Amino Acids Supplied through the Autophagy/Endocytosis Pathway Promote Starch Synthesis in Physcomitrella Protonemal Cells
Abstract
:1. Introduction
2. Results
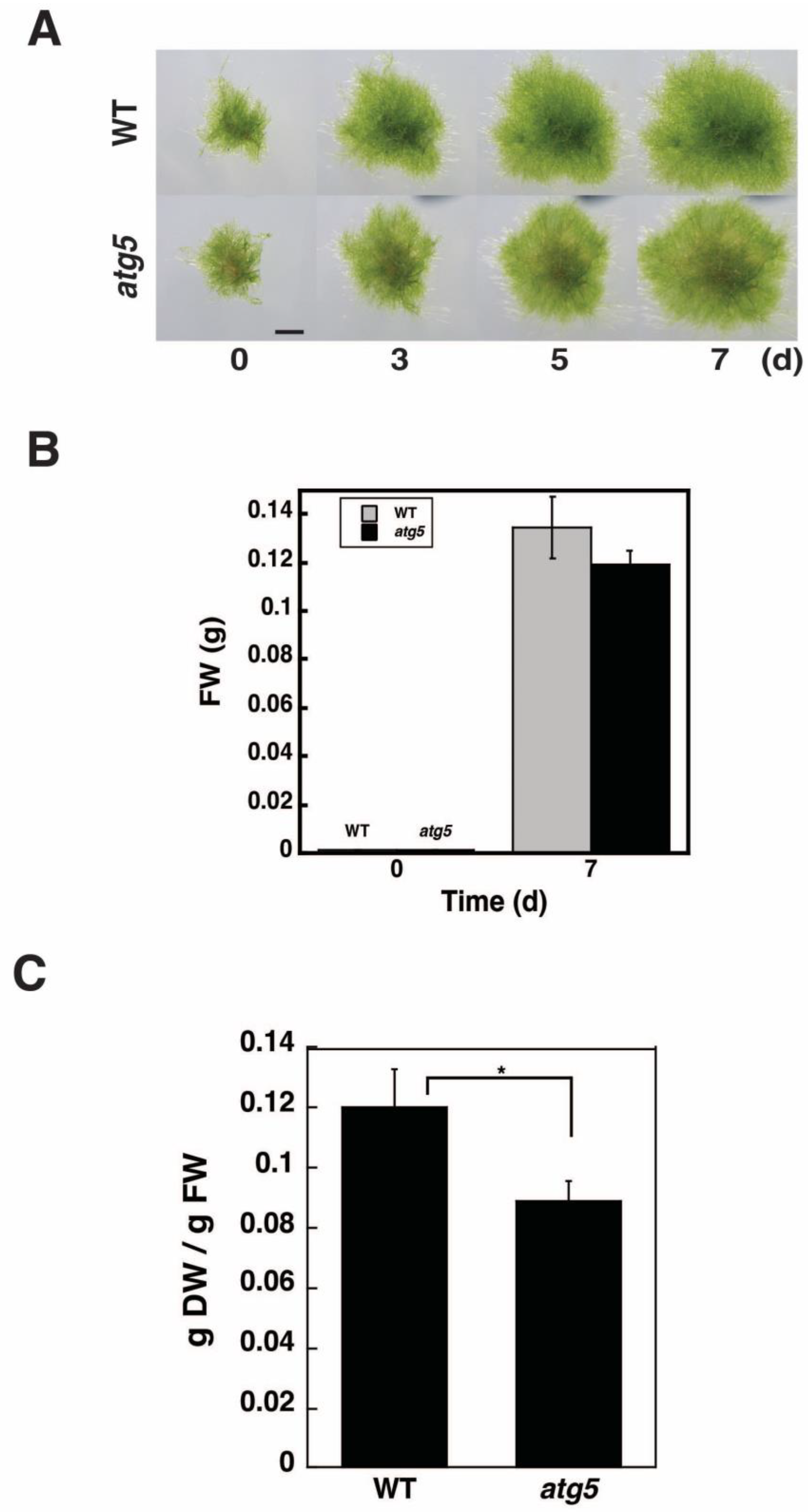
2.1. DW/FW of atg5 Cells Is Lower than That of WT Cells
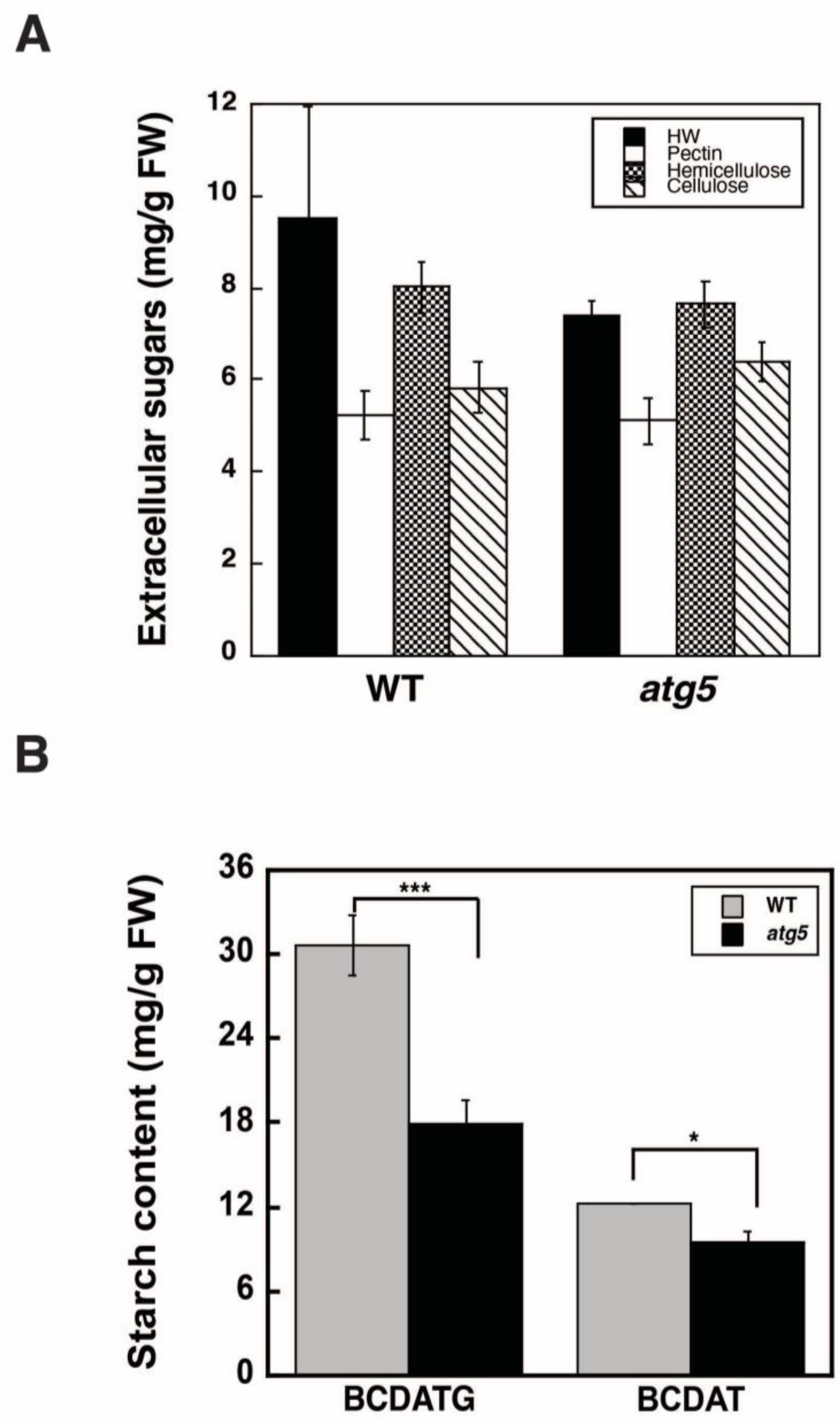
2.2. Autophagy Deficiency Leads to a Decrease in Starch Accumulation
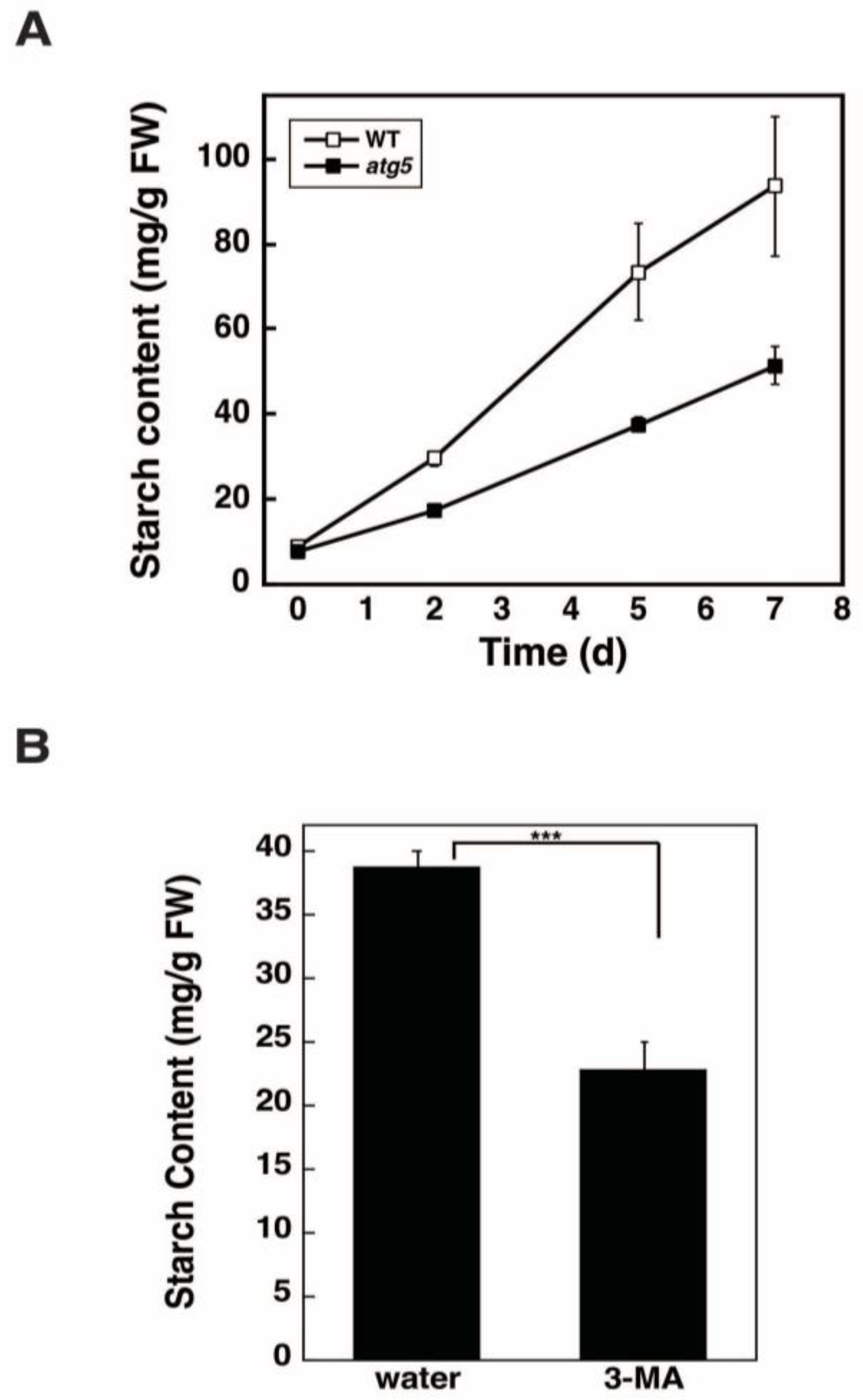
2.3. Starch Synthesis Rate Is Higher in WT Cells than in atg5 Cells
2.4. Inhibition of Autophagy via 3-Methyladenine Decreases the Starch Synthesis Rate
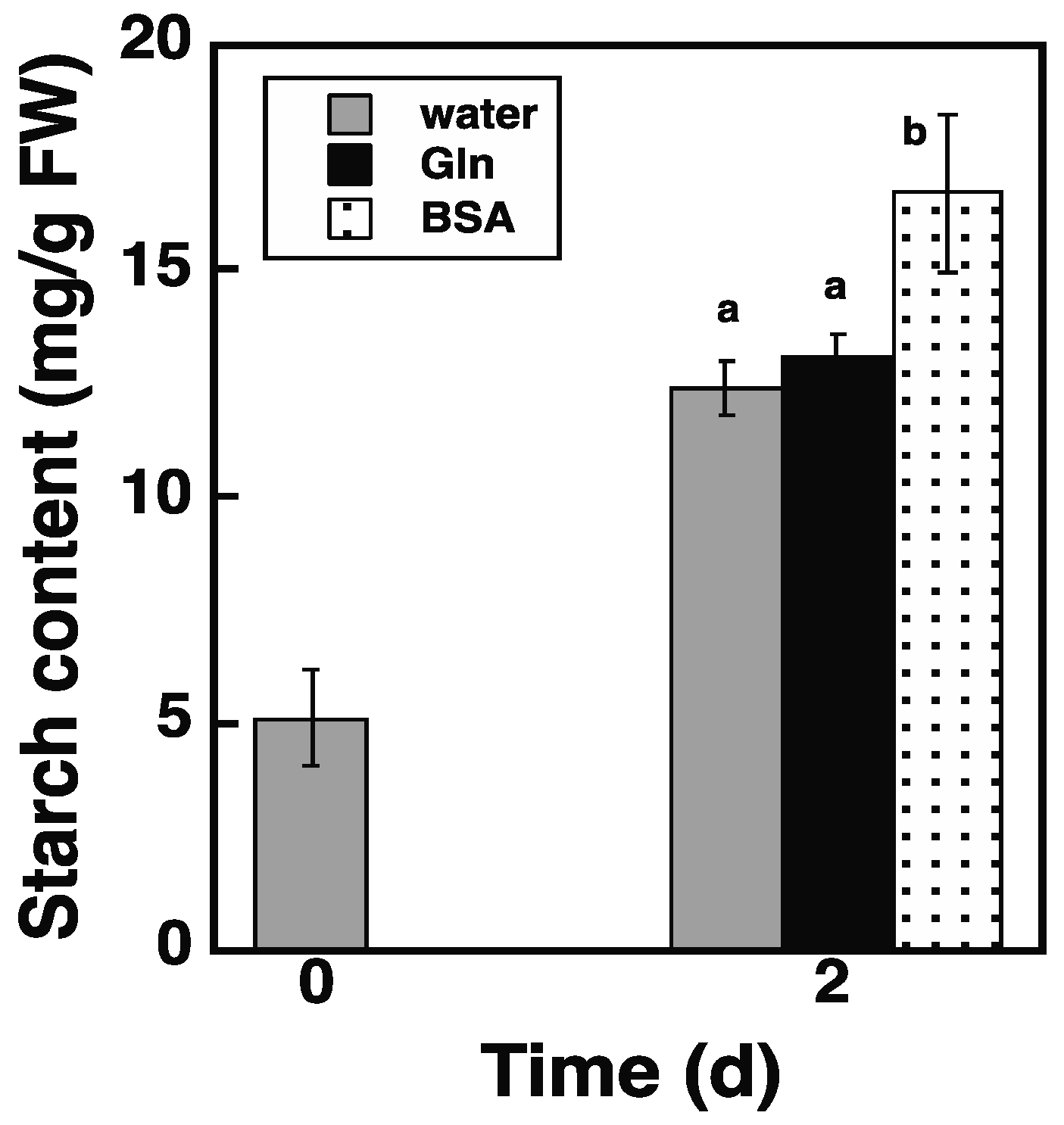
2.5. Loading of Protein via the Endocytosis Pathway Promotes Starch Synthesis in atg5 Cells
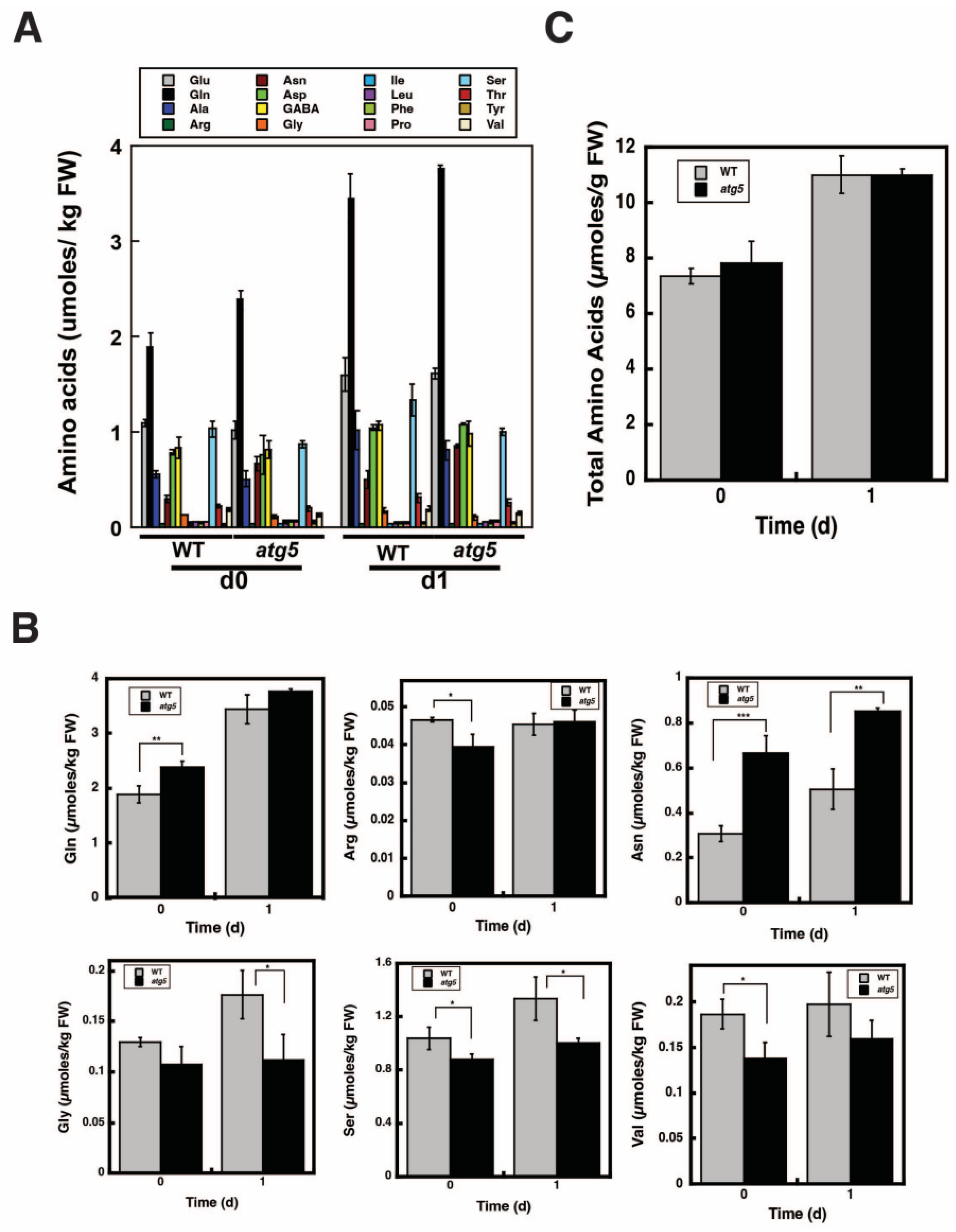
2.6. Change in Total Amino Acid Content before and after Starch Synthesis Did Not Differ between WT and atg5 Cells
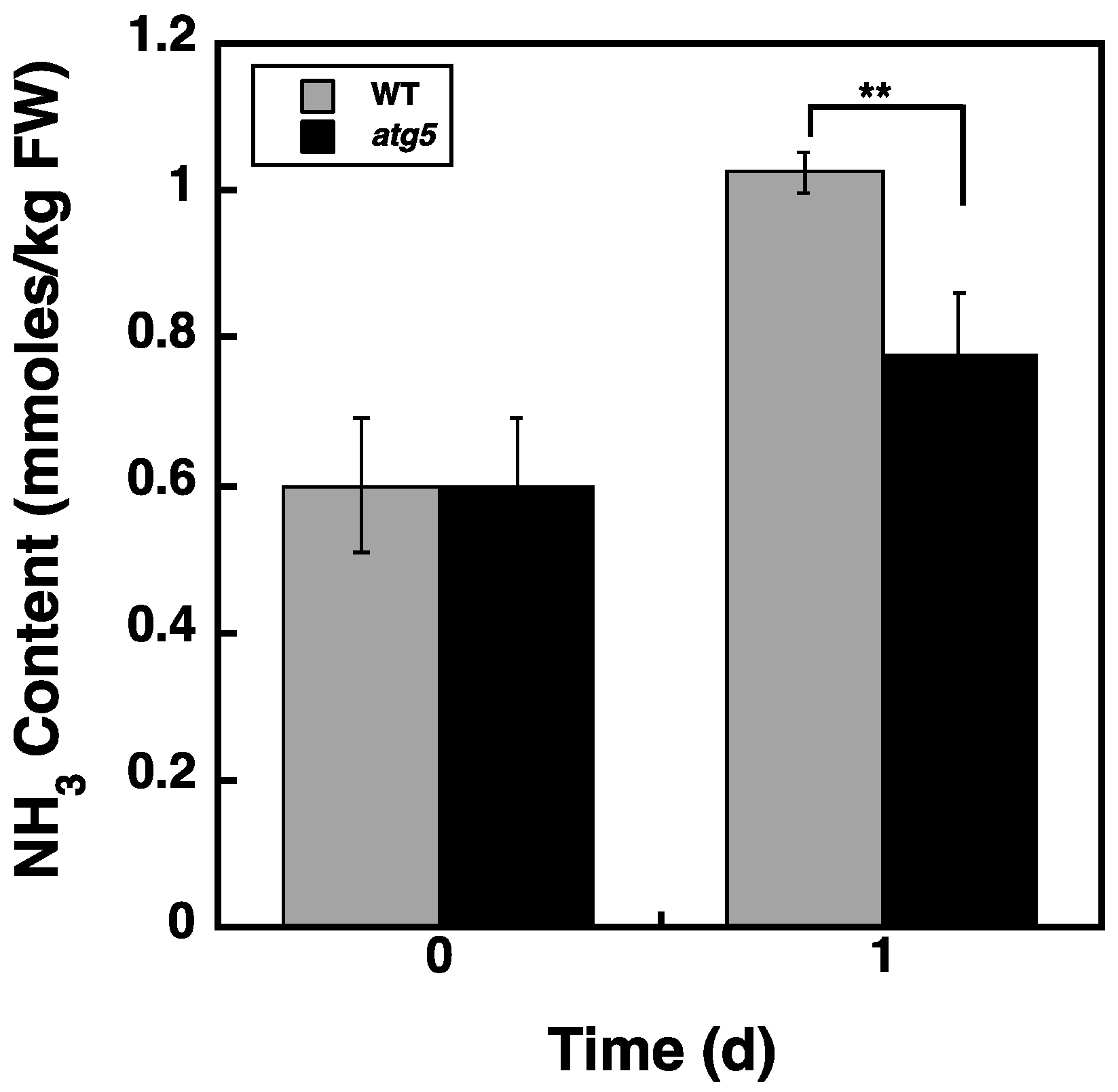
2.7. Ammonia Content Is Higher in WT Cells than in atg5 Cells
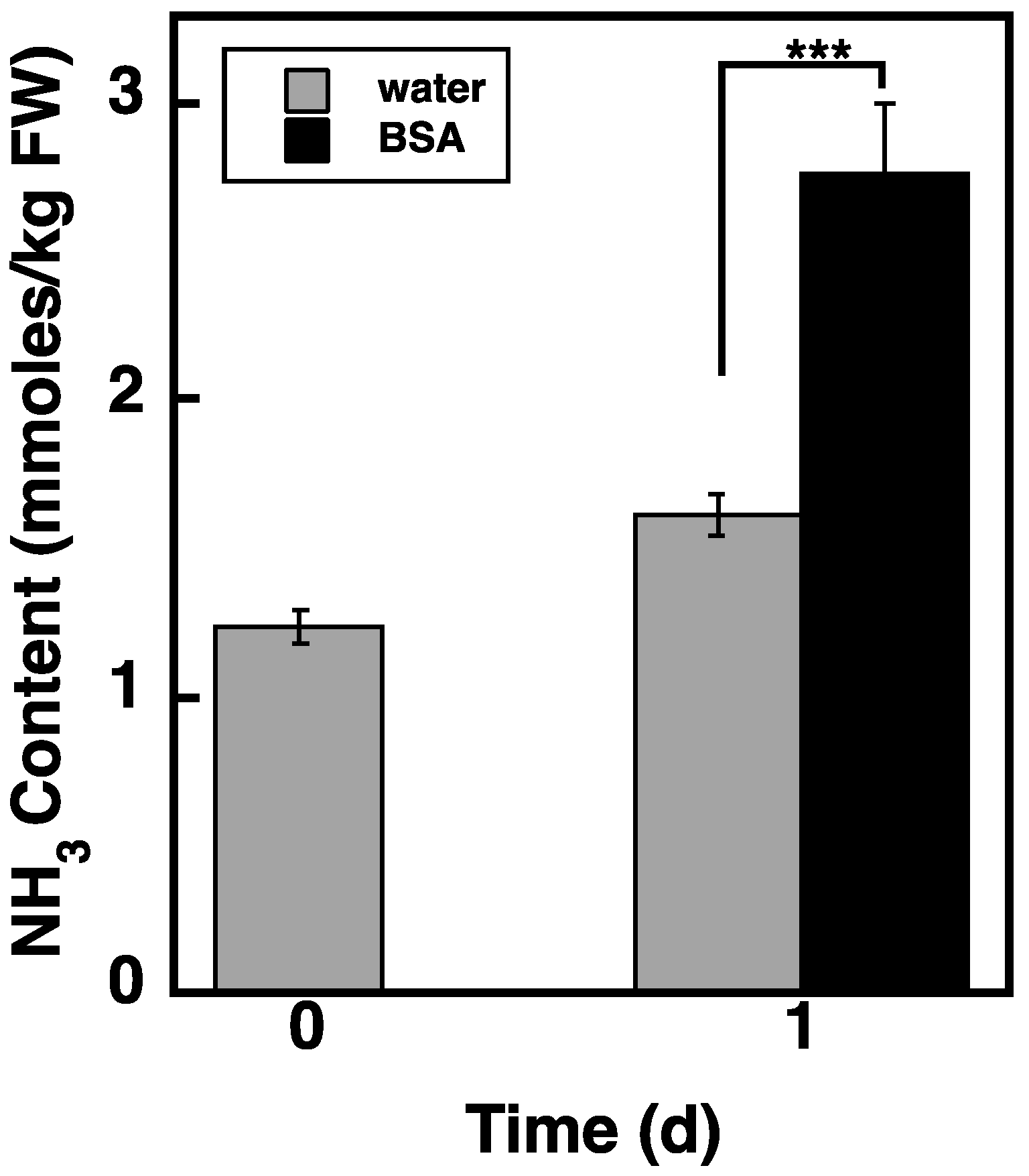
2.8. Loading of BSA via the Endocytosis Pathway Increases NH3 Content in atg5 Cells
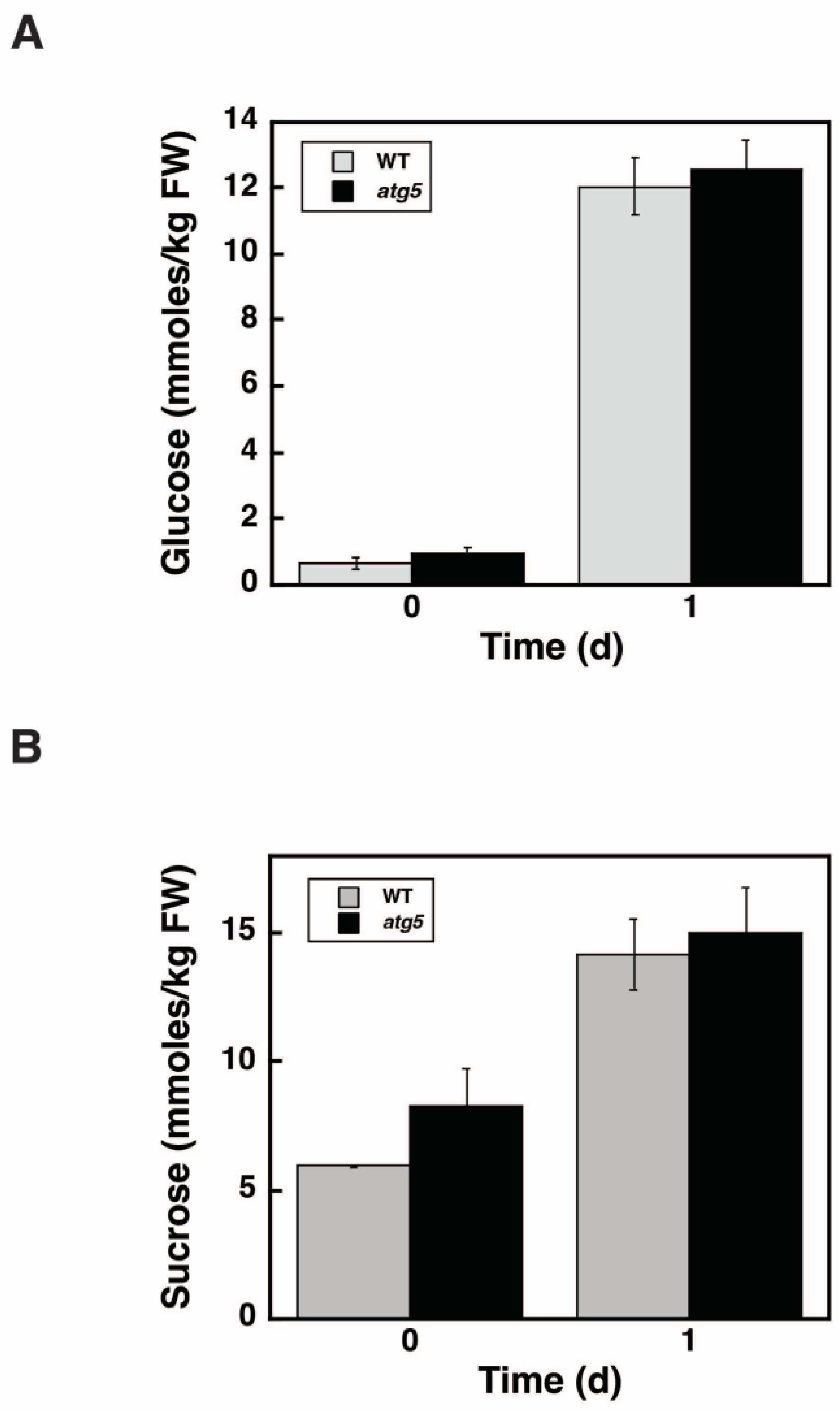
2.9. Glucose and Sucrose Levels Do Not Differ between WT and atg5 Cells during Starch Synthesis
3. Discussion
3.1. Contribution of Autophagy to Starch Synthesis
3.2. Amino Acid Supply Route
3.3. Involvement of Autophagy in Starch Degradation
4. Materials and Methods
4.1. Biological Materials
4.2. Measurement of FW and DW
4.3. Analysis of Cell Wall Polysaccharides
4.4. Measurement of Starch Content
4.5. Measurement of Amino Acid Levels
4.6. Measurement of Glucose and Sucrose Levels
4.7. Measurement of NH3 Content
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Duve, C. The lysosome. Sci. Am. 1963, 208, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, M.; Ohsumi, Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993, 333, 169–174. [Google Scholar] [CrossRef]
- Hanaoka, H.; Noda, T.; Shirano, Y.; Kato, T.; Hayashi, H.; Shibata, D.; Tabata, S.; Ohsumi, Y. Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiol. 2002, 129, 1181–1193. [Google Scholar] [CrossRef]
- Doelling, J.H.; Walker, J.M.; Friedman, E.M.; Thompson, A.R.; Vierstra, R.D. The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J. Biol. Chem. 2002, 277, 33105–33114. [Google Scholar] [CrossRef]
- Mizushima, N.; Klionsky, D.J. Protein turnover via autophagy: Implications for metabolism. Annu. Rev. Nutr. 2007, 27, 19–40. [Google Scholar] [CrossRef]
- Ishida, H.; Yoshimoto, K.; Izumi, M.; Reisen, D.; Yano, Y.; Makino, A.; Ohsumi, Y.; Hanson, M.R.; Mae, T. Mobilization of rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagic process. Plant Physiol. 2008, 148, 142–155. [Google Scholar] [CrossRef]
- Izumi, M.; Hidema, J.; Wada, S.; Kondo, E.; Kurusu, T.; Kuchitsu, K.; Makino, A.; Ishida, H. Establishment of monitoring methods for autophagy in rice reveals autophagic recycling of chloroplasts and root plastids during energy limitation. Plant Physiol. 2015, 167, 1307–1320. [Google Scholar] [CrossRef]
- Shibata, M.; Oikawa, K.; Yoshimoto, K.; Kondo, M.; Mano, S.; Yamada, K.; Hayashi, M.; Sakamoto, W.; Ohsumi, Y.; Nishimura, M. Highly oxidized peroxisomes are selectively degraded via autophagy in Arabidopsis. Plant Cell 2013, 25, 4967–4983. [Google Scholar] [CrossRef]
- Wada, S.; Ishida, H.; Izumi, M.; Yoshimoto, K.; Ohsumi, Y.; Mae, T.; Makino, A. Autophagy plays a role in chloroplast degradation during senescence in individually darkened leaves. Plant Physiol. 2009, 149, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, B.; Zhao, J.; Guo, J.; Li, Y.; Han, S.; Huang, L.; Du, Y.; Hong, Y.; Tang, D. Autophagy contributes to leaf starch degradation. Plant Cell 2013, 25, 1383–1399. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Shibata, M.; Kondo, M.; Oikawa, K.; Sato, M.; Toyooka, K.; Shirasu, K.; Nishimura, M.; Ohsumi, Y. Quality control of plant peroxisomes in organ specific manner via autophagy. J. Cell Sci. 2014, 127 Pt 6, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Jikumaru, Y.; Kamiya, Y.; Kusano, M.; Consonni, C.; Panstruga, R.; Ohsumi, Y.; Shirasu, K. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell 2009, 21, 2914–2927. [Google Scholar] [CrossRef]
- Liou, W.; Geuze, H.J.; Geelen, M.J.; Slot, J.W. The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J. Cell Biol. 1997, 136, 61–70. [Google Scholar] [CrossRef]
- Yano, K.; Matsui, S.; Tsuchiya, T.; Maeshima, M.; Kutsuna, N.; Hasezawa, S.; Moriyasu, Y. Contribution of the plasma membrane and central vacuole in the formation of autolysosomes in cultured tobacco cells. Plant Cell Physiol. 2004, 45, 951–957. [Google Scholar] [CrossRef]
- Barros, J.A.; Cavalcanti, J.H.F.; Medeiros, D.B.; Nunes-Nesi, A.; Avin-Wittenberg, T.; Fernie, A.R.; Araújo, W.L. Autophagy deficiency compromises alternative pathways of respiration following energy deprivation in Arabidopsis thaliana. Plant Physiol. 2017, 175, 62–76. [Google Scholar] [CrossRef]
- Hirota, T.; Izumi, M.; Wada, S.; Makino, A.; Ishida, H. Vacuolar protein degradation via autophagy provides substrates to amino acid catabolic pathways as an adaptive response to sugar starvation in Arabidopsis thaliana. Plant Cell Physiol. 2018, 59, 1363–1376. [Google Scholar] [CrossRef]
- Nishiyama, T.; Hiwatashi, Y.; Sakakibara, K.; Kato, M.; Hasebe, M. Tagged mutagenesis and gene-trap in the moss, Physcomitrella patens by shuttle mutagenesis. DNA Res. 2000, 7, 9–17. [Google Scholar] [CrossRef]
- Mukae, K.; Inoue, Y.; Moriyasu, Y. ATG5-knockout mutants of Physcomitrella provide a platform for analyzing the involvement of autophagy in senescence processes in plant cells. Plant Signal. Behav. 2015, 10, e1086859. [Google Scholar] [CrossRef]
- Aohara, T.; Kotake, T.; Kaneko, Y.; Takatsuji, H.; Tsumuraya, Y.; Kawasaki, S. Rice BRITTLE CULM 5 (BRITTLE NODE) is involved in secondary cell wall formation in the sclerenchyma tissue of nodes. Plant Cell Physiol. 2009, 50, 1886–1897. [Google Scholar] [CrossRef] [PubMed]
- Kotake, T.; Aohara, T.; Hirano, K.; Sato, A.; Kaneko, Y.; Tsumuraya, Y.; Takatsuji, H.; Kawasaki, S. Rice Brittle culm 6 encodes a dominant-negative form of CesA protein that perturbs cellulose synthesis in secondary cell walls. J. Expt. Bot. 2011, 62, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Liu, H.; Ueno, S.; Shimada, R.; Araki, T. The effect of high hydrostatic pressure on taste substance distribution in fresh green tea leaves. High Press. Res. 2019, 39, 408–416. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakil, M.A.; Mukae, K.; Funada, R.; Kotake, T.; Ueno, S.; Aktar, M.M.; Roni, M.S.; Inoue-Aono, Y.; Moriyasu, Y. Amino Acids Supplied through the Autophagy/Endocytosis Pathway Promote Starch Synthesis in Physcomitrella Protonemal Cells. Plants 2022, 11, 2157. https://doi.org/10.3390/plants11162157
Sakil MA, Mukae K, Funada R, Kotake T, Ueno S, Aktar MM, Roni MS, Inoue-Aono Y, Moriyasu Y. Amino Acids Supplied through the Autophagy/Endocytosis Pathway Promote Starch Synthesis in Physcomitrella Protonemal Cells. Plants. 2022; 11(16):2157. https://doi.org/10.3390/plants11162157
Chicago/Turabian StyleSakil, Md. Arif, Kyosuke Mukae, Ryo Funada, Toshihisa Kotake, Shigeaki Ueno, Most Mohoshena Aktar, Md. Shyduzzaman Roni, Yuko Inoue-Aono, and Yuji Moriyasu. 2022. "Amino Acids Supplied through the Autophagy/Endocytosis Pathway Promote Starch Synthesis in Physcomitrella Protonemal Cells" Plants 11, no. 16: 2157. https://doi.org/10.3390/plants11162157
APA StyleSakil, M. A., Mukae, K., Funada, R., Kotake, T., Ueno, S., Aktar, M. M., Roni, M. S., Inoue-Aono, Y., & Moriyasu, Y. (2022). Amino Acids Supplied through the Autophagy/Endocytosis Pathway Promote Starch Synthesis in Physcomitrella Protonemal Cells. Plants, 11(16), 2157. https://doi.org/10.3390/plants11162157






