Abstract
The objective of this study was to determine uranium (U) and other metal(loid) concentrations (As, Cd, Cs, Pb, Mo, Se, Th, and V) in eight species of plants that are commonly used for medicinal purposes on Diné (Navajo) lands in northwestern New Mexico. The study setting was a prime target for U mining, where more than 500 unreclaimed abandoned U mines and structures remain. The plants were located within 3.2 km of abandoned U mines and structures. Plant biota samples (N = 32) and corresponding soil sources were collected. The samples were analyzed using Inductively Coupled Plasma–Mass Spectrometry. In general, the study findings showed that metal(loid)s were concentrated greatest in soil > root > aboveground plant parts, respectively. Several medicinal plant samples were found to exceed the World Health Organization Raw Medicinal Plant Permissible Level for As and Cd; however, using the calculated human intake data, Reference Dietary Intakes, Recommended Dietary Allowances, and tolerable Upper Limits, the levels were not exceeded for those with established food intake or ingestion guidelines. There does not appear to be a dietary food rise of metal(loid) ingestion based solely on the eight medicinal plants examined. Food intake recommendations informed by research are needed for those who may be more sensitive to metal(loid) exposure. Further research is needed to identify research gaps and continued surveillance and monitoring are recommended for mining-impacted communities.
1. Introduction
Diverse populations are disproportionately exposed to toxic materials by virtue of proximity [1]. American Indian (AI) communities are at risk for worsened health burdens, which may be compounded by environmental exposures [2]. One-half of the uranium (U) in the United States (US) is found on AI lands, where mining, milling, and processing commonly occur [3], as well as the storage of remaining waste. In the Western US, more than 160,000 abandoned mines exist on or are adjacent to AI homelands [4]. The study setting was a prime target for U mining for military purposes, where northwestern New Mexico (NM) alone contributed 40% of the US U production [5].
Diné (Navajo) lands were one of the prime targets for mining, contributing thirteen million tons of U ore for military use from 1945 to 1988 [6] and leaving more than 550 abandoned and partially unreclaimed U mines, mills, and waste piles [7].
The extent of the health impacts on the Diné community exposed to these sites is a public health concern. Uranium enters the body primarily by inhalation or ingestion (via contaminated water or food) and is deposited in tissues, primarily the kidneys and bones [8]. High U exposure studies in mammals have shown kidney chemical toxicity [9]. Uranium and metal(loid)s were examined in this study as they may co-occur environmentally with other metal(loid)s and/or may be associated by way of its decay series. Addressing U and its associated co-contaminant exposures is a challenge for rural communities experiencing a myriad of socioeconomic barriers [10]. Arsenic (As) is a teratogen [11]. Cadmium (Cd) can accumulate in organs and is associated with liver and renal problems [12,13]. Long-term neurodevelopmental, renal, and reproductive problems are associated with lead (Pb) [14]. Toxicosis can occur with high doses of selenium (Se) [15]; semen quality and testosterone have been shown to have an inverse association with molybdenum (Mo) [16,17], which also has a negative effect on renal function [18]. Carcinogenicity has been reported for several metal(loid)s, including As [19,20,21], Cd [19], and Pb [22], whereas others have permanent and/or long-term health sequelae (Mo, Thorium (Th), Vanadium (V), and Cesium (Cs)) [23].
The interaction between humans and plants is known as ethnobotany [24] or rather it is how people of a particular region or culture utilize local (indigenous) plants. It is common knowledge that modern medicines are a direct result of traditional ethnobotanical knowledge and information. Globally, up to 80% of the population relies on traditional medicine for their healthcare needs [14]. Yet, traditional medicines are poorly regulated and monitored in many countries including the US. Medicinal plants are assumed to be safe due to ease of accessibility and availability; they are commonly self-administered or self-prescribed without medical consultation.
In the US Southwest, there are more than 3000 known plant species, of which the Diné were said to utilize about 450 species for medicinal purposes [25]. In AI communities, local plants are relied upon for their medicinal or healing properties, are consumed as foods/additives, or are relied upon for innumerable cultural purposes. In this community, the primary categories of plant use are medicine, food/beverage, creating dye, paint, ceremonial objects (baskets, paints), and other uses (such as construction, fuel, and implements such as textiles) [25], respectively. Cultural protocol passed down through generations dictates that all parts of the plant should be used without waste, that the plant was selected using strict environmentally sustainable practices (i.e., the strongest and most robust plants are left unharvested to perpetuate the species), and that the harvester has requested permission to use the plant and has practiced thankfulness and respectfulness for its use [26,27].
Medicinal plant pharmacological indications, routes, and dosages vary and its administration may include drinking it as a tea or as a concentrated decoction or used in combination with other ingredients (concoction), it may be applied by direct dermal application (as a poultice, salve) and may be inhaled via incense or by steam (sweat bath). Also, plant roots may be directly chewed and ingested (e.g., Bouteloua gracilis (Wildenou ex Kunth) Lagasca ex Griffiths). Human exposure can occur through contact with local plant branches, stems, and roots that can be used for cooking and heating (e.g., Juniperus monosperma Engelmann), serve as construction materials (e.g., J. monosperma), and be used in the creation of numerous cultural implements (baskets, cordage). This could include items integral to traditional healing ceremonies. Plants and their contaminants may be ingested indirectly by humans when locally raised meat (via forage, water, and soil ingestion) is consumed. Phytotherapy self-administration/prescription is commonplace. However, laypersons, herbalists, or traditional practitioners or specialists may provide directions or prescriptions for various indications. A summary of the information on the eight study plant species names/taxonomies, descriptions, and ethnobotanical indications can be found in Table 1.

Table 1.
Plant names (scientific, common, and Diné names), biota description/distribution, and ethnobotanical indications.
The purpose of this study was to determine if eight abundant and readily accessible species of plants, a locally harvested resource on Diné lands in northwestern NM, were contaminated with U and other associated metal(loid)s. Food-chain contamination in locally harvested food in the Diné community in NM was reported as a plausible exposure pathway [42]; harvesting and gathering were found to be common practices [43]. The current study was undertaken to characterize the use of eight common local medicinal plants and contribute novel metal(loid) uptake data. The objective of this study was to compare plant-part concentrations to the World Health Organization (WHO) Raw Medicinal Plant Permissible Level (RMPPL) guidelines and calculate an estimated ingestion risk exposure and compare it to the established food intake guidelines according to the Provisional Tolerable Weekly Intake (PTWI) or Reference Dietary Intake (RDI) or Recommended Dietary Allowance (RDA) and Upper Limit (UL) in eight commonly used medicinal plants in a community impacted by the U mining legacy.
2. Results and Discussion
2.1. Data from the Human Harvester Questionnaire
The medicinal plant harvesters (n = 6) were evenly divided between genders and the mean (M ± Standard Deviation (SD)) age was 57.25 ± 1.84 (range 53–62). On average, the calculated weekly intake of herbal medicine was 1.17 for ingestion of at least one plant for a mean consumption of 56.5 ± 3.32 years. Per participant reports, plants were located in the wild and did not benefit from artificial watering, soil amendments, or the application of pesticides. All study participants reported sharing the herbs for free with community members on and off Diné tribal lands. No participants reported selling the herbs. The majority of participants self-prescribed and administered the medicinal plants and reported not having consulted a traditional practitioner for their use. Plant harvesting, preparation, storage, and consumption information was passed down from previous generations via elders; they were often laypersons, herbalists, or other traditional practitioners or specialists.
A study by Tsuji et al. [27] found food-sharing behavior to be common in a North American Indigenous community impacted by mining in a traditional-use territory in Canada. These types of food-sharing behaviors were found to be related to the harvesting of subsistence-type of foods that were found to have a direct exposure impact beyond the mining communities and were considered to be important for assessing and monitoring impacted communities [44], with a special interest in vulnerable groups (children and older tribal members) [45]. Using Geographic Information System (GIS) mapping, the above study [27] demonstrated that longstanding harvesting areas overlapped significantly with contaminated areas and that several important potential routes of exposure were identified and characterized (e.g., ingestion of contaminated foods and drinking water). Using GIS, the current study demonstrated an overlap of medicinal plant gathering and harvesting areas in proximity to mining sites and features; overlap was commonplace and samples fell within a 3.2 km buffer zone of high-risk areas. Proximity (median 3.54 km ((IQR 1.81–8.0)) to U mine and milling sites was found to be a potential contributor to cardiovascular disease [46] in a local GIS study.
2.2. Medicinal Plant Parts
Twenty-seven percent of the medicinal plant species in the study areas consisted of the B. gracilis plant, with 12% each of A. hymenoides, A. purpurea, P. smithii, P. jamesii, and S. cryptandrus and 6% each of J. monosperma and A. tridentate plants. The availability and distribution of the sampled plants were representative of the local flora reported in the literature [29,31,32,34,35,36,37,39]. The majority of the plants had greater concentrations in their aboveground parts than their roots. The metal(loid)s that met statistical significance (p < 0.05) were Cd, Se, Th, and U (Table 2). The largest metal(loid) concentration differences were found between the aboveground A. purpurea plant and its roots for Se (3.50 mg/kg vs. 2.31 mg/kg) and the P. jamesii plant and its roots (3.69 mg/kg vs. 2.41 mg/kg). Others that differed by more than 1 mg/kg were A. tridentate (2.67 mg/kg vs. 1.55 mg/kg) and S. cryptandrus (3.36 mg/kg vs. 2.28 mg/kg).

Table 2.
Concentrations of Arsenic, Cadmium, Cesium, Lead, Molybdenum, Selenium, Thorium, Uranium, and Vanadium in eight species of medicinal plants and soil (Mean ± SD mg/kg, Range).
In general, comparable results were found (or lower plant concentrations) with herbal plant metal(loid) levels in the study area [42,48], including international studies [49,50,51,52], except there were higher concentrations found with a Th [52] plant study (Table 3). Forage grasses reported for U ranged from 0.5 to 7.7 mg/kg (U in root M = 5.0 mg/kg and grass blades 2.4 mg/kg) [42] (Table 3). The plant species reported by local and international studies were dissimilar to the plants reported in this study. Shi et al. [53] reported that various plants are prone to concentrating contaminants in their main roots as they seem to function as a buffer to the aboveground parts of the plant. Similarly, Anke et al. and Soudek et al. found that there were greater metal(loid) concentrations in the plant roots than in the above-ground portions [54,55]; this was particular to U [55]. The uptake of metal(loid)s appeared to differ between species of plants [49,50,51,52].

Table 3.
Similar plant and soil studies examining metal(loid) concentrations. Metal(loid) concentrations are reported as mg/kg from high-impact areas unless otherwise specified.
2.3. Soil
In most instances, the study findings showed that metal(loid)s concentrated greatest in soil > root > aboveground plant parts, respectively (Table 2). Vanadium was the only metal that exceeded the concentration range of 15 mg/kg. Those metal(loid)s that fell between 10 and 15 mg/kg were Pb, Th, and As and of that, less than 5 mg/kg were Se, Cs, Mo, U, and Cd. The mean soil pH was weakly acidic to neutral in reaction (6.91 ± 0.97). Statistical significance was found in comparing the soil to the aboveground plant parts (soil > plants): V (p < 0.001), As (p < 0.05), Cs (p < 0.05), Pb (p < 0.05), Mo (p < 0.05), Th (p < 0.05), and U (p < 0.05). The soil concentrations were greater than the plant roots for all sampled plants: As, Cs, Pb, Mo, and Th (p < 0.001), V (p < 0.01), U, Se, and Cd (p < 0.05). These findings were similar to local and international plant studies examining different species of medicinal plants for metal(loid) content (As, Cd, Cs, Pb, Mo, Se, Th, U, V [43,48], Cd, and Pb [50] (Table 3)). In a regional tea soil study [43,48], there were comparable results for As, Cd, Cs, and V but greater concentrations of Pb, Se, and U; there were smaller concentrations of Mo and Th (Table 3). Regional plant and soil studies were also conducted for a different species of herbal plant (T. megapotamicum). A local study found comparable concentrations of Se in high-impact soil areas [56] but greater U soil concentrations were found in non-control areas [42,57].
The soil pH was comparable to other locally harvested plant and soil studies; they ranged from 6.3 to 6.5 (herbal tea and squash studies) [43,48]. More acidic soils have been demonstrated to increase the transfer and uptake of various metals such as Cd [58] and were thought to increase the likelihood of co-occurrence with other metal(loid)s, which also seems to be dependent on the physiochemical make-up of the soil and individual uptake of metals by various plant species [55,59,60]. It was beyond the scope of this study to describe all variables associated with the uptake of metal(loid)s in herbs from the soil.
For the herb-harvesting activities and consumption reported in this study, the main exposure to metal(loid)s appears to be via soil. In general, the current study has demonstrated that soil contained the greatest amounts of metal(loid)s compared to plant part samples (Table 2), which is comparable to local tea [43], vegetation [42], and crop studies [48].
2.4. WHO RMPPL
The soil Cd concentration levels were exceeded for the WHO RMPPL of 0.3 mg/kg by 2.9 times for the aboveground plant parts for P. jamesii (M = 0.87 ± 1.42 mg/kg, Table 2). Five aboveground plant parts (A. hymenoides: M = 1.31 ± 0.19 mg/kg; A. purpurea: M = 1.22 ± 0.32 mg/kg; B. gracilis: M = 1.08 ± 0.47 mg/kg; P. smithii: M = 1.19 ± 0.41 mg/kg; and P. jamesii: M = 1.40 ± 0.28 mg/kg) exceeded the As concentration level of 1 mg/kg for the WHO RMPPL [47]. Of all the plant species sampled, study participants reported consuming B. gracilis root (M = 1.16 ± 0.41 mg/kg) for medicinal purposes; this was found to have exceeded the As WHO RMPPL by more than 3.5 times the recommended level. There were no exceedances for Pb WHO levels (10 mg/kg) for all eight species of plants [47].
The WHO RMPPLs were put in place to evaluate the presence of metals in herbal tea formulations and tinctures [47]. There are no permissible levels for Cs, Mo, Se, Th, U, and V.
A local herbal plant study found that the WHO RMPPL was exceeded for Cd in a popular species of tea, T. megapotamicum (M = 0.35 ± 0.31 mg/kg) and was higher in high-vehicular-traffic areas (M = 0.68 ± 0.11 mg/kg; p < 0.001) than low-traffic areas (M = 0.10 ± 0.06 mg/kg; p < 0.001) [43]. International medicinal plant studies did not find PTWI exceedances in other species of plants [61,62].
2.5. Human Intake Calculations for As, Cd, and Pb
The weekly intake calculations for As for each plant ranged from 0.29 to 0.82 μg/kg, 0.02 to 0.51 μg/kg for Cd, and 0.29 to 1.55 μg/kg for Pb (Table 4). Collectively, the PTWI percentages were low and fell below 7.3% (range 0.29–7.3%) of the weekly intake for all plants examined.

Table 4.
Provisional Tolerable Weekly Intake (PTWI) of As, Cd, and Pb through the ingestion of several species of medicinal plants.
The PTWI limits are 15 μg/kg body weight (BW), 7 μg/kg BW, and 25 μg/kg BW for As, Cd, and Pb, respectively [63,64]. There are currently no PTWI guidelines set for Cs, Mo, Se, Th, U, or V. All metal(loid) PTWI levels were below the level of concern for all plants examined. The PTWIs reported here were generally lower than those reported for squash and herbal tea plants [43,48] in a comparable regional study.
2.6. Human Intake Calculations for Mo, Se, and V
The daily intake calculation for Mo ranged from 0.28 to 0.91 μg, 0.92 to 2.01 μg for Se, and 0.22 to 4.86 μg for V (Table 5). The percentages for the RDA and RDI all fell below 3.7% for each plant studied. The UL percentages were considerably lower and did not exceed 0.5% for all medicinal plants sampled.

Table 5.
Reference Dietary Intake (RDI) or Recommended Dietary Allowance (RDA) and Upper Limit (UL) of Mo, Se, and V through the ingestion of several species of medicinal plants.
For Mo, the RDA is 45 μg/day with a tolerable Upper Limit (UL) of 2000 μg [65]. The RDI for Se for adults is 55 μg/day with a tolerable UL of 400 μg/day [66]. The UL for V is 1800 μg/day but there are no RDA or RDI guidelines [65]. There are no set RDIs/RDAs for As, Cs, Pb, Th, U, or V. There are no UL guidelines for As, Cd, Pb, Th, or U. In a local study area report, the RDAs, RDIs, and ULs were lower than those reported for squash and herbal tea biota [43,48]. The calculated RDIs/RDAs for Mo, Se, and V were small; however, these may not be completely reflective of the overall diet. It is likely that the Mo, Se, and V RDIs/RDAs were met by the consumption of other foods in the regular overall diet. This study only focused on a small portion of the entire food intake. For this cohort, supplemental Se and Mo in the diet may be needed (if not met by the regular overall diet) and is available in foods such as meat, legumes, grains (Se), and nuts (Mo) [65]. The advice of a dietitian and healthcare provider is recommended for any dietary changes in similar settings.
2.7. Human Implications for Intake Calculations
The intake estimates demonstrate that the consumption of each herbal medicine individually may not be of concern in the current cohort with an intake of 1.17 times per week. Upon direct comparison to the WHO RPPML, several plant species’ concentration levels were found to exceed the permissible levels for As and Cd. When the calculation incorporated a reference to body weight (60 kg) for the cohorts’ weekly intake (1.17 times a week), the PTWI (As, Cd, and Pb), RDAs/RDIs (Mo and Se), and ULs (Mo, Se, and V) were not exceeded for all eight species of medicinal plants. The former guidelines are based on Acceptable Daily Intake and the latter guidelines are more appropriate for long-term or chronic exposure to metal(loid)s [63]. More recent recommendations by the WHO [67] support the use of PTWI for measuring accurate medicinal plant material metal(loid) intake exposure. In this study cohort, participants reported extensive years of exposure to medicinal plant harvesting consumption (56.5 ± 3.32) as well as participation in other related outdoor harvesting activities. This provides support for the use of the PTWI guidelines as an accurate measure of chronic or long-term exposure.
For this study, we only reported individual plant concentration intake estimates consumed on a weekly basis and examined only a portion of the overall dietary intake. In some instances, there was a potential for guideline exceedances if several medicinal plants were used in mixtures or consumed on a more frequent basis. Further, if study participants were consuming additional locally raised and harvested foods (including local water) the combination may exceed the estimates reported here. For a more accurate intake estimate, collective food intake assessments that examine all aspects of one’s dietary intake are recommended. It was beyond the scope of this study to report the estimates of all conceivable mixtures of phytotherapies or to consider every route of administration. In most study case scenarios, medicinal herbs were typically consumed for short periods or were reserved specifically for special albeit infrequent curative ceremonies and their over-consumption was uncommon. Lastly, examining metal(loid) uptake and their calculated intake from other medicinal plants that were not examined in this study is warranted.
This population group has disproportionately high rates of hypertension, diabetes, cancer, cardiovascular disease [2,46], renal disease, and other comorbidities [68]. Metal(loid) exposures are known to worsen these comorbidities. Further, there is little research on the collective bioeffects of co-occurrent contaminants. More research is needed for high-risk groups as they may be more susceptible to the effects of metal(loid)s. High-risk groups include the very young, lactating or pregnant women, older adults, and those with cardiac, renal, and immune function problems. The level to which exposure is a danger to high-risk persons and other interrelated factors are unknown and need further investigation. It is recommended for individuals that consume traditional medicinal plants to consult with their healthcare provider when consuming alternative therapies to avoid untoward medication interactions.
2.8. Limitations
There were several study limitations. There was ample literature documenting the indications for various medicinal plant remedies in this community; however, there was significantly less documentation in relation to dosage information. Several sources of information were available documenting the use of various medicinal plants during pregnancy and the postpartum period [28,33] and for the treatment of infants and children [28,37], but for all age groups, there was no dosage information available. As there was scant dosage information to glean for the study calculations, we relied upon comparable studies. For instance, we provided an estimate of oral intake by using the equivalency of one cup of tea containing one g of plant material [61,69]. Also, exposure in terms of routes of administration was not examined in this study due to the lack of detailed pharmacological information. For example, inhalation (via incense (e.g., P. smithii, A. tridentate) or sweat bath steam or other exposures by smoke or mist or aerosolization) and dermal exposure (e.g., B. gracilis, A. tridentate, J. Monosperma) were not calculated for this report. Future examination is needed to establish dosages and to include various routes of administration such as inhalation and dermal skin exposures.
Other locally derived environmental sources of exposure may add or compound the risks. For instance, it is common practice for people to use local water (regulated and unregulated) to steep the teas or medicinal concoctions possibly using a suite of plant mixtures. Further, such plant mixtures introduce several complexities; without detailed pharmacologic information, synergistic, additive, and antagonistic effects are difficult to determine. In fact, some studies have identified that metal(oid)s may dissociate in water at certain water temperatures [70,71] and that the pH of infusion water may be a factor in uptake [61,72]. These factors warrant further investigation.
A plausible reason for the lack of dosage-specific or other detailed phytotherapy information may be that some tribal communities are protective of this information. Researchers and other experts have reported a general reluctance by tribal members or informants to report on healing ceremonies/medicinal plants as this knowledge is seen as sacred and such esoteric medicinal and ceremonial knowledge is exclusively for the dispensation/treatment/handling by Diné medicine-people with extensive training or who have undertaken apprenticeships [28]. For this paper, the researchers have not reported any new information in this study on medicinal plant indications (including references to specific ceremony names) that has not been published elsewhere [28,29,31,32,33,34,35,36,37,38,39,40,41]. It is rather an organized compilation and report of existing medicinal plant information published by researchers in direct consultation with expert Diné informants [28,29,31,32,33,34,35,36,37,38,39,40,41] who are vetted specialists such as herbalists, medicine-people, or healers. The resource is meant to be a reference source for researchers, healthcare providers, traditional medicine healers, and tribal community members and leaders. Further, it is not the purpose of this paper to report on pharmacokinetics but rather to inform and identify knowledge gaps in this area of medicinal plant research. Pharmacological intake, absorption, bioavailability, distribution, metabolism, and excretion are very complex processes and require extensive research. Further, there are many complicated individualistic (e.g., age, chronic and acute health problems, metabolism, genetics, diet/nutritional status, etc.) and interrelated environmental variables to be considered.
3. Materials and Methods
This was a descriptive, comparative study examining contamination levels in locally harvested medicinal plants and soils from reservation areas within a 3.2 km radius of previously U-mined and disrupted areas. Data obtained from the Diné Network for Environmental Health (DiNEH) study cohort [42] served as one of the sources for identifying the subjects and samples of food, herbs, water, and soil. Additional participants were recruited using snowball methods (word-of-mouth), home visits, and advertising at public tribal community events. Of the DiNEH cohort [42] respondents, those individuals who reported harvesting plant foods were recruited for participation in the present study. Plant biota were selected based on active use by study participants and their proximity to mining structures. The medicinal plant data were compared and reported to reflect an accurate estimated measure of metal(loid) intake in humans via medicinal plant ingestion.
3.1. Study Setting
This study was reviewed and approved by the dual Institutional Review Board (IRB)s, the Navajo Nation Human Research Review Board, and the University of California, Los Angeles, (UCLA) IRB. The eight species of plants identified in this study are not listed as endangered or threatened according to the Navajo Nation Department of Natural Resources [73] and the NM Energy, Minerals, and Natural Resources Department [74].
The field research area is a semi-arid to arid region of the US Southwest in northwestern NM on Diné reservation lands (Figure 1). The average precipitation was found to be <25 cm per year according to meteorological data for NM (Western Regional Climate Center Western US Climatic Historic Summaries) during the study period. The Mariano Lake Chapter is 272 km2 of land mass and the Churchrock Chapter is 233 km2 (total land mass of 505 km2). Recruitment was initiated in May 2012 and enrollment began in July 2012. All samples were collected from 10 November to 13 December 2012. This study focused on locally harvested plant biota and was part of a larger research project that examined subsistence farming on the reservation, including the metal(loid) contamination of herbs, sheep, crops, and associated data [43,48].
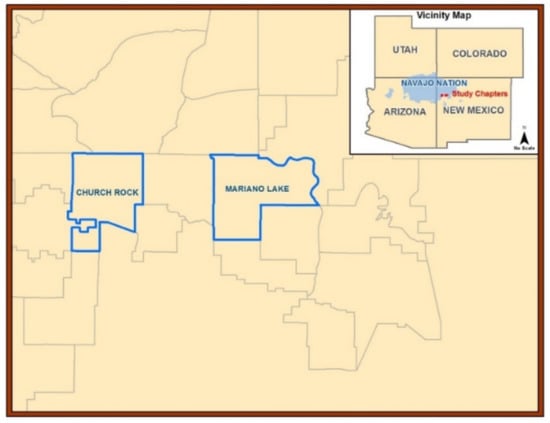
Figure 1.
Research area map. Cartographic map of the Navajo Nation in the Four Corners region of the US Southwest. New Mexico communities or “Chapters”: Churchrock (land mass 233 km2) and Mariano Lake (land mass 272 km2) provided biota and soil samples.
3.2. Human Harvester Questionnaire Data
The Diné Plant-Animal-Human-Questionnaire was administered to collect demographic information and collect overall local food harvesting data. Information on specific harvesting exposure activities was obtained. The Diné Wild Plant/Herb Intake Questionnaire was used to collect information on herbal plant harvesting and consumption. Data collected included plant use; indications; the amount, frequency, and duration of consumption; incidences and the extent of herb sharing and sales; relevant cultural uses for the medicinal plants; and traditional practitioner information.
3.3. Plant Identification and Nomenclature
Live parallel plants were collected, dried, and pressed for identification and archival. A plant collection description log was collected. Color photographs were taken of each plant. The University of New Mexico (UNM) Herbarium identified and archived the plant samples. Global Positioning System (GPS) instrumentation (Trimble Navigation Limited, Westminster, CO, USA) was utilized to collect location data and conduct spatial proximity analysis. Data differential correction was completed within 72 h of data capture using Pathfinder Office version 5.30 (Trimble Navigation Limited, Westminster, CO, USA).
3.4. Medicinal Plant Samples
Eight species of medicinal plants were collected and identified. Live medicinal plant samples were collected from wild, non-cultivated sources within a 3.2 km radius of the central part of abandoned U mines and features (mine portals, pits, rim strips, vertical mine shafts, and prospect areas). The above-ground portions and roots of live plants were stored in polyethylene (PE) plastic Ziplock® bags. The plant samples were photographed, weighed, bagged, and placed on dry ice for shipment for analysis by the UNM Analytical Chemistry Laboratory Earth and Planetary Sciences Department. The medicinal plant flowers, leaves, stems, and roots were analyzed for metal(loid)s (As, Cd, Cs, Pb, Mo, Se, T, U, and V) using inductively coupled plasma–mass spectrometry (ICP-MS).
3.5. Soil Samples
For each medicinal plant sample, parallel soil samples were collected. To avoid cross-contamination, a silicon-coated core sampler (Art’s Manufacturing and Supply Inc. (AMS), American Falls, ID, USA) was utilized. A slide hammer with a stainless-steel hand auger was employed to collect soil samples using a PE liner (AMS Core Sampling Mini-kit, American Falls, ID, USA). One hundred grams (g) of soil were collected for each plant from a 0–25 cm depth. The soil samples were analyzed for metal(loid)s (As, Cd, Cs, Pb, Mo, Se, Th, U, and V) using ICP-MS.
3.6. Sample Analysis
Medicinal plant and environmental sample preparation and analysis are reported in detail in previous publications [43,48]. The biota and soil samples were stored in a −20 °C freezer before preparation and analyses. The organic plant samples were first washed thoroughly with 18 mega Ohm water to remove any suspended materials on the plants’ surfaces. In addition, the samples were then soaked in a very dilute solution (0.001 M HCl) to ensure the removal of clay particles and any pollutants on the plants’ surfaces. The samples were then oven dried at 65 °C until the samples’ weight stabilized. The samples were prepared by weighing 2 g of dry mass into the digestion tube. Two mL of Hydrogen Peroxide (H2O2) and 5 mL of ultra-high purity nitric acid (HNO3) were added, and the solid plant and soil samples were gradually heated to 95 °C and digested for two hours. The digested samples were transferred into 50 mL volumetric flasks and brought to volume using 18 mega Ohm water. Three mL of HNO3 (reagent blank) was run with each batch of samples.
A PerkinElmer NexION 300D ICP–MS (Waltham, MA, USA) coupled with an ESI SeaFast SP3 auto-sampler were used to analyze the digested samples in both direct (Anhydrous Ammonia for trace metals) and hydride (Oxygen for Arsenic) modes to significantly minimize mass interferences. The instrument detection limits are as follows: As 0.3 μg/L, Cd 0.1 μg/L, Mo 0.02 μg/L, Pb, 0.008 μg/L, Se 1.3 μg/L, and U 0.008 μg/L.
For each sample, three replicates (including Certified Reference Materials (CRMs)) were measured. Certified National Institute of Standards and Technology (NIST) Standard Reference Materials were used and include: 2709 San Joaquin soil (NIST, Gaithersburg, MD, USA) and 1573a (tomato leaves, NIST Gaithersburg, MD, USA) and yielded the following values: for Cd 1.474 ± 0.11 mg/kg (1.52 ± 0.04 mg/kg (CRM tomato leaves)) and V 0.94 ± 0.07 mg/kg (0.84 ± 0.01 mg/kg (CRM tomato leaves)) and Cd 0.64 ± 0.09 mg/kg (0.37 ± 0.02 mg/kg (CRM soil value)) and V 83.2 ± 7.7 mg/kg (110 ± 11 mg/kg (CRM soil value)). The relative standard deviation was found to be within the range of 7.1–13.8%.
3.7. Provisionable Tolerable Weekly Intake (PTWI) Calculation Equation
The metal(loid) PTWI calculations were derived by utilizing this equation [61,68]:
PTWI = daily intake of metal(loid)s = ∑[concentration of metal(loid) in herb × mean of herbal intake (grams per person per day)];
weekly intake of metal(loid)s = daily intake × seven days a week;
weekly intake per body weight (kg) (PTWIs) = weekly intake or reference body weight (60 kg).
Consumption based on the number of grams per day that herbal medicines were consumed (5 g) based on comparable data.
weekly intake of metal(loid)s = daily intake × seven days a week;
weekly intake per body weight (kg) (PTWIs) = weekly intake or reference body weight (60 kg).
Consumption based on the number of grams per day that herbal medicines were consumed (5 g) based on comparable data.
3.8. Statistical Analysis
Statistical analysis was undertaken to utilize the Statistical Package for the Social Sciences (SPSS) for Windows (version 28, IBM, Armonk, NY, USA). Metal(loid) concentration levels in the medicinal plants and corresponding soil samples were reported as milligrams per kilogram (mg/kg). The summary data included means, standard deviations, medians, ranges, and percentages. The differences between the metal(loid) levels in the medicinal plant parts and soil were compared, with significance determined by Student’s t-tests. A p-value of <0.05 was considered significant. The absolute value of the t-statistic was reported along with the relevant means and the interpretation of the directions of differences.
4. Conclusions
The WHO RMPPLs were exceeded for As for five aboveground plant parts (A. hymenoides, A. purpurea, B. gracilis (including plant root), P. smithii, P. jamesii) and two plant roots for Cd (P. jamesii and A. Tridentate); however, when the PTWI were calculated using the study participant intake data, all plant concentrations fell below the level of concern for metal(loid)s that have established food intake guidelines. There are no established intake guidelines for Cs, U, and Th. The current data do not appear to demonstrate a risk of metal(loid) ingestion above the average ingestion intake in this study cohort for the eight species of medicinal plants examined. Further study is needed to address the study limitations and the identified research gaps. The limitations to be addressed include further characterizations of medicinal plant dosages, indications and administration routes, and the health effects on high-risk groups. Continued research, surveillance, and monitoring are needed in uranium mining-impacted communities.
Author Contributions
Conceptualization, C.S.-N.; methodology, C.S.-N. and A.-M.S.A.; validation, C.S.-N.; formal analysis, C.S.-N. and A.-M.S.A.; investigation, C.S.-N.; resources, C.S.-N. and A.-M.S.A.; data curation, C.S.-N.; writing—original draft preparation, C.S.-N.; writing—review and editing, C.S.-N.; A.-M.S.A.; supervision, C.S.-N.; project administration, C.S.-N.; funding acquisition, C.S.-N. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the National Institute of Nursing Research (NINR) of the National Institutes of Health (NIH) under Award Numbers F31NR013102 and NIH National Cancer Institute (NCI) K01CA249042. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by the University of California, Los Angeles (UCLA) National Institute for Occupational Safety and Health (NIOSH) 2 T42 OH 8412-8, the UCLA Institute of American Cultures Grant (IAC), and the 2011-13 Navajo Nation Grant.
Institutional Review Board Statement
This study was reviewed and approved by the dual Institutional Review Board (IRB)s, the Navajo Nation Human Research Review Board (NNHRRB) and the University of California, Los Angeles, (UCLA) IRB.
Informed Consent Statement
This study was reviewed and approved by the Navajo Nation Human Research Review Board and the UCLA IRB. Community and individual informed consent were obtained.
Data Availability Statement
Restrictions apply to the availability of these data. Data was obtained from The Navajo Nation and are available with the permission from The Navajo Nation Human Research Review Board.
Acknowledgments
We would like to acknowledge and thank the four participating Diné communities in New Mexico and the Navajo Nation Human Research Review Board (NNHRRB). We appreciate the assistance of the University of New Mexico (UNM) Community Environmental Health Program (CEHP); College of Pharmacy and Bob Sivinski of the UNM Herbarium; and the UNM Analytical Chemistry Laboratory Earth and Planetary Sciences Department staff.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Amiri, A.; Zhao, S. Environmental justice screening tools: Implications for nursing. Public Health Nurs. 2019, 36, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, F.M. Water (in)security and American Indian health: Social and environmental justice implications for policy, practice, and research. Public Health 2019, 176, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Trask, H. From a Native Daughter: Colonialism and Sovereignty in Hawaii; University of Hawaii Press: Honolulu, HI, USA, 1999; p. 44. [Google Scholar]
- Lewis, J.; Hoover, J.; MacKenzie, D. Mining and Environmental Health Disparities in Native American Communities. Curr. Environ. Health Rep. 2017, 4, 130–141. [Google Scholar] [CrossRef] [PubMed]
- McLemore, V.T. Uranium Industry in New Mexico—History, Production and Present Status; New Mexico Bureau of Geology & Mineral Resources: Socorro, NM, USA, 1983; Volume 5, pp. 45–51. [Google Scholar]
- Brugge, D.; Panikkar, B. The ethical issues in uranium mining research in the Navajo Nation. Acc. Res. 2007, 12, 121–153. [Google Scholar]
- Hoover, J.; Erdei, E.; Nash, J.; Gonzales, M. A review of metal exposure studies conducted in the rural southwestern and mountain region of the United States. Curr. Epidemiol. Rep. 2019, 6, 34–49. [Google Scholar] [CrossRef]
- Taylor, D.M.; Taylor, S.K. Environmental uranium and human health. Rev. Environ. Health 1997, 12, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Gilman, A.P.; Villenueve, D.C.; Secours, V.E.; Yagminas, A.P.; Tracy, B.L.; Quinn, J.M.; Valli, V.E.; Willes, R.J.; Moss, M.A. Uranyl nitrate: 28-day and 91-day toxicity studies in the Sprague-Dawley rat. Toxicol. Sci. 1998, 41, 117–128. [Google Scholar]
- Hoover, J.; Gonzales, M.; Shuey, C.; Barney, Y.; Lewis, J. Elevated arsenic and uranium contamination in unregulated water sources on the Navajo Nation, USA. Expo. Health 2017, 9, 113–124. [Google Scholar] [CrossRef]
- Eisler, R. Arsenic hazards to fish, wildlife, and invertebrates: A synoptic view. US Fish. Wildl. Serv. Biol. Rep. 1988, 85, 12. [Google Scholar]
- Fan, X.T. Effects of Soil Amendments on Growth, Metabolism and Cadmium Uptake by Panax Notoginseng; Beijing Normal University: Beijing, China, 2016. [Google Scholar]
- Han, R.; Zhou, B.; Huang, Y.; Lu, X.; Li, S.; Li, N. Bibliometric overview of research trends on heavy metal health risks and impacts in 1989–2018. J. Clean. Prod. 2020, 276, 123249. [Google Scholar] [CrossRef]
- Mikulski, M.A.; Wichman, M.D.; Simmons, D.L.; Pham, A.N.; Clottey, V.; Fuortes, L.J. Toxic metals in ayurvedic preparations from a public health lead poisoning cluster investigation. Int. J. Occup. Environ. Health 2017, 23, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Kumkrong, P.; LeBlanc, K.L.; Mercier, P.H.; Mester, Z. Selenium analysis in waters. Part 1: Regulations and standard methods. Sci. Total Environ. 2018, 640–641, 1611–1634. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Rossano, M.G.; Protas, B.; Diamond, M.P.; Puscheck, E.; Daly, D.; Paneth, N.; Wirth, J.J. Cadmium, lead, and other metals in relation to semen quality: Human evidence for molybdenum as a male reproductive toxicant. Environ. Health Perspect. 2008, 116, 1473–1479. [Google Scholar] [CrossRef]
- Meeker, J.D.; Rossano, M.G.; Protas, B.; Padmanahban, V.; Diamond, M.P.; Puscheck, E.; Daly, D.; Paneth, N.; Wirth, J.J. Environmental exposure to metals and male reproductive hormones: Circulating testosterone is inversely associated with blood molybdenum. Fertil. Steril. 2010, 93, 130–140. [Google Scholar] [CrossRef]
- Yang, F.; Yi, X.; Guo, J.; Shuaishuai, X.; Xiao, Y.; Huang, X.; Duan, Y.; Luo, D.; Xiao, S.; Huang, Z.; et al. Association of plasma and urine metals levels with kidney function: A population-based cross-sectional study in China. Chemosphere 2019, 226, 321–328. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC, 2012). Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, Metals, Fibres and Dusts. Lyon (FR): International Agency for Research on Cancer; 2012. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100C.). Available online: https://www.ncbi.nlm.nih.gov/books/NBK304375/ (accessed on 15 February 2022).
- Pyrzynska, K. Nanomaterials in speciation analysis of metals and metalloids. Talanta 2020, 212, 120784. [Google Scholar] [CrossRef]
- Yu, H.-S.; Liao, W.-T.; Chai, C.-Y. Arsenic carcinogenesis in the skin. J. Biomed. Sci. 2006, 13, 657–666. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC, 2006). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 87, Inorganic and Organic Lead Compounds. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Inorganic-And-Organic-Lead-Compounds-2006 (accessed on 15 February 2022).
- Charen, E.; Harbord, N. Toxicity of herbs, vitamins, and supplements. Adv. Chronic Kidney Dis. 2020, 27, 67–71. [Google Scholar] [CrossRef]
- Rahman, I.U.; Afzal, A.; Iqbal, Z.; Ijaz, F.; Ali, N.; Shah, M.; Ullah, S.; Bussmann, R.W. Historical perspectives o ethnobotany. Clin. Dermatol. 2019, 37, 382–388. [Google Scholar] [CrossRef]
- Dunmire, W.W.; Tierney, G.D. Wild Plants and Native Peoples of the Four Corners; Museum of New Mexico Press: Santa Fe, NM, USA, 1997; pp. 20, 44, 76, 126–129, 191–195. [Google Scholar]
- Hosen, N.; Nakamura, H.; Hamzah, A. Adaptation to climate change: Does traditional ecological knowledge hold the key? Sustainability 2020, 12, 676. [Google Scholar] [CrossRef]
- Tsuji, L.J.S.; Manson, H.; Wainman, B.C.; Vanspronsen, E.P.; Shecapio-Blacksmith, J.; Rabbitskin, T. Identifying potential receptors and routes of contaminant exposure in the traditional territory of the Ouje-Bougoumou Cree: Land use and a geographical information system. Environ. Monit. Assess. 2007, 127, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Mayes, V.O.; Lacy, B.B. Nanise’ A Navajo Herbal One Hundred Plants from the Navajo Reservation; Five Star Publications, Inc.: Chandler, AZ, USA, 2012; pp. 39, 45–46, 55, 102–103, 106–107, 128, 132. [Google Scholar]
- United States Department of Agriculture (USDA, 2000). Indian Ricegrass Plant Guide. Available online: https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_achy.pdf (accessed on 10 December 2021).
- Arkush, B.S.; Arkush, D. Aboriginal plant use in the central Rocky Mountains: Macrobotanical records from three prehistoric sites in Birch Creek Valley, eastern Idaho. N. Am. Archaeol. 2021, 42, 66–108. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA, 2020). Purple Threeawn Plant Guide. Available online: https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_arpu9.pdf (accessed on 11 December 2021).
- United States Department of Agriculture (USDA, 2002). Big Sagebrush Plant Fact Sheet. Available online: https://plants.usda.gov/DocumentLibrary/factsheet/pdf/fs_artr2.pdf (accessed on 10 December 2021).
- Shemluck, M. Medicinal and other uses of the compositae by Indians in the United States and Canada. J. Ethnopharmacol. 1982, 5, 303–358. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA, 2007). Blue Grama Plant Guide. Available online: https://plants.usda.gov/home/plantProfile?symbol=BOGR2 (accessed on 4 January 2022).
- United States Department of Agriculture (USDA, 2014). Plants. Juniperus monosperma (Englm). Sarg. General. Website: United States Department of Agriculture (USDA, 2014). Available online: https://www.fs.fed.us/database/feis/plants/tree/junmon/all.html (accessed on 18 December 2021).
- United States Department of Agriculture (USDA, 2000). Western Wheatgrass Plant Guide. Available online: https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_pasm.pdf (accessed on 4 January 2022).
- United States Department of Agriculture (USDA, 2012). James’ Galleta Plant Guide. Available online: https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_plja.pdf (accessed on 3 January 2022).
- Matthews, W. Navajo names for plants. Am. Nat. 1886, 20, 767–777. [Google Scholar] [CrossRef][Green Version]
- United States Department of Agriculture (USDA, 2010). Sand Dropseed Plant guide. Available online: https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_spcr.pdf (accessed on 5 January 2022).
- Hocking, G.M. Some plant materials used medicinally and otherwise by the Navaho Indians in the Chaco Canyon, New Mexico. El Palacio 1956, 63, 146–165. Available online: http://www.chacoarchive.org/docs/000846complete.pdf (accessed on 20 January 2022).
- Vestal, P.A. Ethnobotony of the Ramah Navajo. In Papers of the Peabody Museum of American Archeology and Ethnology; Harvard University: Cambridge, MA, USA, 1952; Volume 40. [Google Scholar]
- de Lemos, J.L.; Brugge, D.; Cajero, M.; Downs, M.; Durant, J.L.; George, C.M.; Henio-Adeky, S.; Nez, T.; Manning, T.; Rock, T.; et al. Development of risk maps to minimize uranium exposures in the Navajo Churchrock mining district. Environ. Health 2009, 8, 29. [Google Scholar] [CrossRef]
- Samuel-Nakamura, C.; Hodge, F.S. Occurrence and risk of meta(loid)s in Thelesperma megapotamicum. Plants 2019, 9, 21. [Google Scholar] [CrossRef]
- Fromberg, E.; Goble, R.; Sanchez, V.; Quigley, D. The assessment of radiation exposure in Native American communities from nuclear weapons testing in Nevada. Soc. Risk Analysis 2000, 20, 101–111. [Google Scholar] [CrossRef]
- Wolfley, J. Ecological risk assessment and management: Their failure to value Indigenous traditional ecological knowledge and protect tribal homelands. Am. Indian Cult. Res. J. 1998, 22, 151–169. [Google Scholar] [CrossRef]
- Harmon, M.E.; Lewis, J.; Miller, C.; Hoover, J.; Shuey, C.; Cajero, M.; Lucas, S.; Zychowski, K.; Pacheco, B.; Erdie, E.; et al. Residential proximity to abandoned uranium mines and serum inflammatory potential in chronically exposed Navajo communities. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 365–371. [Google Scholar] [CrossRef]
- World Health Organization. Quality Control Methods for Medicinal Plant Materials; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Samuel-Nakamura, C.; Hodge, F.S.; Sokolow, S.; Ali, A.S.; Robbins, W.A. Metal(loid)s in Cucurbita pepo in a Uranium Mining Impacted Area in Northwestern New Mexico, USA. Int. J. Environ. Res. Public Health 2019, 16, 2569. [Google Scholar] [CrossRef] [PubMed]
- Antal, D.; Dehelean, C.; Dobrea, C.; Manfred, A. Vanadium in medicinal plants: New data on the occurrence of an element both essential and toxic to plants and man. An. Univ. Din Oradea Fasc. Biol. 2009, 16, 5–10. [Google Scholar]
- Isa, P.A.; Hitler, L.; Joseph, I.A.; Oyetola, O. Evaluation of heavy metals concentrations in selected medicinal plants (Sterculia setigera Del. And Sclerocarya birrea (A. rich.) Hochst) collected from Bwabul Spring, Bambuka and Jalingo Low-lands, Traba State. J. Environ. Anal. Chem. 2017, 4, 1000225. [Google Scholar] [CrossRef]
- Kolachi, N.F.; Kazi, T.G.; Afridi, H.I.; Khan, S.; Wadhwa, S.K.; Shah, A.Q.; Shah, F.; Baig, J.A.; Sirajuddin. Determination of selenium content in aqueous extract of medicinal plants used as herbal supplement for cancer patients. Food. Chem. Toxicol. 2010, 48, 3327–3332. [Google Scholar] [CrossRef]
- Oprea, E.M.; Pintilie, V.; Bufnea, V.; Aprotosoaie, A.C.; Cioanca, O.; Trifan, A.; Hancianu, M. Radionuclides content in some medicinal plants commonly used in Romania. Farmacia 2014, 62, 658–663. [Google Scholar]
- Shi, Y.-z.; Ruan, J.-y.; Ma, L.-f.; Han, W.-y.; Wang, F. Accumulation and distribution of arsenic and cadmium by tea plants. J. Zhejiang Univ. Sci. B 2008, 9, 265–270. [Google Scholar] [CrossRef]
- Anke, M.; Seeber, O.; Muller, R.; Schafer, U.; Zerull, J. Uranium transfer in the food chain from soil to plants, animals, and man. Chemie Erde-Geochem. 2009, 69, 75–90. [Google Scholar] [CrossRef]
- Soudek, P.; Petrova, S.; Benesova, D.; Dvorakova, M.; Vanek, T. Uranium uptake by hydroponically cultivated crop plants. J. Environ. Radioact. 2011, 102, 598–604. [Google Scholar] [CrossRef]
- Dreesen, D.R.; Cokal, E.J. Plant uptake assay to determine bioavailability of inorganic contaminants. Water Air Soil Pollut. 1984, 22, 85–93. [Google Scholar] [CrossRef]
- deLemos, J.L.; Bostick, B.C.; Quicksall, A.N.; Landis, J.D.; George, C.C.; Slagowski, N.L.; Rock, T.; Brugge, D.; Lewis, J.; Durant, J.L. Rapid dissolution of soluble uranyl phases in arid, mine-impacted catchments near Church Rock, NM. Environ. Sci. Technol. 2008, 42, 3951–3957. [Google Scholar] [CrossRef]
- Wu, J.; Zou, Y.; Zhan, X.; Chen, S.; Lu, G.; Lai, F. Survey of heavy metal pollution in four Chinese crude drugs and their cultivated soils. Bull. Environ. Contam. Toxicol. 2008, 81, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Barthwal, J.; Nair, S.; Kakkar, P. Heavy metal accumulation in medicinal plants collected from environmentally different sites. Biomed. Environ. Sci. 2008, 21, 319–324. [Google Scholar] [CrossRef]
- Sarma, H.; Deka, S.; Deka, H.; Saikia, R.R. Accumulation of heavy metals in selected medicinal plants. Rev. Environ. Contam. Toxicol. 2011, 214, 63–86. [Google Scholar] [CrossRef] [PubMed]
- Arpadjan, S.; Celik, G.; Taskesen, S.; Gucer, S. Arsenic, cadmium and lead in medicinal herbs and their fractionation. Food Chem. Toxicol. 2008, 46, 2871–2875. [Google Scholar] [CrossRef]
- Naithani, V.; Kakkar, P. Effect of ecological variation on heavy metal content of some medicinal plants used as herbal tea ingredients in India. Bull. Environ. Contam. Toxicol. 2006, 76, 285–292. [Google Scholar] [CrossRef]
- Joint Expert Committee on Food Additives (JECFA). Evaluation of Certain Food Additives and Contaminants. In Thirty-Third Report of the Joint Food and Agriculture Organization (FAO)/World Health Organization (WHO) Expert Committee on Food Additives; WHO Technical Report Series No. 776; World Health Organization: Geneva, Switzerland, 1989. [Google Scholar]
- Joint Expert Committee on Food Additives (JECFA). Joint FAO/WHO Expert Committee on food additives. In Sixty-First Meeting: Summary and Conclusions; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids; The National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- World Health Organization. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Harmon, M.E.; Campen, M.J.; Miller, C.; Shuey, C.; Cajero, M.; Lucas, S.; Pacheco, B.; Erdei, E.; Ramone, S.; Nez, T.; et al. Associations of circulating oxidized LDL and conventional biomarkers of cardiovascular disease in a cross-sectional study of the Navajo population. PLoS ONE 2016, 11, e0143102. [Google Scholar] [CrossRef]
- Salahinejad, M.; Aflaki, F. Toxic and essential mineral elements content of black tea leaves and their tea infusions consumed in Iran. Biol. Trace Elem. Res. 2010, 134, 109–117. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, E.; He, B.; Jiang, G. Arsenic species and leaching characteristics in tea (Camellia sinensis). Food Chem. Toxicol. 2007, 45, 2381–2389. [Google Scholar] [CrossRef]
- Basgel, S.; Erdemoglu, S.B. Determination of mineral and trace elements in some medicinal herbs and their infusions consumed in Turkey. Sci. Total Environ. 2006, 359, 82–89. [Google Scholar] [CrossRef]
- Navajo Nation Division of Natural Resources (NNDNR). Navajo Endangered Species List; Navajo Nation Division of Natural Resources (NNDNR): Window Rock, AZ, USA, 2008.
- New Mexico Energy, Minerals, and Natural Resources Department (NMEMNRD). New Mexico Rare Plant. Conservation Strategy; Forestry Division, Ed.; New Mexico Energy, Minerals, and Natural Resources Department (NMEMNRD): Santa Fe, NM, USA, 2017.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).