Diversification of Vascular Occlusions and Crystal Deposits in the Xylem Sap Flow of Five Tunisian Grapevines
Abstract
1. Introduction
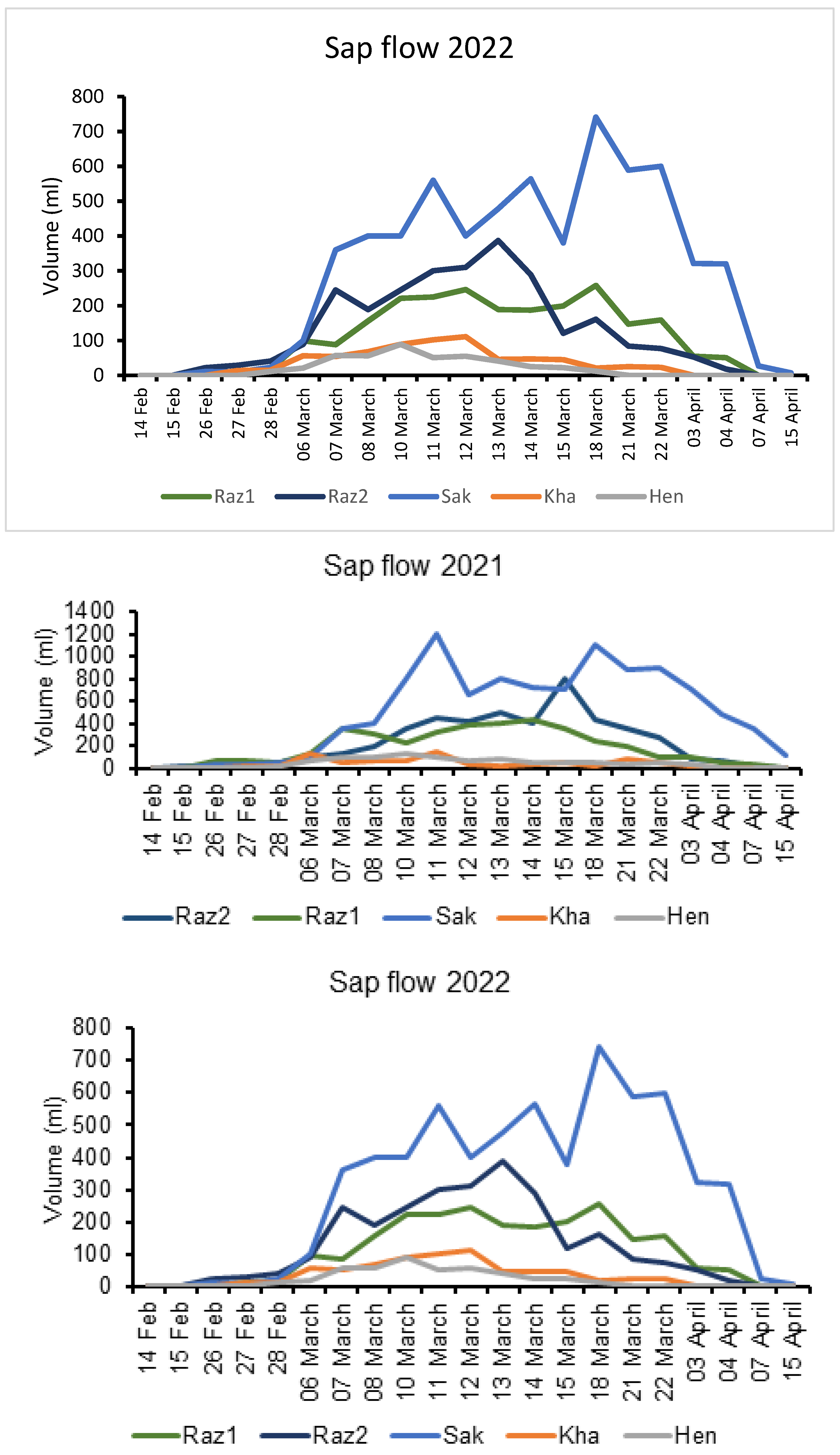
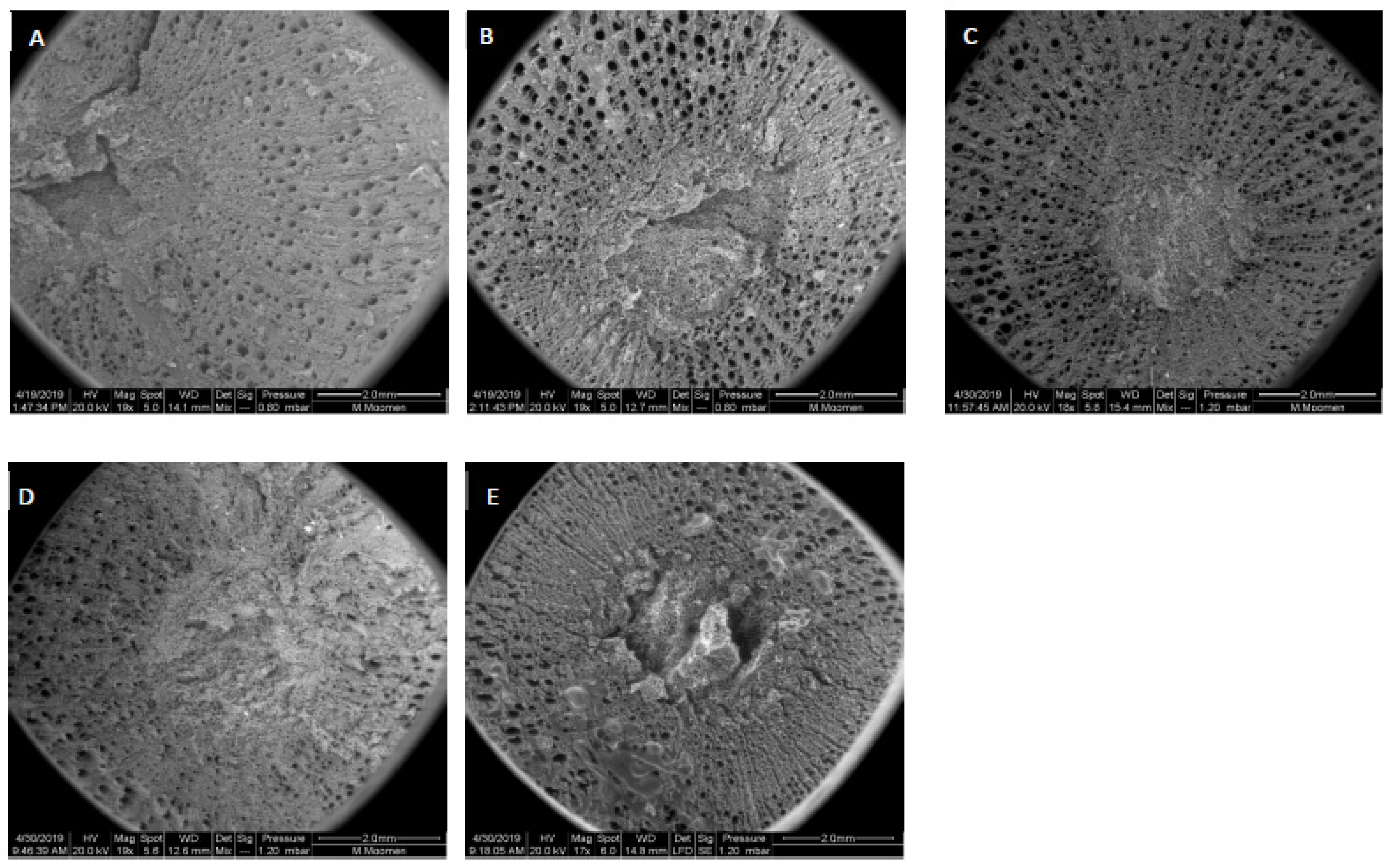
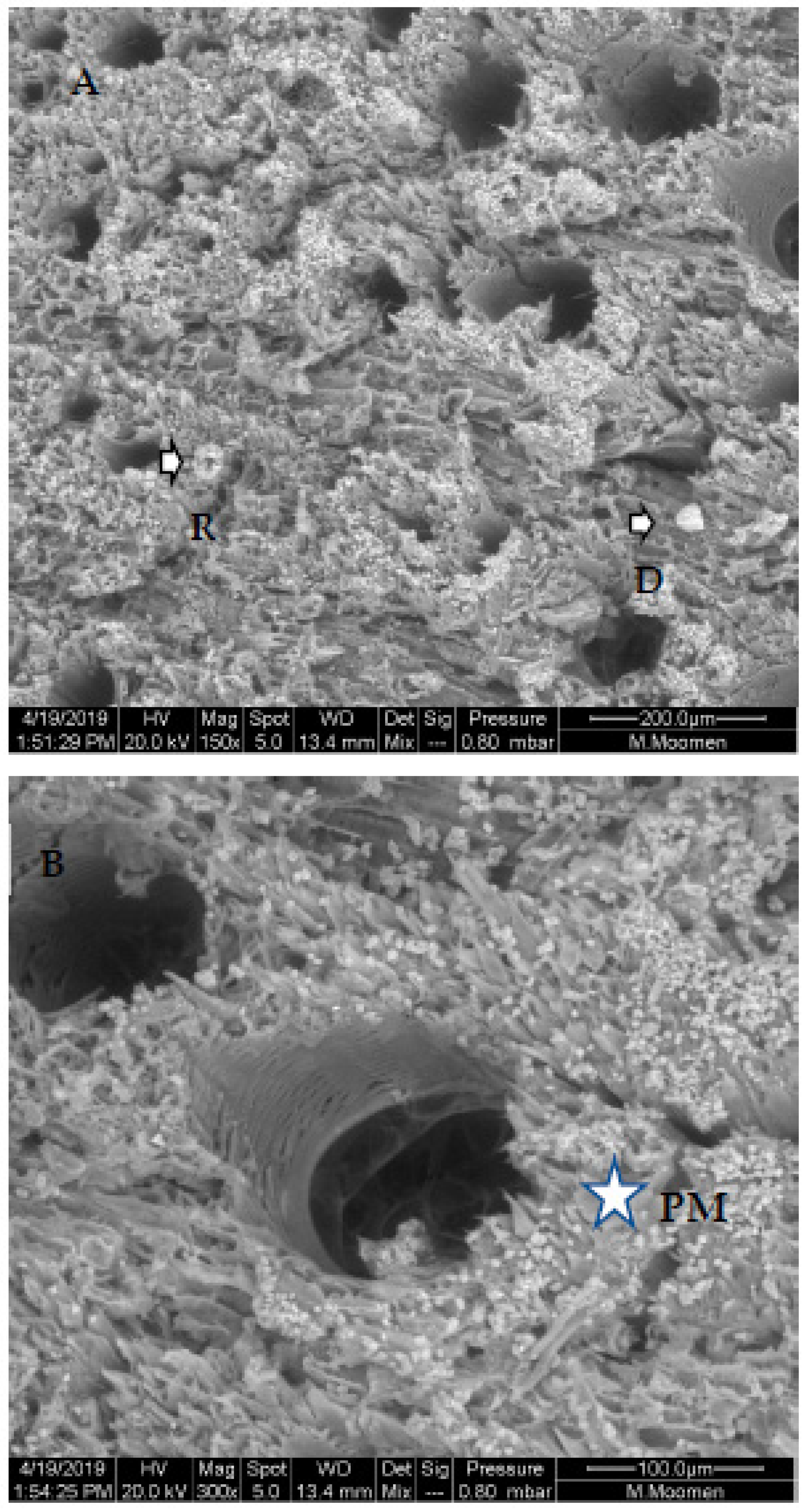
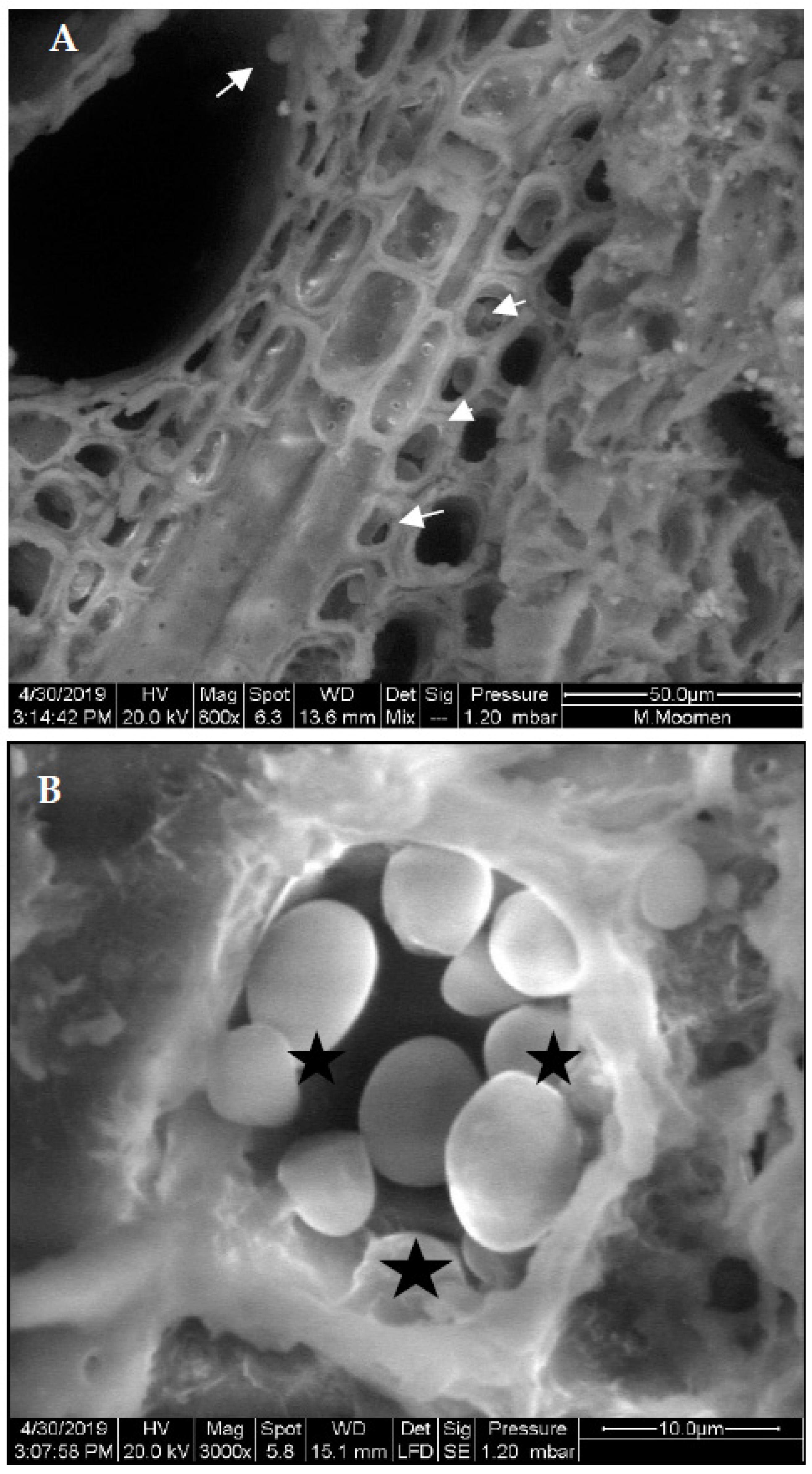
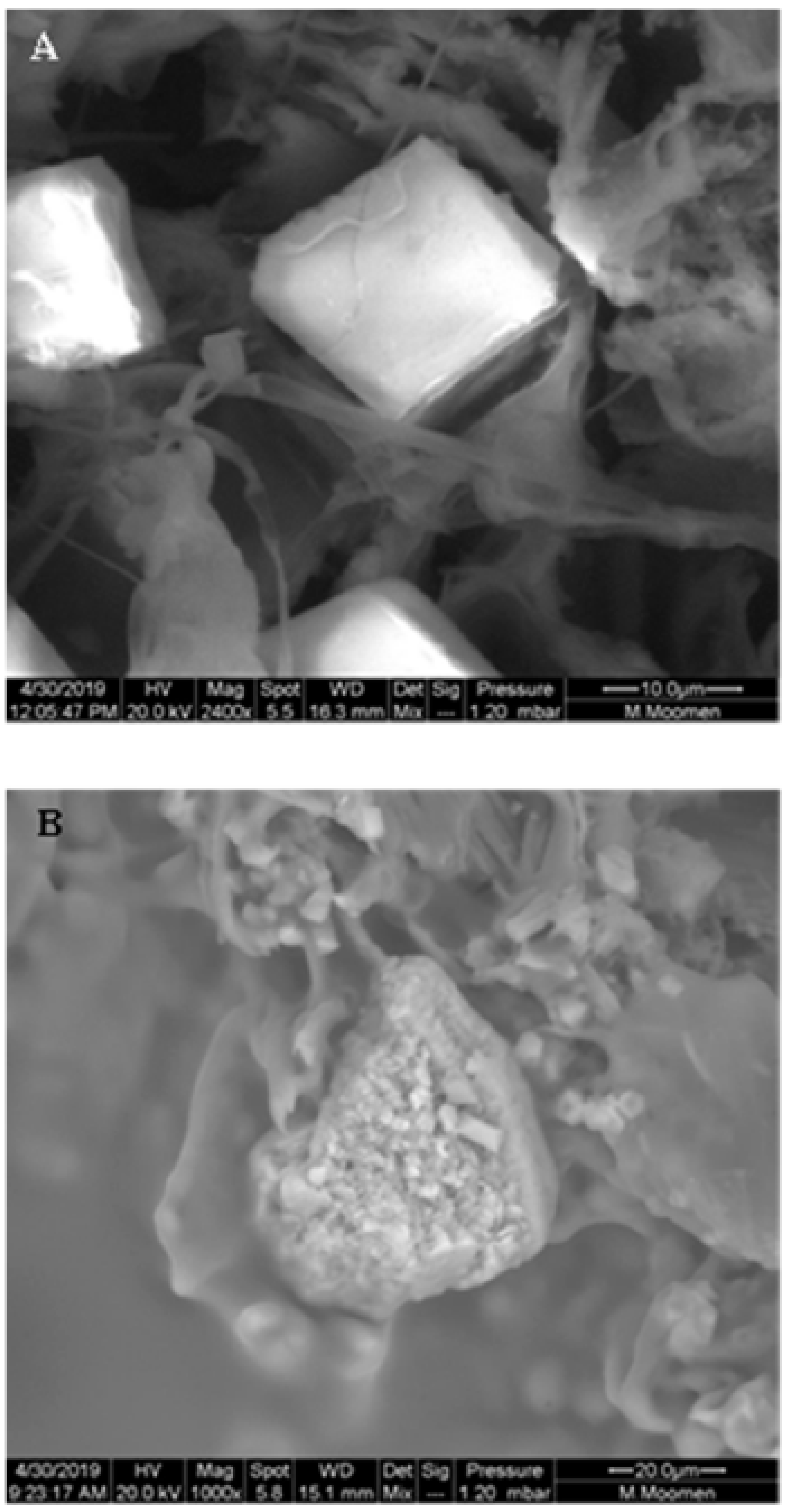
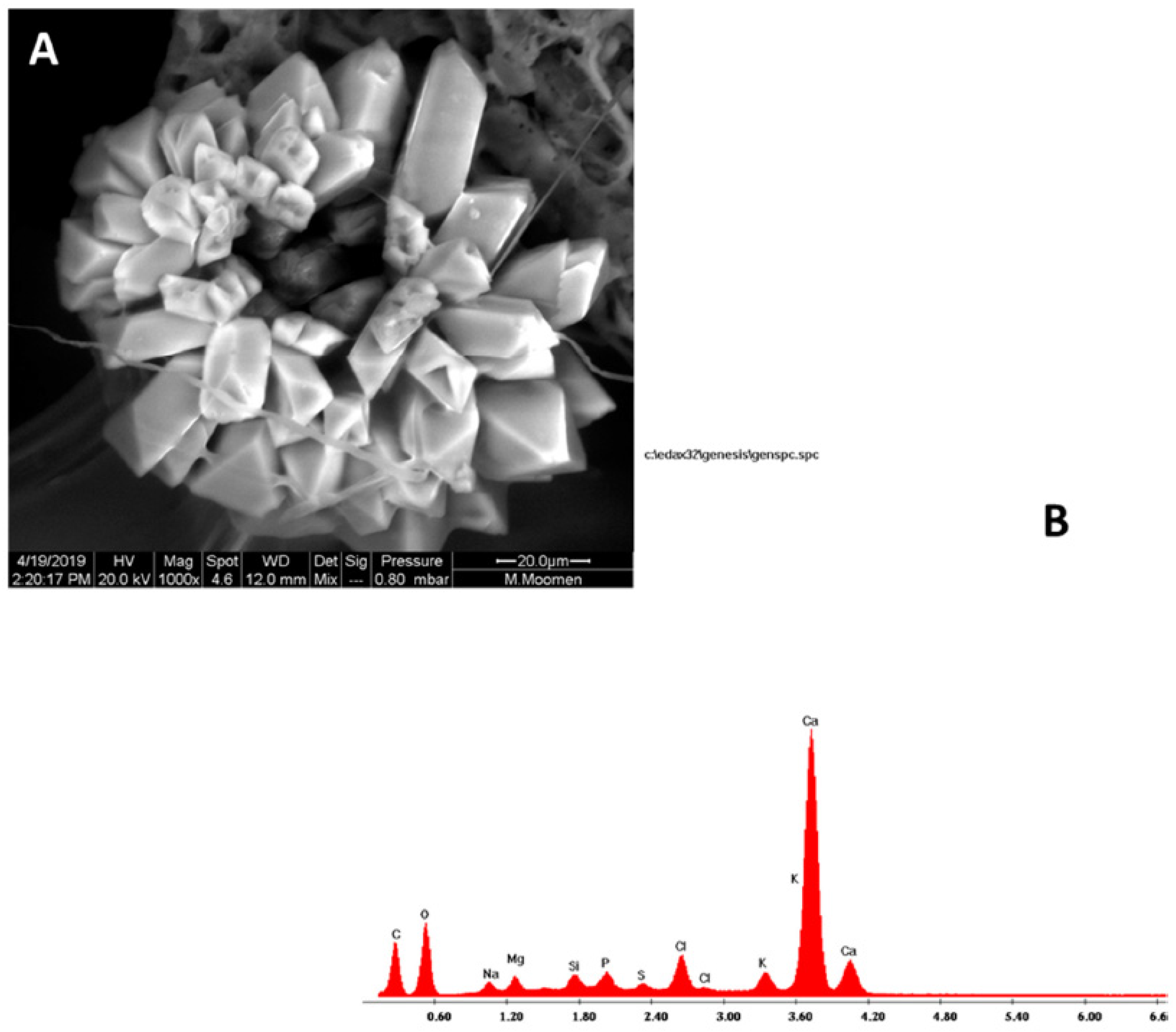
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material and Sampling
4.2. Scanning Electron Microscopy
4.3. Vessel Occlusion Analysis and Mineral Composition
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bois, B.; Zito, S.; Calonnec, A. Climate vs. grapevine pests and diseases worldwide: The first results of a global survey. OENO One 2017, 51, 133–139. [Google Scholar] [CrossRef]
- Foglia, R.; Landi, L.; Gianfranco, R. Analyses of xylem vessel size on grapevine cultivars and relationship with incidence of Esca disease, a threat to grape quality. Appl. Sci. 2022, 12, 1177. [Google Scholar] [CrossRef]
- Yacoub, A.; Gerbore, J.; Magnin, N.; Chambon, P.; Dufour, M.-C.; Corio-Costet, M.F.; Guyoneaud, R.; Rey, P. Ability of Pythium oligandrum strains to protect Vitis vinifera L.; by inducing plant resistance against Phaeomoniella chlamydospora, a pathogen involved in Esca, a grapevine trunk disease. Biol. Control. 2016, 92, 7–16. [Google Scholar] [CrossRef]
- Wang, Y.; Xin, H.; Fan, P.; Zhang, J.; Liu, Y.; Dong, Y.; Wang, Z.; Yang, Y.; Zhang, Q.; Ming, R.; et al. The genome of Shanputao (Vitis amurensis) provides a new insight into cold tolerance of grapevine. Plant. J. 2020, 105, 1495–1506. [Google Scholar] [CrossRef]
- Dries, L.; Hendgen, M.; Schnell, S.; Löhnertz, O.; Vortkamp, A. Rhizosphere engineering: Leading towards a sustainable viticulture? OENO One 2021, 55, 353–363. [Google Scholar] [CrossRef]
- Fedorina, J.; Tikhonova, N.; Ukhatova, Y.; Ivanov, R.; Khlestkina, E. Grapevine gene systems for resistance to Gray Mold Botrytis cinerea and Powdery Mildew Erysiphe necator. Agronomy 2022, 12, 499. [Google Scholar] [CrossRef]
- Lukšić, K.; Zdunić, G.; Hančević, K.; Mihaljević, M.Ž.; Mucalo, A.; Maul, E.; Riaz, S.; Pejić, I. Identification of powdery mildew resistance in wild grapevine (Vitis vinifera subsp. sylvestris Gmel Hegi) from Croatia and Bosnia and Herzegovina. Sci. Rep. 2022, 12, 2128. [Google Scholar] [CrossRef]
- Bove, F.; Bavaresco, L.; Caffi, T.; Rossi, T. Assessment of resistance components for improved phenotyping of grapevine varieties resistant to Downy Mildew. Front Plant Sci. 2019, 10, 1559. [Google Scholar] [CrossRef]
- Zheng, T.; Haider, M.S.; Zhang, K.; Jia, H.; Fang, J. Biological and functional properties of xylem sap extracted from grapevine (cv. Rosario Bianco). Sci. Hortic. 2020, 272, 109563. [Google Scholar] [CrossRef]
- Losso, A.; Beikircher, B.; Dämon, B.; Kikuta, S.; Schmid, P.; Mayr, S. Xylem sap surface tension may be crucial for hydraulic safety. Plant Physiol. 2017, 175, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Andreini, L.; Caruso, G.; Bertollo, C.; Scalabrelli, G.; Viti, R.; Gucci, R. Gas exchange, stem water potential and xylem flux on some grape vine cultivars affected by Esca disease. S. Afr. Entol. Vitic. 2009, 30, 142–147. [Google Scholar]
- Jacobsen, A.L.; Valdovinos-Ayala, J.; Pratt, R.B. Functional lifespans of xylem vessels: Development, hydraulic function, and post-function of vessels in several species of woody plants. Am. J. Bot. 2018, 105, 142–150. [Google Scholar] [CrossRef]
- Nikolaou, N.; Koukouricou, M.; Karagiannidis, N. Effects of various rootstocks on xylem exudates cytokinin content, nutrient uptake and growth patterns of grapevine V. vinifera L. cv. Thompson seedless. Agronomie 2000, 20, 363–373. [Google Scholar] [CrossRef]
- Pouzoulet, J.; Pivovaroff, A.L.; Santiago, L.S.; Rolshausen, P.E. Can vessel dimension explain tolerance toward fungal vascular wilt diseases in woody plants? Lessons from Dutch elm disease and esca disease in grapevine. Front Plant Sci. 2014, 5, 253. [Google Scholar] [CrossRef]
- Bortolami, G.; Farolfi, E.; Badel, E.; Burlett, R.; Cochard, H.; Ferrer, N.; Delmas, C.E. Seasonal and long-term consequences of esca on grapevine stem xylem integrity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Falchi, R.; Petrussa, E.; Braidot, E.; Sivilotti, P.; Boscutti, F.; Vuerich, M.; Calligaro, C.; Antonio Filippi, A.; Herrera, J.C.; Sabbatini, P.; et al. Analysis of non-structural carbohydrates and xylem anatomy of leaf petioles offer new insights in the drought response of two grapevine cultivars. Int. J. Mol. Sci. 2020, 21, 1457. [Google Scholar] [CrossRef]
- Komatsu, T.; Kondo, N. Winter habitat of Xylophilus ampelinus, the cause of bacterial blight of grapevine, in Japan. J. Gen. Plant Pathol. 2015, 81, 237–242. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, Y.; Walker, M.A.; Labavitch, J.M. Vascular occlusions in grapevine with Pierce’s disease make disease symptom development worse. Plant Physiol. 2013, 161, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Fritschi, F.B.; Lin, H.; Walker, A. Scanning electron microscopy reveals different response pattern of four Vitis genotypes to Xylella fastidiosa infection. Plant Dis. 2008, 92, 276–286. [Google Scholar] [CrossRef]
- Perez-Donoso, A.G.; Greve, L.C.; Walton, J.H.; Shackel, K.A.; Labavitch, J.M. X. fastidiosa infection and ethylene exposure result in xylem and water movement disruption in grapevine Shoots. Plant Physiol. 2007, 143, 1024–1036. [Google Scholar] [CrossRef]
- Ifrim, C.; Jitareanu, C.D.; Slabu, C.; Marta, A.E. Aspects regarding the Calcium Oxalate crystals at the grapevines cultivated in Iasi and Cotnari vineyards. LucrăriŞtiinţifice 2012, 55, 73–78. [Google Scholar]
- Konyar-Tütüncü, S.; Öztürk, N.; Feruzan, D. Occurrence, types and distribution of calcium oxalate crystals in leaves and stems of some species of poisonous plants. Bot. Stud. 2014, 55, 32. [Google Scholar] [CrossRef] [PubMed]
- Meric, C. Calcium oxalate crystals in some species of the tribe Inuleae (Asteraceae). Acta Biol. Crac. Ser. Bot. 2009, 51, 105–110. [Google Scholar]
- Bernardo, S.; Dinis, L.T.; Machado, N.; Moutinho-Pereira, J. Grapevine abiotic stress assessment and search for sustainable adaptation strategies in Mediterranean-like climates. A review. Agron. Sustain. Dev. 2018, 38, 66. [Google Scholar] [CrossRef]
- Sabir, A.; Kucukbasmaci, A.; Taytak, M.; Bilgin, O.F.; Jawshle, A.I.M.; Mohammed, M.M.; Gayretli, Y. Sustainable viticulture practices on the face of climate change. Agric. Res. Technol. Open Access J. 2018, 17, 556033. [Google Scholar] [CrossRef]
- Sevanto, S.; Nikinmaa, E.; Riikonen, A.; Daley, M.; Pettijohn, J.C.; Mikkelsen, T.N.; Phillips, N.; Holbrook, N.M. Linking xylem diameter variations with sap flow measurements. Plant Soil 2008, 305, 77–90. [Google Scholar] [CrossRef]
- Inch, S.; Ploetz, R.; Held, B.; Blanchette, R. Histological and anatomical responses in avocado, Persea americana, induced by the vascular wilt pathogen, Raffaelea lauricola. Botany 2012, 90, 627–635. [Google Scholar] [CrossRef]
- Campbell, A.S.; Ploetz, R.C.; Rollins, J.A. Comparing Avocado, swamp bay, and camphortree as hosts of Raffaelea lauricola using a green fluorescent protein (GFP)-labeled strain of the pathogen. Phytopathology 2016, 107, 70–74. [Google Scholar] [CrossRef][Green Version]
- Fraedrich, S.W.; Harrington, T.C.; Best, G.S. Xyleborus glabratus attacks and systemic colonization by Raffaelea lauricola associated with dieback of Cinnamomum camphora in the southeastern United States. For. Pathol. 2015, 45, 60–70. [Google Scholar] [CrossRef]
- Castillo-Argaez, R.; Vazquez, A.; Konkol, J.L.; Vargas, A.I.; Ploetz, R.C.; Etxeberria, E.; Schaffer, B. Sap flow, xylem anatomy and photosynthetic variables of three Persea species in response to laurel wilt. Tree Physiol. 2020, 41, 1004–1018. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Martel, A.B.; Dixon, S.L. Environmental Factors Influence Plant Vascular System and Water Regulation. Plants 2019, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Brodersten, C.R.; McElerone, A.J. Maintenance of xylem transport capacity: A review of embolism repair in vascular plant. Front. Plant Sci. 2013, 108, 1–12. [Google Scholar]
- Sabella, E.; Aprile, A.; Genga, A.; Siciliano, T.; Nutricati, E.; Nicolì, F.; Vergine, M.; Negro, C.; De Bellis, L.; Luvisi, A. Xylem cavitation susceptibility and refilling mechanisms in olive trees infected by X. fastidiosa. Sci. Rep. 2019, 9, 9602. [Google Scholar] [CrossRef]
- Ouadi, L.; Bruez, E.; Bastien, S.; Yacoub, A.; Coppin, C.; Guerin-Dubrana, L.; Fontaine, F.; Domec, J.C.; Rey, P. Sap flow disruption in grapevine is the early signal predicting the structural, functional, and genetic responses to Esca disease. Front. Plant Sci. 2021, 12, 695846. [Google Scholar] [CrossRef]
- Sun, Q.; Rost, T.L.; Matthews, M.A. Wound-induced vascular occlusions in Vitis vinifera (Vitaceae): Tyloses in summer and gels in winter. Am. J. Bot. 2008, 95, 1498–1505. [Google Scholar] [CrossRef]
- Pouzoulet, J.; Scudiero, E.; Schiavon, M.; Santiago, L.; Rolshausen, P.E. Modelling of xylem vessel occlusion in grapevine. Tree Physiol. 2019, 39, 1438–1445. [Google Scholar] [CrossRef]
- Sun, Q.; Rost, T.L.; Matthews, M.A. Pruning-induced tylose development in stems of current-year shoots of Vitis vinifera (Vitaceae). Am. J. Bot. 2006, 93, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Balzano, A.; Wheeler, E.A.; Baas, P. Tyloses and gums: A review of structure, function and occurrence of vessel occlusions. IAWA J. 2016, 37, 186–205. [Google Scholar] [CrossRef]
- Pouzoulet, J.; Scudiero, E.; Schiavon, M.; Rolshausen, P.E. Xylem vessel diameter affects the compartmentalization of the vascular pathogen Phaeomoniella chlamydospore in grapevine. Front Plant Sci. 2017, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Valtaud, C.; Foyer, C.H.; Fleurat-Lessard, P.; Bourbouloux, A. Systemic effects on leaf glutathione metabolism and defence protein expression caused by esca infection in grapevines. Funct. Plant Biol. 2009, 36, 260–279. [Google Scholar] [CrossRef] [PubMed]
- Jáuregui-Zúñiga, D.; Reyes-Grajeda, J.P.; Sepúlveda-Sánchez, J.D.; Whitaker, J.R.; Moreno, A. Crystallochemical characterization of calcium oxalate crystals isolated from seed coats of Phaseolus vulgaris and leaves of Vitis vinifera. J. Plant Physiol. 2003, 160, 239–245. [Google Scholar] [CrossRef]
- Morrow, A.C.; Dute, R.R. Crystals associated with the intertracheid pit membrane of the woody fern Botrychium multifidum. Am. Fern J. 2002, 92, 10–19. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, S.; Vishnuprasad, V. Druses in the secondary xylem of Mangifera indica collected from coal mines, India. J. Trop. For. Sci. 2017, 29, 179–184. [Google Scholar]
- Stambouli-Essassi, S.; Mejri, F.; Dhoueibi, M.; Mrabet, Y.; Harzallah-Skhiri, F.; Hosni, K. Anatomical features, fatty acid profile and tocopherol content of the Tunisian Cakile maritima subsp. maritima Scop. Fruit. J. Anim. Plant Sci. 2020, 43, 7366–7379. [Google Scholar] [CrossRef]
- Bertsch, C.; Ramírez-Suero, M.; Magninrobert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef]
- Eskalen, A.; Feliciano, A.J.; Gubler, W.A. Susceptibility of grapevine pruning wounds and symptom development in response to infection by Phaeoacremonium aleophilum and Phaeomoniella chlamydospora. Plant Dis. 2007, 91, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, U.; Rockwell, F.; Holbrook, N.; Cochard, H. Iso/anisohydry: A plant—Environment interaction rather than a simple hydraulic trait. Trends Plant Sci. 2018, 23, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Intrigliolo, D.S.; Pérez, D.; Risco, D.; Yeves, A.; Castel, J.R. Yield components and grape composition responses to seasonal water deficits in Tempranillo grapevines. Irrig. Sci. 2012, 30, 339–349. [Google Scholar] [CrossRef]
- Zoghlami, N.; Riahi, L.; Laucou, V.; Lacombe, T.; Mliki, A.; Ghorbel, A.; This, P. Origin and genetic diversity of Tunisian grapes as revealed by microsatellite markers. Sci. Hortic. 2009, 120, 479–486. [Google Scholar] [CrossRef]
- Souid, I.; Zemni, H.; Sanchez-Palomo, E.; Perez-Coello, M.S.; Ghorbel, A. Varietal aroma compound of Vitis vinifera cv Khamri grown in Tunisia. J. Food Qual. 2007, 30, 718–730. [Google Scholar] [CrossRef]
- Habib, A.; Ben Maachia, S.; Salhi, A.; Harbi-Ben Slimene, M. Berry quality of principal grapevines in the Oasis of El Jerid, Tunisa. J. Hortic. Postharvest Res. 2020, 3, 141–150. [Google Scholar]
- Ben Abdallah, F.; Chibani, F.; Fnayou, A.; Ghorbel, A.; Boursiquot, J.M. Biochemical characterization of Tunisian grapevine varieties. Int. J. Wine Vine 1998, 32, 17–25. [Google Scholar] [CrossRef]
- Bouamama-Gzara, B.; Selmi, I.; Chebil, S.; Melki, I.; Mliki, A.; Ghorbel, A.; Carra, A.; Carimi, F.; Mahfoudhi, N. Elimination of Grapevine leafroll associated virus-3, Grapevine rupestris stem pitting associated virus and Grapevine virus A from a Tunisian cultivar by somatic embryogenesis and characterization of the somaclones using ampelographic descriptors. Plant Pathol. J. 2017, 33, 561–571. [Google Scholar] [CrossRef]




| Cultivars | Tyloses | Gums | Gels | Starch Grains | Occlusion Sums |
|---|---|---|---|---|---|
| Sakasly | 25 | 33 | 0 | 75 | 133 |
| Razegui1 | 13 | 22 | 0 | 55 | 90 |
| Razegui2 | 11 | 9 | 11 | 39 | 70 |
| Hencha | 21 | 6 | 21 | 21 | 69 |
| Khamri | 27 | 5 | 17 | 11 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouamama-Gzara, B.; Zemni, H.; Sleimi, N.; Ghorbel, A.; Gzara, L.; Mahfoudhi, N. Diversification of Vascular Occlusions and Crystal Deposits in the Xylem Sap Flow of Five Tunisian Grapevines. Plants 2022, 11, 2177. https://doi.org/10.3390/plants11162177
Bouamama-Gzara B, Zemni H, Sleimi N, Ghorbel A, Gzara L, Mahfoudhi N. Diversification of Vascular Occlusions and Crystal Deposits in the Xylem Sap Flow of Five Tunisian Grapevines. Plants. 2022; 11(16):2177. https://doi.org/10.3390/plants11162177
Chicago/Turabian StyleBouamama-Gzara, Badra, Hassene Zemni, Noomene Sleimi, Abdelwahed Ghorbel, Lassaad Gzara, and Naima Mahfoudhi. 2022. "Diversification of Vascular Occlusions and Crystal Deposits in the Xylem Sap Flow of Five Tunisian Grapevines" Plants 11, no. 16: 2177. https://doi.org/10.3390/plants11162177
APA StyleBouamama-Gzara, B., Zemni, H., Sleimi, N., Ghorbel, A., Gzara, L., & Mahfoudhi, N. (2022). Diversification of Vascular Occlusions and Crystal Deposits in the Xylem Sap Flow of Five Tunisian Grapevines. Plants, 11(16), 2177. https://doi.org/10.3390/plants11162177






