Application of Silica Nanoparticles in Combination with Two Bacterial Strains Improves the Growth, Antioxidant Capacity and Production of Barley Irrigated with Saline Water in Salt-Affected Soil
Abstract
:1. Introduction
2. Results
2.1. Response of Soil Chemical Properties to the Application of PGPR and SiNPs
2.2. Soil Microbiota and Enzyme Activities
2.3. Physiological Responses of Barley Plants Irrigated with Saline Water to PGPR and SiNP Application
2.3.1. Relative Water Content
2.3.2. Na+, K+, and K+/Na+ in Barley Leaves
2.3.3. Hydrogen Peroxide and Lipid Peroxidation
2.3.4. Relative Chlorophyll Content (SPAD) and Stomatal Conductance
2.3.5. Electrolyte Leakage (EL) and Proline Content
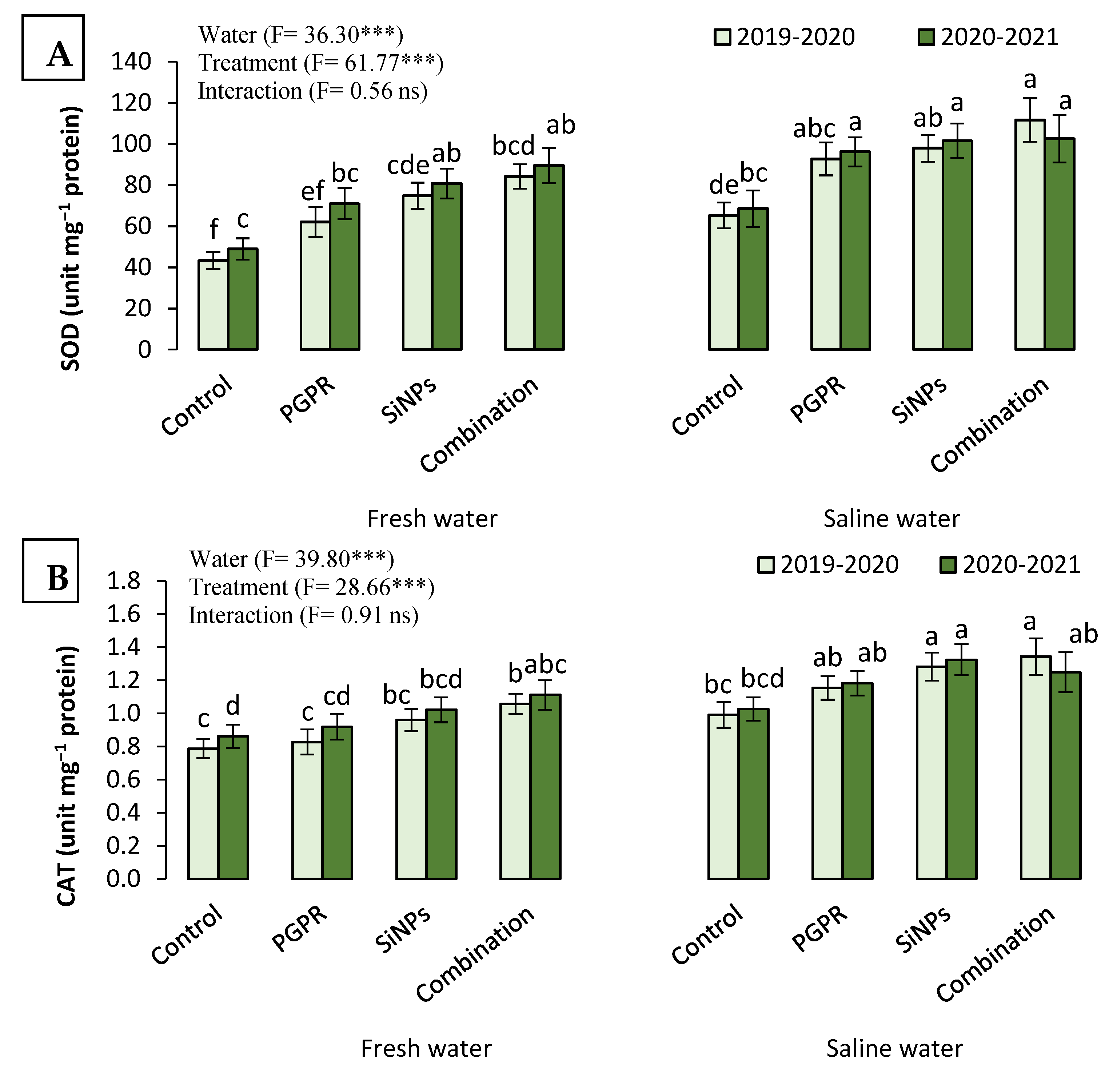
2.3.6. The Antioxidant Capacity of Barley Plants Irrigated with Saline Water in the Presence of PGPR and SiNPs
2.4. Productivity of Barley Watered with Saline Water in the Presence of PGPR and SiNPs
2.4.1. Yield and Yield-Related Traits of Barley
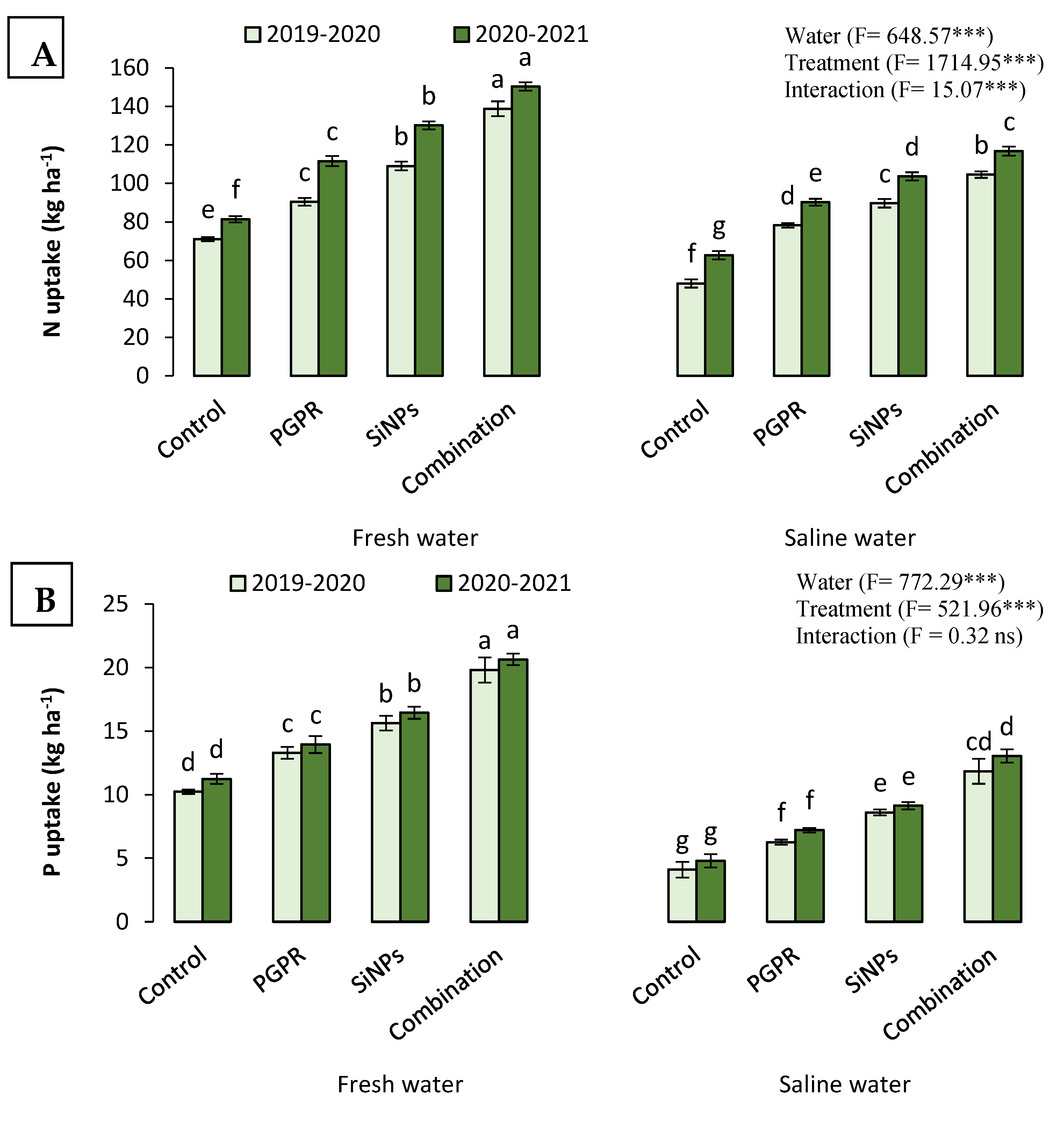
2.4.2. N, P, and K Uptake
3. Discussion
4. Materials and Methods
4.1. Experimental Layout and Growth Conditions
4.2. Measurements
4.2.1. Soil Analysis
4.2.2. Microbiological Analysis
4.2.3. Determination of Soil Enzymes Activity
4.3. Physiological Characteristics of Barley Plants
4.3.1. Relative Water Content (RWC)
4.3.2. Na+ and K+ Ions in Leaves
4.4. Analysis of Oxidative Stress Indicators
4.4.1. Hydrogen Peroxide
4.4.2. Lipid Peroxidation
4.5. Chlorophyll Content
4.6. Stomatal Conductance
4.7. Electrolyte Leakage
4.8. Proline Content
4.9. Antioxidant System
4.10. Crop Yield
4.11. Nutrient Uptake
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAOSTAT. Food and Agriculture Organization of the United Nations. 2021. Available online: https://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 13 February 2020).
- Ko, J.; Jeong, S.; Kim, J.; Lee, B.; Kim, H. Impacts of regional climate change on barley yield and its geographical variation in South Korea. Int. Agrophysics 2019, 33, 81–96. [Google Scholar] [CrossRef]
- Thalooth, T.A.; Bahr, A.; Tawfik, M.M. Productivity of some barley cultivars as affected by inoculation under water stress conditions. Elixir Appl. Bot. 2012, 51, 10743–10749. [Google Scholar]
- Hafez, E.M.; Abou El-Hassan, W.H. Nitrogen and water utilization efficiency of barley subjected to desiccated conditions in moderately salt-affected soil. Egypt. J. Agron. 2015, 37, 231–249. [Google Scholar] [CrossRef]
- Hafez, E.M.; Seleiman, M.F. Response of barley quality traits, yield and antioxidant enzymes to water-stress and chemical inducers. Int. J. Plant Prod. 2017, 11, 477–490. [Google Scholar]
- Ullah, N.; Ditta, A.; Khalid, A.; Mehmood, S.; Rizwan, M.S.; Ashraf, M.; Mubeen, F.; Imtiaz, M.; Iqbal, M.M. Integrated Effect of Algal Biochar and Plant Growth Promoting Rhizobacteria on Physiology and Growth of Maize Under Deficit Irrigations. J. Soil Sci. Plant Nutr. 2019, 20, 346–356. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Climate Change and Salinity Effects on Crops and Chemical Communication between Plants and Plant Growth-Promoting Microorganisms under Stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Hafez, E.M.; Omara, A.E.D.; Alhumaydhi, F.A.; El-Esawi, M.A. Minimizing hazard impacts of soil salinity and water stress on wheat plants by soil application of vermicompost and biochar. Physiol. Plant. 2021, 172, 587–602. [Google Scholar] [CrossRef]
- Hafez, E.M.; El Hassan, W.H.A.; Gaafar, I.A.; Seleiman, M.F. Effect of Gypsum Application and Irrigation Intervals on Clay Saline-Sodic Soil Characterization, Rice Water Use Efficiency, Growth, and Yield. J. Agric. Sci. 2015, 7, 208. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Kheir, A.M.S.; Ali, O.A.M.; Hafez, E.M.; ElShamey, E.A.; Zhou, Z.; Wang, B.; Lin, X.; Ge, Y.; Fahmy, A.E.; et al. A vermicompost and deep tillage system to improve saline-sodic soil quality and wheat productivity. J. Environ. Manage 2021, 277, 111388. [Google Scholar] [CrossRef]
- Hafez, E.M.; Kheir, A.M.S.; Badawy, S.A.; Rashwan, E.; Farig, M.; Osman, H.S. Differences in Physiological and Biochemical Attributes of Wheat in Response to Single and Combined Salicylic Acid and Biochar Subjected to Limited Water Irrigation in Saline Sodic Soil. Plants 2020, 9, 1346. [Google Scholar] [CrossRef]
- Niranjana, S.R.; Hariprasad, P. Understanding the Mechanism Involved in PGPR-Mediated Growth Promotion and Suppression of Biotic and Abiotic Stress in Plants. In Future Challenges in Crop Protection Against Fungal Pathogens; Springer: Berlin/Heidelberg, Germany, 2014; pp. 59–108. [Google Scholar] [CrossRef]
- Omara, A.E.D.; Hafez, E.M.; Osman, H.S.; Rashwan, E.; El-Said, M.A.; Alharbi, K.; Abd El-Moneim, D.; Gowayed, S.M. Collaborative Impact of Compost and Beneficial Rhizobacteria on Soil Properties, Physiological Attributes, and Productivity of Wheat Subjected to Deficit Irrigation in Salt Affected Soil. Plants 2022, 11, 877. [Google Scholar] [CrossRef] [PubMed]
- Osman, H.S.; Gowayed, S.M.; Elbagory, M.; Omara, A.E.; Abd El-Monem, A.M.; Abd El-Razek, U.A.; Hafez, E.M. Interactive impacts of beneficial microbes and Si-Zn nano-composite on growth and productivity of soybean subjected to water deficit under salt-affected soil conditions. Plants 2021, 10, 1396. [Google Scholar] [CrossRef]
- Nehela, Y.; Mazrou, Y.S.; Alshaal, T.; Rady, A.M.; El-Sherif, A.M.; Omara, A.E.D.; Abd El-Monem, A.M.; Hafez, E.M. The Integrated Amendment of Sodic-Saline Soils Using Biochar and Plant Growth-Promoting Rhizobacteria Enhances Maize (Zea mays L.) Resilience to Water Salinity. Plants 2021, 10, 1960. [Google Scholar] [CrossRef] [PubMed]
- Hafez, E.M.; Osman, H.S.; Gowayed, S.M.; Okasha, S.A.; Omara, A.E.; Sami, R.; Abd El-Monem, A.M.; Abd El-Razek, U.A. Minimizing the adversely impacts of water deficit and soil salinity on maize growth and productivity in response to the application of plant growth-promoting rhizobacteria and silica nanoparticles. Agronomy 2021, 11, 676. [Google Scholar] [CrossRef]
- Hafez, E.M.; Osman, H.S.; El-Razek, U.A.; Elbagory, M.; Omara, A.E.; Eid, M.A.; Gowayed, S.M. Foliar-applied potassium silicate coupled with plant growth-promoting rhizobacteria improves growth, physiology, nutrient uptake and productivity of faba bean (Vicia faba L.) irrigated with saline water in salt-affected soil. Plants 2021, 10, 894. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Qin, H.; Ding, H.; Li, Y.; Guo, T. Silicon Application Alleviates Drought Stress in Wheat Through Transcriptional Regulation of Multiple Antioxidant Defense Pathways. J. Plant Growth Regul. 2016, 35, 1–10. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Ashraf, M. Influence of foliar application of silicon on chlorophyll fluorescence, photosynthetic pigments, and growth in water-stressed wheat cultivars differing in drought tolerance. Turk. J. Bot. 2015, 39, 625–634. [Google Scholar] [CrossRef]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawany, M.; Elhawat, N.; Al-Otaibi, A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef]
- Li, B.; Tao, G.; Xie, Y.; Cai, X. Physiological effects under the condition of spraying nano-SiO2 onto the Indocalamus barbatus McClure leaves. J Nanjing Forest Univ. 2012, 4, 161–164. [Google Scholar]
- Esmaili, S.; Tavallali, V.; Amiri, B. Nano-Silicon Complexes Enhance Growth, Yield, Water Relations and Mineral Composition in Tanacetum parthenium under Water Deficit Stress. Silicon 2020, 13, 2493–2508. [Google Scholar] [CrossRef]
- El-Shamy, M.A.; Alshaal, T.; Mohamed, H.H.; Rady, A.M.; Hafez, E.M.; Alsohim, A.S.; Abd El-Moneim, D. Quinoa Response to Application of Phosphogypsum and Plant Growth-Promoting Rhizobacteria under Water Stress Associated with Salt-Affected Soil. Plants 2022, 11, 872. [Google Scholar] [CrossRef]
- Hafez, E.M.; Alsohim, A.S.; Farig, M.; Omara, A.-D.; Rashwan, E.; Kamara, M.M. Synergistic Effect of Biochar and Plant Growth Promoting Rhizobacteria on Alleviation of Water Deficit in Rice Plants under Salt-Affected Soil. Agronomy 2019, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Diaz, R.M.; Lobo, P.A.E.; Rigobelo, E.C. Use of plant growth-promoting rhizobacteria in maize and sugarcane: Characteristics and applications. Front. Sustain. Food Syst. 2020, 4, 136. [Google Scholar] [CrossRef]
- Abou El Hassan, W.H.; Hafez, E.M.; Ghareib, A.A.A.; Ragab, M.F.; Seleiman, M.F. Impact of nitrogen fertilization and irrigation on N accumulation, growth and yields of Zea mays L. J. Food Agric. Environ. 2014, 12, 217–222. [Google Scholar]
- Kamara, M.M.; Rehan, M.; Ibrahim, K.M.; Alsohim, A.S.; Elsharkawy, M.M.; Kheir, A.M.S.; Hafez, E.M.; El-Esawi, M.A. Genetic Diversity and Combining Ability of White Maize Inbred Lines under Different Plant Densities. Plants 2020, 9, 1140. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Hafez, E.M. Optimizing Inputs Management for Sustainable Agricultural Development. In Mitigating Environmental Stresses for Agricultural Sustainability in Egypt; Awaad, H., Abu-hashim, M., Negm, A., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- El-Saadony, F.M.A.; Mazrou, Y.S.A.; Khalaf, A.E.A.; El-Sherif, A.M.A.; Osman, H.S.; Hafez, E.M.; Eid, M.A.M. Utilization Efficiency of Growth Regulators in Wheat under Drought Stress and Sandy Soil Conditions. Agronomy 2021, 11, 1760. [Google Scholar] [CrossRef]
- Gupta, A.; Vandana, P. Effect of PGPR isolates on plant growth promotion in relation to salinity stress. Bull. Environ. Pharmacol. Life Sci. 2019, 8, 18–26. [Google Scholar]
- Ilangumaran, G.; Smith, D.L. Plant Growth Promoting Rhizobacteria in Amelioration of Salinity Stress: A Systems Biology Perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture - A review. Agron. Sustain. Dev. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Abdelrasheed, K.G.; Mazrou, Y.; Omara, A.E.-D.; Osman, H.S.; Nehela, Y.; Hafez, E.M.; Rady, A.M.S.; El-Moneim, D.A.; Alowaiesh, B.F.; Gowayed, S.M. Soil Amendment Using Biochar and Application of K-Humate Enhance the Growth, Productivity, and Nutritional Value of Onion (Allium cepa L.) under Deficit Irrigation Conditions. Plants 2021, 10, 2598. [Google Scholar] [CrossRef] [PubMed]
- Rangaraj, S.; Gopalu, K.; Rathinam, Y.; Periasamy, P.; Venkatachalam, R.; Narayanasamy, K. Effect of silica nanoparticles on microbial biomass and silica availability in maize rhizosphere. Biotechnol. Appl. Biochem. 2014, 61, 668–675. [Google Scholar] [CrossRef]
- Akhayere, E.; Kavaz, D.; Vaseashta, A. Synthesizing Nano Silica Nanoparticles from Barley Grain Waste: Effect of Temperatureon Mechanical Properties. Pol. J. Environ. Stud. 2019, 28, 2513–2521. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Naveed, M.; Zahir, Z.A.; Liu, F.; Akhtar, S.S.; Andersen, M.N.; Naveed, M.; Zahir, Z.A.; Liu, F. Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Funct. Plant Biol. 2015, 42, 770–781. [Google Scholar] [CrossRef]
- Osman, H.S.; Rady, A.; Awadalla, A.; Omara, A.E.D.; Hafez, E.M. Improving the Antioxidants System, Growth, and Sugar Beet Quality Subjected to Long-Term Osmotic Stress by Phosphate Solubilizing Bacteria and Compost Tea. Int. J. Plant Prod. 2021, 16, 119–135. [Google Scholar] [CrossRef]
- Kamara, M.M.; Rehan, M.; Mohamed, A.M.; El Mantawy, R.F.; Kheir, A.M.S.; Abd El-Moneim, D.; Safhi, F.A.; ALshamrani, S.M.; Hafez, E.M.; Behiry, S.I. Genetic Potential and Inheritance Patterns of Physiological, Agronomic and Quality Traits in Bread Wheat under Normal and Water Deficit Conditions. Plants 2022, 11, 952. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Refay, Y.; Al-Suhaibani, N.; Al-Ashkar, I.; El-Hendawy, S.; Hafez, E.M. Integrative Effects of Rice-Straw Biochar and Silicon on Oil and Seed Quality, Yield and Physiological Traits of Helianthus annuus L. Grown under Water Deficit Stress. Agronomy 2019, 9, 637. [Google Scholar] [CrossRef] [Green Version]
- Gharib, H.; Hafez, E.; El-Sabagh, A. Optimized potential of utilization efficiency and productivity in wheat by integrated chemical nitrogen fertilization and simulative compounds. Cercet. Agron. Mold. 2016, 49, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Li, Y.; Zhang, Y.; Bi, Y.; Sun, Z. Responses of saline soil properties and cotton growth to different organic amendments. Pedosphere 2018, 28, 521–529. [Google Scholar] [CrossRef]
- .Hafez, E.M.; Gowayed, S.M.; Nehela, Y.; Sakran, R.M.; Rady, A.M.S.; Awadalla, A.; Omara, A.E.-D.; Alowaiesh, B.F. Incorporated Biochar-Based Soil Amendment and Exogenous Glycine Betaine Foliar Application Ameliorate Rice (Oryza sativa L.) Tolerance and Resilience to Osmotic Stress. Plants 2021, 10, 1930. [Google Scholar] [CrossRef] [PubMed]
- Kheir, A.M.S.; Abouelsoud, H.M.; Hafez, E.M.; Ali, O.A.M. Integrated effect of nano-Zn, nano-Si, and drainage using crop straw–filled ditches on saline sodic soil properties and rice productivity. Arab. J. Geosci. 2019, 12, 471. [Google Scholar] [CrossRef]
- Ashour, M.M.; Soliman, I.; Mabrouk, M.; Behere, H.H.; Tohamy, K. Silica nanoparticles as a potential carrier for doxycycline hyclate. Egy. J. Biomed. Engineer. Biophys. 2020, 21, 33–41. [Google Scholar] [CrossRef]
- Ullah, S.; Bano, A. Isolation of plant-growth-promoting rhizobacteria from rhizospheric soil of halophytes and their impact on maize (Zea mays L.) under induced soil salinity. Can. J. Microbiol. 2015, 61, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Chouhan, R.; Bakshi, P.; Gandhi, S.G.; Kaur, R.; Sharma, A.; Bhardwaj, R. Amelioration of Chromium-Induced Oxidative Stress by Combined Treatment of Selected Plant-Growth-Promoting Rhizobacteria and Earthworms via Modulating the Expression of Genes Related to Reactive Oxygen Species Metabolism in Brassica juncea. Front. Microbiol. 2022, 13, 802512. [Google Scholar] [CrossRef]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawani, M.; Elhawat, N.; Al-Otaibi, A. Exogenous nanosilica improves germination and growth of cucumber by maintaining K+/Na+ ratio under elevated Na+ stress. Plant Physiol. Biochem. 2018, 125, 164–171. [Google Scholar] [CrossRef]
- Habib, S.H.; Kausar, H.; Saud, H.M. Plant Growth-Promoting Rhizobacteria Enhance Salinity Stress Tolerance in Okra through ROS-Scavenging Enzymes. Biomed Res. Int. 2016, 2016, 6284547. [Google Scholar] [CrossRef] [Green Version]
- Sushanto, G.; Rout, G.K.; Gitishree, D.; Spiros, P.; Han-Seung, S.; Jayanta, K.P.; Yang, A.Z.; Akhtar, S.S.; Iqbal, S.; Amjad, M.; et al. Enhancing salt tolerance in quinoa by halotolerant bacterial inoculation. Funct. Plant Biol. 2016, 43, 632–642. [Google Scholar]
- Saravanakumar, D.; Samiyappan, R. ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J Appl Microbiol. 2007, 102, 1283–1292. [Google Scholar] [CrossRef]
- Al-Enazy, A.A.; Al-Barakah, F.; Al-Oud, S.; Usman, A. Effect of phosphogypsum application and bacteria co-inoculation on biochemical properties and nutrient availability to maize plants in a saline soil. Arch. Agron. Soil Sci. 2018, 64, 1394–1406. [Google Scholar] [CrossRef]
- Ayuso-Calles, M.; Flores-Félix, J.D.; Rivas, R. Overview of the Role of Rhizobacteria in Plant Salt Stress Tolerance. Agronomy 2021, 11, 1759. [Google Scholar] [CrossRef]
- Ismail, L.M.; Soliman, M.I.; Abd El-Aziz, M.H.; Abdel-Aziz, H.M.M. Impact of Silica Ions and Nano Silica on Growth and Productivity of Pea Plants under Salinity Stress. Plants 2022, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.F.; Ajaj, R.; Gaballah, M.S.; Ogbaga, C.C.; Kalaji, H.M.; Hatterman-Valenti, H.M.; Alam-Eldein, S.M. Foliar Application of Nano-Silicon Improves the Physiological and Biochemical Characteristics of ‘Kalamata’ Olive Subjected to Deficit Irrigation in a Semi-Arid Climate. Plants 2022, 11, 1561. [Google Scholar] [CrossRef] [PubMed]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef]
- Hafez, E.; Omara, A.E.D.; Ahmed, A. The Coupling Effects of Plant Growth Promoting Rhizobacteria and Salicylic Acid on Physiological Modifications, Yield Traits, and Productivity of Wheat under Water Deficient Conditions. Agronomy 2019, 9, 524. [Google Scholar] [CrossRef] [Green Version]
- Seilsepour, M.; Rashidi, M.; Khabbaz, B.G. Prediction of soil exchangeable sodium percentage based on soil sodium adsorption ratio. Am. J. Agric. Environ. Sci. 2009, 5, 1–4. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils (Agriculture Handbook No. 60); Richards, L.A., Ed.; United States Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Cochran, W.G. Estimation of Bacterial Densities by Means of the “Most Probable Number. Biometrics 1950, 6, 105. [Google Scholar] [CrossRef]
- Casida, L.E.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Urease activity. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1998; pp. 316–320. [Google Scholar]
- Mersi, V.W. Enzymes Involved in Intracellular Metabolism: Dehydrogenase Activity with the Substrate INT. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 243–245. [Google Scholar]
- Barrs, H.; Weatherley, P. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413. [Google Scholar] [CrossRef] [Green Version]
- Temminghoff, E.E.; Houba, V.J. (Eds.) Plant Analysis Procedures, 2nd ed.; Kluwer Academic Publishers: London, UK, 2004. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Pro-tective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Ling, Q.; Huang, W.; Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth. Res. 2010, 107, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajji, M.; Kinet, J.-M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analyt. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: London, UK, 1984; pp. 121–126. [Google Scholar]
- Vetter, J.L.; Steinberg, M.P.; Nelson, A.I. Enzyme assay, quantitative determination of peroxidase in sweet corn. J. Agric. Food Chemist. 1958, 6, 39–41. [Google Scholar] [CrossRef]
- A.O.A.C. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis: Part 3 Chemical Methods; Soil Science Society of America, Inc., American Society of Agronomy, Inc.: Madison, WI, USA, 1996. [Google Scholar]

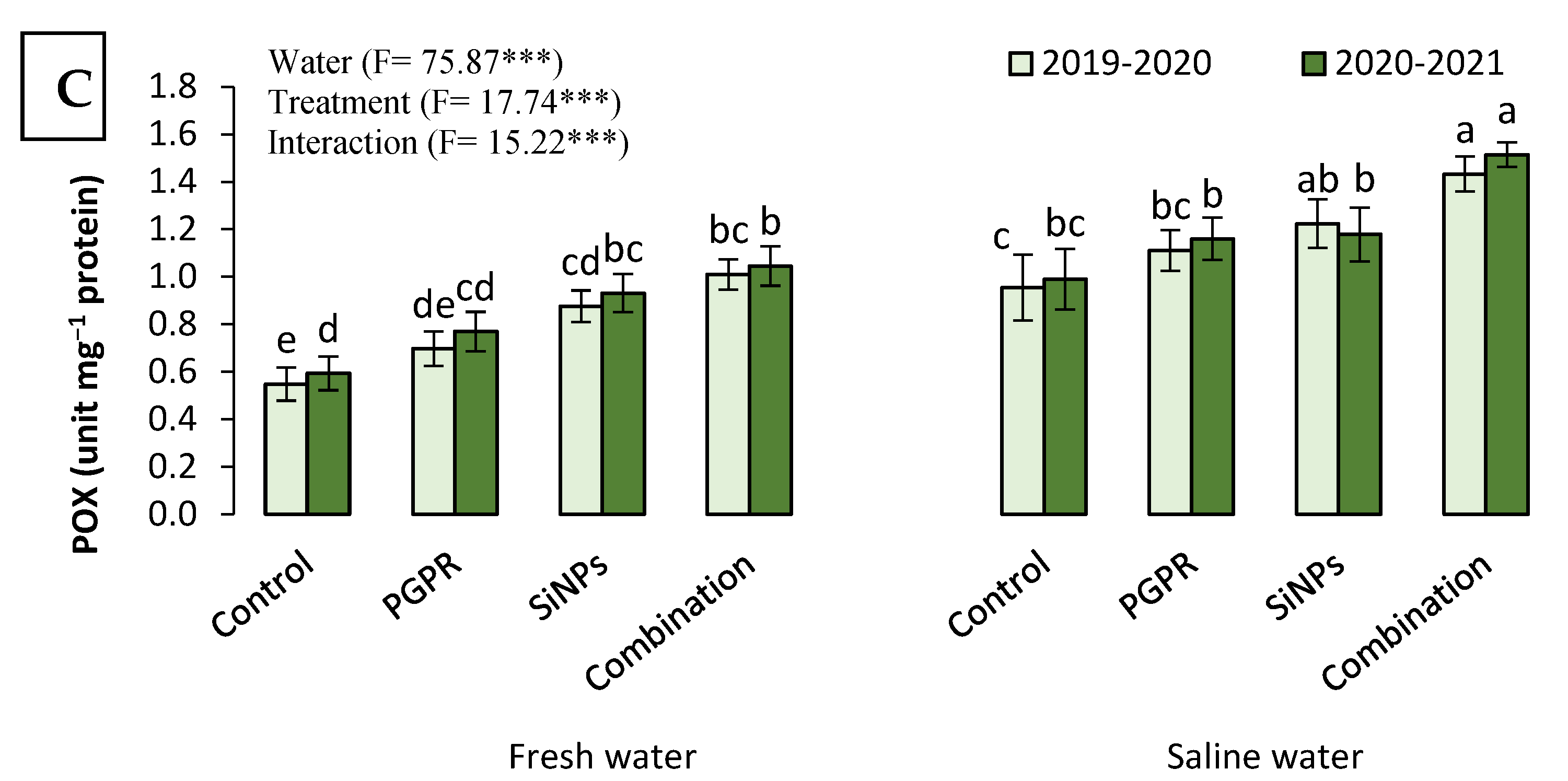
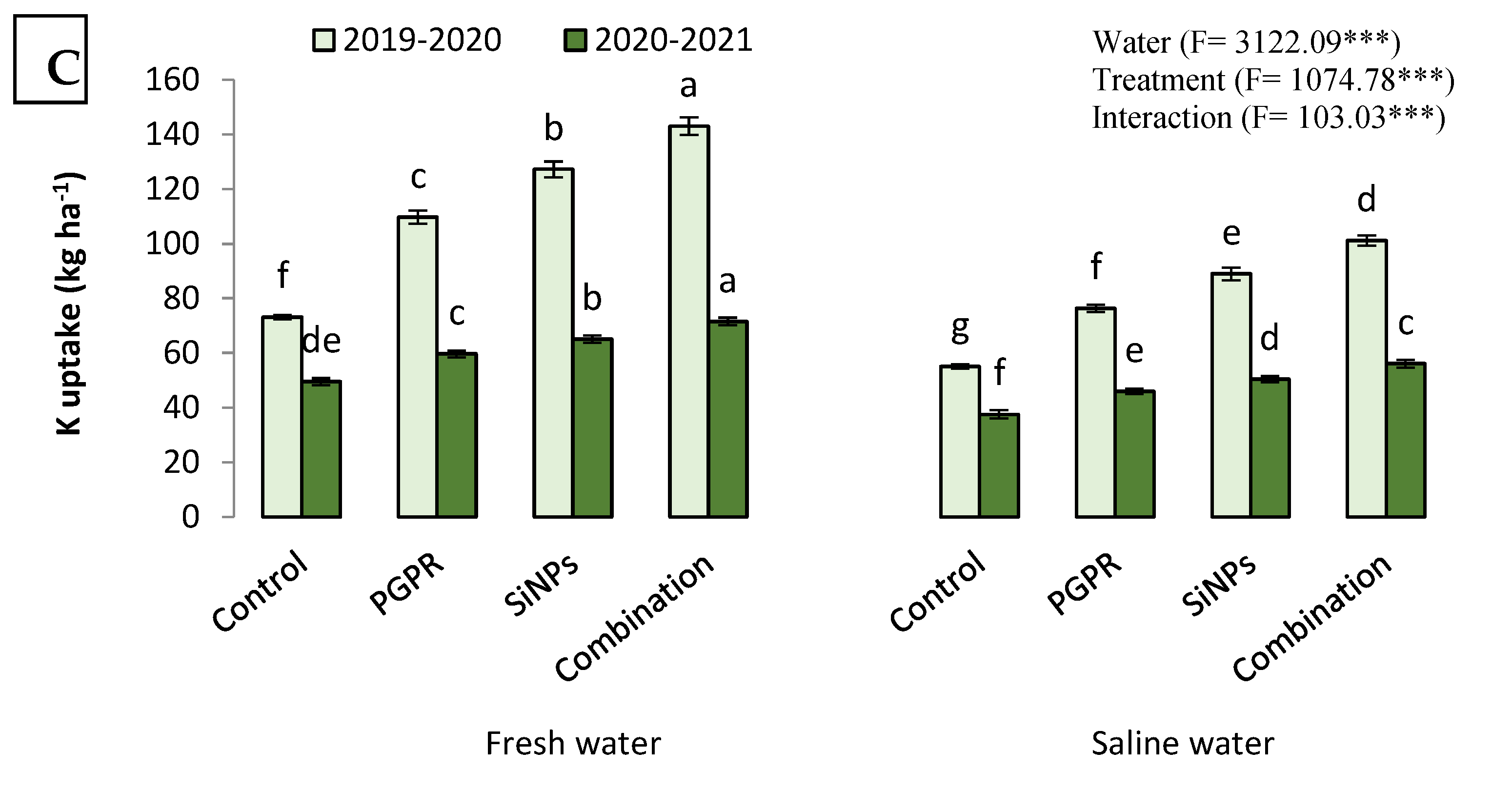
| pH * | ECe (dS m−1) ‡ | SAR ¥ | ESP (%) † | |||
|---|---|---|---|---|---|---|
| Water type | ||||||
| Fresh water_2019–2020 | 8.06 ± 0.05 c | 4.54 ± 0.68 c | 7.16 ± 1.48 c | 15.88 ± 2.80 c | ||
| Saline water_2019–2020 | 8.13 ± 0.05 a | 5.71 ± 0.38 a | 9.69 ± 1.05 a | 20.91 ± 2.14 a | ||
| Fresh water_2020–2021 | 8.02 ± 0.06 d | 4.12 ± 0.79 d | 6.54 ± 1.43 d | 14.50 ± 2.96 d | ||
| Saline water_2020–2021 | 8.11 ± 0.07 b | 5.38 ± 0.51 b | 8.80 ± 1.11 b | 18.80 ± 1.99 b | ||
| Treatment | ||||||
| Control | 8.15 ± 0.05 a | 5.77 ± 0.45 a | 9.65 ± 1.12 a | 20.71 ± 2.27 a | ||
| PGPR | 8.09 ± 0.00 b | 4.88 ± 0.80 b | 8.26 ± 1.50 b | 17.78 ± 2.80 b | ||
| SiNPs | 8.07 ± 0.05 c | 4.69 ± 0.84 c | 7.64 ± 1.64 c | 16.75 ± 3.41 c | ||
| Combination | 8.01 ± 0.06 d | 4.42 ± 0.83 d | 6.64 ± 1.58 d | 14.85 ± 3.14 d | ||
| Interaction | ||||||
| Year | Water type | Treatment | ||||
| 2019–2020 | Fresh water | Control | 8.11 ± 0.01 c BC | 5.50 ± 0.05 c C | 9.15 ± 0.48 bc BC | 19.82 ± 0.23 c CD |
| PGPR | 8.06 ± 0.01 de EF | 4.52 ± 0.07 e F | 7.26 ± 0.32 d E | 15.72 ± 0.15 e GH | ||
| SiNPs | 8.05 ± 0.01 e FG | 4.18 ± 0.034 f G | 6.52 ± 0.46 de EFG | 14.61 ± 0.10 f I | ||
| Combination | 7.99 ± 0.01 f I | 3.97 ± 0.03 g H | 5.70 ± 0.30 e GH | 13.35 ± 0.20 g J | ||
| Saline water | Control | 8.19 ± 0.01 a A | 6.18 ± 0.04 a A | 10.96 ± 0.38 a A | 23.61 ± 0.42 a A | |
| PGPR | 8.13 ± 0.01 b B | 5.78 ± 0.04 b B | 9.96 ± 0.44 ab AB | 20.99 ± 0.39 b B | ||
| SiNPs | 8.13 ± 0.01 bc B | 5.60 ± 0.03 c C | 9.36 ± 0.36 bc BC | 20.63 ± 0.21 b BC | ||
| Combination | 8.08 ± 0.01 d DE | 5.27 ± 0.06 d D | 8.46 ± 0.43 c CD | 18.40 ± 0.32 d EF | ||
| 2020–2021 | Fresh water | Control | 8.09 ± 0.01 bc CD | 5.28 ± 0.04 bc D | 8.38 ± 0.35 b CD | 18.25 ± 0.35 c F |
| PGPR | 8.03 ± 0.01 d GH | 3.93 ± 0.04 e H | 6.81 ± 0.43 cd EF | 15.19 ± 0.18 e HI | ||
| SiNPs | 8.01 ± 0.01 d HI | 3.79 ± 0.07 f I | 5.99 ± 0.35 d FGH | 13.25 ± 0.24 f J | ||
| Combination | 7.94 ± 0.01 e j | 3.49 ± 0.07 g J | 5.00 ± 0.28 e H | 11.31 ± 0.40 g K | ||
| Saline water | Control | 8.19 ± 0.01 a A | 6.12 ± 0.01 a A | 10.09 ± 0.36 a AB | 21.15 ± 0.22 a B | |
| PGPR | 8.11 ± 0.01 b BC | 5.30 ± 0.06 b D | 9.03 ± 0.24 b BC | 19.22 ± 0.28 b DE | ||
| SiNPs | 8.09 ± 0.01 c D | 5.17 ± 0.03 c D | 8.69 ± 0.38 b C | 18.52 ± 0.28 bc EF | ||
| Combination | 8.03 ± 0.01 d H | 4.94 ± 0.02 d E | 7.39 ± 0.00 c DE | 16.32 ± 0.00 d G | ||
| Two-way ANOVA | ||||||
| Water type | *** | *** | *** | *** | ||
| Treatment | *** | *** | *** | *** | ||
| Interaction | *** | *** | ns | *** | ||
| Total Microbial Count (Log cfu g−1 Soil) | Soil Enzymes | ||||||
|---|---|---|---|---|---|---|---|
| Bacteria (×107) | Azotobacter (×106) | Bacillus spp. (×105) | Urease (mg NH4+ g−1 dry soil d−1) | Dehydrogenase (mg TPF g−1 dry soil d−1) | |||
| Water type | |||||||
| Fresh water_2019–2020 | 3.72 ± 1.37 b | 1.45 ± 0.51 a | 2.81 ± 0.88 a | 163.2 ± 41.5 b | 98.7 ± 36.2 b | ||
| Saline water_2019–2020 | 2.39 ± 0.72 d | 1.08 ± 0.51 d | 1.64 ± 0.76 d | 117.7 ± 28.2 d | 73.9 ± 33.1 d | ||
| Fresh water_2020–2021 | 4.75 ± 1.25 a | 1.27 ± 0.54 b | 2.51 ± 0.92 b | 174.0 ± 42.0 a | 104.2 ± 38.6 a | ||
| Saline water_2020–2021 | 3.52 ± 1.21 c | 1.11 ± 0.52 c | 1.72 ± 0.77 c | 124.4 ± 27.0 c | 82.6 ± 29.5 c | ||
| Treatment | |||||||
| Control | 2.40 ± 0.80 d | 0.59 ± 0.17 d | 1.21 ± 0.40 d | 106.0 ± 18.0 d | 49.4 ± 11.6 d | ||
| PGPR | 3.74 ± 1.34 b | 1.35 ± 0.19 b | 2.33 ± 0.83 b | 149.0 ± 32.0 b | 97.7 ± 14.7 b | ||
| SiNPs | 3.26 ± 0.70 c | 1.15 ± 0.28 c | 2.01 ± 0.71 c | 135.0 ± 25.0 c | 80.8 ± 11.5 c | ||
| Combination | 4.98 ± 1.27 a | 1.82 ± 0.15 a | 3.13 ± 0.49 a | 189.2 ± 37.0 a | 131.6 ± 19.0 a | ||
| Interaction | |||||||
| Year | Water type | Treatment | |||||
| 2019–2020 | Fresh water | Control | 2.66 ± 0.05 c G | 0.73 ± 0.02 f H | 1.54 ± 0.02 d FG | 116.9 ± 2.39 de GH | 56.7 ± 3.06 g H |
| PGPR | 3.25 ± 0.03 b EF | 1.58 ± 0.01 c C | 3.14 ± 0.03 b B | 168.7 ± 1.84 b D | 107.5 ± 2.11 c D | ||
| SiNPs | 3.23 ± 0.04 b EF | 1.56 ± 0.02 c C | 3.04 ± 0.09 b BC | 150.7 ± 3.74 c F | 87.7 ± 1.15 d E | ||
| Combination | 5.74 ± 0.10 a AB | 1.94 ± 0.02 a A | 3.53 ± 0.04 a A | 216.4 ± 2.41 a B | 143.0 ± 4.07 a B | ||
| Saline water | Control | 1.41 ± 0.05 e I | 0.44 ± 0.03 g I | 0.84 ± 0.01 e H | 86.6 ± 1.74 f J | 34.4 ± 2.04 h J | |
| PGPR | 2.61 ± 0.12 c G | 1.18 ± 0.01 d E | 1.56 ± 0.04 d FG | 119.9 ± 3.42 d G | 80.6 ± 0.74 e F | ||
| SiNPs | 2.40 ± 0.04 d H | 1.04 ± 0.02 e F | 1.48 ± 0.02 d G | 110.0 ± 2.18 e H | 66.5 ± 0.77 f G | ||
| Combination | 3.14 ± 0.08 b F | 1.67 ± 0.03 b B | 2.67 ± 0.04 c D | 154.4 ± 2.37 c EF | 114.1 ± 1.85 b C | ||
| 2020–2021 | Fresh water | Control | 3.31 ± 0.05 e EF | 0.75 ± 0.01 g H | 1.56 ± 0.02 e FG | 125.1 ± 3.60 d G | 60.3 ± 1.52 e GH |
| PGPR | 5.69 ± 0.07 b B | 1.45 ± 0.01 c D | 2.95 ± 0.03 b C | 183.6 ± 2.16 b C | 112.2 ± 1.79 b CD | ||
| SiNPs | 4.10 ± 0.06 d D | 0.93 ± 0.01 f G | 1.96 ± 0.10 d E | 161.5 ± 2.65 c DE | 92.1 ± 1.60 c E | ||
| Combination | 5.89 ± 0.07 a A | 1.95 ± 0.01 a A | 3.57 ± 0.04 a A | 225.6 ± 3.51 a A | 152.4 ± 3.34 a A | ||
| Saline water | Control | 2.22 ± 0.04 f H | 0.45 ± 0.02 h I | 0.89 ± 0.02 f H | 95.5 ± 2.10 e I | 46.2 ± 2.41 f I | |
| PGPR | 3.41 ± 0.05 e E | 1.21 ± 0.01 d E | 1.67 ± 0.03 e F | 123.9 ± 2.66 d G | 90.5 ± 1.64 c E | ||
| SiNPs | 3.32 ± 0.05 e EF | 1.05 ± 0.02 e F | 1.57 ± 0.02 e FG | 117.6 ± 3.57 d GH | 76.9 ± 2.65 d F | ||
| Combination | 5.14 ± 0.05 c C | 1.72 ± 0.03 b B | 2.76 ± 0.04 c D | 160.5 ± 1.61 c DE | 117.0 ± 1.42 b C | ||
| Two-way ANOVA | |||||||
| Water type | *** | *** | *** | *** | *** | ||
| Treatment | *** | *** | *** | *** | *** | ||
| Interaction | *** | *** | *** | *** | *** | ||
| RWC † (%) | K/Na Ratio | H2O2 (µmol g−1 FW) | MDA ‡ (nmol g−1 FW) | |||
|---|---|---|---|---|---|---|
| Water type | ||||||
| Fresh water_2019–2020 | 81.42 ± 6.22 b | 1.61 ± 0.88 b | 2.34 ± 0.92 b | 5.51 ± 2.87 b | ||
| Saline water_2019–2020 | 62.19 ± 9.62 d | 0.52 ± 0.37 d | 3.03 ± 1.07 a | 12.47 ± 4.05 a | ||
| Fresh water_2020–2021 | 83.57 ± 7.71 a | 1.85 ± 1.08 a | 3.22 ± 1.28 a | 6.50 ± 2.84 b | ||
| Saline water_2020–2021 | 65.61 ± 10.50 c | 0.62 ± 0.46 c | 3.32 ± 1.40 a | 12.97 ± 4.95 a | ||
| Treatment | ||||||
| Control | 61.83 ± 11.89 d | 0.29 ± 0.20 d | 4.26 ± 0.54 a | 13.71 ± 4.79 a | ||
| PGPR | 73.50 ± 13.80 c | 1.07 ± 0.70 c | 3.27 ± 0.60 b | 10.35 ± 4.20 b | ||
| SiNPs | 76.13 ± 10.97 b | 1.29 ± 0.79 b | 2.89 ± 0.57 b | 8.40 ± 4.33 c | ||
| Combination | 81.33 ± 6.96 a | 1.97 ± 1.01 a | 1.48 ± 0.13 c | 4.99 ± 2.45 d | ||
| Interaction | ||||||
| Year | Water type | Treatment | ||||
| 2019–2020 | Fresh water | Control | 72.2 ± 1.70 b CD | 0.48 ± 0.02 d FG | 3.58 ± 0.46 ab ABCD | 9.1 ± 1.1 cd DEFG |
| PGPR | 83.9 ± 1.47 a A | 1.57 ± 0.12 b D | 2.34 ± 0.39 bcde CDEFG | 6.2 ± 1.8 def FGHI | ||
| SiNPs | 84.0 ± 0.70 a A | 1.80 ± 0.11 b D | 2.10 ± 0.31 cde DEFG | 4.2 ± 1.1 ef HI | ||
| Combination | 85.6 ± 1.22 a A | 2.61 ± 0.22 a B | 1.35 ± 0.25 e G | 2.4 ± 0.2 f I | ||
| Saline water | Control | 50.9 ± 2.77 e G | 0.13 ± 0.02 e H | 4.19 ± 0.85 a AB | 17.4 ± 1.5 a AB | |
| PGPR | 58.9 ± 2.50 d F | 0.39 ± 0.05 de G | 3.37 ± 0.40 abc BCD | 13.2 ± 0.20 b BCD | ||
| SiNPs | 65.4 ± 1.86 c E | 0.56 ± 0.02 d FG | 2.91 ± 0.51 abcd BCDEF | 11.6 ± 0.4 bc CDE | ||
| Combination | 73.5 ± 1.80 b BC | 1.01 ± 0.09 c E | 1.64 ± 0.67 de EFG | 7.7 ± 1.9 cde EFGH | ||
| 2020–2021 | Fresh water | Control | 72.1 ± 1.66 c CD | 0.45 ± 0.02 f FG | 4.41 ± 0.56 ab AB | 10.0 ± 0.0 cd DEF |
| PGPR | 86.6 ± 1.25 a A | 1.79 ± 0.10 c D | 3.64 ± 0.49 ab ABC | 7.4 ± 1.8 de EFGHI | ||
| SiNPs | 87.1 ± 0.35 a A | 2.11 ± 0.03 b C | 3.43 ± 0.29 b ABCD | 5.1 ± 1.7 e GHI | ||
| Combination | 88.6 ± 0.70 a A | 3.06 ± 0.08 a A | 1.40 ± 0.59 c G | 3.5 ± 0.5 e HI | ||
| Saline water | Control | 52.2 ± 2.04 f G | 0.10 ± 0.01 g H | 4.88 ± 0.37 a A | 18.2 ± 2.4 a A | |
| PGPR | 64.6 ± 1.26 e E | 0.52 ± 0.04 ef FG | 3.74 ± 0.67 ab ABC | 14.6 ± 0.7 ab ABC | ||
| SiNPs | 68.1 ± 0.20 d DE | 0.67 ± 0.02 e F | 3.13 ± 0.36 b BCDE | 12.7 ± 1.6 bc CD | ||
| Combination | 77.6 ± 0.76 b B | 1.21 ± 0.09 d E | 1.53 ± 0.38 c FG | 6.4 ± 1.1 de FGHI | ||
| Two-way ANOVA | ||||||
| Water type | *** | *** | *** | *** | ||
| Treatment | *** | *** | *** | *** | ||
| Interaction | *** | *** | ns | ns | ||
| Spike Length (cm) | Grain Per Spike | 1000-Grain Weight (g) | Grain Yield (kg ha−1) | Straw Yield (kg ha−1) | Biological Yield (kg ha−1) | HI † (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Water type | |||||||||
| Fresh water_2019–2020 | 8.25 ± 0.87 b | 54.89 ± 4.98 b | 50.07 ± 4.37 b | 3188 ± 410 a | 5447 ± 101 a | 8634 ± 501 b | 36.8 ± 2.69 a | ||
| Saline water_2019–2020 | 6.79 ± 0.75 d | 46.44 ± 5.07 d | 43.66 ± 5.94 d | 2599 ± 395 b | 5007 ± 439 b | 7606 ± 831 c | 34.1 ± 1.55 c | ||
| Fresh water_2020–2021 | 8.38 ± 0.91 a | 56.47 ± 5.49 a | 51.67 ± 5.07 a | 3232 ± 351 a | 5542 ± 158 a | 8774 ± 502 a | 36.8 ± 1.95 a | ||
| Saline water_2020–2021 | 6.93 ± 0.79 c | 47.75 ± 4.64 c | 44.92 ± 3.56 c | 2624 ± 355 b | 4783 ± 222 c | 7406 ± 572 d | 35.3 ± 2.13 b | ||
| Treatment | |||||||||
| Control | 6.54 ± 0.63 d | 45.27 ± 4.99 d | 42.59 ± 3.36 d | 2430 ± 335 d | 4877 ± 505 c | 7306 ± 837 d | 33.2 ± 0.84 d | ||
| PGPR | 7.48 ± 1.00 c | 50.12 ± 4.50 c | 46.48 ± 3.80 c | 2836 ± 379 c | 5224 ± 378 b | 8060 ± 750 c | 35.1 ± 1.55 c | ||
| SiNPs | 7.82 ± 0.96 b | 52.87 ± 5.21 b | 48.53 ± 3.60 b | 3055 ± 328 b | 5266 ± 325 b | 8322 ± 636 b | 36.7 ± 1.38 b | ||
| Combination | 8.51 ± 0.78 a | 57.29 ± 5.42 a | 52.73 ± 4.82 a | 3321 ± 346 a | 5411 ± 296 a | 8732 ± 605 a | 38.0 ± 1.68 a | ||
| Interaction | |||||||||
| Year | Water type | Treatment | |||||||
| 2019–2020 | Fresh water | Control | 7.03 ± 0.02 d E | 48.9 ± 0.46 d HI | 44.77 ± 0.38 d HI | 2644 ± 39 de EF | 5296 ± 89 ab BCDE | 7939 ± 85 d E | 33.3 ± 0.58 d EF |
| PGPR | 8.31 ± 0.03 b C | 53.3 ± 0.48 c EF | 49.22 ± 0.53 c EF | 3161 ± 67 bc CD | 5499 ± 81 a ABC | 8660 ± 124 bc CD | 36.5 ± 0.48 bc BCD | ||
| SiNPs | 8.55 ± 0.12 b BC | 56.7 ± 0.41 b CD | 50.96 ± 0.56 b D | 3324 ± 70 b BC | 5492 ± 102 a ABC | 8816 ± 35 b C | 37.7 ± 0.93 ab ABC | ||
| Combination | 9.09 ± 0.03 a A | 60.6 ± 1.09 a B | 55.32 ± 0.39 a B | 3622 ± 90 a A | 5500 ± 226 a ABC | 9122 ± 145 a AB | 39.7 ± 1.57 a A | ||
| Saline water | Control | 5.95 ± 0.07 f H | 40.0 ± 1.62 f K | 38.86 ± 0.56 f L | 2115 ± 45 f G | 4407 ± 78 c G | 6522 ± 114 f G | 32.4 ± 0.34 d F | |
| PGPR | 6.48 ± 0.13 e G | 45.6 ± 0.1.42 e J | 42.58 ± 0.42 e J | 2473 ± 36 e F | 4989 ± 23 b EF | 7462 ± 44 e F | 33.1 ± 0.33 d F | ||
| SiNPs | 6.98 ± 0.09 d EF | 48.2 ± 0.59 de HI | 45.03 ± 0.26 d GHI | 2774 ± 71 d E | 5199 ± 155 ab CDE | 7973 ± 114 d E | 34.8 ± 1.18 cd DEF | ||
| Combination | 7.73 ± 0.16 c D | 52.0 ± 0.69 c FG | 48.19 ± 0.48 c F | 3033 ± 52 c D | 5434 ± 136 a ABC | 8467 ± 118 c D | 35.8 ± 0.85 bc CDE | ||
| 2020–2021 | Fresh water | Control | 7.14 ± 0.05 e E | 50.1 ± 0.43 d GH | 46.00 ± 0.24 e G | 2785 ± 60 d E | 5331 ± 114 b BCD | 8116 ± 76 c E | 34.3 ± 0.91 cd DEF |
| PGPR | 8.39 ± 0.15 c C | 54.7 ± 0.60 c DE | 50.24 ± 0.11 c DE | 3166 ± 80 c CD | 5591 ± 133 ab AB | 8757 ± 53 b C | 36.2 ± 1.14 bc CD | ||
| SiNPs | 8.74 ± 0.06 b B | 58.0 ± 0.51 b C | 52.23 ± 0.30 b C | 3355 ± 49 b B | 5540 ± 133 ab AB | 8896 ± 86 b BC | 37.7 ± 0.90 ab ABC | ||
| Combination | 9.27 ± 0.05 a A | 63.1 ± 0.74 a A | 58.20 ± 0.55 a A | 3620 ± 35 a A | 5708 ± 54 a A | 9328 ± 56 a A | 38.8 ± 0.37 a AB | ||
| Saline water | Control | 6.04 ± 0.08 g H | 42.1 ± 0.56 f K | 40.71 ± 0.44 g K | 2174 ± 77 f G | 4474 ± 67 d G | 6648 ± 142 f G | 32.7 ± 0.48 d F | |
| PGPR | 6.74 ± 0.14 f FG | 46.9 ± 0.72 e IJ | 43.88 ± 0.20 f I | 2544 ± 50 e F | 4819 ± 100 c F | 7363 ± 56 e F | 34.6 ± 0.90 cd DEF | ||
| SiNPs | 6.99 ± 0.08 ef EF | 48.6 ± 0.70 de HI | 45.90 ± 0.11 e GH | 2768 ± 57 d E | 4834 ± 88 c F | 7602 ± 75 d F | 36.4 ± 0.79 bc BCD | ||
| Combination | 7.95 ± 0.08 d D | 53.4 ± 0.82 c EF | 49.19 ± 0.34 d EF | 3009 ± 68 c D | 5003 ± 15 c DEF | 8012 ± 55 c E | 37.6 ± 0.59 ab ABC | ||
| Two-way ANOVA | |||||||||
| Water type | *** | *** | *** | *** | *** | *** | *** | ||
| Treatment | *** | *** | *** | *** | *** | *** | *** | ||
| Interaction | *** | ns | *** | ns | *** | *** | ns | ||
| Year Month | 2019/2020 | 2020/2021 | ||||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Rainfall (mm) | Relative Humidity (%) | Temperature (°C) | Rainfall (mm) | Relative Humidity (%) | |||
| Max | Min | Max | Min | |||||
| November | 27.8 | 19.6 | 0.87 | 34.3 | 24.3 | 15.2 | 0.88 | 32.6 |
| December | 26.7 | 18.7 | 0.76 | 35.4 | 23.9 | 16.3 | 0.99 | 33.2 |
| January | 25.6 | 16.6 | 1.65 | 32.6 | 22.2 | 14.4 | 0.76 | 34.7 |
| February | 23.4 | 15.4 | 3.54 | 34.7 | 21.3 | 10.1 | 3.08 | 45.4 |
| March | 22.3 | 10.3 | 6.43 | 45.8 | 21.6 | 11.7 | 6.35 | 45.1 |
| April | 24.1 | 11.2 | 0.57 | 46.9 | 23.5 | 11.5 | 0.57 | 43.8 |
| Character | 2019/20 | 2020/21 |
|---|---|---|
| pH (1:2.5 soil:water suspension) | 8.14 ± 0.01 † | 8.15 ± 0.02 |
| Electrical conductivity (EC, dS m−1) ¥ | 5.23 ± 0.02 | 5.34 ± 0.01 |
| Soil organic matter (g kg−1) | 11.33 ± 0.02 | 11.4 ± 0.04 |
| ESP # (%) | 22.12 ± 0.21 | 21.34 ± 0.22 |
| Particle size distribution (%) | ||
| Sand | 27.22 ± 1.34 | 27.22 ± 1.12 |
| Silt | 25.23 ± 2.01 | 25.32 ± 1.44 |
| Clay | 47.55 ± 2.34 | 47.13 ± 2.12 |
| Texture grade | clayey | clayey |
| Soluble cations (meq L−1) ¥ | ||
| Ca++ | 7.43 ± 0.65 | 9.12 ± 0.54 |
| Mg++ | 5.23 ± 1.33 | 6.32 ± 1.32 |
| Na+ | 26.23 ± 2.12 | 22.43 ± 3.54 |
| K+ | 0.54 ± 0.12 | 0.44 ± 0.12 |
| Soluble anions (meq L−1) ¥ | ||
| CO3− − | nd ‡ | nd |
| HCO3− | 4.22 ± 0.54 | 3.65 ± 0.43 |
| Cl− | 24.33 ± 1.21 | 18.43 ± 1.12 |
| SO4− − | 15.43 ± 3.45 | 11.21 ± 3.22 |
| Available macronutrients (mg kg−1) | ||
| N | 9.43± 0.54 | 10.12 ± 1.54 |
| P | 8.12 ± 1.32 | 8.23 ± 1.32 |
| K | 354 ± 26.12 | 367 ± 24.12 |
| Total counts of soil microbes | ||
| Bacteria (CFU ×107 g−1 dry soil) | 32 ± 1.34 | 37 ± 1.45 |
| Fungi (CFU ×104 g−1 dry soil) | 11 ± 0.45 | 16 ± 1.76 |
| Actinomycetes (CFU ×105 g−1 dry soil) | 22 ± 1.76 | 25 ± 1.23 |
| Character | Fresh Water | Saline Water * | ||
|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | |
| pH | 7.32 ± 0.65 | 7.24 ± 0.66 | 8.45 ± 0.16 | 8.26 ± 0.12 |
| EC (dS m−1) | 0.45 ± 0.12 | 0.49 ± 0.01 | 3.87 ± 0.05 | 3.73 ± 0.11 |
| SAR | 1.36 ± 0.12 | 1.41 ± 0.06 | 7.65 ± 0.33 | 7.66 ± 0.22 |
| Na+ (mq L−1) | 1.78 ± 0.13 | 1.91 ± 0.04 | 16.54 ± 1.45 | 16.75 ± 1.21 |
| Cl− (mq L−1) | 3.47 ± 0.02 | 3.37 ± 0.05 | 11.33 ± 0.76 | 11.75 ± 0.43 |
| SO4− (mq L−1) | 0.25 ± 0.03 | 0.13 ± 0.02 | 7.87 ± 0.24 | 8.44 ± 0.05 |
| NH4+ (mq L−1) | 1.36 ± 0.04 | 1.82 ± 0.04 | 2.16 ± 0.05 | 2.33 ± 0.03 |
| COD (mq L−1) | 12.45 ± 0.88 | 11.11 ± 1.06 | nd ¥ | nd |
| BOD (mq L−1) | 5.36 ± 0.27 | 5.24 ± 0.87 | nd | nd |
| SS (mq L−1) | 174 ± 12.48 | 183 ± 13.4 | 18 ± 1.3 | 15 ± 1.4 |
| DS (mq L−1) | 365 ± 33 | 387 ± 36 | 2976 ± 154 | 2944 ± 123 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, K.; Rashwan, E.; Mohamed, H.H.; Awadalla, A.; Omara, A.E.-D.; Hafez, E.M.; Alshaal, T. Application of Silica Nanoparticles in Combination with Two Bacterial Strains Improves the Growth, Antioxidant Capacity and Production of Barley Irrigated with Saline Water in Salt-Affected Soil. Plants 2022, 11, 2026. https://doi.org/10.3390/plants11152026
Alharbi K, Rashwan E, Mohamed HH, Awadalla A, Omara AE-D, Hafez EM, Alshaal T. Application of Silica Nanoparticles in Combination with Two Bacterial Strains Improves the Growth, Antioxidant Capacity and Production of Barley Irrigated with Saline Water in Salt-Affected Soil. Plants. 2022; 11(15):2026. https://doi.org/10.3390/plants11152026
Chicago/Turabian StyleAlharbi, Khadiga, Emadeldeen Rashwan, Hossam Hussein Mohamed, Abdelmoniem Awadalla, Alaa El-Dein Omara, Emad M. Hafez, and Tarek Alshaal. 2022. "Application of Silica Nanoparticles in Combination with Two Bacterial Strains Improves the Growth, Antioxidant Capacity and Production of Barley Irrigated with Saline Water in Salt-Affected Soil" Plants 11, no. 15: 2026. https://doi.org/10.3390/plants11152026
APA StyleAlharbi, K., Rashwan, E., Mohamed, H. H., Awadalla, A., Omara, A. E.-D., Hafez, E. M., & Alshaal, T. (2022). Application of Silica Nanoparticles in Combination with Two Bacterial Strains Improves the Growth, Antioxidant Capacity and Production of Barley Irrigated with Saline Water in Salt-Affected Soil. Plants, 11(15), 2026. https://doi.org/10.3390/plants11152026








