genotypes of cassava (Manihot esculenta Crantz) under irrigation conditions in a tropical savanna climate. Agronomy 2019, 9, 206. [Google Scholar] [CrossRef]
- Dongsansuk, A.; Lutz, C.; Neuner, G. Effects of temperature and irradiance on quantum yield of PSII photochemistry and xanthophyll cycle in a tropical and a temperate species. Photosynthetica 2013, 51, 13–21. [Google Scholar] [CrossRef]
- Fu, J.; Gates, R.N.; Xu, Y.; Hu, T. Diffusion limitations and metabolic factors associated with inhibition and recovery of photosynthesis following cold stress in Elymus nutans Griseb. J. Photochem. Photobiol. B 2016, 163, 30–39. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, K.; Gu, Y.; Zhang, L.; Li, W.; Li, Z. Effects of low-temperature stress and brassinolide application on the photosynthesis and leaf structure of Tung Tree seedlings. Front. Plant Sci. 2020, 10, 1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, P.; Liu, P.; Shao, J.; Li, C.; Wang, B.; Guo, X.; Yan, B.; Xia, Y.; Peng, M. Analysis of different strategies adapted by two cassava cultivars in response to drought stress: Ensuring survival or continuing growth. J. Exp. Bot. 2015, 66, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Luo, X.; Wei, M.; Huang, T.; Khan, A.; Zhu, Y. Physiological and proteomic analysis on long-term drought resistance of cassava (Manihot esculenta Crantz). Sci. Rep. 2018, 8, 17982. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Tie, W.; Fu, L.; Yan, Y.; Liu, G.; Yan, W.; Li, Y.; Wu, C.; Zhang, J.; Hu, W. Strand-specific RNA-seq based identification and functional prediction of drought-responsive lncRNAs in cassava. BMC Genom. 2019, 20, 214. [Google Scholar] [CrossRef]
- An, F.; Li, G.; Li, Q.X.; Li, K.; Carvalho, L.J.B.C.; Ou, W.; Chen, S. The comparatively proteomic analysis in response to cold strees in cassava plantlets. Plant Mol. Biol. Rep. 2016, 34, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Chen, T.; Stephanie, D.M.A.; Li, K.; Li, Q.X.; Carvalho, L.J.C.B.; Tomlins, K.; Li, J.; Gu, B.; Chen, S. Domestication syndrome is investigated by proteomic analysis between cultivated cassava (Manihot esculenta Crantz) and Its wild relative. PLoS ONE 2016, 11, e0152154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, W.; Bian, J.; Xie, H.; Li, Y.; Xu, C.; Ma, J.; Guo, S.; Chen, J.; Cai, X.; et al. Proteomics and Phosphoproteomics of Heat Stress-Responsive Mechanisms in Spinach. Front. Plant Sci. 2018, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Okogbenin, E.; Setter, T.L.; Ferguson, M.; Mutegi, R.; Ceballos, H.; Olasanmi, B.; Fregene, M. Phenotypic approaches to drought in cassava: Review. Front. Physiol. 2013, 4, 93. [Google Scholar] [CrossRef]
- Rosenthal, D.M.; Ort, D.R. Examining cassava’s potential to enhance food security under climate change. Trop. Plant Biol. 2011, 4, 30–38. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; De Tafur, S.M.D. Comparative photosynthesis, growth, productivity, and nutrient use efficiency among tall- and short-stemmed rain-fed cassava cultivars. Photosynthetica 2010, 48, 173–188. [Google Scholar] [CrossRef]
- Cock, J.H. Cassava. In Symposium on Potential Productivity of Field Crops under Different Environments; International Rice Research Institute, Ed.; Los Baños Laguna: Manila, Philippine, 1983; pp. 341–345. [Google Scholar]
- Lebot, V. Temperature. In Tropical Root and Tuber Crops: Cassava, Sweet Potato, Yams and Aroids; Lebot, V., Ed.; CABI Publishers: Wallingforg, UK, 2009; pp. 39–47. [Google Scholar]
- Mahakosee, S.; Jogloy, S.; Vorasoot, N.; Theerakulpisut, P.; Banterng, P.; Kesmala, T.; Holbrook, C.C.; Kvien, C. Seasonal variations in canopy size and yield of Rayong 9 cassava genotype under rainfed and irrigated condition. Agronomy 2019, 9, 362. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.C. Cassava botany and physiology. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 67–72. [Google Scholar]
- El-Sharkawy, M.A. Cassava biology and physiology. Plant Mol. Biol. 2004, 56, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Gago, J.; Daloso, D.M.; Figueroa, C.M.; Fernie, J.F.A.R.; Nikoloski, Z. Relationships of leaf net photosynthesis, stomatal conductance, and mesophyll conductance to primary metabolism: A multispecies meta-Analysis approach. Plant Physiol. 2016, 171, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.; McAdam, S.; Rose, M.; Murphy, C. Xylem and Stomata, Coordinated Through Time and Space. Plant Cell Environ. 2016, 40, 872–880. [Google Scholar] [CrossRef]
- Chovancek, E.; Zivcak, M.; Botyanszka, L.; Hauptvogel, P.; Yang, X.; Misheva, S.; Hussain, S.; Brestic, M. Transient heat waves may affect the photosynthetic capacity of susceptible wheat genotypes due to insufficient photosystem I photoprotection. Plants 2019, 8, 282. [Google Scholar] [CrossRef]
- Pérez-Romero, J.A.; Duarte, B.; Barcia-Piedras, J.; Matos, A.R.; Redondo-Gómez, S.; Caçador, I.; Mateos-Naranjo, E. Investigating the physiological mechanisms underlying Salicornia ramosissima response to atmospheric CO2 enrichment under coexistence of prolonged soil flooding and saline excess. Plant Physiol. Biochem. 2019, 135, 149–159. [Google Scholar] [CrossRef]
- Yan, K.; Chen, P.; Shao, H.; Zhang, L.; Xu, G. Effects of short-term high temperature on photosynthesis and photosystem II performance in sorghum. J. Agron. Crop Sci. 2011, 197, 400–408. [Google Scholar] [CrossRef]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Derks, A.; Schaven, K.; Bruce, D. Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim. Biophys. Acta 2015, 1847, 468–485. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, S.; Jajoo, A. Alterations in photochemical efficiency of photosystem II in wheat plant on hot summer day. Physiol. Mol. Biol. Plants 2014, 20, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, X.; Liu, L.; Guan, L. Evaluating the performance of the SCOPE model in simulating canopy solar-Induced chlorophyll fluorescence. Remote Sens. 2018, 10, 250. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Hou, W.; Sun, A.H.; Chen, H.L.; Yang, F.S.; Pan, J.L.; Guan, M.Y. Effects of chilling and high temperatures on photosynthesis and chlorophyll fluorescence in leaves of watermelon seedlings. Biol. Plant. 2016, 60, 148–154. [Google Scholar] [CrossRef]
- Alric, J.; Johnson, X. Alternative electron transport pathways in photosynthesis: A confluence of regulation. Curr. Opin. Plant Biol. 2017, 37, 78–86. [Google Scholar] [CrossRef]
- Sagun, J.V.; Badger, M.R.; Chow, W.S.; Ghannoum, O. Mehler reaction plays a role in C3 and C4 photosynthesis under shade and low CO2. Photosynth. Res. 2021, 149, 171–185. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Li, M.; Kim, C. Chloroplast ROS and stress signaling. Plant Commun. 2022, 3, 100264. [Google Scholar] [CrossRef]
- Guidi1, L.; Piccolo, E.L.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 Species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- An, D.; Yang, J.; Zhang, P. Transcriptome profiling of low temperature treated cassava apical shoots showed dynamic responses of tropical plant to cold stress. BMC Genet. 2012, 13, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aazami, M.A.; Asghari-Aruq, M.; Hassanpouraghdam, M.B.; Ercisli, S.; Baron, M.; Sochor, J. Low temperature stress mediates the antioxidants pool and chlorophyll fluorescence in Vitis vinifera L. cultivars. Plants 2021, 10, 1877. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.; Kandpal, G.; Kumar, P. Proline accumulation and its defensive role under diverse stress condition in plants: An overview. J. Pure Appl. Microbiol. 2018, 12, 1655–1659. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Mahabub Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Harsh, A.; Sharma, Y.K.; Joshi, U.; Rampuria, S.; Singh, G.; Kumar, S.; Sharma, R. Effect of short-term heat stress on total sugars, proline and some antioxidant enzymes in moth bean (Vigna aconitifolia). Ann. Agric. Sci. 2016, 61, 57–64. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savoure, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Kumari, P.H.; Sunita, M.S.L.; Sreenivasulu, N. Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front. Plant Sci. 2015, 6, 544. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Lizarbe, M.A.; Barrasa, J.I.; Olmo, N.; Gavilanes, F.; Turnay, J. Annexin-Phospholipid Interactions. Functional implications. Int. J. Mol. Sci. 2013, 14, 2652–2683. [Google Scholar] [CrossRef]
- Gao, F.; Han, X.; Wu, J.; Zheng, S.; Shang, Z.; Sun, D.; Zhou, R.; Li, B. A heat-activated calcium-permeable channel–Arabidopsis cyclic nucleotide-gated ion channel 6–is involved in heat shock responses. Plant J. 2012, 70, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, X.; Wang, H.; Li, B.; Clark, G.; Guo, Y.; Roux, S.; Sun, D.; Tang, W. Proteomic Study of Microsomal Proteins Reveals a Key Role for Arabidopsis Annexin 1 in Mediating Heat Stress-Induced Increase in Intracellular Calcium Levels. MCP 2015, 14, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Zhang, Q.; Liu, D.; Wang, H.; Yin, J.; Wang, R.; He, M.; Cui, M.; Shang, Z.; Wang, D.; et al. A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J. Exp. Bot. 2015, 66, 5853–5866. [Google Scholar] [CrossRef]
- Konopka-Postupolska, D.; Clark, G.; Goch, G.; Debski, J.; Floras, K.; Cantero, A.; Fijolek, B.; Roux, S.; Hennig, J. The Role of Annexin 1 in Drought Stress in Arabidopsis. Plant Physiol. 2009, 150, 1394–1410. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, T.; Guan, C.; Gao, Y.; Ma, J.; Gu, X.; Qi, Z.; Wang, X.; Zhu, Z. OsANN4 modulates ROS production and mediates Ca2+ influx in response to ABA. BMC Plant Biol. 2021, 21, 474. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; Ohme-Takagi, M.; Perata, P. APETALA2/Ethylene Responsive Factor (AP2/ERF) transcriptionfactors: Mediators of stress responses and developmental programs. New Phytol. 2013, 199, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Ritonga, F.N.; Ngatia, J.N.; Wang, Y.; Khoso, M.A.; Umar Farooq, U.; Chen, S. AP2/ERF, an important cold stress-related transcription factor family in plants: A review. Physiol. Mol. Biol. Plants 2021, 27, 1953–1968. [Google Scholar] [CrossRef]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef]
- An, D.; Ma, Q.; Wang, H.; Jun Yang, J.; Zhou, W.; Zhang, P. Cassava C-repeat binding factor 1 gene responds to low temperature and enhances cold tolerance when overexpressed in Arabidopsis and cassava. Plant Mol. Biol. 2017, 94, 109–124. [Google Scholar] [CrossRef]
- Li, S. Redox modulation matters: Emerging functions for glutaredoxins in plant development and stress responses. Plants 2014, 3, 559–582. [Google Scholar] [CrossRef]
- Wu, H.; Tito, N.; Giraldo, J.P. Anionic cerium oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS Nano 2017, 11, 11283–11297. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, A.; Dietz, K. Reactive oxygen species and the redox-regulatory network in cold stress acclimation. Antioxidants 2018, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.B.; Yang, Y.L.; Li, K.M.; Guo, X.; Wang, B.; Yu, X.; Peng, M. Identification and characterization of drought-responsive CC-type glutaredoxins from cassava cultivars reveals their involvement in ABA signalling. BMC Plant Biol. 2018, 18, 329. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, Q.; Sprague, S.A.; Park, J.; Oh, M.; Rajashekar, C.B.; Koiwa, H.; Nakata, P.A.; Ninghui Cheng, N.; Hirschi, K.D.; et al. Tomato expressing Arabidopsis glutaredoxin gene AtGRXS17 confers tolerance to chilling stress via modulating cold responsive components. Hortic. Res. 2015, 2, 15051. [Google Scholar] [CrossRef]
- Huangi, L.; Ye, Z.; Bell, R.W.; Dell, B. Boron nutrition and chilling tolerance of warm climate crop species. Ann. Bot. 2005, 96, 755–767. [Google Scholar] [CrossRef]
- Kjellsen, T.D.; Shiryaevab, L.; Wolfgang, P.; Schröderb, G.; Strimbecka, R. Proteomics of extreme freezing tolerance in Siberian spruce (Picea obovata). J. Proteom. 2010, 73, 965–975. [Google Scholar] [CrossRef]
- Aslama, M.; Beenish Fakher, B.; Greaves, J.G.; Jakada, B.H.; Rongjuan Qin, R.; Qina, Y. A CBL-interacting protein kinase, AcCIPK18, from Ananas comosus regulates tolerance to salt, drought, heat stress and Sclerotinia sclerotiorum infection in Arabidopsis. Environ. Exp. Bot. 2022, 194, 104728. [Google Scholar] [CrossRef]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef]
- Liu, J.; He, Z. Small DNA methylation, big player in plant abiotic stress responses and memory. Front. Plant Sci. 2020, 11, 595603. [Google Scholar] [CrossRef]
- Phosaengsri, W.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P. Leaf performance of cassava genotypes in different seasons and its relationship with biomass. Turk. J. Field Crops 2019, 24, 54–64. [Google Scholar] [CrossRef]
- Prammanee, S.; Kamprerasart, K.; Salakan, S.; Sriroth, K. Growth and starch content evaluation on newly released cassava cultivars, Rayong 9, Rayong 7 and Rayong 80 at different harvest times. Kasetsart J. (Nat. Sci.) 2010, 44, 558–563. [Google Scholar]
- Santanoo, S.; Vongcharoen, K.; Banterng, P.; Vorasoot, N.; Joyloy, S.; Roytrakul, S.; Theerakulpisult, P. Canopy structure and photosynthetic performance of irrigated cassava genotypes growing in different seasons in a tropical savanna climate. Agronomy 2020, 10, 2018. [Google Scholar] [CrossRef]
- Howeler, R.H. Cassava mineral nutrition and fertilization. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 149–166. [Google Scholar]
- Bajji, M.; Kinet, J.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treted bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Losuwannarak, N.; Maiuthed, A.; Kitkumthorn, N.; Leelahavanichkul, A.; Roytrakul, S.; Chanvorachote, P. Gigantol targets cancer stem cells and destabilizes tumors via the suppression of the PI3K/AKT and JAK/STAT pathways in ectopic lung cancer xenografts. Cancers 2019, 11, 2032. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S.; Roytrakul, S.; Settachaimongkon, S.; Wattanasiritham, L.S.; Boonbumrung, S.; Mookdasanit, J.; Sithtisarn, S. Arthrospira platensis mutagenesis for protein and C-Phycocyanin improvement and proteomics approaches. Life 2022, 12, 911. [Google Scholar] [CrossRef]
- Johansson, C.; Samskog, J.; Sundström, L.; Wadensten, H.; Björkesten, L.; Flensburg, J. Differential expression analysis of Escherichia coli proteins using a novel software for relative quantitation of LC-MS/MS data. Proteomics 2006, 6, 4475–4485. [Google Scholar] [CrossRef]
- Thorsell, A.; Portelius, E.; Blennow, K.; Westman-Brinkmalm, A. Evaluation of sample fractionation using micro-scale liquid-phase isoelectric focusing on mass spectrometric identification and quantitation of proteins in a SILAC experiment. Rapid Commun. Mass Spectrom. 2007, 21, 771–778. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.A.; Gomez, A.A. Single-factor experiments. In Statistical Procedures for Agricultural Research; Gomez, K.A., Gomez, A.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NY, USA, 1984; pp. 8–13. [Google Scholar]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Genotype | Protein ID | Protein Names | Gene Ontology (Biological Process) | Gene Ontology (GO) | Cellular Component |
|---|---|---|---|---|---|
| KU50-15 °C|RY9-15 °C | A0A2C9V0E9 | AP2/ERF domain-containing protein | nucleus; DNA binding; DNA-binding transcription factor activity | nucleus | |
| A0A2C9UPP7 | Glutaredoxin domain-containing protein | cytoplasm; protein disulfide oxidoreductase activity | cytoplasm | ||
| RY9-15 °C | A0A2C9WDC0 | Thymidine kinase | DNA biosynthetic process; thymidine metabolic process | ATP binding; thymidine kinase activity; DNA biosynthetic process; thymidine metabolic process | |
| A0A2C9VTU6 | Uncharacterized protein | integral component of membrane | integral component of membrane | ||
| A0A2C9UA68 | Cytokinin dehydrogenase | cytokinin metabolic process; oxidation-reduction process | cytokinin dehydrogenase activity; FAD binding; oxidoreductase activity; cytokinin metabolic process; oxidation-reduction process | ||
| KU50-15 °C | A0A2C9VH14 | Uncharacterized protein | |||
| KU50-45 °C|RY9-45 °C | A0A0C5A1R1 | Annexin | calcium ion transmembrane transport; phloem sucrose unloading; primary root development; response to abscisic acid; response to cadmium ion; response to cold; response to heat; response to salt stress; response to water deprivation | apoplast; cell wall; chloroplast stroma; cytosol; mitochondrion; plasma membrane; plasmodesma; thylakoid; vacuolar membrane; ATP binding; calcium ion binding; calcium-dependent phospholipid binding; peroxidase activity; protein homodimerization activity; zinc ion binding; calcium ion transmembrane transport; phloem sucrose unloading; primary root development; response to abscisic acid; response to cadmium ion; response to cold; response to heat; response to salt stress; response to water deprivation | apoplast; cell wall; chloroplast stroma; cytosol; mitochondrion; plasma membrane; plasmodesma; thylakoid; vacuolar membrane |
| KU50-45 °C | A0A2C9U0M7 | Uncharacterized protein | |||
| RY9-45 °C | — | — | — | — | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santanoo, S.; Vongcharoen, K.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Roytrakul, S.; Theerakulpisut, P. Physiological and Proteomic Responses of Cassava to Short-Term Extreme Cool and Hot Temperature. Plants 2022, 11, 2307. https://doi.org/10.3390/plants11172307
Santanoo S, Vongcharoen K, Banterng P, Vorasoot N, Jogloy S, Roytrakul S, Theerakulpisut P. Physiological and Proteomic Responses of Cassava to Short-Term Extreme Cool and Hot Temperature. Plants. 2022; 11(17):2307. https://doi.org/10.3390/plants11172307
Chicago/Turabian StyleSantanoo, Supranee, Kochaphan Vongcharoen, Poramate Banterng, Nimitr Vorasoot, Sanun Jogloy, Sittiruk Roytrakul, and Piyada Theerakulpisut. 2022. "Physiological and Proteomic Responses of Cassava to Short-Term Extreme Cool and Hot Temperature" Plants 11, no. 17: 2307. https://doi.org/10.3390/plants11172307
APA StyleSantanoo, S., Vongcharoen, K., Banterng, P., Vorasoot, N., Jogloy, S., Roytrakul, S., & Theerakulpisut, P. (2022). Physiological and Proteomic Responses of Cassava to Short-Term Extreme Cool and Hot Temperature. Plants, 11(17), 2307. https://doi.org/10.3390/plants11172307








