1. Introduction
HCPro is a non-structural, multifunctional dimeric protein of
Potyviruses that among its functions performs two that are essential to the virus infection cycle: it suppresses gene silencing, neutralizing selectively plant antiviral defenses based on small RNAs (sRNAs) of viral sequence [
1,
2,
3]. This facilitates the systemic infection of the plant and allows viruses to accumulate in systemic tissues. On the other hand, HCPro is mechanistically required to spread infection to other plants through aphid vectors [
4,
5]. There is a strong correlation between systemic viral titers in infected plants and the ability of insects to disperse potyviruses in a non-persistent way [
6].
Since HCPro can determine viral titers in compatible infections, and those titers affect the ability of vectors to disperse the virus, it is possible that HCPro could act as a regulator that assures in the longer term that the virulence of viral isolates of different geographic origins and climates is adequate to allow optimal dispersal of infection within and between plants at the minimum possible cost for the virus, and thus the permanence of infection in such hosts and environments. HCPro could thus be playing a role in the adaptation of a virus strain to a particular host and environment condition.
Studies on the adaptation of natural potyvirus isolates to changing environmental conditions, to new hosts, or to both, found that the non-structural genome-linked protein Vpg from turnip mosaic virus (TuMV), accumulated alterations as the virus adapted to the new conditions [
7]. This correlation suggests that highly variable regions within the Vpg play a significant role in adaptation. The Vpg is an intrinsically disordered protein that self-interacts, and also interacts with most other potyviral proteins, as well as with many host factors [
8,
9]. The Vpg is a second potyviral suppressor of RNA silencing [
10], together with HCPro. The same studies on adaptation of a TuMV isolate naive to different arabidopsis accessions and under different environments through mechanical passages showed that the second protein accumulating mutations after the Vpg was HCPro, although at a distance from the former [
7].
In contrast to the overall disorganized Vpg, HCPro has hypervariable regions limited to the protein N and C termini, and the number of sites under positive selection for the occurrence of non-synonymous substitutions that could help the virus adapt is lower than it would be expected randomly [
11]. HCPro contains several functional domains that may not accumulate alterations easily, without having negative or even deleterious effects on essential functions of this protein, and thus on viral fitness. This limitation does not exclude that HCPro could acquire mutations that help the virus adapt.
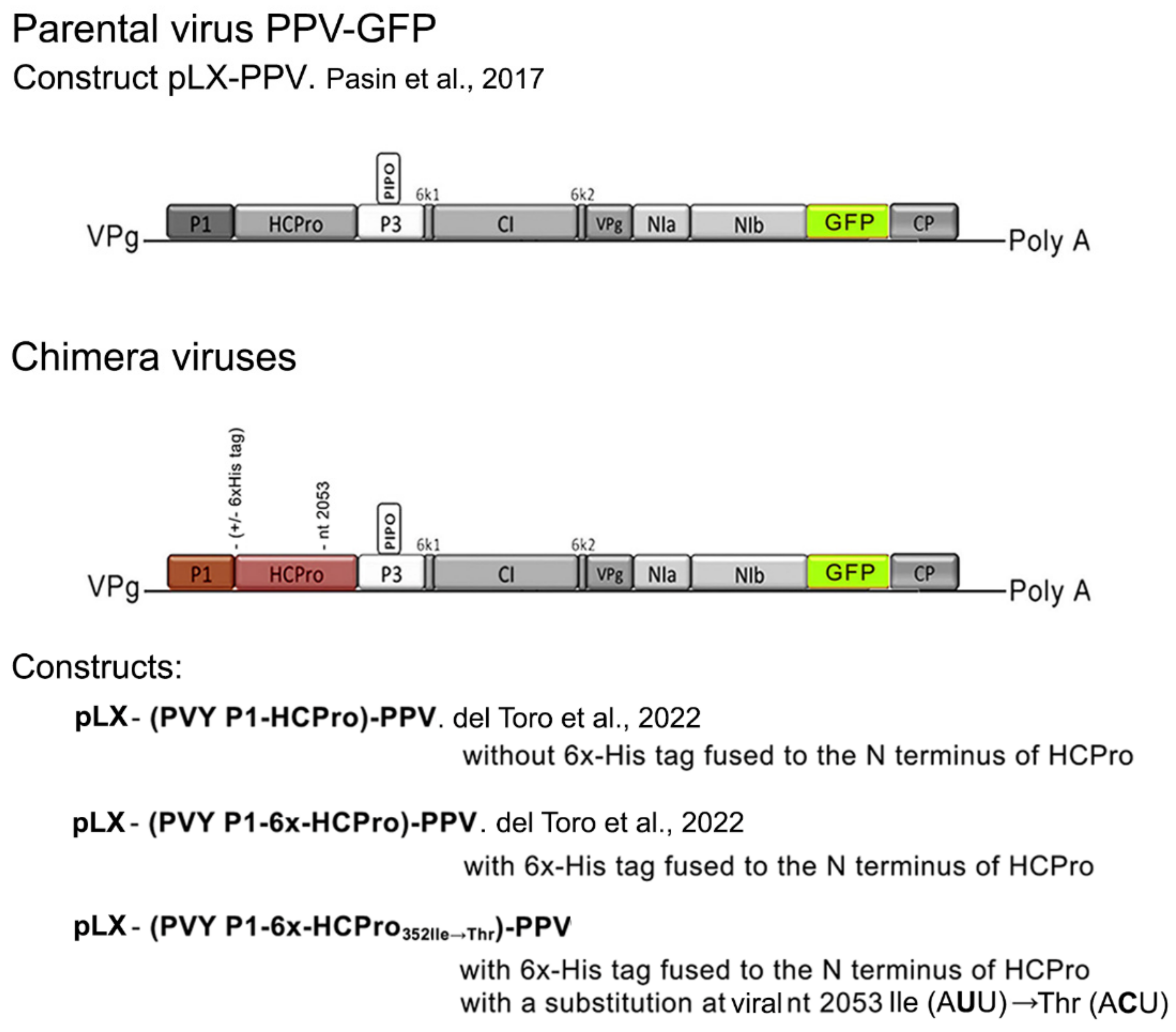
In a previous study on how potyviral HCPro performs its silencing suppression, we had created a binary construct that expressed an infectious chimera [
3] based on a plum pox virus (PPV) construct that had an added cistron that encoded green fluorescent protein (PPV-GFP) [
12]. In the chimera, the
P1-HCPro bi-cistron of PPV had been replaced by that of PVY. Viral titers of the chimera in infected
Nicotiana benthamiana plants were low, around 5-to–10% of those of the parental PPV-GFP, and symptoms were very mild [
3]. The artificial chimera was therefore more poorly adapted to this host than either of the parental viruses.
In this work, we assessed whether this infectious but artificial chimera, which lacks any nucleotide diversity, would yet possess the capability to adapt further to this compatible host increasing in virulence, and how this adaptation would relate to alterations in its genome. We found that the chimera could increase in virulence through mechanical passages. The two adapted chimeras analyzed had very few, and different, substitutions: combined, we found two in HCPro, two in the Vpg and one CP. We showed that mutations found in HCPro were likely induced under selective pressures that were occurring already in the agroinfiltrated plants (generation 1 viruses), previous to any mechanical passage bottleneck. Furthermore, we show that one single substitution in HCPro can alone increase virulence dramatically.
We have investigated additional properties of the chimera, relative to the parental viruses: its host range, and whether the chimera is transmitted by aphids.
2. Materials and Methods
2.1. Plants, Viruses and Plant Growing Conditions
Wild type (wt)
N. benthamiana plants, as well as
N. benthamiana transgenic plants that have their dicer-like
DCL 2 and
4 genes silenced (DCL2/4.5i plants) [
13] were used in this study.
Nicotiana tabacum cv. Xanthi nc and
Arabidopsis thaliana ecotype Col 0 plants were also used.
N. benthamiana and tobacco plants were grown in growth chambers at 25 °C with a 16/8-h day/night photoperiod. Arabidopsis plants were grown at 21/18 °C and 16/8-h day/night photoperiod.
The potyviruses used in this study were: a Scottish PVY isolate (PVYO-SCRI-O; Genbank accession number AJ585196) [
14]. Nucleotide annotations to its published
HCPro cistron sequence are shown in [
15]; a modified PPV derived from an infectious clone with an added cistron to express GFP (PPV-GFP) cloned in binary construct pLX-PPV [
12], donated by Juan Antonio García (CNB, Cantoblanco, Madrid); two chimeric potyviruses in which the P1-HCPro bi-cistron of the Scottish PVY isolate replaced that of PPV-GFP [constructs pLX-(PVY P1-6x-HCPro)-PPV or pLX-(PVY P1-HCPro)-PPV] [
3], expressing, respectively, chimeric viruses with (6x) or without a histidine tag (6xHis tag) fused to the N-terminus of PVY HCPro, respectively; a third chimeric construct was also used in which viral nt 2053 was altered (U→C), resulting in HCPro amino acid 352 substitution from Ile (A
UU) to Thr (A
CU). Cloning of this construct was performed as follows: a PCR fragment was amplified using template plasmid pLX (PVY P1-6x-HCPro)-PPV and oligo Fw: AGATTAATTAAGTACTAGTCCAG and Rv: ATGTCGCGAACTTTCTTTGTGAAATCCTTTGCATCCTCCTCGCTAATGTTA
GTCAGCATT. The reverse oligo contains the nt 2053 (U→C) substitution (highlighted). The PCR fragment was digested with
BlpI and
NruI, and cloned into construct pLX (PVY P1-6x-HCPro)-PPV linearized with the same enzymes, resulting in construct pLX (PVY P1-6x-HCPro
352Ile→Thr)-PPV that expressed chimera 352Ile→Thr. The general structure of the parental virus, and of the three chimeric viruses is shown in
Figure 1.
2.2. Infection of Plants with Viruses Using the Agroinfiltration Technique, or by Mechanical Inoculation
Plants (
N. benthamiana, tobacco, arabidopsis) were infected with PPV-GFP or with the chimeric viruses using the infectious binary plasmids in which they were cloned. These binary plasmids were delivered into plant leaf tissues by agroinfiltration: in the case of
N. benthamiana and tobacco plants, agrobacterium cell cultures carrying the binary constructs were grown exponentially at 28 °C in liquid LB media under shaking at 200 rpm, with the appropriate antibiotic selection (kanamycin resistance provided by the pROK2-based vectors, and rifampicin), pelleted, and re-suspended to an optical density at 600 nm of 0.3 in 2-N-morpholino ethanesulfonic acid (MES)-based infiltration buffer containing acetosyringone 0.2 mM to activate T-DNA transfer. After incubation at room temperature for 2 h, bacterial solutions were co-infiltrated together (1/1
V/V mixtures, each at OD 600 of 03). In the case of arabidopsis plants, the previous protocol, as well as the one described by [
16], was used.
In mechanical passages of infections, N. benthamiana plants were also infected using extracts made from already infected plants: plant leaves were dusted with carborundum and inoculated mechanically with an ice-cold extract from an infected plant made with 0.1 M sodium phosphate buffer, pH 8 (1/1 weight/volume). The extract (25-to−50 μL/leaf) was rubbed lightly on the leaf surface with gloved hands, and the leaves were washed with distilled water immediately afterwards.
2.3. Assessment of Viral Infections
The presence of local or systemic viral infections, as well as a measure of relative viral titers, was made in two ways:
(a) Measuring viral protein levels in infected plant tissues by western blot, using anti-PPV coat protein (CP) and anti-GFP antibodies: leaf discs were mechanically ground in extraction buffer (0.1 M Tris-HCl PH 8, 10 mM EDTA, 0.1 M LiCl, 1% β-mercaptoethanol and 1% SDS), boiled for 5 min, clarified by bench centrifugation and fractionated in 15% SDS-PAGE gels, and transferred by western blot to PVDF membranes. To detect and quantify the viral CP, a rabbit polyclonal antiserum to the PPV CP was used (Loewe Biochemica, Sauerlach, Germany). A rabbit GFP polyclonal antiserum donated by G. Cowan and L. Torrance (The James Hutton Institute, Dundee, UK) was also used. A commercial alkaline phosphatase-labeled goat anti-rabbit secondary antibody (Sigma-Aldrich, St. Louis, MO, USA) and BCIP/NBT substrate solutions (Duchefa, Haarlem, The Netherlands) were also used. Western blot membranes were scanned, and relative band densities were quantified using Image J software (
www.imagej.nih.gov, accessed on 10 July 2022).
(b) Measuring viral genomic RNA levels by RT-qPCR using one-step reverse transcription plus real-time quantitative polymerase chain reactions (RT-qPCR). Briefly, total RNA was extracted from leaf tissue using TRIzol reagent (Invitrogen, Waltham, MA, USA) following the manufacturer’s instructions, and contaminant DNA was removed by treatment with TURBO DNA-free kit (Ambion, Austin, TX, USA). RT-qPCR was performed using a final reaction volume of 15 uL that contained 7.5 μL of Brilliant III Ultra-Fast RT-qPCR Master Mix (Agilent, Santa Clara, CA, USA), 1.8 μL of RNase-free water, 0.75 μL of reverse transcriptase (Agilent), 0.15 μL of 100 mM dithiothreitol (Agilent), 0.3 μM each primer, and 3 μL of total RNA extract (approximately 15 ng RNA/μL). Relative quantifications were calculated by the ΔΔCt method.
A pair of oligos of PPV sequence was specifically designed and tested for the qPCR amplification of PPV-GFP and of the chimera: qPCR PPV Fw: AAGTCGATGGGCGAACTATG, and qPCR PPV Rv: AAACCGAAGTCCACAACCAC. In addition, oligos 18SrRNA-Fw (5′-GCCCGTTGCTGCGATGATTC-3′) and 18SrRNA-Rv (5′- GCTGCCTTCCTTGGATGTGG-3′) for 18S rRNA amplification were used for normalization. RT-qPCRs were performed in a Rotor-Gene Q thermal cycler (Qiagen, Hilden, Germany) using the following thermal protocol: 50 °C for 10 min; 95 °C for 3 min; 40 cycles of 95 °C for 10 s and 60 °C for 20 s; and a final ramp for melting analysis from 60 °C to 95 °C rising 1 °C every 5 s.
Results from either the serological quantitation of CP/GFP viral protein levels or from the RT-qPCR quantitation of viral genome levels were analyzed with SPSS STATISTICS (IBM, Armonk, NY, USA).
In addition to measuring viral titers, the virulence of infections in N. benthamiana plants was assessed visually, looking for the presence of chlorotic mosaic and leaf curling (typical PPV symptoms in this host), relative to non-infected plants.
2.4. Transmission Assays with Aphids
Aphid transmission assays were performed using a clone of the peach aphid Myzus persicae Sulz. Systemic N. benthamiana fully expanded leaves infected with the virus were used as donors to feed aphids that had been starved for the previous 2 h. Detached leaves were washed with mild detergent to remove the aphid repellants present in this plant species, rinsed with water and gently dried. Aphids fed on the abaxial side of the leaves placed inside wet glass container chambers. Feeding time was ~15 min, after which the insects were individually transferred to small, healthy N. benthamiana plantlets. Each plantlet received 10 aphids. Insects were killed 24 h after the transmission assay, and plants were allowed to grow for 20 days. At that time, leaf samples were taken, and the presence of the virus was determined by western blot with an antibody against the CP of PPV, as already described.
2.5. Analysis of sRNA Populations Present in Systemic Tissue of Plants Infected with the Chimeras
Data from four next-generation sequencing (NGS) libraries of sRNA populations present in systemically infected tissues of
N. benthamiana plants that had been agroinfiltrated with viral chimeras were available to us [
3]. These high-throughput sequencing data are deposited and are accessible at the Gene Expression Omnibus (GEO,
http://www.ncbi.nlm.nih.gov/geo; accession no. GSE135651, accessed on 10 July 2022). Using MISIS-2 [
17] and our own Perl scripts, we identified single nucleotide polymorphisms (SNPs) in reads of sRNAs that corresponded to the viral genome sequence. To be considered, an SNP would have to have at least 100 reads, and the nt of reference would have to be detected in less than 95% of the reads in at least one of the four libraries analyzed.
2.6. Analysis of Genomic Regions of the Chimeras after Several Mechanical Passages in N. benthamiana Plants
Three genomic regions of two viral chimeras present in two different plants after they had undergone several consecutive mechanical passages (generations) through
N. benthamiana plants were amplified by RT-PCR from total RNAs extracted from those two plants, to sequence them and to look for nucleotide changes/deletions, relative to the original sequence. Appropriate primers were used (
Table 1). They produced fragment 1 (2403 nt in length), fragment 2 (900 nt), and fragment 3 (1929 nt). Fragment 1 amplified the
P1-HCPro bi-cistron + flanking areas; Fragment 2 amplified the
Vpg cistron + flanking areas; Fragment 3 amplified the
GFP and
CP cistrons + flanking areas. Together they covered 5232 nt, just over half of the 10,452 nt of the (PVY P1-HCPro)-PPV-GFP genome (without the polyA tail).
To amplify these three fragments, cDNAs were generated from total RNAs extracted from three discs obtained from two systemic leaves of each of the two infected
N. benthamiana plants, using SuperscriptIII DNA polymerase, following the manufacturer’s instructions (Invitrogen). As reverse oligos primers D, F and B were used (
Table 1). Amplification by PCR of the three fragments from the cDNAs was performed using high-fidelity Phusion DNA polymerase (Invitrogen): fragment 1 (primers A and D), fragment 2 (primers E and F), fragment 3 (primers H and B). PCR amplification conditions were [98 °C 1 min] 1 cycle; [98 °C 10 sc/50 °C 10 sc/72 °C 25 sc] 35 cycles. In addition to these three genomic coding regions, we amplified by RT-PCR in the same way the 5′ untranslated region (UTR) of the two viral chimeras, which is limited to 35 nt in the cloned virus, plus the start of the P1 (146 nt in total) using primers A′ and C′ (
Table 1).
Amplified PCR fragments were agarose gel-fractionated, excised and extracted with QIAEX DNA gel extraction kit (Qiagen). Cleaned fragments were then sequenced (Secugen SA). Contigs of the sequence reads were assembled into the three fragments and compared to the original clone sequence using Vector NTI software (Thermofisher Scientific/Invitrogen).
4. Discussion
In our previous studies on two functions of HCPro (silencing suppression inside the plant, and mediation of insect transmission between plants) we created mutant and tagged forms of PVY HCPro that we expressed in plants either from binary vectors in a virus-free context or from a heterologous potato virus X (PVX) vector [
2,
4,
15,
20,
21] since we lacked an infectious clone of PVY amenable to manipulation in our hands. Recently we created infectious potyviral chimeras based on a PPV-GFP that had its
P1-HCPro bi-cistron replaced by that of PVY (
Figure 1) and [
3]. This allows us to express PVY HCPro mutants and variants in the context of a potyviral infection. However, the chimeras spread and accumulate poorly (
Figure 2b) and [
3], and this fact handicaps their use in research. We had observed that in DCL2/4.5i plants with their silencing suppression mechanisms compromised [
13] the chimeras accumulated more, suggesting that the antiviral silencing machinery was contributing to the chimera’s reduced virulence (viral titers and symptoms), whereas this effect of these plants was not observed for the parental PPV-GFP [
3]. The reason for the latter is not clear.
On the other hand, how plant viruses adapt and evolve in response to new hosts and environments, and vectors, is currently a very interesting topic of research, as climate change and increased global trade multiply the opportunities for those situations to occur [
22]. Studies on
Potyviruses indicate that mutations leading to adaptation do not distribute uniformly along the viral genome but target instead specific variable regions of some of the encoded viral proteins [
7,
11].
We wanted therefore to test whether we could increase the virulence of our chimera for practical purposes, but also to study how an un-adapted virus could evolve towards becoming better adapted to its host. The fundamentals of adaptation of natural viruses to new contexts may or may not differ from those of adaptation to a compatible experimental host (N. benthamiana) of an intrinsically un-adapted, artificial virus lacking any starting sequence diversity.
We agroinfiltrated plants with the chimera constructs (generation 1 plants) and after several consecutive mechanical passages that lasted around four months (
Figure 2a) we selected two plants, A and D (generations 3 and 4, respectively) that we used to inoculate either wt or DCL2/4.5i plants (generations 4 and 5). We analyzed systemic viral titers in these inoculated plants and found that they were several-fold higher than those of generation 1 plants. Interestingly, these “adapted chimeras” did not accumulate to higher levels in DCL2/4.5i plants than in wt plants (
Figure 2b). In this aspect, they now behaved like the parental PPV-GFP, rather than like the original chimeras.
We sequenced three fragments covering together over half of the genomes of the chimeras infecting plants A and D (
Figure 3a). The reasons for selecting those regions are that the
P1HCPro bi-cistron (in fragment 1) is a heterologous sequence that originates from another potyvirus, PVY; that the
Vpg-NIa (in fragment 2) contains hyper-variable regions (also
HCPro), where non-synonymous substitutions have been shown to occur under positive selection, facilitating the genetic adaptation of viral isolates [
7,
11]; and for fragment 3, there are two reasons, the first one is that the
GFP cistron is a non-viral sequence that could be lost, and the second one that the
CP has been also reported as a potential target for viral adaptation and evolution [
11,
23].
We found that the heterologous
GFP cistron had been maintained through the months of passages, suggesting that its fitness cost to the virus in this host is low, or lower than the cost of deleting it. Overall, we could find only a few non-synonymous substitutions, but different among the two chimeras, indicating that divergent chimera evolution in different plants had occurred (
Figure 3b) that led both to increased titers. Amino acid mutations were two in the chimera infecting plant A (in the C-terminal third of HCPro, and the Vpg) and three in the other one (at the C-terminus of HCPro, the Vpg and the N-terminal third of the CP). Interestingly, they all fell within proteins or protein domains that have been associated with potential sites for mutations leading to adaptation, as mentioned. The mutation in amino acid 352 of HCPro encoded by the chimera infecting plant A is non-conservative (Ile→Thr). On the other hand, the mutation in HCPro found in the chimera infecting plant D restored the exact sequence of PVY HCPro (
Figure 4). The
Vpg mutations in the chimeras infecting plants A and D were not the same but were only two nucleotides apart. When aligning with TuMV YC5, the amino acid changes in HCPro and in the Vpg found in the chimeras infecting plants A and D did not correspond with mutations in the TuMV isolate after adapting to different arabidopsis accessions [
7].
Four NGS libraries of reads of sRNA populations from systemic leaves of wt plants infected with generation 1 chimeras were available to us. We looked for the presence of SNPs in those sRNA reads, and found 120 along the viral genome (
Figure 5), including the two nucleotide positions that cause the mutations in the HCPro of the chimeras infecting plants A and D. Remarkably, mutation at nucleotide 2053 that causes HCPro amino acid 352 Ile→Thr substitution in the chimera infecting plant A was found in the four libraries. It is the only position along the whole protein-encoding genome with that property. On the other hand, mutations in the Vpg of the chimeras infecting plants A and D, or in the CP of the chimera infecting plant D did not correlate with SNPs found in any of the four libraries from generation 1 infections.
These data indicate that mutations in specific sites of the viral genome are occurring repeatedly, prior to any passage bottlenecks, and that selective pressures are already operating in generation 1 infections that will lead to some becoming eventually prevalent, as happened to those in positions 2053 and 2359 in the HCPro cistron in the chimeras infecting plants A and D. Generation 1 viral genomic RNAs with the mutations in HCPro may perhaps be advantaged in their replication/accumulation, and/or local or systemic spread (the libraries originate from systemically infected tissues). In the case of the mutation in HCPro of the chimera infecting plant D, restoration of the intact PVY HCPro sequence may have led to a slightly more efficient polyprotein processing, although western blot data shows that the original unmodified chimera processes HCPro properly (not shown). In the case of mutation of HCPro amino acid 352, we have no indication of why it could be selected.
To test the effect on virulence of the nucleotide 2053 mutation leading to HCPro amino acid substitution 352 Ile→Thr, we incorporated it into the original chimera, creating chimera 352Ile→Thr, and compared both viruses. We found that chimera 352Ile→Thr titers were several-fold higher in agroinfiltrated tissues as well as in systemic tissues than those of the unmodified chimera, and also that the virus moved much faster (
Figure 6). Therefore, a single nucleotide substitution in HCPro dramatically increased the virulence of the chimera.
We finally tested the host range of our chimera relative to those of the parental viruses. PVY infects many solanaceous plants of economic importance such as potato, pepper or tomato. PPV has also devastating effects on bone fruit trees (
Prunus). With regard to experimental plants, PVY infects
N. benthamiana and tobacco, but not arabidopsis Col 0 plants. PPV-GFP by contrast infects
N. benthamiana, arabidopsis Col 0, and the agroinfiltrated leaves of tobacco leaves but does not spread systemically [
20]. We found that the host range of the chimeras is more limited than that of each parental virus (
Figure 7). We tested the transmissibility by aphids of the chimeras but could not transmit them, even chimera 352Ile→Thr that had higher titers. This was somewhat unexpected, as it is known that PVY HCPro can mediate the transmission of PPV in trans and
in vitro [
24], and the CP of PPV-GFP and the chimeras contain the DAG motif. Why transmission failed in cis and
in vivo is a matter for further research.
In conclusion, we show that an un-adapted artificial chimeric potyvirus lacking any initial sequence variability can adapt to a compatible host and increase in virulence through mutations in its genome. We found that different mutations can lead to increased virulence in different chimera lineages (plants A vs. D). We show that in generation 1 infections, selective pressures created genomic variability at specific nucleotides, which with regard at least to those in
HCPro would lead eventually to their incorporation prevalence in later passages. In the case of the single non-conservative amino acid substitution in HCPro, 352 Ile→Thr, we demonstrate that it can dramatically increase the virulence of the chimera. This happens in spite of this substitution not restoring any natural HCPro sequence known to us, as remarkably, the Thr at this position seems to be not found among natural potyviruses (
Figure S2). This could suggest that this novel substitution is caused by the chimeric nature of the virus, perhaps then related to the need for interactions between HCPro (from PVY) and other viral components (from PPV). We also do not know how this substitution affects specific molecular properties of the protein and its functions. For example, does it increase or diminish its suppression activity? [
25]; or does it affect dimerization, or the ability of dimers to relocate to the microtubules in response to osmotic stress? [
21], but all this will be a matter for our future research.