A New Stilbene Glucoside from Biotransformation-Guided Purification of Chinese Herb Ha-Soo-Oh
Abstract
:1. Introduction
2. Results and Discussion
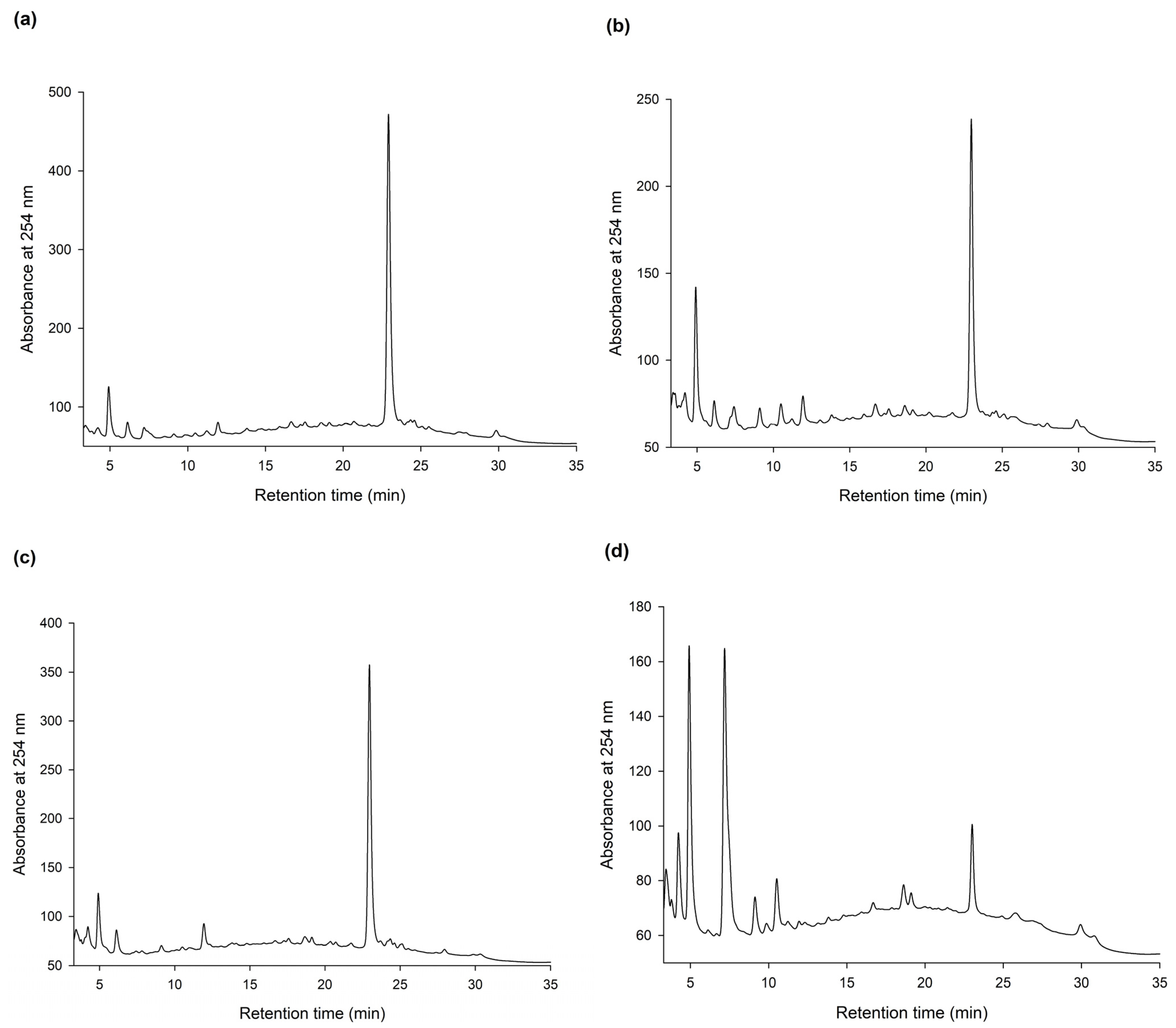
2.1. Biotransformation of Ha-Soo-Oh Extracts by BmTYR
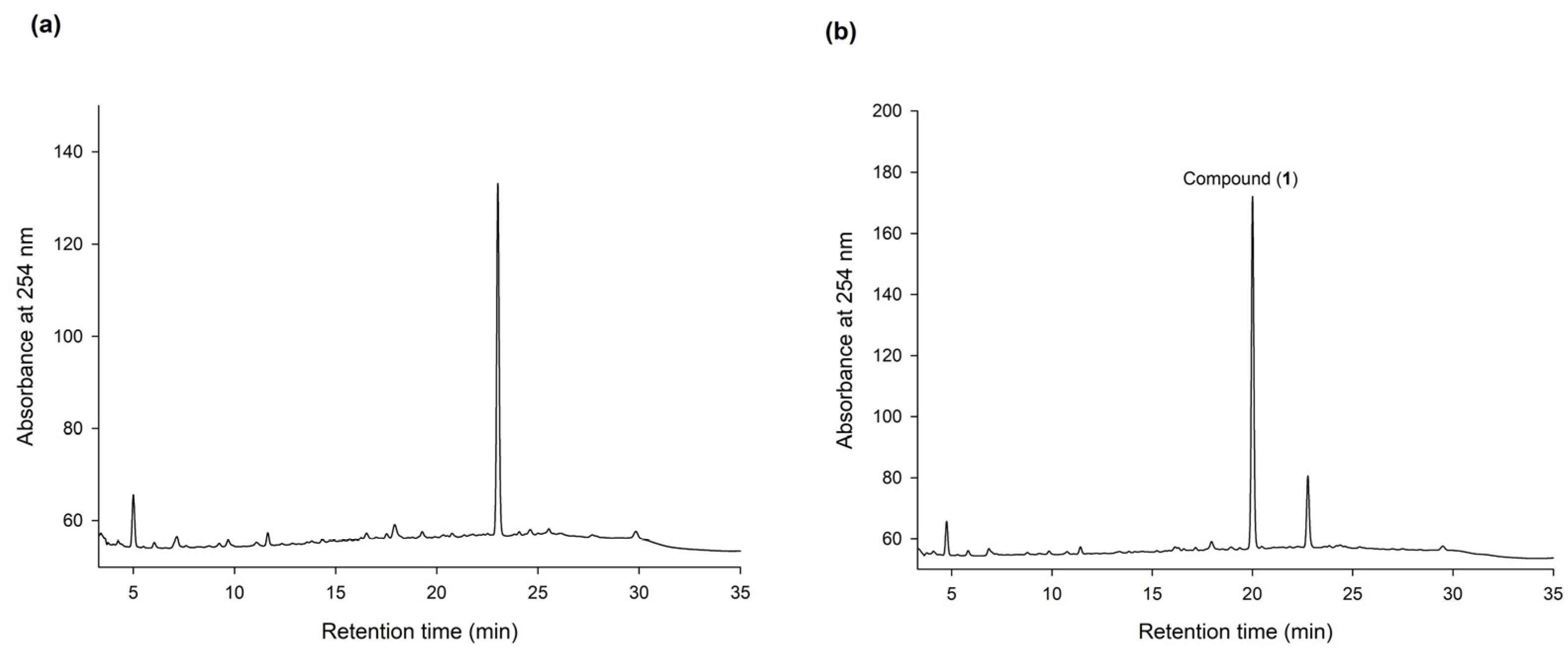
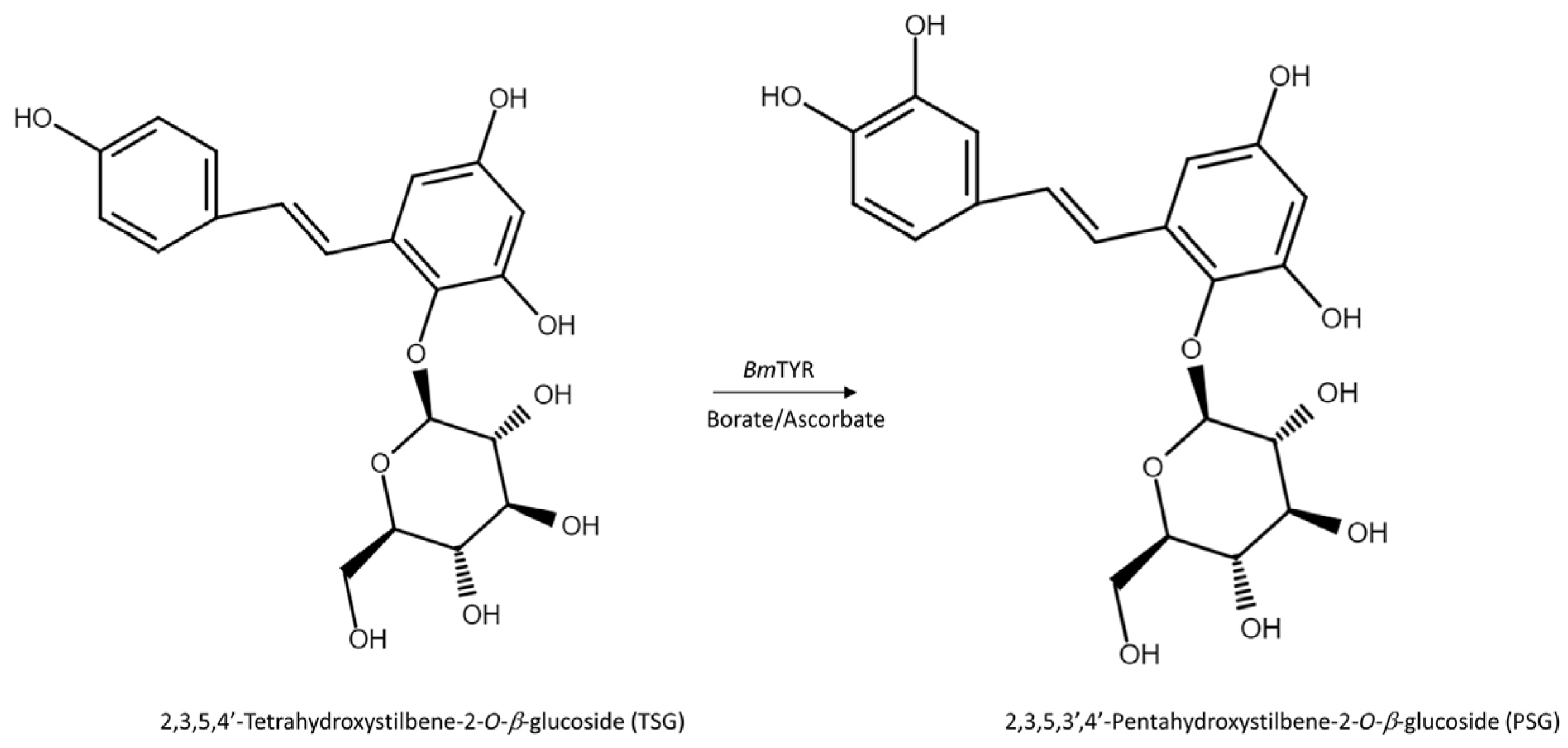
2.2. Purification and Identification of the Biotransformation Product from the Ha-Soo-Oh Extract
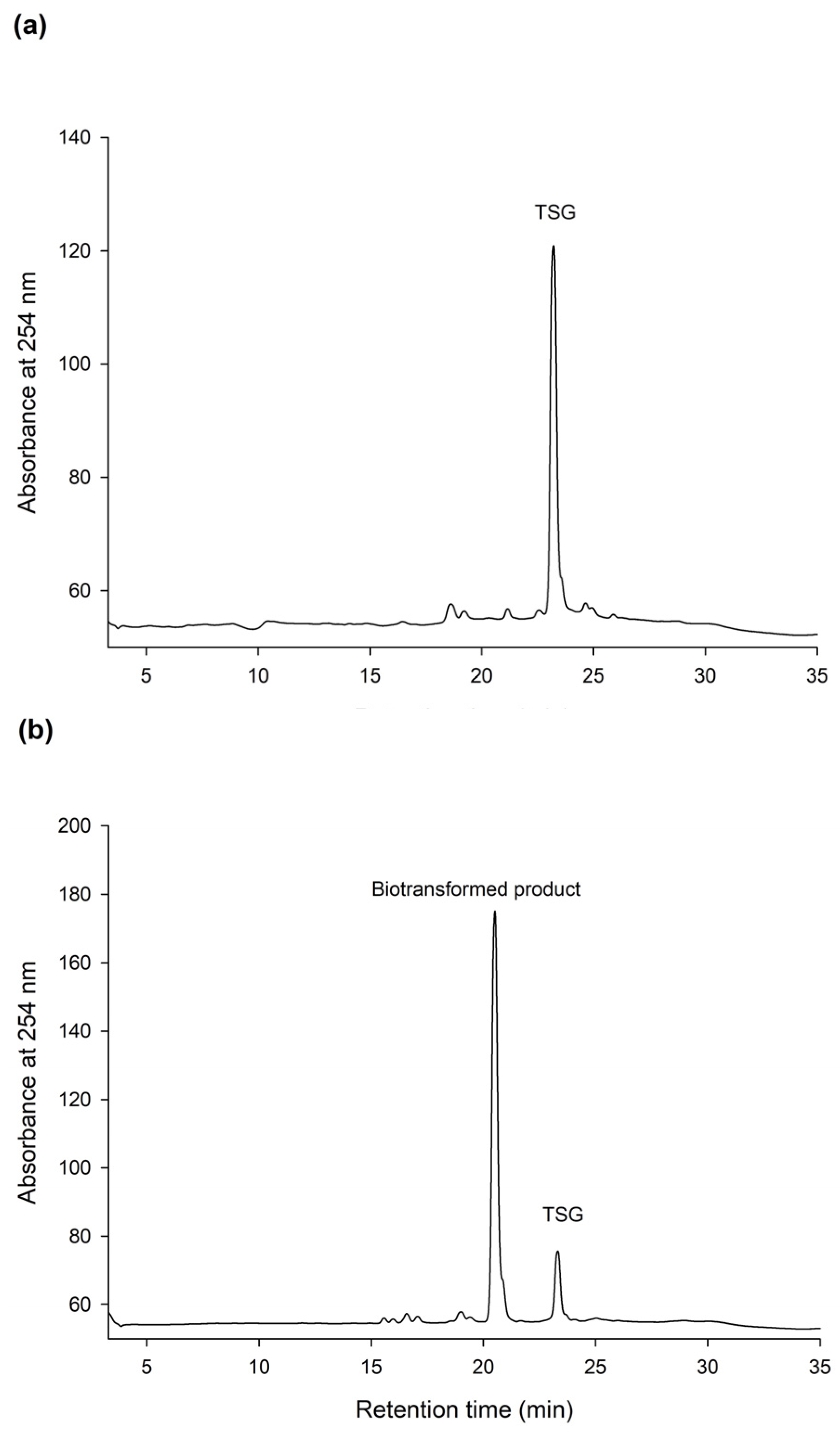
2.3. Confirming the Biotransformation Process
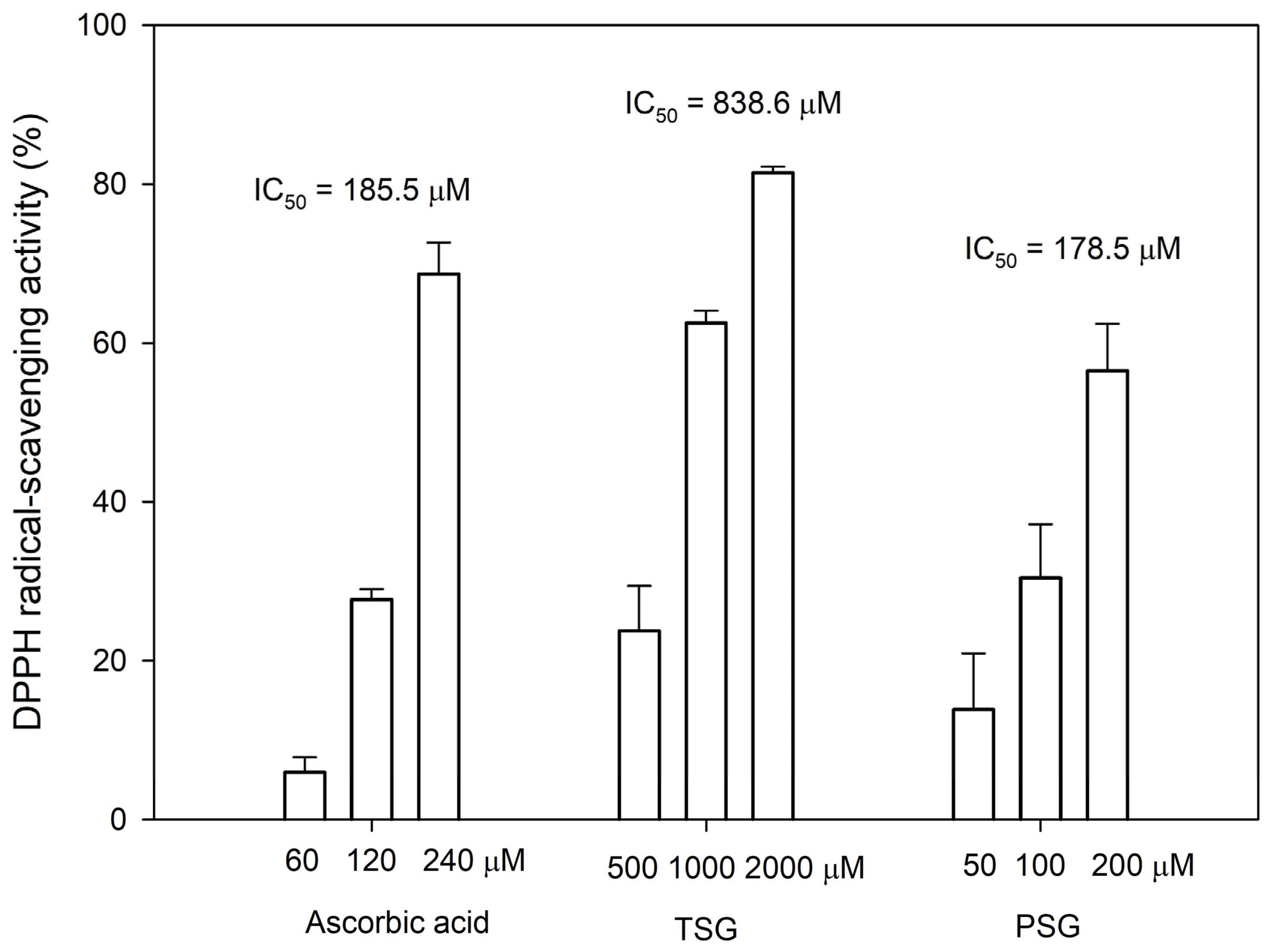
2.4. Antioxidant Activity of TSG and PSG
3. Materials and Methods
3.1. Enzymes and Chemicals
3.2. Preparation of Ha-Soo-Oh Extract
3.3. Biotransformation Using BmTYR
3.4. HPLC
3.5. Purification and Identification of the Biotransformation Metabolite
3.6. Determination of DPPH Free Radical-Scavenging Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sultana, N.; Saify, Z.S. Enzymatic biotransformation of terpenes as bioactive agents. J. Enzym. Inhib. Med. Chem. 2013, 28, 1113–1128. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Tan, H.L.; Sultan, S.; Faridz, M.A.B.M.; Shah, M.A.B.M.; Nurfazilah, S.; Hussain, M. Microbial-catalyzed biotransformation of multifunctional triterpenoids derived from phytonutrients. Int. J. Mol. Sci. 2014, 15, 12027–12060. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef]
- Chang, T.-S. Isolation, bioactivity, and production of ortho-hydroxydaidzein and ortho-hydroxygenistein. Int. J. Mol. Sci. 2014, 15, 5699–5716. [Google Scholar] [CrossRef]
- Ramsden, C.A.; Riley, P.A. Tyrosinase: The four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorgan. Med. Chem. 2014, 22, 2388–2395. [Google Scholar] [CrossRef]
- Lee, S.-H.; Baek, K.; Lee, J.-E.; Kim, B.-G. Using tyrosinase as a monophenol monooxygenase: A combined strategy for effective inhibition of melanin formation. Biotechnol. Bioeng. 2016, 113, 735–743. [Google Scholar] [CrossRef]
- Chiang, C.-M.; Wang, D.-S.; Chang, T.-S. Improving free radical scavenging activity of soy isoflavone glycosides daidzin and genistin by 3’-hydroxylation using recombinant Escherichia coli. Molecules 2016, 21, 1723. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Ding, H.-Y.; Wang, T.-Y.; Cai, C.-Z.; Chang, T.-S. Application of biotransformation-guided purification in Chinese medicine: An example to produce butin from licorice. Catalysts 2022, 12, 718. [Google Scholar] [CrossRef]
- Lin, L.; Ni, B.; Lin, H.; Zhang, M.; Li, X.; Yin, X.; Qu, C.; Ni, J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: A review. J. Ethnopharmacol. 2015, 159, 158–183. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Yang, J.; Guo, X.; Liu, W.; Ma, S.; Li, S. Polygonum multiflorum Thunb.: A review on chemical analysis, processing mechanism, quality evaluation, and hepatotoxicity. Front. Pharmacol. 2018, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Ho, T.-T.; Murthy, H.N.; Dalawai, D.; Bhat, M.A.; Paek, K.-Y.; Park, S.-Y. Attributes of Polygonum multiflorum to transfigure red biotechnology. Appl. Microbiol. Biotechnol. 2019, 103, 3317–3326. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-G.; Chen, L.-L.; Huang, X.-J.; Zhao, B.-X.; Wang, Y.; Ye, W.-C. Five new stilbene glycosides from the roots of Polygonum multiflorum. J. Asian Nat. Prod. Res. 2013, 15, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.-L.; Su, Y.-F.; Chen, L.; Que, M.; Gao, X.-M.; Chang, J.-B. Polygonumosides A–D, stilbene derivatives from processed roots of Polygonum multiflorum. J. Nat. Prod. 2014, 77, 397–401. [Google Scholar] [CrossRef]
- Dai, L.; Li, J.; Yao, P.; Zhu, Y.; Men, Y.; Zeng, Y.; Yang, J.; Sun, Y. Exploiting the aglycon promiscuity of glycosyltransferase Bs-YjiC from Bacillus subtilis and its application in synthesis of glycosides. J. Biotechnol. 2017, 248, 69–76. [Google Scholar] [CrossRef]
- Ling, S.; Xu, J.W. Biological Activities of 2,3,5,4’-Tetrahydroxystilbene-2-O-beta-D-Glucoside in Antiaging and Antiaging-Related Disease Treatments. Oxidative Med. Cell. Longev. 2016, 2016, 4973239. [Google Scholar] [CrossRef]
- Wu, J.; Hu, W.; Gong, Y.; Wang, P.; Tong, L.; Chen, X.; Chen, Z.; Xu, X.; Yao, W.; Zhang, W.; et al. Current pharmacological developments in 2,3,4’,5-tetrahydroxystilbene 2-O-beta-D-glucoside (TSG). Eur. J. Pharmacol. 2017, 811, 21–29. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J. Biological effects of tetrahydroxystilbene glucoside: An active component of a rhizome extracted from Polygonum multiflorum. Oxidative Med. Cell. Longev. 2018, 2018, 3641960. [Google Scholar] [CrossRef]
- Shimoda, K.; Kubota, N.; Uesugi, D.; Kobayashi, Y.; Hamada, H.; Hamada, H. Glycosylation of Stilbene Compounds by Cultured Plant Cells. Molecules 2020, 25, 1437. [Google Scholar] [CrossRef]
- Lin, Y.; Peng, X.; Xia, B.; Zhang, Z.; Li, C.; Wu, P.; Lin, L.; Liao, D. Investigation of toxicity attenuation mechanism of tetrahydroxy stilbene glucoside in Polygonum multiflorum Thunb. by Ganoderma lucidum. J. Ethnopharmacol. 2021, 280, 114421. [Google Scholar] [CrossRef] [PubMed]
- Treml, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Qian, J.; Hou, M.; Wu, X.; Dai, C.; Sun, J.; Dong, L. A review on the extraction, purification, detection, and pharmacological effects of 2,3,5,4’-tetrahydroxystilbene-2-O-beta-d-glucoside from Polygonum multiflorum. Biomed. Pharmacother. 2020, 124, 109923. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Su, W.; Wang, N.; Li, P.; Wang, Y. A potent tyrosinase activator from Radix Polygoni multiflori and its melanogenesis stimulatory effect in B16 melanoma cells. Phytother. Res. 2008, 22, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xu, J.; Long, M.; Tu, Z.; Yang, G.; He, G. 2, 3, 5, 4’-tetrahydroxystilbene-2-O-beta-D-glucoside (THSG) induces melanogenesis in B16 cells by MAP kinase activation and tyrosinase upregulation. Life Sci. 2009, 85, 345–350. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-Y.; Ding, H.-Y.; Wang, T.-Y.; Hsu, M.-H.; Chang, T.-S. A New Stilbene Glucoside from Biotransformation-Guided Purification of Chinese Herb Ha-Soo-Oh. Plants 2022, 11, 2286. https://doi.org/10.3390/plants11172286
Wu J-Y, Ding H-Y, Wang T-Y, Hsu M-H, Chang T-S. A New Stilbene Glucoside from Biotransformation-Guided Purification of Chinese Herb Ha-Soo-Oh. Plants. 2022; 11(17):2286. https://doi.org/10.3390/plants11172286
Chicago/Turabian StyleWu, Jiumn-Yih, Hsiou-Yu Ding, Tzi-Yuan Wang, Min-Hui Hsu, and Te-Sheng Chang. 2022. "A New Stilbene Glucoside from Biotransformation-Guided Purification of Chinese Herb Ha-Soo-Oh" Plants 11, no. 17: 2286. https://doi.org/10.3390/plants11172286
APA StyleWu, J.-Y., Ding, H.-Y., Wang, T.-Y., Hsu, M.-H., & Chang, T.-S. (2022). A New Stilbene Glucoside from Biotransformation-Guided Purification of Chinese Herb Ha-Soo-Oh. Plants, 11(17), 2286. https://doi.org/10.3390/plants11172286







