Mummified Wood of Juniperus (Cupressaceae) from the Late Miocene of Taman Peninsula, South Russia
Abstract
:1. Introduction
2. Results
2.1. Systematic Description
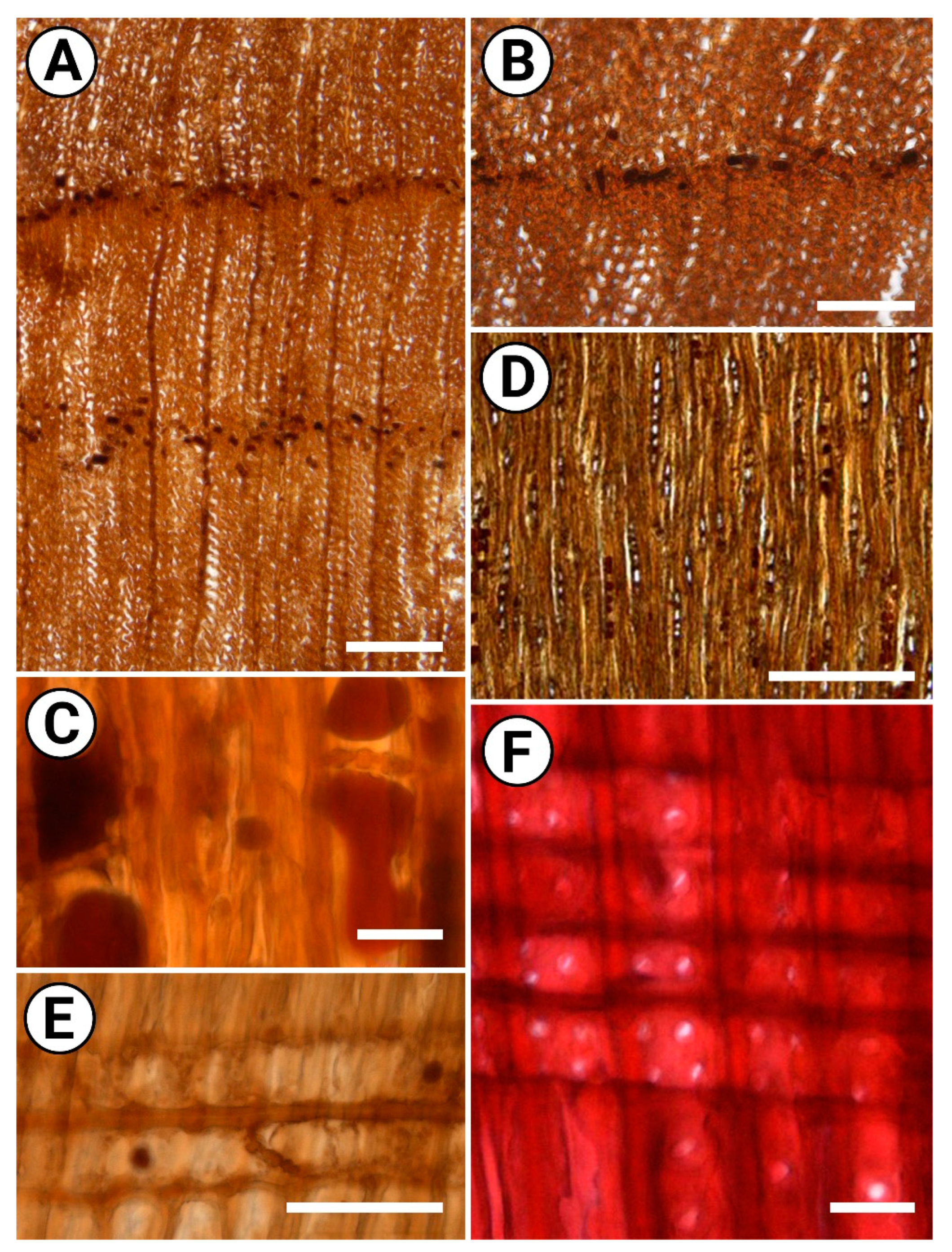
2.2. Description
2.3. Comparison with Modern Woods
2.4. Comparison with Fossil Woods
3. Discussion
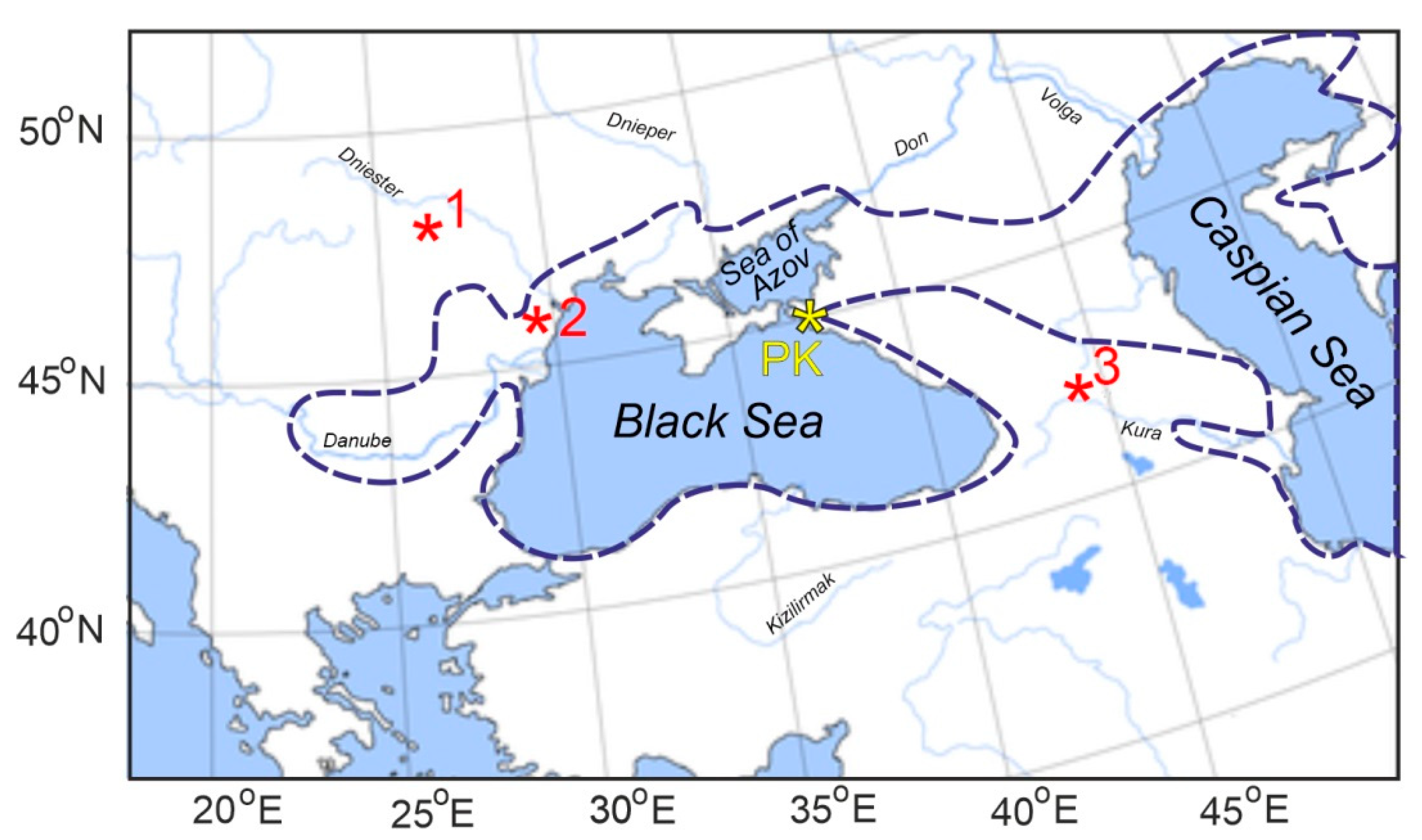
4. Materials and Methods
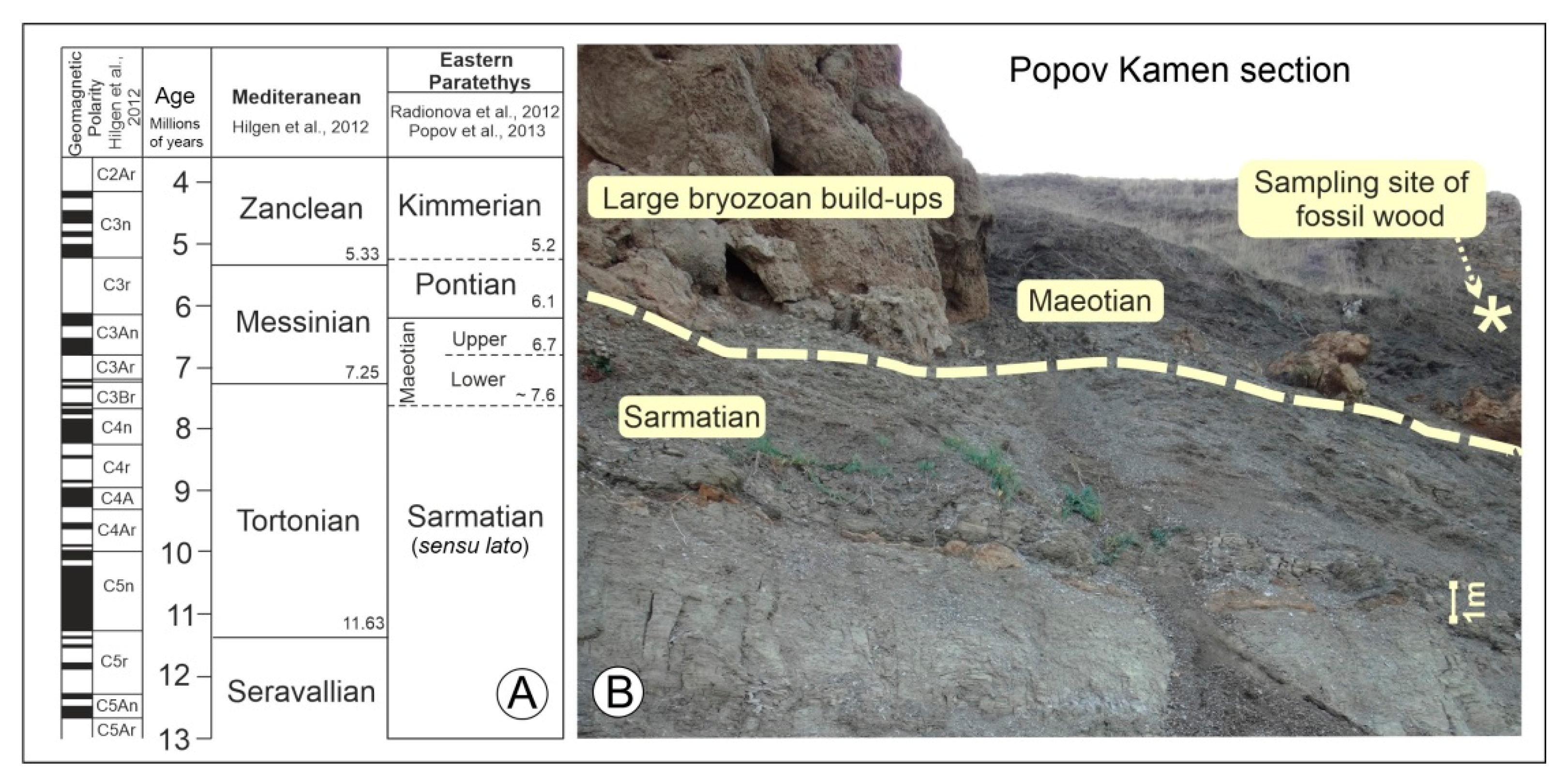
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, R.P. Junipers of the World: The Genus Juniperus, 4th ed.; Trafford Publishing: Victoria, Australia, 2014; pp. 1–422. [Google Scholar]
- Farjon, A.; Filer, D. An Atlas of the World’s Conifers: An Analysis of Their Distribution, Biogeography, Diversity and Conservation Status; Brill: Leiden, The Netherlands, 2013; pp. 1–524. [Google Scholar]
- Farjon, A. A Monograph of Cupressaceae and Sciadopitys; Royal Botanic Gardens: Kew, Australia, 2005; pp. 1–648. [Google Scholar]
- Mao, K.S.; Hao, G.; Liu, J.Q.; Adams, R.P.; Milne, R.I. Diversification and biogeography of Juniperus (Cupressaceae): Variable diversification rates and multiple intercontinental dispersals. New Phytol. 2010, 188, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Leslie, A.B.; Beaulieu, J.; Holman, G.; Campbell, C.S.; Mei, W.B.; Raubeson, L.R.; Mathews, S. An overview of extant conifer evolution from the perspective of the fossil record. Am. J. Bot. 2018, 105, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D.I. Evolution and biogeography of Madrean-Tethyan sclerophyll vegetation. Ann. Mo. Bot. Gard. 1975, 62, 280–334. [Google Scholar] [CrossRef]
- Kvaček, Z. A new juniper from the Palaeogene of Central Europe. Feddes Repert. 2002, 113, 492–502. [Google Scholar] [CrossRef]
- Axelrod, D.I. Mio-Pliocene Floras from West-Central Nevada; University California Press: Oakland, CA, USA, 1956; Volume 33, pp. 1–316. [Google Scholar]
- Axelrod, D.I. The Late Oligocene Creede Flora, Colorado; University California Press: Oakland, CA, USA, 1987; Volume 130, pp. 1–235. [Google Scholar]
- Axelrod, D.I. The Early Miocene Buffalo Canyon Flora of Western Nevada; University California Press: Oakland, CA, USA, 1991; Volume 135, pp. 1–76. [Google Scholar]
- Wolfe, J.A. Miocene Floras from Fingerrock Wash, Southwestern Nevada; US Government Printing Office: Washington, DC, USA, 1964; Volume 454–N, pp. 1–36.
- Palamarev, E.; Bozukov, V.; Uzunova, K.; Petkova, A.; Kitanov, G. Catalogue of the Cenozoic plants of Bulgaria (Eocene to Pliocene). Phytol. Balc. 2005, 11, 215–364. [Google Scholar]
- Dorofeev, P.I. On the Pliocene flora of Bashkiria. Botanicheskii Zhurnal 1962, 47, 787–801. (In Russian) [Google Scholar]
- Negru, A.G. Lower Sarmatian Flora of Northeastern Moldavia; Shtiintsa: Kishinev, Moldova, 1972; pp. 1–203. (In Russian) [Google Scholar]
- Palamarev, E. Paleobotanical evidences of the Tertiary history and origin of the Mediterranean sclerophyll dendroflora. Plant. Syst. Evol. 1989, 162, 93–107. [Google Scholar] [CrossRef]
- Kovar-Eder, J.; Kvaček, Z.; Martinetto, E.; Roiron, P. Late Miocene to Early Pliocene vegetation of southern Europe (7–4Ma) as reflected in the megafossil plant record. Palaeogeogr. Palaeoclim. Palaeoecol. 2006, 238, 321–339. [Google Scholar] [CrossRef]
- InsideWood. 2004–Onwards. Available online: http://insidewood.lib.ncsu.edu/ (accessed on 28 March 2022).
- Román-Jordán, E.; Esteban, L.G.; de Palacios, P.; Fernández, F.G. Comparative wood anatomy of the Cupressaceae and correspondence with phylogeny, with special reference to the monotypic taxa. Plant. Sys. Evol. 2017, 303, 203–219. [Google Scholar] [CrossRef]
- Esteban, L.G.; de Palacios, P.; Casasús, A.G.; Fernández, F.G. Characterisation of the xylem of 352 conifers. Investig. Agrar. Sist. Recur. For. 2004, 13, 152–178. [Google Scholar]
- Akkemik, Ü. A new species of Juniperoxylon from the Early Miocene of Northwestern Turkey. Acta Palaeontol. Pol. 2021, 17, 15–26. [Google Scholar] [CrossRef]
- Greguss, P. Xylotomische Bestimmung der heute Lebenden Gymnospermen; Akadémiai Kiadó: Budapest, Hungary, 1955; pp. 1–700. [Google Scholar]
- Philippe, M.; Bamford, M.K. A key to morphogenera used for Mesozoic conifer-like woods. Rev. Palaeobot. Palyno. 2008, 148, 184–207. [Google Scholar] [CrossRef]
- Ruiz, D.P.; Bodnar, J. The oldest record of Juniperoxylon, a cupressaceous fossil wood from the Middle Triassic of Argentina. Acta Palaeontol. Pol. 2019, 64, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Akkemik, Ü.; Arslan, M.; Poole, I.; Tosun, S.; Köse, N.; Karlıoğlu Kılıç, N.; Aydın, A. Silicified woods from two previously undescribed early Miocene forest sites near Seben, northwest Turkey. Rev. Palaeobot. Palynol. 2016, 235, 31–50. [Google Scholar] [CrossRef]
- Stopes, M.C. Catalogue of the Mesozoic Plants in the British Museum (Natural History); Part II: Lower Greensand (Aptian) Plants of Britain; Trustees of British Museum: London, UK, 1915; pp. 1–360. [Google Scholar]
- Kräusel, R. Die fossilen Koniferen-Hölzer (Unter Ausschluß von Araucarioxylon Kraus). II: Kritische Untersuchungen zur Diagnostik lebender und fossiler Koniferen-Hölzer. Palaeontogr. B 1949, 89, 83–203. [Google Scholar]
- Süss, H.; Rathner, U. Ein neues fossiles Holz, Juniperoxylon wagneri sp. nova, aus der miozänen Braunkohle von Wetro (Oberlausitdsachsen, Deutschland). Feddes Repert. 1998, 109, 15–24. [Google Scholar] [CrossRef]
- Bodnar, J.; Ruiz, D.P.; Artabe, A.E.; Morel, E.M.; Ganuza, D. Voltziales y Pinales (=Coniferales) de la Formación Cortaderita (Triásico Medio), Argentina, y su implicancia en la reconstrucción de las coníferas triásicas. Rev. Bras. Paleontol. 2015, 18, 141–160. [Google Scholar] [CrossRef]
- Watari, S.; Nishida, M. Juniperoxylon from the Tertiary of Hokkaido. J. Jpn. Bot. 1973, 48, 154–159. [Google Scholar]
- Van der Burgh, J. Hölzer der niederrheinischen Braunkohlen formation, 2. Hölzer der Braunkohlengruben “Maria Theresia” zu Herzogenrath, “Zukunft West” zu Eschweiler und “Victor” (Zülpich Mitte) zu Zülpich. Nebst einer systematisch– anatomischen Bearbeitung der Gattung pinus L. Rev. Palaeobot. Palynol. 1973, 15, 73–275. [Google Scholar]
- Dolezych, M. A remarkable extinct wood from Lusatia (central Europe)—Juniperoxylon schneiderianum sp. nov. with affinity to Cupressospermum saxonicum Mai. Palaeontographica B 2016, 295, 5–31. [Google Scholar] [CrossRef]
- Acarca Bayam, N.N.; Akkemik, Ü.; Poole, I.; Akarsu, F. Further Contributions to the early Miocene forest vegetation of the Galatean Volcanic Province, Turkey. Palaeobot. Electron. 2018, 21, 1–42. [Google Scholar]
- Shchekina, N.A. History of Flora and Vegetation of the Southern European Part of the USSR in the Late Miocene and Early Pliocene; Naukova Dumka: Kyiv, Ukraine, 1979; pp. 1–198. (In Russian) [Google Scholar]
- Shatilova, I.; Maissuradze, L.; Koiava, K.; Mchedlishvili, N.; Rukhadze, L.; Spezzaferri, S.; Strasser, A. Foraminifers and palynomorphs in the Sarmatian deposits of Kartli (Eastern Georgia): Stratigraphical and palaeoclimatological implications. Proc. Georgian Acad. Sci. Biol. Ser. B 2008, 6, 65–76. [Google Scholar]
- Philippova, N.J. Spores, pollen and organic-walled phytoplankton from Neogene deposits of the Zheleznyi Rog Key Section (Taman Peninsula). Stratigr. Geol. Correl. 2002, 10, 80–92. (In Russian) [Google Scholar]
- Razumkova, E.S. Palynological record of the Sarmatian deposits of the Eastern Paratethys (Section Zelenskii Mountain-Cape Panagiya, Taman Peninsula). Stratigr. Geol. Correl. 2012, 20, 108–119. (In Russian) [Google Scholar] [CrossRef]
- Ananova, E.N. Pollen in the Neogene Deposits of the Southern Part of Russian Plain; Leningrad State University: Leningrad, Russia, 1974; pp. 1–196. (In Russian) [Google Scholar]
- Ivanov, D. Climate and vegetation change during the late Miocene in southwest Bulgaria based on pollen data from the Sandanski Basin. Rev. Palaeobot. Palynol. 2015, 221, 128–137. [Google Scholar] [CrossRef]
- Shatilova, I.I.; Maissuradze, L.S.; Kokolashvili, I.M.; Bruch, A.A. The palaeobiological basis of the stratigraphcal subdivision of Meotian deposits of Abkhazia. Bull. Georgian Natl. Acad. Sci. 2019, 13, 118–125. [Google Scholar]
- Shatilova, I.I.; Maissuradze, L.S.; Koiava, K.P.; Kokolashvili, I.M.; Bukhsianidze, M.G.; Bruch, A.A. The Environmental History of Georgia During the Late Miocene Based of Foraminifera and Pollen; Georgian National Museum: Tbilisi, Georgia, 2020; pp. 1–84. [Google Scholar]
- Kutuzkina, E.F. The Sarmatian flora of Armavir. Paleobotanika 1964, 5, 148–229. (In Russian) [Google Scholar]
- Uznadze, M.D. Neogene Flora of Georgia; Metsniereba: Tbilisi, Georgia, 1965; pp. 1–180. (In Russian) [Google Scholar]
- Uznadze, M.D.; Tsagareli, E.A. The Sarmatian Flora of the Dzindza River Ravine (Goderdzi Flora); Metsniereba: Tbilisi, Georgia, 1979; pp. 1–165. (In Russian) [Google Scholar]
- Murphy, E.; Nistor, I.; Cornett, A.; Wilson, J.; Pilechi, A. Fate and transport of coastal driftwood: A critical review. Mar. Pollut. Bull. 2021, 170, 112649. [Google Scholar] [CrossRef]
- Andrusov, N. Geological researches at the Taman Peninsula. Mater. Geol. Russ. 1903, 21, 257–383. (In Russian) [Google Scholar]
- Popov, S.V.; Zastrozhnov, A. Neogene Key Sections of the Eastern Paratethys (Taman Peninsula); Tour Guide: Volgograd-Taman, Russia, 1998; pp. 1–20. (In Russian) [Google Scholar]
- Rostovtseva, Y.V.; Goncharova, I. The structure of relatively deep-water sediments of the Lower Maeotian Black Sea region (Taman Peninsula: Section Popov Kamen), Biostratigraphic basis for the development of stratigraphic patterns in Ukraine. Proc. Inst. Geol. Sci. NAS Ukr. 2008, 4, 270–275. (In Russian) [Google Scholar]
- Rostovtseva, Y.V. Lower Maeotian facies of the Taman trough. Lithol. Miner. Resour. 2009, 44, 451–464. [Google Scholar] [CrossRef]
- Radionova, E.P.; Golovina, L.A. Upper Maeotian–Lower Pontian “Transitional Strata” in the Taman Peninsula: Stratigraphic position and paleogeographic interpretations. Geol. Carpath. 2011, 2, 62–100. [Google Scholar] [CrossRef] [Green Version]
- Trubikhin, V.M.; Pilipenko, O.V. Rock magnetism and paleomagnetism of Maeotian deposits of the Popov Kamen Reference Section (Taman Peninsula). Izvestiya. Phys. Solid Earth 2011, 47, 233–245. (In Russian) [Google Scholar] [CrossRef]
- Radionova, E.P.; Golovina, L.A.; Filippova, N.Y.; Trubikhin, V.M.; Popov, S.V.; Goncharova, I.A.; Vernigorova, Y.V.; Pinchuk, T.N. Middle–Upper Miocene stratigraphy of the Taman Peninsula, Eastern Paratethys. Cent. Eur. J. Geosci. 2012, 4, 188–204. [Google Scholar] [CrossRef]
- Popov, S.V.; Akhmetiev, M.A.; Golovina, L.A.; Goncharova, I.A.; Radionova, E.P.; Filippova, N.Y.; Trubichin, V.M. Neogene regiostage stratigraphic scale of the South Russia: Current state and perspectives. In General Stratigraphic Scale of Russia: Current State and Ways of Perfection; Fedonkin, M.A., Ed.; Geological Institute of RAS: Moscow, Russia, 2013; pp. 356–360. (In Russian) [Google Scholar]
- Iljina, L.B.; Nevesskaya, L.A.; Paramonova, N.P. Patterns of the Mollusks Development in Brackish Sea Basins of Eurasia; Nedra: Moscow, Russia, 1976; pp. 1–288. (In Russian) [Google Scholar]
- Hilgen, F.J.; Lourens, L.J.; Van Dam, J.A. The Neogene Period. In The Geological Time Scale 2012; Gradstein, F., Ogg, J., Schmitz, M., Ogg, G., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 923–979. [Google Scholar]
- Popov, S.V.; Rostovtseve, Y.V.; Fillippova, N.Y.; Golovina, L.A.; Radionova, E.P.; Vernyhorvoa, Y.V.; Dykan, N.I.; Pinchuk, T.N.; Iljina, L.B.; Koromyslova, A.V.; et al. Paleontology and stratigraphy of the Middle-Upper Miocene of the Taman Peninsula: Part 1. Description of key sections and benthic fossil groups. Paleontol. J. 2016, 50, 1039–1206. [Google Scholar] [CrossRef]
- Jansen, S.; Choat, B.; Vinckier, S.; Lens, F.; Schols, P.; Smets, E. Intervascular pit membranes with a torus in the wood of Ulmus (Ulmaceae) and related genera. New Phytol. 2004, 163, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IAWA Committee. IAWA List of microscopic features for softwood identification. IAWA J. 2004, 25, 1–70. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, A.V.; Odintsova, A.A.; Rybkina, A.I.; Rostovtseva, Y.V.; Oskolski, A.A. Mummified Wood of Juniperus (Cupressaceae) from the Late Miocene of Taman Peninsula, South Russia. Plants 2022, 11, 2050. https://doi.org/10.3390/plants11152050
Stepanova AV, Odintsova AA, Rybkina AI, Rostovtseva YV, Oskolski AA. Mummified Wood of Juniperus (Cupressaceae) from the Late Miocene of Taman Peninsula, South Russia. Plants. 2022; 11(15):2050. https://doi.org/10.3390/plants11152050
Chicago/Turabian StyleStepanova, Anna V., Anastasia A. Odintsova, Alena I. Rybkina, Yuliana V. Rostovtseva, and Alexei A. Oskolski. 2022. "Mummified Wood of Juniperus (Cupressaceae) from the Late Miocene of Taman Peninsula, South Russia" Plants 11, no. 15: 2050. https://doi.org/10.3390/plants11152050
APA StyleStepanova, A. V., Odintsova, A. A., Rybkina, A. I., Rostovtseva, Y. V., & Oskolski, A. A. (2022). Mummified Wood of Juniperus (Cupressaceae) from the Late Miocene of Taman Peninsula, South Russia. Plants, 11(15), 2050. https://doi.org/10.3390/plants11152050





