Roots’ Drought Adaptive Traits in Crop Improvement
Abstract
:1. Introduction
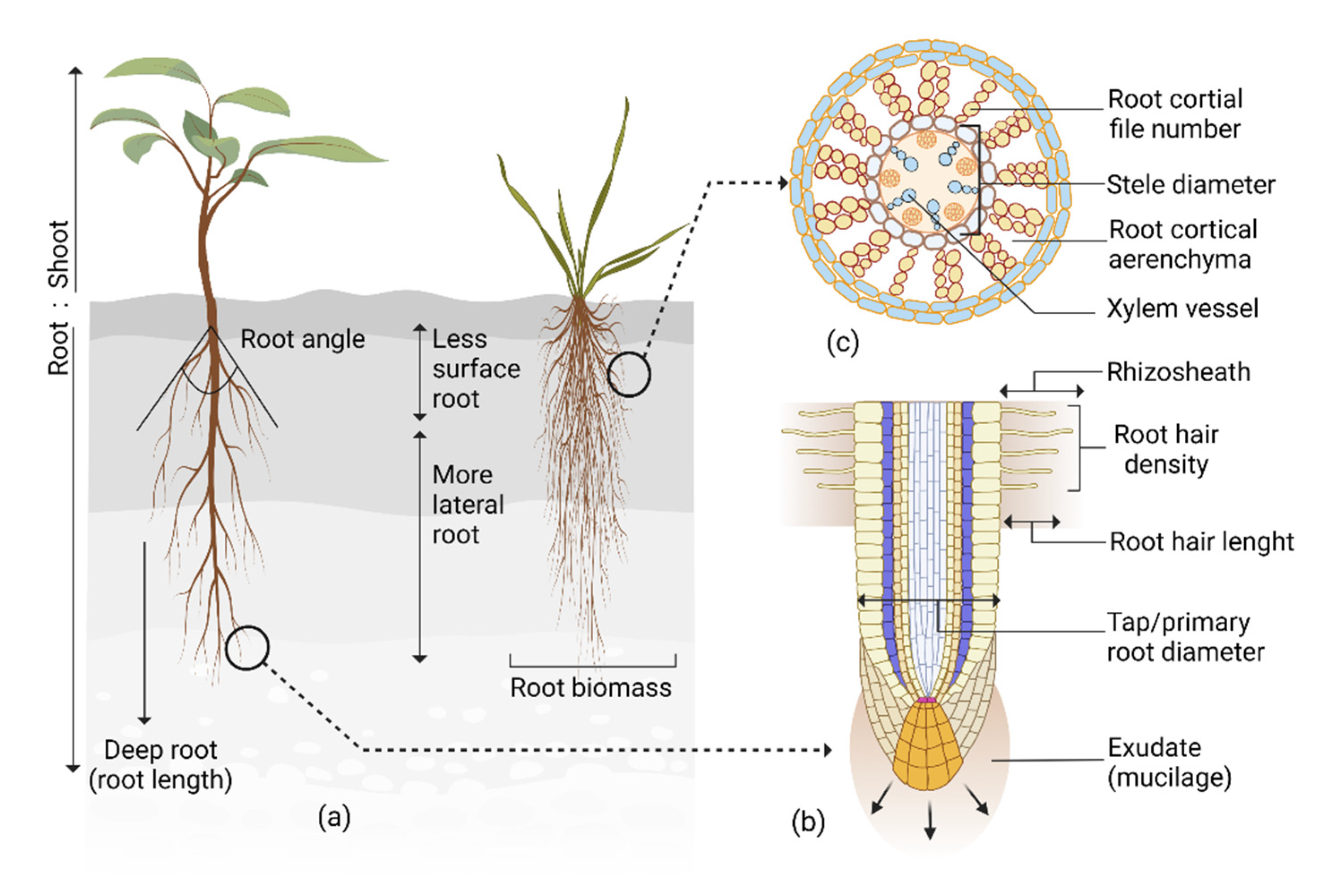
2. Root System Architecture and Its Interaction with the Shoot in Response to Drought
3. Roots’ Structural Response to Drought
4. Root Anatomical Responses to Drought
4.1. Anatomical Adaptation in Reducing Metabolic Cost
4.2. Anatomical Response Improving Root Penetration
4.3. Anatomical Attributes Facilitating Microbial Symbiosis
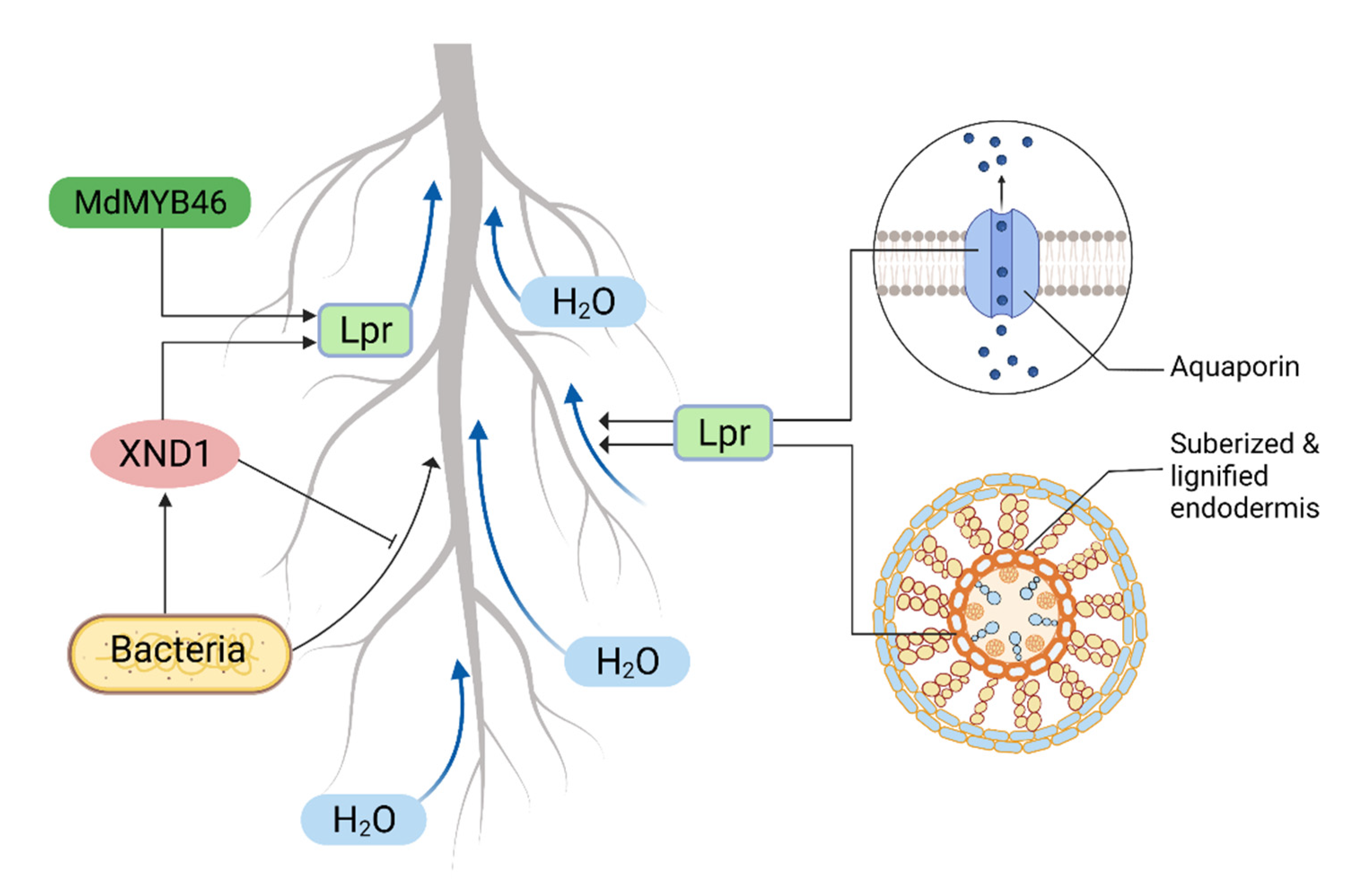
4.4. Anatomical Adaptation in Regulating Water Transport
5. Root Hydraulics
6. Interaction among Drought Response Root Traits
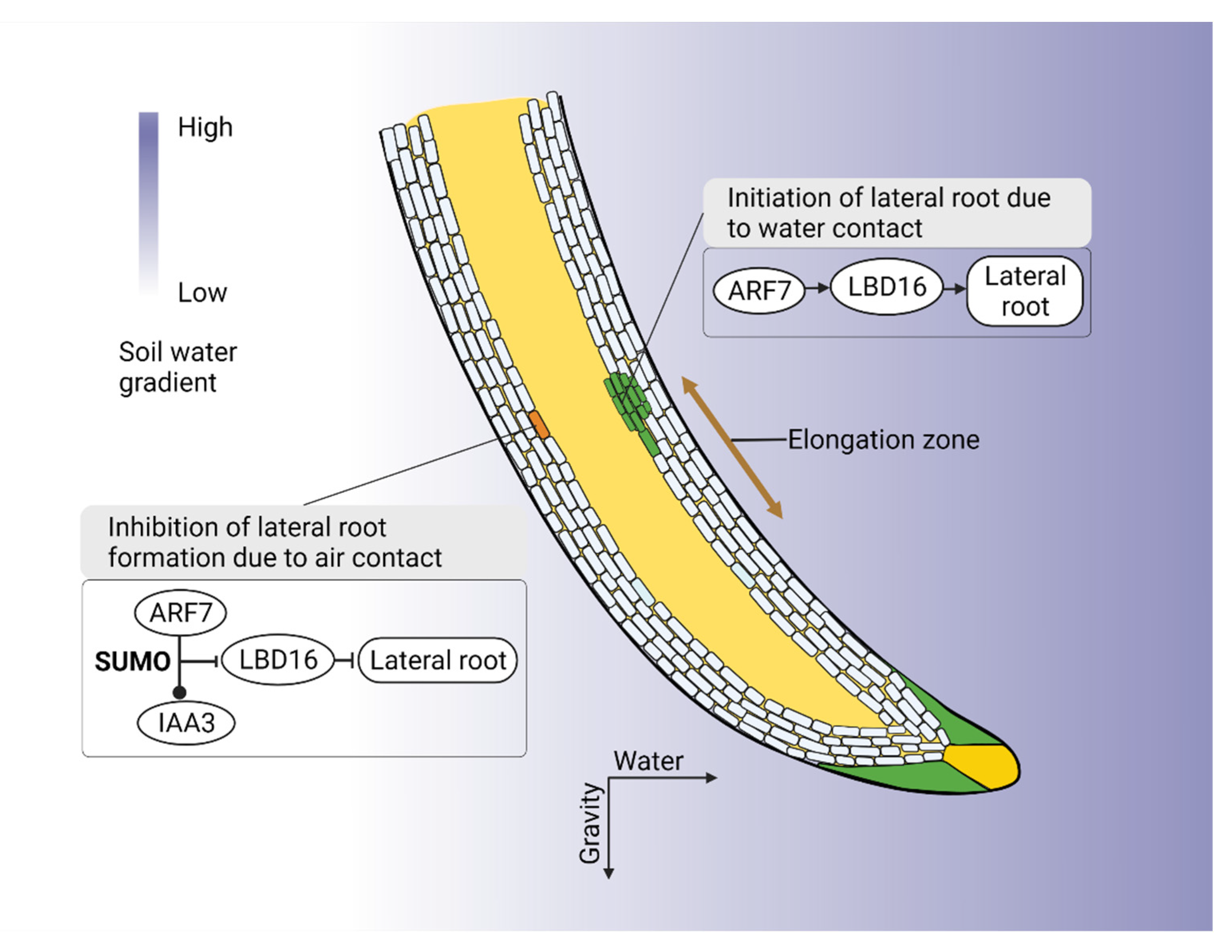
7. Root Plasticity in Drought Adaptation
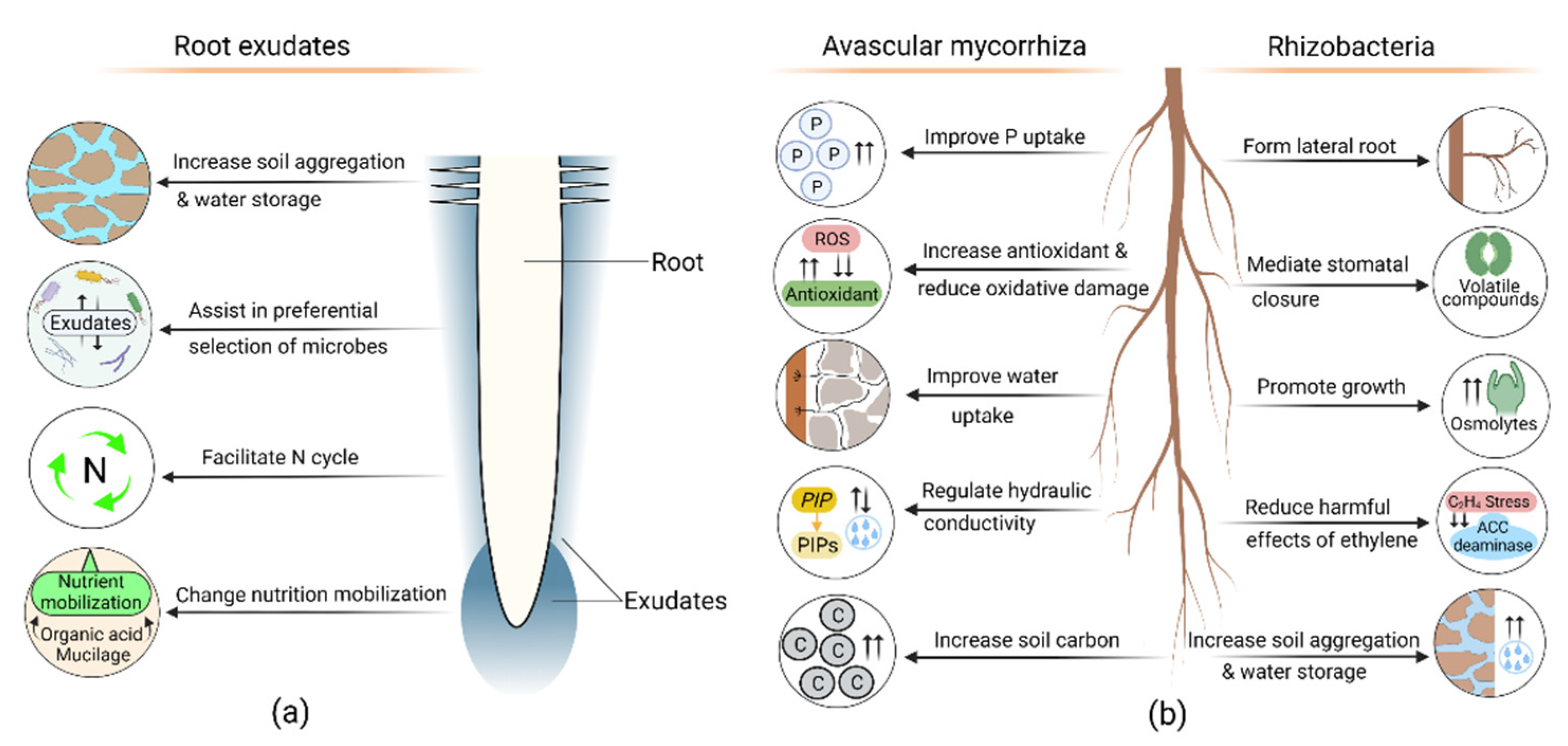
8. Root Exudates and Microbial Symbiosis in Drought Adaptation
8.1. Root Exudate Role in Drought Adaptation
8.2. Contribution of Avascular Mycorrhizal Symbiosis in Drought Response
8.3. Role of Rhizobacteria in Drought Adaptation
9. Root Soil Building Attributes in Drought Adaptation
10. Drought Adaptive Root Ideotypes
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Den Herder, G.; Van Isterdael, G.; Beeckman, T.; De Smet, I. The roots of a new green revolution. Trends Plant Sci. 2010, 15, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Nacry, P. Root architecture and hydraulics converge for acclimation to changing water availability. Nat. Plants 2020, 6, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Delory, B.M.; Hernandez-Soriano, M.C.; Wacker, T.S.; Dimitrova, A.; Ding, Y.; Greeley, L.A.; Ng, J.L.P.; Mesa-Marín, J.; Xie, L.; Zheng, C. A snapshot of the root phenotyping landscape in 2021. bioRxiv 2022. [Google Scholar] [CrossRef]
- Wasson, A.P.; Nagel, K.A.; Tracy, S.; Watt, M. Beyond Digging: Noninvasive Root and Rhizosphere Phenotyping. Trends Plant Sci. 2020, 25, 119–120. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Zhan, A.; Schneider, H.; Lynch, J.P. Reduced Lateral Root Branching Density Improves Drought Tolerance in Maize. Plant Physiol. 2015, 168, 1603–1615. [Google Scholar] [CrossRef]
- Rabbi, S.M.F.; Tighe, M.K.; Flavel, R.J.; Kaiser, B.N.; Guppy, C.N.; Zhang, X.; Young, I.M. Plant roots redesign the rhizosphere to alter the three-dimensional physical architecture and water dynamics. New Phytol. 2018, 219, 542–550. [Google Scholar] [CrossRef]
- Gao, Y.; Lynch, J.P. Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). J. Exp. Bot. 2016, 67, 4545–4557. [Google Scholar] [CrossRef]
- Kiran, B.O.; Karabhantanal, S.S.; Patil, S.B.; Ashwathama, V.H.; M, G.; Sajjanar; Jolli, R.B.; Tonapi, V.A. Phenotyping sorghum for drought-adaptive physiological and root architectural traits under water-limited environments. Cereal Res. Commun. 2022, 50, 1–9. [Google Scholar] [CrossRef]
- Chai, Y.N.; Schachtman, D.P. Root exudates impact plant performance under abiotic stress. Trends Plant Sci. 2022, 27, 80–91. [Google Scholar] [CrossRef]
- Thirkell, T.J.; Charters, M.D.; Elliott, A.J.; Sait, S.M.; Field, K.J. Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J. Ecol. 2017, 105, 921–929. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol. 2007, 173, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A.; et al. A starting guide to root ecology: Strengthening ecological concepts and standardising root classification, sampling, processing and trait measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Singla-Pareek, S.L.; Pareek, A.; Singh, A.K. Shaping the Root System Architecture in Plants for Adaptation to Drought Stress. Physiol. Plant. 2022, 174, e13651. [Google Scholar] [CrossRef]
- Rongsawat, T.; Peltier, J.B.; Boyer, J.C.; Véry, A.A.; Sentenac, H. Looking for Root Hairs to Overcome Poor Soils. Trends Plant Sci. 2021, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Armstrong, R.; Silva-Perez, V.; Tefera, A.T.; Rosewarne, G.M. Pulse Root Ideotype for Water Stress in Temperate Cropping System. Plants 2021, 10, 692. [Google Scholar] [CrossRef]
- Giehl, R.F.H.; Von Wiren, N. Hydropatterning-how roots test the waters. Science 2018, 362, 1358–1359. [Google Scholar] [CrossRef]
- Orman-Ligeza, B.; Morris, E.C.; Parizot, B.; Lavigne, T.; Babé, A.; Ligeza, A.; Klein, S.; Sturrock, C.; Xuan, W.; Novák, O.; et al. The Xerobranching Response Represses Lateral Root Formation When Roots Are Not in Contact with Water. Curr. Biol. 2018, 28, 3165–3173.e3165. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, Y. Adaptive Growth: Shaping Auxin-Mediated Root System Architecture. Trends Plant Sci. 2020, 25, 121–123. [Google Scholar] [CrossRef]
- Ogura, T.; Goeschl, C.; Filiault, D.; Mirea, M.; Slovak, R.; Wolhrab, B.; Satbhai, S.B.; Busch, W. Root System Depth in Arabidopsis Is Shaped by EXOCYST70A3 via the Dynamic Modulation of Auxin Transport. Cell 2019, 178, 400–412.e416. [Google Scholar] [CrossRef] [PubMed]
- Waidmann, S.; Ruiz Rosquete, M.; Scholler, M.; Sarkel, E.; Lindner, H.; LaRue, T.; Petrik, I.; Dunser, K.; Martopawiro, S.; Sasidharan, R.; et al. Cytokinin functions as an asymmetric and anti-gravitropic signal in lateral roots. Nat. Commun. 2019, 10, 3540. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Testerink, C.; Zhang, Y. How roots and shoots communicate through stressful times. Trends Plant Sci. 2021, 26, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Bacher, H.; Sharaby, Y.; Walia, H.; Peleg, Z. Modifying root-to-shoot ratio improves root water influxes in wheat under drought stress. J. Exp. Bot. 2021, 73, 1643–1654. [Google Scholar] [CrossRef]
- Becker, S.R.; Byrne, P.F.; Reid, S.D.; Bauerle, W.L.; McKay, J.K.; Haley, S.D. Root traits contributing to drought tolerance of synthetic hexaploid wheat in a greenhouse study. Euphytica 2015, 207, 213–224. [Google Scholar] [CrossRef]
- Eziz, A.; Yan, Z.; Tian, D.; Han, W.; Tang, Z.; Fang, J. Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, T.; Li, X.; Song, C.P.; Zhu, J.K.; Chen, L.; Zhao, Y. Phosphorylation of SWEET sucrose transporters regulates plant root:shoot ratio under drought. Nat. Plants 2022, 8, 68–77. [Google Scholar] [CrossRef]
- Caradus, J.R.; Woodfield, D.R. Genetic control of adaptive root characteristics in white clover. In Root Demographics and Their Efficiencies in Sustainable Agriculture, Grasslands and Forest Ecosystems; Springer: Berlin/Heidelberg, Germany, 1998; pp. 651–662. [Google Scholar]
- Chimungu, J.G.; Brown, K.M.; Lynch, J.P. Large Root Cortical Cell Size Improves Drought Tolerance in Maize. Plant Physiol. 2014, 166, 2166–2178. [Google Scholar] [CrossRef]
- Prince, S.J.; Murphy, M.; Mutava, R.N.; Durnell, L.A.; Valliyodan, B.; Shannon, J.G.; Nguyen, H.T. Root xylem plasticity to improve water use and yield in water-stressed soybean. J. Exp. Bot. 2017, 68, 2027–2036. [Google Scholar] [CrossRef]
- Galindo-Castañeda, T.; Brown, K.M.; Kuldau, G.A.; Roth, G.W.; Wenner, N.G.; Ray, S.; Schneider, H.; Lynch, J.P. Root cortical anatomy is associated with differential pathogenic and symbiotic fungal colonization in maize. Plant Cell Environ. 2019, 42, 2999–3014. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Dong, X. Anatomical structures of fine roots of 91 vascular plant species from four groups in a temperate forest in Northeast China. PLoS ONE 2019, 14, e0215126. [Google Scholar]
- Schneider, H.M.; Strock, C.F.; Hanlon, M.T.; Vanhees, D.J.; Perkins, A.C.; Ajmera, I.B.; Sidhu, J.S.; Mooney, S.J.; Brown, K.M.; Lynch, J.P. Multiseriate cortical sclerenchyma enhance root penetration in compacted soils. Proc. Natl. Acad. Sci. USA 2021, 118, e2012087118. [Google Scholar] [CrossRef] [PubMed]
- Colombi, T.; Herrmann, A.M.; Vallenback, P.; Keller, T. Cortical Cell Diameter Is Key To Energy Costs of Root Growth in Wheat. Plant Physiol. 2019, 180, 2049–2060. [Google Scholar] [CrossRef]
- Lynch, J.P.; Chimungu, J.G.; Brown, K.M. Root anatomical phenes associated with water acquisition from drying soil: Targets for crop improvement. J. Exp. Bot. 2014, 65, 6155–6166. [Google Scholar] [CrossRef] [PubMed]
- Chimungu, J.G.; Brown, K.M.; Lynch, J.P. Reduced Root Cortical Cell File Number Improves Drought Tolerance in Maize. Plant Physiol. 2014, 166, 1943–1955. [Google Scholar] [CrossRef]
- Karlova, R.; Boer, D.; Hayes, S.; Testerink, C. Root plasticity under abiotic stress. Plant Physiol. 2021, 187, 1057–1070. [Google Scholar] [CrossRef]
- Vanhees, D.J.; Loades, K.W.; Bengough, A.G.; Mooney, S.J.; Lynch, J.P. Root anatomical traits contribute to deeper rooting of maize under compacted field conditions. J. Exp. Bot. 2020, 71, 4243–4257. [Google Scholar] [CrossRef]
- Zhu, J.; Brown, K.M.; Lynch, J.P. Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell Environ. 2010, 33, 740–749. [Google Scholar] [CrossRef]
- Lynch, J.P.; Mooney, S.J.; Strock, C.F.; Schneider, H.M. Future roots for future soils. Plant Cell Environ. 2022, 45, 620–636. [Google Scholar] [CrossRef]
- Chimungu, J.G.; Loades, K.W.; Lynch, J.P. Root anatomical phenes predict root penetration ability and biomechanical properties in maize (Zea Mays). J. Exp. Bot. 2015, 66, 3151–3162. [Google Scholar] [CrossRef]
- Jansa, J.; Mozafar, A.; Kuhn, G.; Anken, T.; Ruh, R.; Sanders, I.R.; Frossard, E. Soil Tillage Affects the Community Structure of Mycorrhizal Fungi in Maize Roots. Ecol. Appl. 2003, 13, 1164–1176. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Nutrition and Growth: New Paradigms from Cellular to Ecosystem Scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Chareesri, A.; De Deyn, G.B.; Sergeeva, L.; Polthanee, A.; Kuyper, T.W. Increased arbuscular mycorrhizal fungal colonization reduces yield loss of rice (Oryza sativa L.) under drought. Mycorrhiza 2020, 30, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the Impact of Arbuscular Mycorrhizal Symbiosis on Tomato Tolerance to Water Stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wang, S.; Wang, W.; Wang, N.; Gu, J. Variations in Arbuscular Mycorrhizal Colonization Associated with Root Diameter and Hypodermis Passages Cells across Temperate and Tropical Woody Species. Forests 2022, 13, 140. [Google Scholar] [CrossRef]
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef]
- Dreyer, B.; Morte, A.; López, J.Á.; Honrubia, M. Comparative study of mycorrhizal susceptibility and anatomy of four palm species. Mycorrhiza 2010, 20, 103–115. [Google Scholar] [CrossRef]
- Lynch, J.P.; Strock, C.F.; Schneider, H.M.; Sidhu, J.S.; Ajmera, I.; Galindo-Castañeda, T.; Klein, S.P.; Hanlon, M.T. Root anatomy and soil resource capture. Plant Soil 2021, 466, 21–63. [Google Scholar] [CrossRef]
- Strock, C.F.; Burridge, J.D.; Niemiec, M.D.; Brown, K.M.; Lynch, J.P. Root metaxylem and architecture phenotypes integrate to regulate water use under drought stress. Plant Cell Environ. 2021, 44, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Ben-Gal, A.; Shtein, I.; Bustan, A.; Dag, A.; Erel, R. Root structural plasticity enhances salt tolerance in mature olives. Environ. Exp. Bot. 2020, 179, 104224. [Google Scholar] [CrossRef]
- Shahzad, Z.; Canut, M.; Tournaire-Roux, C.; Martinière, A.; Boursiac, Y.; Loudet, O.; Maurel, C. A Potassium-Dependent Oxygen Sensing Pathway Regulates Plant Root Hydraulics. Cell 2016, 167, 87–98.e14. [Google Scholar] [CrossRef] [PubMed]
- Vadez, V. Root hydraulics: The forgotten side of roots in drought adaptation. Field Crops Res. 2014, 165, 15–24. [Google Scholar] [CrossRef]
- Cai, G.; Ahmed, M.A.; Abdalla, M.; Carminati, A. Root hydraulic phenotypes impacting water uptake in drying soils. Plant Cell Environ. 2022, 45, 650–663. [Google Scholar] [CrossRef]
- Tang, N.; Shahzad, Z.; Lonjon, F.; Loudet, O.; Vailleau, F.; Maurel, C. Natural variation at XND1 impacts root hydraulics and trade-off for stress responses in Arabidopsis. Nat. Commun. 2018, 9, 3884. [Google Scholar] [CrossRef]
- Matsuo, N.; Ozawa, K.; Mochizuki, T. Genotypic differences in root hydraulic conductance of rice (Oryza sativa L.) in response to water regimes. Plant Soil 2009, 316, 25–34. [Google Scholar] [CrossRef]
- Fennell, A.; Markhart, A.H. Rapid acclimation of root hydraulic conductivity to low temperature. J. Exp. Bot. 1998, 49, 879–884. [Google Scholar] [CrossRef]
- Lo Gullo, M.A.; Nardini, A.; Salleo, S.; Tyree, M.T. Changes in root hydraulic conductance (KR) of Olea oleaster seedlings following drought stress and irrigation. New Phytol. 1998, 140, 25–31. [Google Scholar] [CrossRef]
- Saliendra, N.Z.; Meinzer, F.C. Genotypic, Developmental and Drought-Induced Differences in Root Hydraulic Conductance of Contrasting Sugarcane Cultivars. J. Exp. Bot. 1992, 43, 1209–1217. [Google Scholar] [CrossRef]
- Rieger, M.; Litvin, P. Root system hydraulic conductivity in species with contrasting root anatomy. J. Exp. Bot. 1999, 50, 201–209. [Google Scholar] [CrossRef]
- Fan, M.; Bai, R.; Zhao, X.; Zhang, J. Aerenchyma formed under phosphorus deficiency contributes to the reduced root hydraulic conductivity in maize roots. J. Integr. Plant Biol. 2007, 49, 598–604. [Google Scholar] [CrossRef]
- Muhsin, T.M.; Zwiazek, J.J. Ectomycorrhizas increase apoplastic water transport and root hydraulic conductivity in Ulmus americana seedlings. New Phytol. 2002, 153, 153–158. [Google Scholar] [CrossRef]
- Geng, D.; Chen, P.; Shen, X.; Zhang, Y.; Li, X.; Jiang, L.; Xie, Y.; Niu, C.; Zhang, J.; Huang, X.; et al. MdMYB88 and MdMYB124 Enhance Drought Tolerance by Modulating Root Vessels and Cell Walls in Apple. Plant Physiol. 2018, 178, 1296–1309. [Google Scholar] [CrossRef] [PubMed]
- Javot, H.; Lauvergeat, V.; Santoni, V.; Martin-Laurent, F.; Güçlü, J.; Vinh, J.; Heyes, J.; Franck, K.I.; Schäffner, A.R.; Bouchez, D.; et al. Role of a Single Aquaporin Isoform in Root Water Uptake. Plant Cell 2003, 15, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Postaire, O.; Tournaire-Roux, C.; Grondin, A.; Boursiac, Y.; Morillon, R.; Schäffner, A.R.; Maurel, C. A PIP1 Aquaporin Contributes to Hydrostatic Pressure-Induced Water Transport in Both the Root and Rosette of Arabidopsis. Plant Physiol. 2009, 152, 1418–1430. [Google Scholar] [CrossRef]
- Siefritz, F.; Tyree, M.T.; Lovisolo, C.; Schubert, A.; Kaldenhoff, R. PIP1 Plasma Membrane Aquaporins in Tobacco. Plant Cell 2002, 14, 869–876. [Google Scholar] [CrossRef]
- Peret, B.; Li, G.; Zhao, J.; Band, L.R.; Voss, U.; Postaire, O.; Luu, D.T.; Da Ines, O.; Casimiro, I.; Lucas, M.; et al. Auxin regulates aquaporin function to facilitate lateral root emergence. Nat. Cell Biol. 2012, 14, 991–998. [Google Scholar] [CrossRef]
- Caldeira, C.F.; Jeanguenin, L.; Chaumont, F.; Tardieu, F. Circadian rhythms of hydraulic conductance and growth are enhanced by drought and improve plant performance. Nat. Commun. 2014, 5, 5365. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Ribeyre, Z.; Dauzat, M.; Reyt, G.; Hidalgo-Shrestha, C.; Diehl, P.; Frenger, M.; Simonneau, T.; Muller, B.; Salt, D.E.; et al. Physiological roles of Casparian strips and suberin in the transport of water and solutes. New Phytol. 2021, 232, 2295–2307. [Google Scholar] [CrossRef]
- Lefebvre, V.; Fortabat, M.-N.; Ducamp, A.; North, H.M.; Maia-Grondard, A.; Trouverie, J.; Boursiac, Y.; Mouille, G.; Durand-Tardif, M. ESKIMO1 Disruption in Arabidopsis Alters Vascular Tissue and Impairs Water Transport. PLoS ONE 2011, 6, e16645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwieniecki, M.A.; Boersma, L. A technique to measure root tip hydraulic conductivity and root water potential simultaneously. J. Exp. Bot. 1997, 48, 333–336. [Google Scholar] [CrossRef]
- Li, Q.-M.; Liu, B.-B. Comparison of three methods for determination of root hydraulic conductivity of maize (Zea mays L.) root system. Agric. Sci. China 2010, 9, 1438–1447. [Google Scholar] [CrossRef]
- Sutka, M.; Li, G.; Boudet, J.; Boursiac, Y.; Doumas, P.; Maurel, C. Natural variation of root hydraulics in Arabidopsis grown in normal and salt-stressed conditions. Plant Physiol. 2011, 155, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Heymans, A.; Couvreur, V.; Lobet, G. Combining cross-section images and modeling tools to create high-resolution root system hydraulic atlases in Zea mays. Plant Direct 2021, 5, e334. [Google Scholar] [CrossRef]
- Ajmera, I.; Henry, A.; Radanielson, A.M.; Klein, S.P.; Ianevski, A.; Bennett, M.J.; Band, L.R.; Lynch, J.P. Integrated root phenotypes for improved rice performance under low nitrogen availability. Plant Cell Environ. 2022, 45, 805–822. [Google Scholar] [CrossRef]
- Haling, R.E.; Brown, L.K.; Bengough, A.G.; Young, I.M.; Hallett, P.D.; White, P.J.; George, T.S. Root hairs improve root penetration, root–soil contact, and phosphorus acquisition in soils of different strength. J. Exp. Bot. 2013, 64, 3711–3721. [Google Scholar] [CrossRef]
- Li, T.; Lin, G.; Zhang, X.; Chen, Y.; Zhang, S.; Chen, B. Relative importance of an arbuscular mycorrhizal fungus (Rhizophagus intraradices) and root hairs in plant drought tolerance. Mycorrhiza 2014, 24, 595–602. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Zou, Y.-N.; Wu, Q.-S. Alleviation of drought stress by mycorrhizas is related to increased root H2O2 efflux in trifoliate orange. Sci. Rep. 2017, 7, 42335. [Google Scholar] [CrossRef]
- Li, J.; Meng, B.; Chai, H.; Yang, X.; Song, W.; Li, S.; Lu, A.; Zhang, T.; Sun, W. Arbuscular Mycorrhizal Fungi Alleviate Drought Stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) Grasses via Altering Antioxidant Enzyme Activities and Photosynthesis. Front. Plant Sci. 2019, 10, 499. [Google Scholar] [CrossRef]
- Guseman, J.M.; Webb, K.; Srinivasan, C.; Dardick, C. DRO 1 influences root system architecture in Arabidopsis and Prunus species. Plant J. 2017, 89, 1093–1105. [Google Scholar] [CrossRef] [Green Version]
- Kitomi, Y.; Hanzawa, E.; Kuya, N.; Inoue, H.; Hara, N.; Kawai, S.; Kanno, N.; Endo, M.; Sugimoto, K.; Yamazaki, T.; et al. Root angle modifications by the DRO1 homolog improve rice yields in saline paddy fields. Proc. Natl. Acad. Sci. USA 2020, 117, 21242–21250. [Google Scholar] [CrossRef] [PubMed]
- Van Der Bom, F.J.T.; Williams, A.; Bell, M.J. Root architecture for improved resource capture: Trade-offs in complex environments. J. Exp. Bot. 2020, 71, 5752–5763. [Google Scholar] [CrossRef] [PubMed]
- Okushima, Y.; Fukaki, H.; Onoda, M.; Theologis, A.; Tasaka, M. ARF7 and ARF19 Regulate Lateral Root Formation via Direct Activation of LBD/ASL Genes in Arabidopsis. Plant Cell 2007, 19, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Wilmoth, J.C.; Wang, S.; Tiwari, S.B.; Joshi, A.D.; Hagen, G.; Guilfoyle, T.J.; Alonso, J.M.; Ecker, J.R.; Reed, J.W. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 2005, 43, 118–130. [Google Scholar] [CrossRef]
- Orosa-Puente, B.; Leftley, N.; von Wangenheim, D.; Banda, J.; Srivastava, A.K.; Hill, K.; Truskina, J.; Bhosale, R.; Morris, E.; Srivastava, M. Root branching toward water involves posttranslational modification of transcription factor ARF7. Science 2018, 362, 1407–1410. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Z.; Huang, K.; Han, Y.; Zhao, A.; Han, H.; Song, L.; Fan, C.; Li, R.; Xin, M.; et al. Three genomes differentially contribute to the seedling lateral root number in allohexaploid wheat: Evidence from phenotype evolution and gene expression. Plant J. 2018, 95, 976–987. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, T.; Wang, X.; Deng, Y.; Ma, H.; Zhang, R.; Zhao, J. OsAUX1 controls lateral root initiation in rice (Oryza sativa L.). Plant Cell Environ. 2015, 38, 2208–2222. [Google Scholar] [CrossRef]
- Shkolnik, D.; Krieger, G.; Nuriel, R.; Fromm, H. Hydrotropism: Root Bending Does Not Require Auxin Redistribution. Mol. Plant 2016, 9, 757–759. [Google Scholar] [CrossRef]
- Dietrich, D.; Pang, L.; Kobayashi, A.; Fozard, J.A.; Boudolf, V.; Bhosale, R.; Antoni, R.; Nguyen, T.; Hiratsuka, S.; Fujii, N. Root hydrotropism is controlled via a cortex-specific growth mechanism. Nat. Plants 2017, 3, 17057. [Google Scholar] [CrossRef]
- Kobayashi, A.; Takahashi, A.; Kakimoto, Y.; Miyazawa, Y.; Fujii, N.; Higashitani, A.; Takahashi, H. A gene essential for hydrotropism in roots. Proc. Natl. Acad. Sci. USA 2007, 104, 4724–4729. [Google Scholar] [CrossRef] [Green Version]
- Vries, F.T.d.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; White, P.J.; Shen, J.; Lambers, H. Linking root exudation to belowground economic traits for resource acquisition. New Phytol. 2022, 233, 1620–1635. [Google Scholar] [CrossRef] [PubMed]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Penuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696. [Google Scholar] [CrossRef] [PubMed]
- Basirat, M.; Mousavi, S.M.; Abbaszadeh, S.; Ebrahimi, M.; Zarebanadkouki, M. The rhizosheath: A potential root trait helping plants to tolerate drought stress. Plant Soil 2019, 445, 565–575. [Google Scholar] [CrossRef]
- Etesami, H. Potential advantage of rhizosheath microbiome, in contrast to rhizosphere microbiome, to improve drought tolerance in crops. Rhizosphere 2021, 20, 100439. [Google Scholar] [CrossRef]
- Young, I.M. Variation in moisture contents between bulk soil and the rhizosheath of wheat (Triticum aestivum L. cv. Wembley). New Phytol. 1995, 130, 135–139. [Google Scholar] [CrossRef]
- Hu, L.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; van der Heijden, M.G.A.; et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Koprivova, A.; Kopriva, S. Pinpointing secondary metabolites that shape the composition and function of the plant microbiome. J. Exp. Bot. 2021, 72, 57–69. [Google Scholar] [CrossRef]
- Huang, A.C.; Jiang, T.; Liu, Y.X.; Bai, Y.C.; Reed, J.; Qu, B.; Goossens, A.; Nutzmann, H.W.; Bai, Y.; Osbourn, A. A specialized metabolic network selectively modulates Arabidopsis root microbiota. Science 2019, 364, eaau6389. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Coleman-Derr, D. Causes and consequences of a conserved bacterial root microbiome response to drought stress. Curr. Opin. Microbiol. 2019, 49, 1–6. [Google Scholar] [CrossRef]
- Henry, A.; Doucette, W.; Norton, J.; Bugbee, B. Changes in crested wheatgrass root exudation caused by flood, drought, and nutrient stress. J. Environ. Qual. 2007, 36, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Vries, F.T. Plant root exudation under drought: Implications for ecosystem functioning. New Phytol. 2020, 225, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Naylor, D.; Degraaf, S.; Purdom, E.; Coleman-Derr, D. Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 2017, 11, 2691–2704. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Darrah, P.R. Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 1994, 166, 247–257. [Google Scholar] [CrossRef]
- Paporisch, A.; Bavli, H.; Strickman, R.J.; Neumann, R.B.; Schwartz, N. Root Exudates Alters Nutrient Transport in Soil. Water Resour. Res. 2021, 57, e2021WR029976. [Google Scholar] [CrossRef]
- Jayne, B.; Quigley, M. Influence of arbuscular mycorrhiza on growth and reproductive response of plants under water deficit: A meta-analysis. Mycorrhiza 2014, 24, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Liu, C.; Guo, X.; Wu, X.; Dai, F.; Wu, Q. The Comprehensive Effects of Rhizophagus intraradices and P on Root System Architecture and P Transportation in Citrus limon L. Agriculture 2022, 12, 317. [Google Scholar] [CrossRef]
- Paszkowski, U.; Boller, T. The growth defect of lrt1, a maize mutant lacking lateral roots, can be complemented by symbiotic fungi or high phosphate nutrition. Planta 2002, 214, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J.M. Enhanced Drought Stress Tolerance by the Arbuscular Mycorrhizal Symbiosis in a Drought-Sensitive Maize Cultivar Is Related to a Broader and Differential Regulation of Host Plant Aquaporins than in a Drought-Tolerant Cultivar. Front. Plant Sci. 2017, 8, 1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Verbruggen, E.; Struyf, E.; Vicca, S. Can arbuscular mycorrhizal fungi speed up carbon sequestration by enhanced weathering? Plants People Planet 2021, 3, 445–453. [Google Scholar] [CrossRef]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef]
- Liu, X.; Duan, B.; Li, L.; Chen, G.; Su-Zhou, C.; Li, Y.; Merkeryan, H.; Liu, W. ACC Deaminase-producing PGPRs Improve Drought Stress Tolerance in Grapevine (Vitis vinifera L.). Front. Plant Sci. 2021, 12, 1849. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Sher, Y.; Baker, N.R.; Herman, D.; Fossum, C.; Hale, L.; Zhang, X.; Nuccio, E.; Saha, M.; Zhou, J.; Pett-Ridge, J. Microbial extracellular polysaccharide production and aggregate stability controlled by switchgrass (Panicum virgatum) root biomass and soil water potential. Soil Biol. Biochem. 2020, 143, 107742. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, H. The effects of bacterial volatile emissions on plant abiotic stress tolerance. Front. Plant Sci. 2015, 6, 774. [Google Scholar] [CrossRef]
- Chen, Y.; Gozzi, K.; Yan, F.; Chai, Y. Acetic acid acts as a volatile signal to stimulate bacterial biofilm formation. MBio 2015, 6, e00392-15. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.-Y.; Lee, Y.-H.; Cho, B.H.; Yang, K.-Y.; Ryu, C.-M.; Kim, Y.C. 2R, 3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef]
- Staudinger, C.; Mehmeti-Tershani, V.; Gil-Quintana, E.; Gonzalez, E.M.; Hofhansl, F.; Bachmann, G.; Wienkoop, S. Evidence for a rhizobia-induced drought stress response strategy in Medicago truncatula. J. Proteom. 2016, 136, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Vurukonda, S.S.; Vardharajula, S.; Shrivastava, M.; Sk, Z.A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Paul, M.J.; Primavesi, L.F.; Jhurreea, D.; Zhang, Y. Trehalose metabolism and signaling. Annu. Rev. Plant Biol. 2008, 59, 417–441. [Google Scholar] [CrossRef]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef]
- Glick, B.R. Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol. Lett. 2005, 251, 1–7. [Google Scholar] [CrossRef]
- Mansour, E.; Mahgoub, H.A.M.; Mahgoub, S.A.; El-Sobky, E.E.A.; Abdul-Hamid, M.I.; Kamara, M.M.; AbuQamar, S.F.; El-Tarabily, K.A.; Desoky, E.M. Enhancement of drought tolerance in diverse Vicia faba cultivars by inoculation with plant growth-promoting rhizobacteria under newly reclaimed soil conditions. Sci. Rep. 2021, 11, 24142. [Google Scholar] [CrossRef]
- Sutulienė, R.; Ragelienė, L.; Samuolienė, G.; Brazaitytė, A.; Urbutis, M.; Miliauskienė, J. The Response of Antioxidant System of Drought-Stressed Green Pea (Pisum sativum L.) Affected by Watering and Foliar Spray with Silica Nanoparticles. Horticulturae 2022, 8, 35. [Google Scholar] [CrossRef]
- Mekureyaw, M.F.; Pandey, C.; Hennessy, R.C.; Nicolaisen, M.H.; Liu, F.; Nybroe, O.; Roitsch, T. The cytokinin-producing plant beneficial bacterium Pseudomonas fluorescens G20-18 primes tomato (Solanum lycopersicum) for enhanced drought stress responses. J. Plant Physiol. 2022, 270, 153629. [Google Scholar] [CrossRef]
- Yu, P.; He, X.; Baer, M.; Beirinckx, S.; Tian, T.; Moya, Y.A.T.; Zhang, X.; Deichmann, M.; Frey, F.P.; Bresgen, V.; et al. Plant flavones enrich rhizosphere Oxalobacteraceae to improve maize performance under nitrogen deprivation. Nat. Plants 2021, 7, 481–499. [Google Scholar] [CrossRef]
- Oburger, E.; Jones, D.L. Sampling root exudates—Mission impossible? Rhizosphere 2018, 6, 116–133. [Google Scholar] [CrossRef]
- Hallett, P.D.; Marin, M.; Bending, G.D.; George, T.S.; Collins, C.D.; Otten, W. Building soil sustainability from root-soil interface traits. Trends Plant Sci. 2022, 27, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Schluter, S.; Vogel, H.J.; Vetterlein, D. Roots compact the surrounding soil depending on the structures they encounter. Sci. Rep. 2019, 9, 16236. [Google Scholar] [CrossRef] [PubMed]
- Kautz, T. Research on subsoil biopores and their functions in organically managed soils: A review. Renew. Agric. Food Syst. 2015, 30, 318–327. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, X. Bio-tillage: A new perspective for sustainable agriculture. Soil Tillage Res. 2021, 206, 104844. [Google Scholar] [CrossRef]
- Marcacci, K.M.; Warren, J.M.; Perfect, E.; Labbé, J.L. Influence of living grass Roots and endophytic fungal hyphae on soil hydraulic properties. Rhizosphere 2022, 22, 100510. [Google Scholar] [CrossRef]
- Galloway, A.F.; Pedersen, M.J.; Merry, B.; Marcus, S.E.; Blacker, J.; Benning, L.G.; Field, K.J.; Knox, J.P. Xyloglucan is released by plants and promotes soil particle aggregation. New Phytol. 2018, 217, 1128–1136. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Chang. Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef]
- Cabal, C.; Martínez-García, R.; Aguilar, A.d.C.; Valladares, F.; Pacala, S.W. The exploitative segregation of plant roots. Science 2020, 370, 1197–1199. [Google Scholar] [CrossRef]
- Poirier, V.; Roumet, C.; Munson, A.D. The root of the matter: Linking root traits and soil organic matter stabilization processes. Soil Biol. Biochem. 2018, 120, 246–259. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef]
- Hinsinger, P.; Gobran, G.R.; Gregory, P.J.; Wenzel, W.W. Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes. New Phytol. 2005, 168, 293–303. [Google Scholar] [CrossRef]
- Goss, M.; Miller, M.; Bailey, L.; Grant, C. Root growth and distribution in relation to nutrient availability and uptake. Eur. J. Agron. 1993, 2, 57–67. [Google Scholar] [CrossRef]
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef]
- Carminati, A.; Javaux, M. Soil Rather Than Xylem Vulnerability Controls Stomatal Response to Drought. Trends Plant Sci. 2020, 25, 868–880. [Google Scholar] [CrossRef]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.V.S.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef] [Green Version]
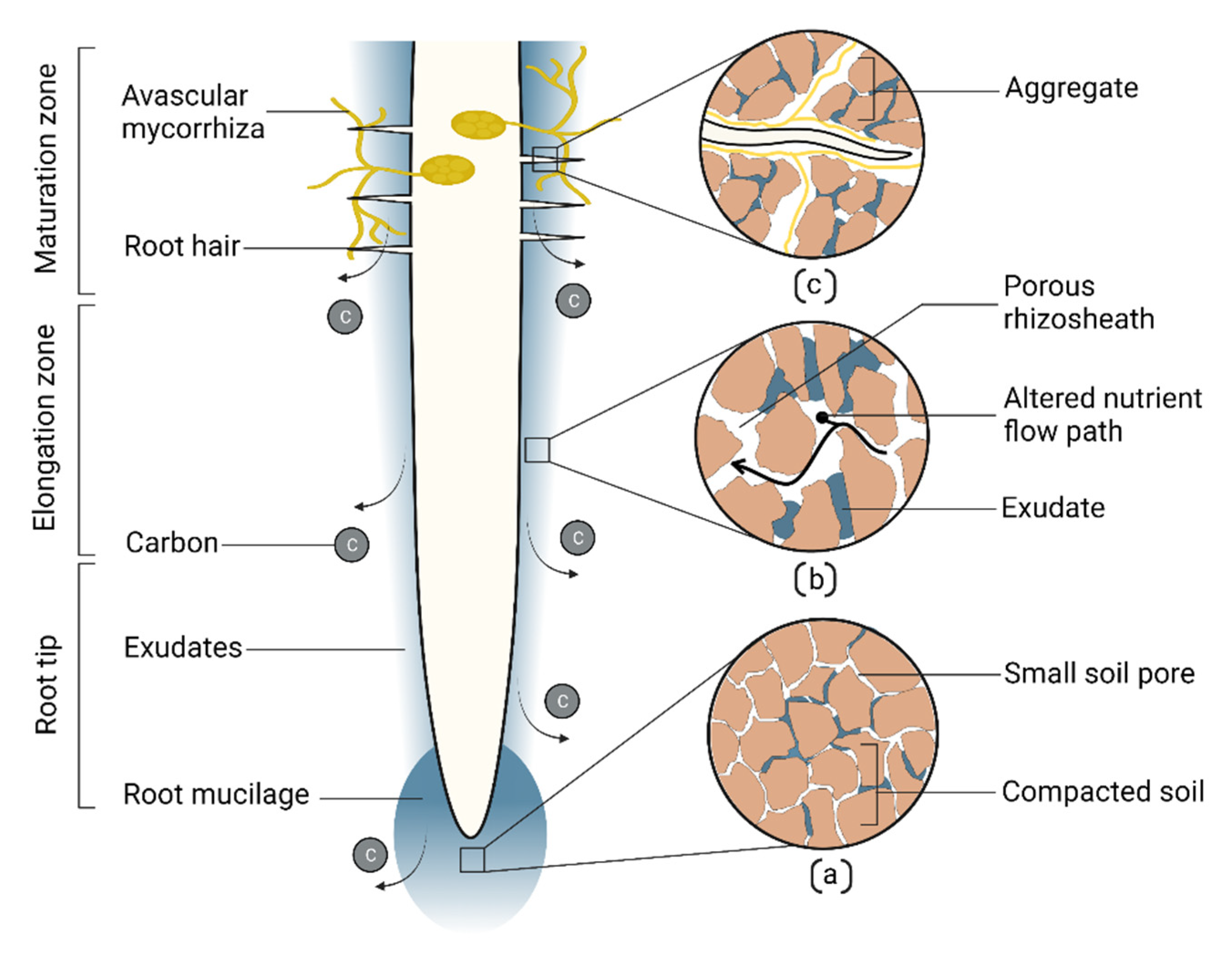
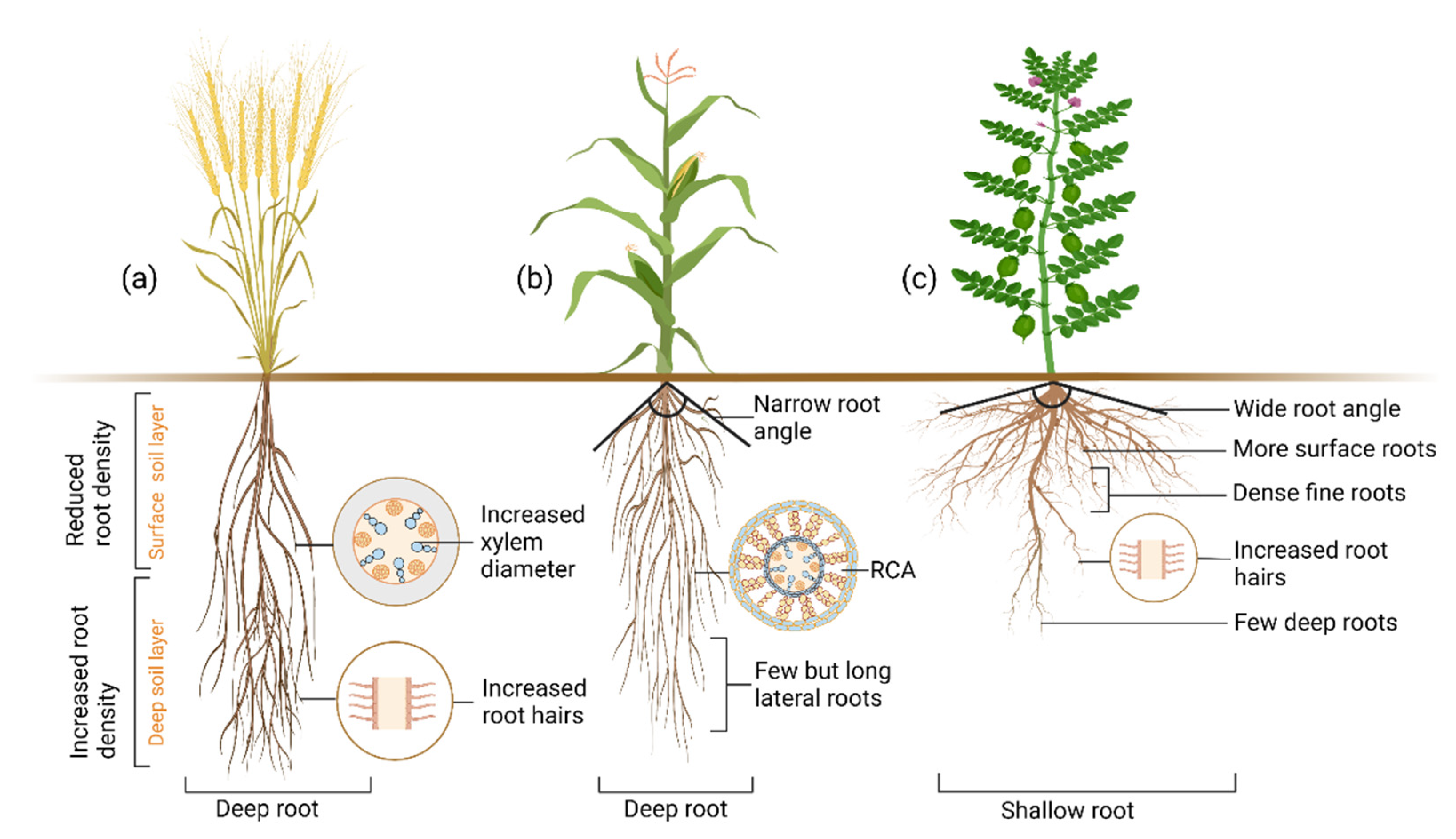
| Structural Root Traits | Drought Adaptive Responses | Crop | Reference |
|---|---|---|---|
| Taproot diameter | Large taproot diameter genotypes had increased yield and drought resistance. | White clover (Trifolium repens L.), Soybean (Glycine max L.), Chickpea (Cicer arietinum L.) | Caradus and Woodfield [28], Fenta et al. [29], Rabbi et al. [7] |
| Taproot length | Long taproot genotypes yielded higher. | Soybean (Glycine max L.) | Jumrani and Bhatia [30] |
| Root hair | Reduced root hair genotype had lower water absorption and decreased drought resistance. | Arabidopsis (Arabidopsis thaliana L.) | Tanaka et al. [31] |
| Root hair production time | Drought-resistant genotypes had faster root hair production. | Barley (Hordeum vulgare L.) | Carter et al. [32] |
| Root hair length and number | Longer and higher root hair genotypes had less negative leaf water potential and improved water status under drought. | Barley (Hordeum vulgare L.) | Marin et al. [33] |
| Rhizosheath size | Large rhizosheath genotypes were drought resistant. Longer and denser root hairs contributed to larger rhizosheath formation. | Barley (Hordeum vulgare L.), Lotus (Lotus japonicus L.), and Maize (Zea mays L.) | Liu et al. [34], Rabbi et al. [7]. |
| Root growth angle and rooting depth | Narrow root angles had downward root growth resulting in deep rooting and better yield under drought. | Rice (Oryza sativa L.), Soybean (Glycine max L.) | Uga et al. [5], Gobu et al. [35], Fenta et al. [29] |
| Seminal and nodal root angle | Steeper seminal and nodal root angle genotypes had a higher yield. | Maize (Zea mays L.) | Ali et al. [36] |
| Tap and lateral root branching intensity | Drought-resistance genotypes had more tap and lateral root branches. | Soybean Glycine max L.) | Fenta et al. [29] |
| Number of crown root | Low crown root number genotypes had better water status and yield. | Maize (Zea mays L.) | Gao and Lynch [8] |
| Quantity of fine-diameter roots | Drought-resistant genotypes had substantial amounts of small-diameter roots in deep soil. | Wheat (Triticum aestivum) | Becker et al. [25] |
| Lateral root branching density | Genotypes with fewer but longer lateral roots had better water status, biomass, and yield. | Maize (Zea mays L.) | Zhan et al. [6] |
| Root length, branching rate and surface area | Drought-resistant genotypes had increased root length, branching rate, larger root surface, and decreased coarse to fine root ratio. | Oat (Avena sativa L.) | Canales et al. [37] |
| Root volume and dry matter | Drought-resistant genotypes had larger root volumes and more root dry weight. | Sorghum (Sorghum bicolor L. Moench) | Kiran et al. [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoaib, M.; Banerjee, B.P.; Hayden, M.; Kant, S. Roots’ Drought Adaptive Traits in Crop Improvement. Plants 2022, 11, 2256. https://doi.org/10.3390/plants11172256
Shoaib M, Banerjee BP, Hayden M, Kant S. Roots’ Drought Adaptive Traits in Crop Improvement. Plants. 2022; 11(17):2256. https://doi.org/10.3390/plants11172256
Chicago/Turabian StyleShoaib, Mirza, Bikram P. Banerjee, Matthew Hayden, and Surya Kant. 2022. "Roots’ Drought Adaptive Traits in Crop Improvement" Plants 11, no. 17: 2256. https://doi.org/10.3390/plants11172256
APA StyleShoaib, M., Banerjee, B. P., Hayden, M., & Kant, S. (2022). Roots’ Drought Adaptive Traits in Crop Improvement. Plants, 11(17), 2256. https://doi.org/10.3390/plants11172256









