Exogenous Melatonin Alleviated Leaf Yellowing via Inhibiting Respiration and Ethylene Biosynthesis during Shelf Life in Pakchoi
Abstract
:1. Introduction
2. Results
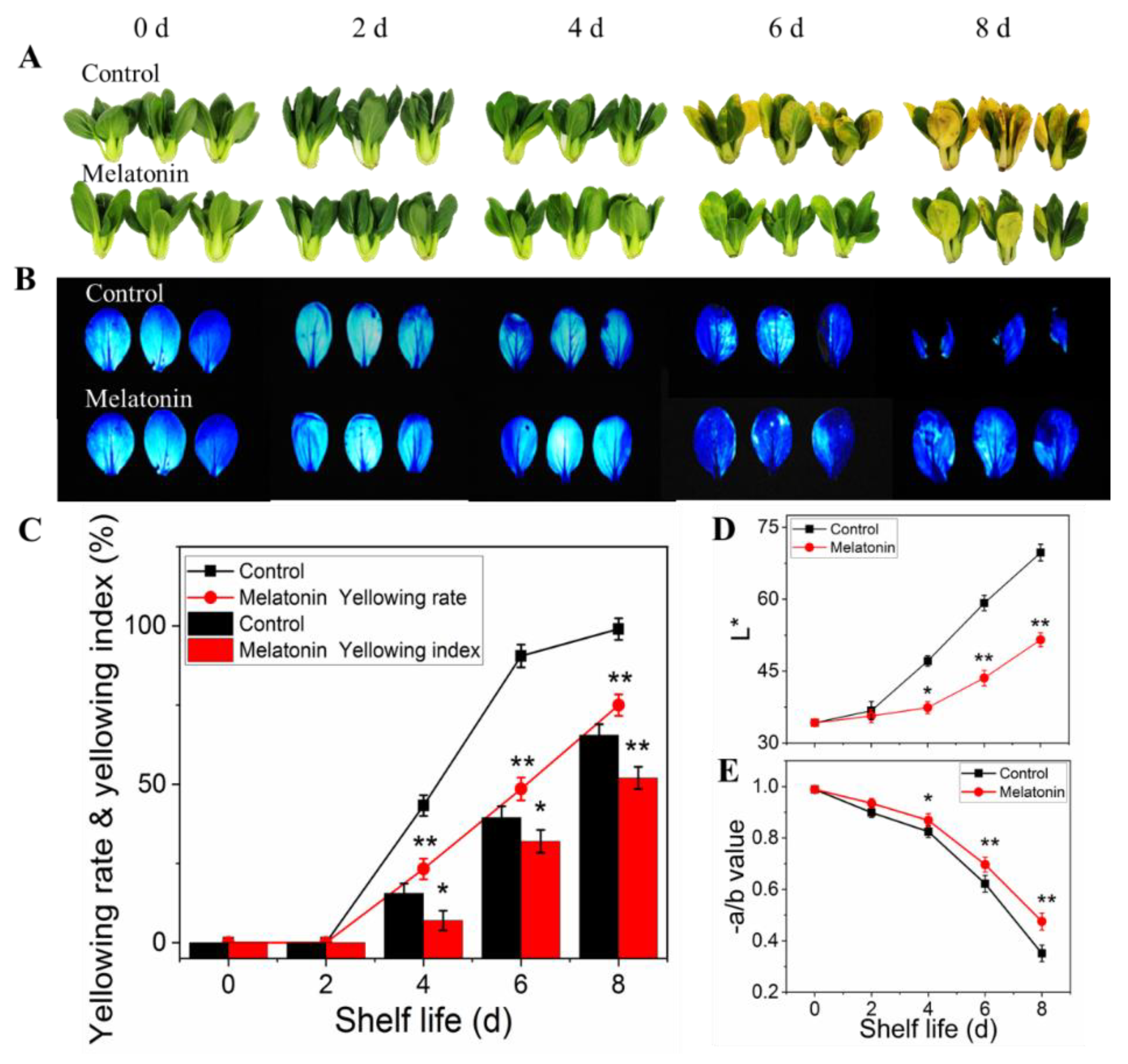
2.1. Exogenous Melatonin Alleviated Leaf Yellowing of Pakchoi
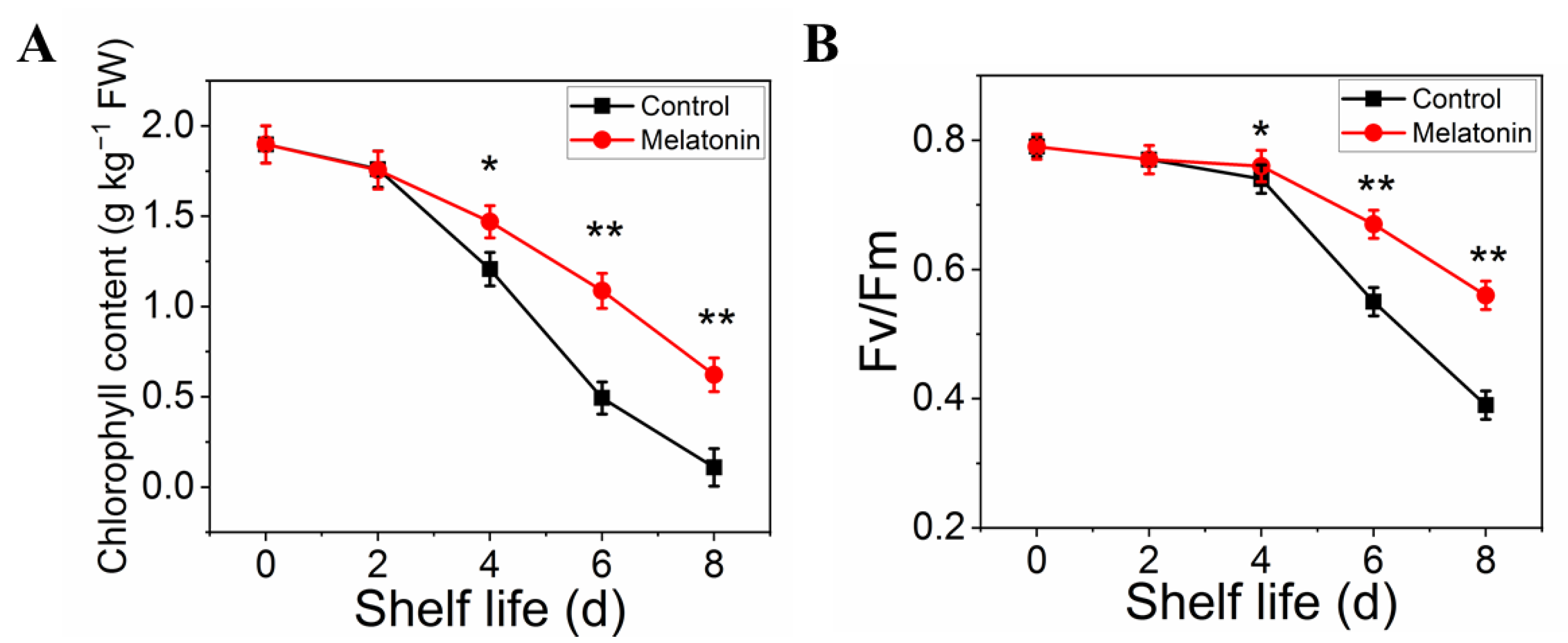
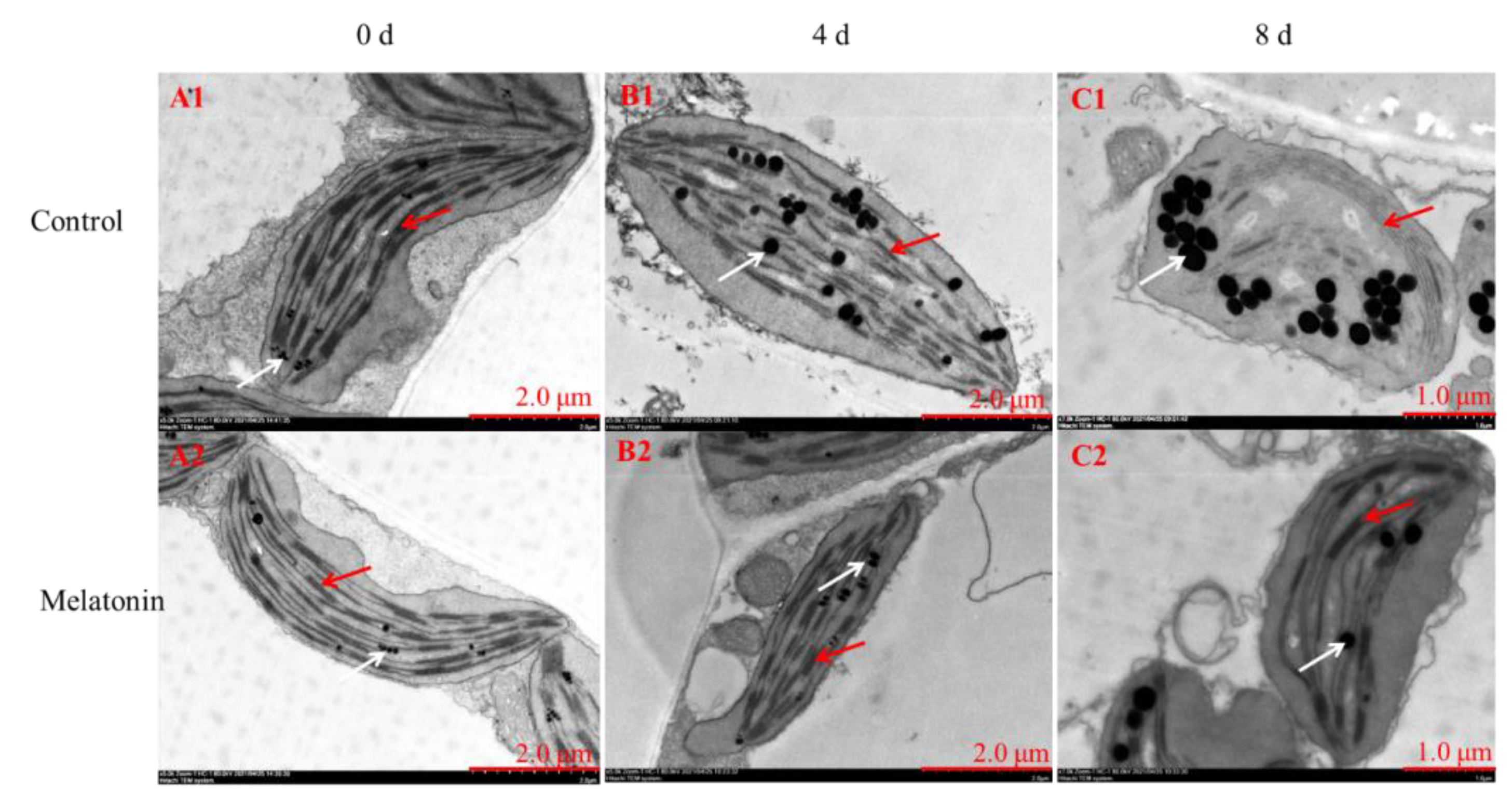
2.2. Exogenous Melatonin Inhibited Chlorophyll Degradation in Pakchoi
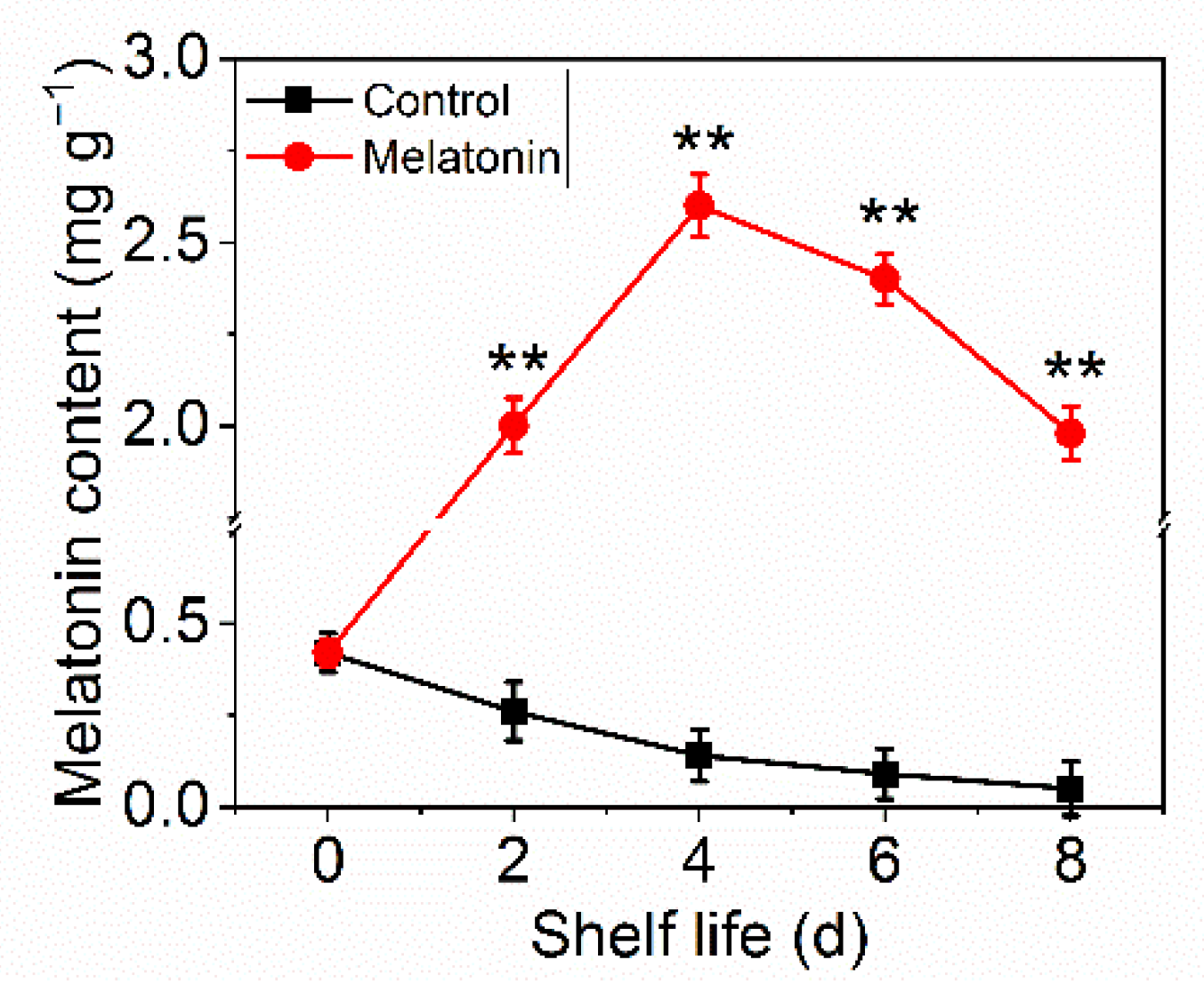
2.3. Exogenous Melatonin Increased Melatonin Accumulation
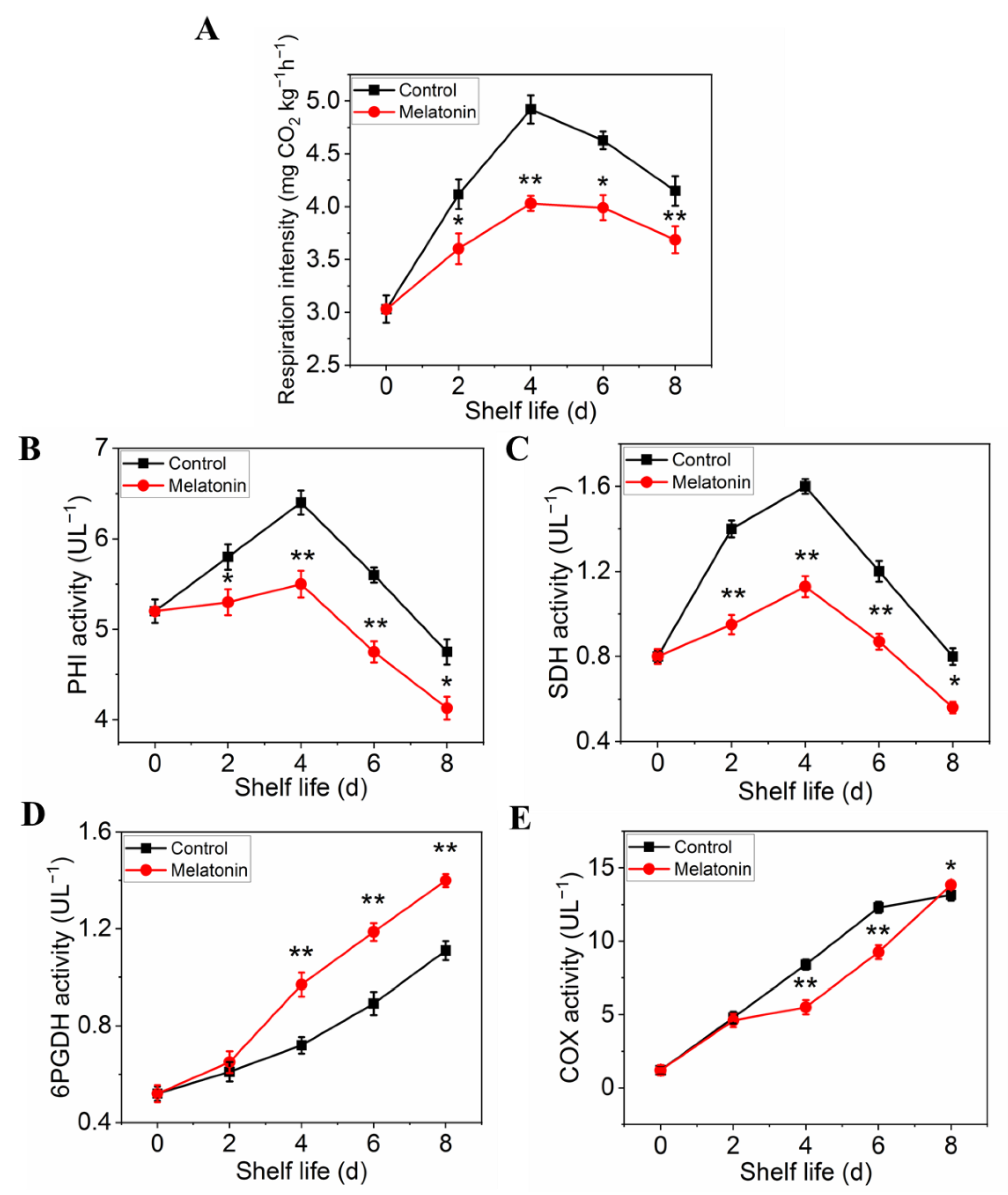
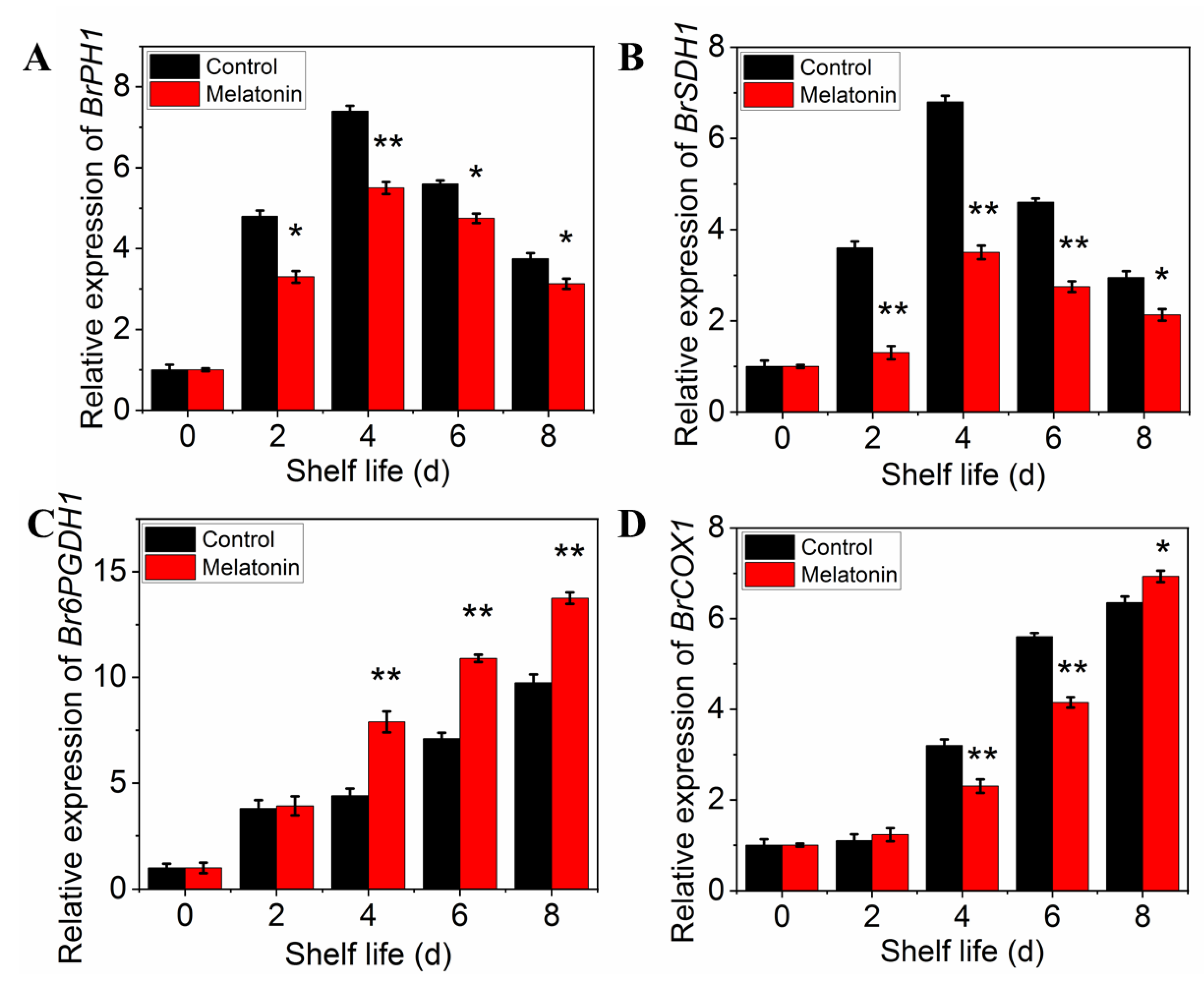
2.4. Exogenous Melatonin Mitigated Respiratory Metabolism in Postharvest Pakchoi
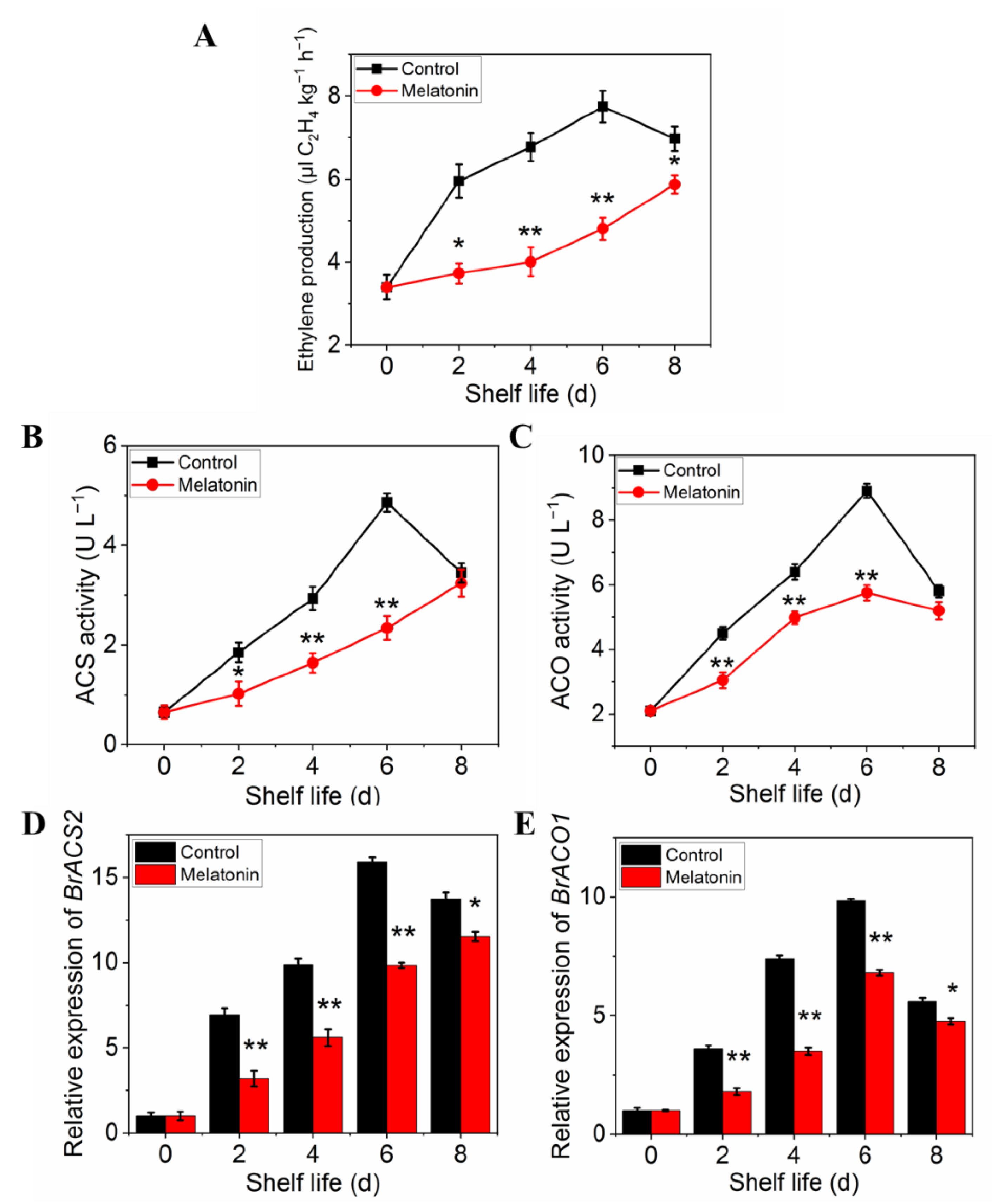
2.5. Exogenous Melatonin Reduced Ethylene Biosynthesis in Postharvest Pakchoi
2.6. Correlation Analysis between the Yellowing Index and the Chlorophyll Content, Respiration Rate and Ethylene Production
3. Discussion
4. Materials and Methods
4.1. Materials and Treatments
4.2. Examination of Leaf Color Changes
4.3. Determination of Chlorophyll Content and Fv/Fm
4.4. Observations of Chloroplast Morphology
4.5. Measurement of Respiration Rate and Ethylene Production
4.6. Assay of Respiratory Metabolism and Ethylene Biosynthesis Related Enzymes Activity
4.7. Quantitative Real-Time PCR
4.8. Quantification of Melatonin Content
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Able, A.J.; Wong, L.S.; Prasad, A.; O’Hare, T.J. 1-MCP is more effective on a floral brassica (Brassica oleracea var. italica L.) than a leafy brassica (Brassica rapa var. chinensis). Postharvest Biol. Technol. 2002, 26, 147–155. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Tan, X.L.; Chen, J.W.; Liu, Z.L.; Kuang, J.F.; Lu, W.J.; Shan, W.; Chen, J.Y. BrNAC055, a novel transcriptional activator, regulates leaf senescence in Chinese flowering cabbage by modulating reactive oxygen species production and chlorophyll degradation. J. Agric. Food Chem. 2018, 66, 9399–9408. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, Y.; Wang, Y.H.; Liu, Z.Y.; Li, C.Y.; Feng, H. Assessment of a stay-green mutant for variety improvement in Pakchoi (Brassica campestris L. ssp. chinensis). Sci. Horticult. 2020, 266, 109261. [Google Scholar] [CrossRef]
- Park, S.; Yu, J.; Park, J.; Li, J.; Yoo, S.; Lee, N.; Lee, S.; Jeong, S.; Seo, H.; Koh, H.; et al. The senescence-induced stay green protein regulates chlorophyll degradation. Plant Cell 2007, 19, 1649–1664. [Google Scholar] [CrossRef] [PubMed]
- Saltveit, M.E. Respiratory metabolism. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 73–91. [Google Scholar] [CrossRef]
- Tan, X.L.; Fan, Z.Q.; Zeng, Z.X.; Shan, W.; Kuang, J.F.; Lu, W.J.; Su, X.G.; Tao, N.G.; Lakshmanan, P.; Chen, J.Y.; et al. Exogenous melatonin maintains leaf quality of postharvest Chinese flowering cabbage by modulating respiratory metabolism and energy status. Postharvest Biol. Technol. 2021, 177, 111524. [Google Scholar] [CrossRef]
- Van Dongen, J.T.; Gupta, K.J.; Ramírez-Aguilar, S.J.; Araújo, W.L.; Nunes-Nesi, A.; Fernie, A.R. Regulation of respiration in plants: A role for alternative metabolic pathways. J. Plant Physiol. 2011, 168, 1434–1443. [Google Scholar] [CrossRef]
- Vanlerberghe, G. Alternative oxidase: A mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef]
- MØller, I.M.; Rasmusson, A.G.; Siedow, J.N.; Vanlerberghe, G.C. The product of the alternative oxidase is still H2O. Arch. Biochem. Biophys. 2010, 495, 93–94. [Google Scholar] [CrossRef]
- Lin, Y.X.; Lin, H.T.; Chen, Y.H.; Wang, H.; Lin, M.S.; Ritenour, M.A.; Lin, Y.F. The role of ROS-induced change of respiratory metabolism in pulp breakdown development of longan fruit during storage. Food Chem. 2019, 305, 125439. [Google Scholar] [CrossRef]
- Liu, G.S.; Zhang, Y.X.; Yun, Z.; Hu, M.J.; Liu, J.L.; Jiang, Y.M.; Zhang, Z.K. Melatonin enhances cold tolerance by regulating energy and proline metabolism in litchi fruit. Foods 2020, 9, 454. [Google Scholar] [CrossRef]
- Soto, I.C.; Fontanesi, F.; Liu, J.J.; Barrientos, A. Biogenesis and assembly of eukaryotic cytochrome c oxidase catalytic core. Biochim. Biophys. Acta BBA Bioenerg. 2012, 1817, 883–897. [Google Scholar] [CrossRef]
- Li, L.; Lv, F.Y.; Guo, Y.Y.; Wang, Z.Q. Respiratory pathway metabolism and energy metabolism associated with senescence in postharvest broccoli (Brassica oleracea L. var. italica) florets in response to O2/CO2 controlled atmospheres. Postharvest Biol. Technol. 2016, 111, 330–336. [Google Scholar] [CrossRef]
- Lin, Y.X.; Lin, Y.F.; Chen, Y.H.; Wang, H.; Shi, J.; Lin, H.T. Hydrogen peroxide induced changes in energy status and respiration metabolism of harvested longan fruit in relation to pericarp browning. J. Agric. Food Chem. 2016, 64, 4627–4632. [Google Scholar] [CrossRef]
- Baclayon, D.P.; Matsui, T. Exposure of Broccoli to different temperatures during storage: Some changes in postharvest physiology and activities of ammonia-assimilating enzymes. Acta Hortic. 2008, 768, 551–558. [Google Scholar] [CrossRef]
- Al Ubeed, H.M.S.; Wills, R.B.H.; Bowyer, M.C.; Golding, J.B. Comparison of hydrogen sulphide with 1-methylcyclopropene (1-MCP) to inhibit senescence of the leafy vegetable, pak choy. Postharvest Biol. Technol. 2017, 137, 129–133. [Google Scholar] [CrossRef]
- Kusaba, M.; Tanaka, A.; Tanaka, R. Stay green plants: What do they tell us about the molecular mechanism of leaf senescence? Photosynth. Res. 2013, 117, 221–234. [Google Scholar] [CrossRef]
- Grbić, V.; Bleecker, A.B. Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant J. 1995, 8, 595–602. [Google Scholar] [CrossRef]
- Amir-Shapira, D.; Goldschmidt, E.E.; Altman, A. Chlorophyll catabolism in senescing plant tissues: In vivo breakdown intermediates suggest different degradative pathways for citrus fruit and parsley leaves. Proc. Natl. Acad. Sci. USA 1987, 84, 1901–1905. [Google Scholar] [CrossRef]
- Yamauchi, N.; Watada, A.E. Regulated chlorophyll degradation in spinach leaves during storage. J. Am. Soc. Hortic. Sci. 1991, 116, 58–62. [Google Scholar] [CrossRef]
- Gong, Y.; Mattheis, J.P. Effect of ethylene and 1-methylcyclopropene on chlorophyll catabolism of broccoli florets. Plant Growth Regul. 2003, 40, 33–38. [Google Scholar] [CrossRef]
- Aharoni, N.; Lieberman, M. Ethylene as a regulator of senescence in tobacco leaf discs. Plant Physiol. 1979, 64, 801–804. [Google Scholar] [CrossRef]
- Able, A.J.; Wong, L.S.; Prasad, A.; O’Hare, T.J. The physiology of senescence in detached pak choy leaves (Brassica rapa var. chinensis) during storage at different temperatures. Postharvest Biol. Technol. 2005, 35, 271–278. [Google Scholar] [CrossRef]
- Gapper, N.E.; Coupe, S.A.; Mckenzie, M.J.; Sinclair, B.K.; Lill, R.E.; Jameson, P.E. Regulation of harvest-induced senescence in broccoli (Brassica oleracea, var. italica) by cytokinin, ethylene, and sucrose. Plant Growth Regul. 2005, 24, 153–165. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, a pineal factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by HPLC-MS. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Bose, S.K.; Howlader, P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review. Environ. Exp. Bot. 2020, 176, 104063. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Synthesis from tryptophan and its role in higher plants. In Amino Acids in Higher Plants; D’Mello, J.P.F., Ed.; CAB International: Boston, MA, USA, 2015; pp. 390–435. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Hameed, S. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Na, Z.; Wang, J.; Cao, Y.; Li, X.; Zhang, H.; Zhang, L.; Tan, D.X.; Guo, Y.D. A label-free differential proteomics analysis reveals the effect of melatonin on promoting fruit ripening and anthocyanin accumulation upon postharvest in tomato. J. Pineal Res. 2016, 61, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria × anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, H.; Sheng, K.; Wei, L.; Lei, Z. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Gao, H.; Lu, Z.M.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, M.M.; Cao, W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chem. 2017, 245, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, T.; Zhang, P.; Wang, Z.Y. Melatonin attenuates postharvest physiological deterioration of cassava storage roots. J. Pineal Res. 2016, 60, 424–434. [Google Scholar] [CrossRef]
- Tan, X.L.; Fan, Z.Q.; Kuang, J.F.; Lu, W.J.; Reiter, R.J.; Lakshmanan, P.; Su, X.G.; Zhou, J.; Chen, J.Y.; Shan, W. Melatonin delays leaf senescence of Chinese flowering cabbage by suppressing ABFs-mediated abscisic acid biosynthesis and chlorophyll degradation. J. Pineal Res. 2019, 67, e12570. [Google Scholar] [CrossRef]
- Tan, X.L.; Zhao, Y.T.; Shan, W.; Kuang, J.F.; Lu, W.J.; Su, X.G.; Tao, N.G.; Lakshmanan, P.; Chen, J.Y. Melatonin delays leaf senescence of postharvest Chinese flowering cabbage through ROS homeostasis. Food Res. Int. 2020, 138, 109790. [Google Scholar] [CrossRef]
- Luo, F.; Cai, J.H.; Zhang, X.; Tao, D.B.; Zhou, X.; Zhou, Q.; Zhao, Y.B.; Wei, B.D.; Cheng, S.C.; Ji, S.J. Effects of methyl jasmonate and melatonin treatments on the sensory quality and bioactive compounds of harvested broccoli. RSC Adv. 2018, 8, 41422–41431. [Google Scholar] [CrossRef]
- Rosa, B.A.; Aharoni, D.; Feygenberg, O.; Aharoni, N.; Keynan, A.; Pesis, E. Effect of modified atmosphere packing on mango ripening. Acta Hortic. 2001, 553, 607–610. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Feng, X.; Lin, L.; Zhao, Y.; Jiang, W. Effects of 1-MCP and exogenous ethylene on fruit ripening and antioxidant in stored mango. Plant Growth Regul. 2009, 57, 185–192. [Google Scholar] [CrossRef]
- Zaharah, S.S.; Singh, Z. Mode of action of nitric oxide in inhibiting ethylene biosynthesis and fruit softening during ripening and cool storage of ‘Kensington Pride’ mango. Postharvest Biol. Technol. 2011, 62, 258–266. [Google Scholar] [CrossRef]
- Li, L.; Kitazawa, H.; Zhang, X.H.; Zhang, L.M.; Sun, Y.; Wang, X.Y.; Liu, Z.L.; Guo, Y.Y.; Yu, S.X. Melatonin retards senescence via regulation of the electron leakage of postharvest white mushroom (Agaricus bisporus). Food Chem. 2020, 340, 127833. [Google Scholar] [CrossRef]
- Wu, C.H.; Cao, S.F.; Xie, K.Q.; Chi, Z.Y.; Wang, J.; Wang, H.F.; Wei, Y.Y.; Shao, X.F.; Zhang, C.D.; Xu, F.; et al. Melatonin delays yellowing of broccoli during storage by regulating chlorophyll catabolism and maintaining chloroplast ultrastructure. Postharvest Biol. Technol. 2021, 172, 111378. [Google Scholar] [CrossRef]
- Wang, C.Y. Effect of short-term carbon dioxide treatment on the market quality of stored broccoli. J. Food Sci. 1979, 44, 1478–1482. [Google Scholar] [CrossRef]
- Hirata, K.; Chachin, K.; Iwata, T. Quality changes of some vegetables used in tropical and subtropical areas during storage at various temperatures. Nippon Shokuhin Kagaku Kogaku Kaishi 2009, 34, 566–573. [Google Scholar] [CrossRef]
- Wang, H.; Herner, R.C. Effect of CA storage on the ultrastructure of chloroplasts and chlorophyll content of Chinese mustard. HortScience 1990, 25, 1127b. [Google Scholar] [CrossRef]
- Li, D.; Li, L.; Ge, Z.; Limwachiranon, J.; Ban, Z.J.; Yang, D.; Luo, Z.S. Effects of hydrogen sulfide on yellowing and energy metabolism in broccoli. Postharvest Biol. Technol. 2017, 129, 136–142. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, J.; Liu, F.; Zhao, Y.; Liu, L.; Fang, C.; Wang, H.; Li, X.; Wang, Z.; Ma, F. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharvest Biol. Technol. 2018, 139, 38–46. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Inhibition of ACC oxidase activity by melatonin and IAA in etiolated lupin hypocotyls. In Advances in Plant Ethylene Research; Ramina, A., Chang, C., Giovannoni, J., Klee, H., Perata, P., Woltering, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 101–103. [Google Scholar]
- Mukherjee, S. Recent advancements in the mechanism of nitric oxide signaling associated with hydrogen sulfide and melatonin crosstalk during ethylene-induced fruit ripening in plants. Nitric Oxide 2018, 82, 25–34. [Google Scholar] [CrossRef]
- Tsuchisaka, A.; Yu, G.; Jin, H.; Alonso, J.M.; Ecker, J.R.; Zhang, X.; Gao, S.; Theologis, A. A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics 2009, 183, 979–1003. [Google Scholar] [CrossRef]
- Oh, S.A.; Park, J.H.; Lee, G.I.; Paek, K.H.; Park, S.K.; Nam, H.G. Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. Plant J. 1997, 12, 527–535. [Google Scholar] [CrossRef]
- Saltveit, M.E. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol. Technol. 1999, 15, 279–292. [Google Scholar] [CrossRef]
- Kays, S.J.; Paull, R.E. Postharvest Biology; Exon Press: Athens, Greece, 2004. [Google Scholar]
- Yang, S.F. The role of ethylene and ethylene synthesis in fruit ripening. In Plant Senescence: Its Biochemistry and Physiology; Thomson, W.W., Nothnagel, E.A., Huffaker, R.C., Eds.; The American Society of Plant Physiologists: Rockville, MD, USA, 1987; pp. 156–166. [Google Scholar]
- Tian, M.S.; Prakash, S.; Elgar, H.J.; Young, H.; Burmeister, D.M.; Ross, G.S. Responses of strawberry fruit to 1-methylcyclopropene (1-MCP) and ethylene. Plant Growth Regul. 2000, 32, 83–90. [Google Scholar] [CrossRef]
- Cefola, M.; Amodioa, M.L.; Rinaldi, R.; Vanadia, S.; Colelli, G. Exposure to 1-methylcyclopropene (1-MCP) delays the effects of ethylene on fresh-cut broccoli raab (Brassica rapa L.). Postharvest Biol. Technol. 2010, 58, 29–35. [Google Scholar] [CrossRef]
- Ku, V.V.V.; Wills, R.B.H. Effect of 1-methylcyclopropene on the storage life of broccoli. Postharvest Biol. Technol. 1999, 17, 127–132. [Google Scholar] [CrossRef]
- Fan, X.T.; Mattheis, J.P. Yellowing of broccoli in storage is reduced by 1-methylcyclopropene. HortScience 2000, 35, 885–887. [Google Scholar] [CrossRef]
- Wang, N.; Kong, X.M.; Luo, M.L.; Sun, Y.Y.; Liu, Z.Y.; Feng, H.; Ji, S.J. SGR mutation in pakchoi prolongs its shelf life by retarding chlorophyll degradation and maintaining membrane function. Postharvest Biol. Technol. 2022, 191, 111986. [Google Scholar] [CrossRef]
- Meng, J.; Zhou, Q.; Zhou, X.; Fang, H.X.; Ji, S.J. Ethylene and 1-MCP treatments affect leaf abscission and associated metabolism of Chinese cabbage. Postharvest Biol. Technol. 2019, 157, 110963. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, B.; Peng, X.Y.; Wang, J.D.; Li, Q.P.; Jin, J.; Ha, Y.M. Effects of chlorine dioxide treatment on respiration rate and ethylene synthesis of postharvest tomato fruit. Postharvest Biol. Technol. 2014, 93, 9–14. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Z.Y.; Zhang, Y.; Li, C.Y.; Feng, H. Identification and fine mapping of a stay-green gene (Brnye1) in pakchoi (Brassica campestris L. ssp. chinensis). Theor. Appl. Gen. 2018, 131, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.Y.; Zhang, Y.; Xu, Y.P.; Qi, Z.Y.; Li, M.Q.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J.; et al. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 2017, 62, e12387. [Google Scholar] [CrossRef] [PubMed]
| Correlation Analysis of the Control Pakchoi | ||||
|---|---|---|---|---|
| YellowingIndex | Chlorophyll Content | Respiration Rate | Ethylene Production | |
| Yellowing index | 1 | −0.992 ** | 0.962 ** | 0.793 ** |
| Chlorophyll content | −0.992 ** | 1 | −0.947 ** | −0.788 ** |
| Respiration rate | 0.962 ** | −0.947 ** | 1 | 0.835 ** |
| Ethylene production | 0.793 ** | −0.788 ** | 0.835 ** | 1 |
| Correlation analysis of the MT-treated pakchoi | ||||
| Yellowing index | 1 | −0.986 ** | 0.957 ** | 0.774 ** |
| Chlorophyll content | −0.986 ** | 1 | −0.914 ** | −0.754 ** |
| Respiration rate | 0.957 ** | −0.914 ** | 1 | 0.787 ** |
| Ethylene production | 0.774 ** | −0.754 ** | 0.787 ** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Fang, H.; Yang, Q.; Liu, Z.; Feng, H.; Ji, S. Exogenous Melatonin Alleviated Leaf Yellowing via Inhibiting Respiration and Ethylene Biosynthesis during Shelf Life in Pakchoi. Plants 2022, 11, 2102. https://doi.org/10.3390/plants11162102
Wang N, Fang H, Yang Q, Liu Z, Feng H, Ji S. Exogenous Melatonin Alleviated Leaf Yellowing via Inhibiting Respiration and Ethylene Biosynthesis during Shelf Life in Pakchoi. Plants. 2022; 11(16):2102. https://doi.org/10.3390/plants11162102
Chicago/Turabian StyleWang, Nan, Huixin Fang, Qingxi Yang, Zhiyong Liu, Hui Feng, and Shujuan Ji. 2022. "Exogenous Melatonin Alleviated Leaf Yellowing via Inhibiting Respiration and Ethylene Biosynthesis during Shelf Life in Pakchoi" Plants 11, no. 16: 2102. https://doi.org/10.3390/plants11162102
APA StyleWang, N., Fang, H., Yang, Q., Liu, Z., Feng, H., & Ji, S. (2022). Exogenous Melatonin Alleviated Leaf Yellowing via Inhibiting Respiration and Ethylene Biosynthesis during Shelf Life in Pakchoi. Plants, 11(16), 2102. https://doi.org/10.3390/plants11162102





