“Hong Long” Lychee (Litchi chinensis Sonn.) Is the Optimal Pollinizer for the Main Lychee Cultivars in Israel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiments
2.2. Greenhouse Experiments
2.3. Initial Fruit Set, Yield, and Fruit Weight
2.4. DNA Purification
2.5. Marker Identification by iPBS
2.6. Statistical Analysis
3. Results
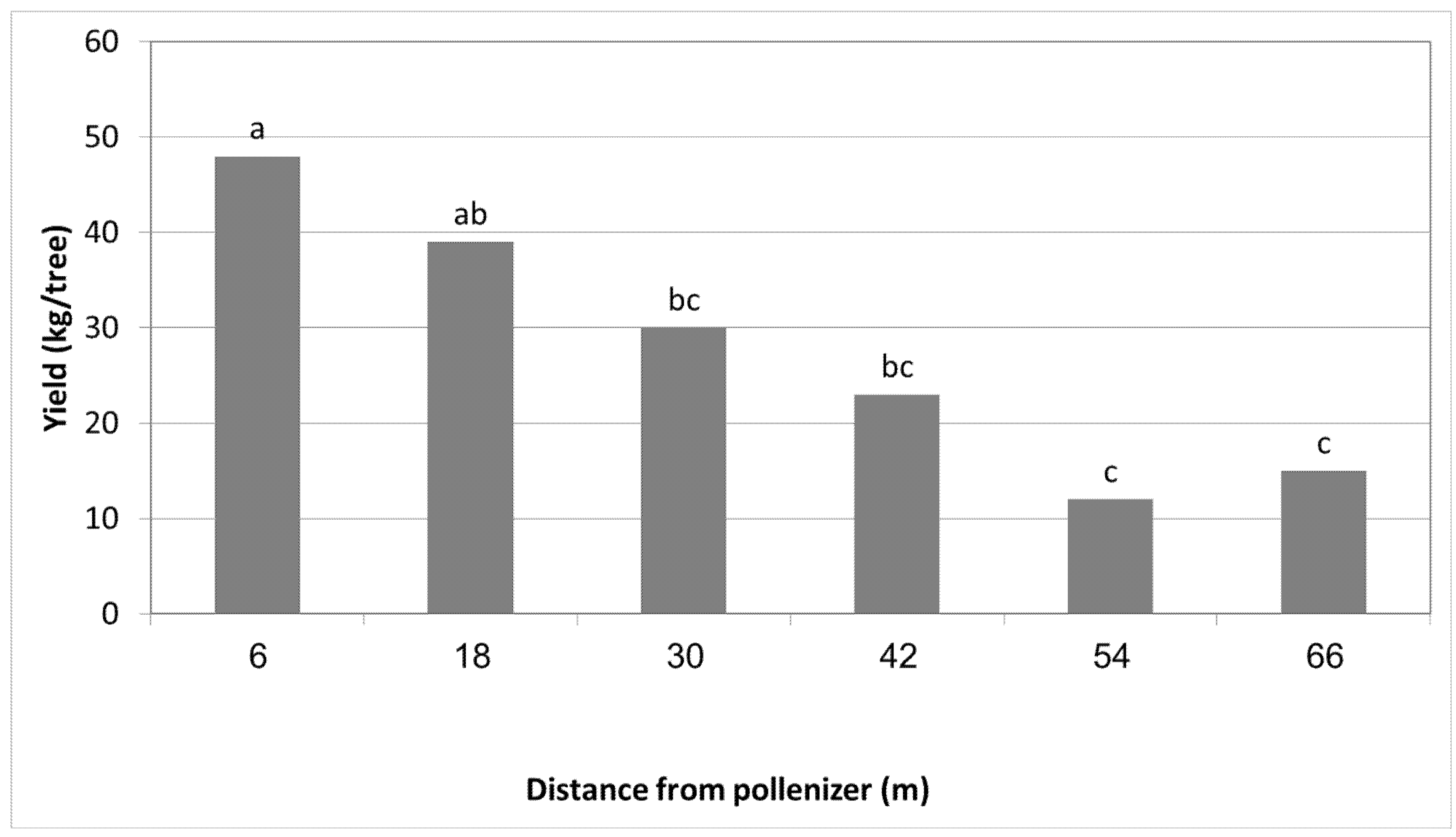
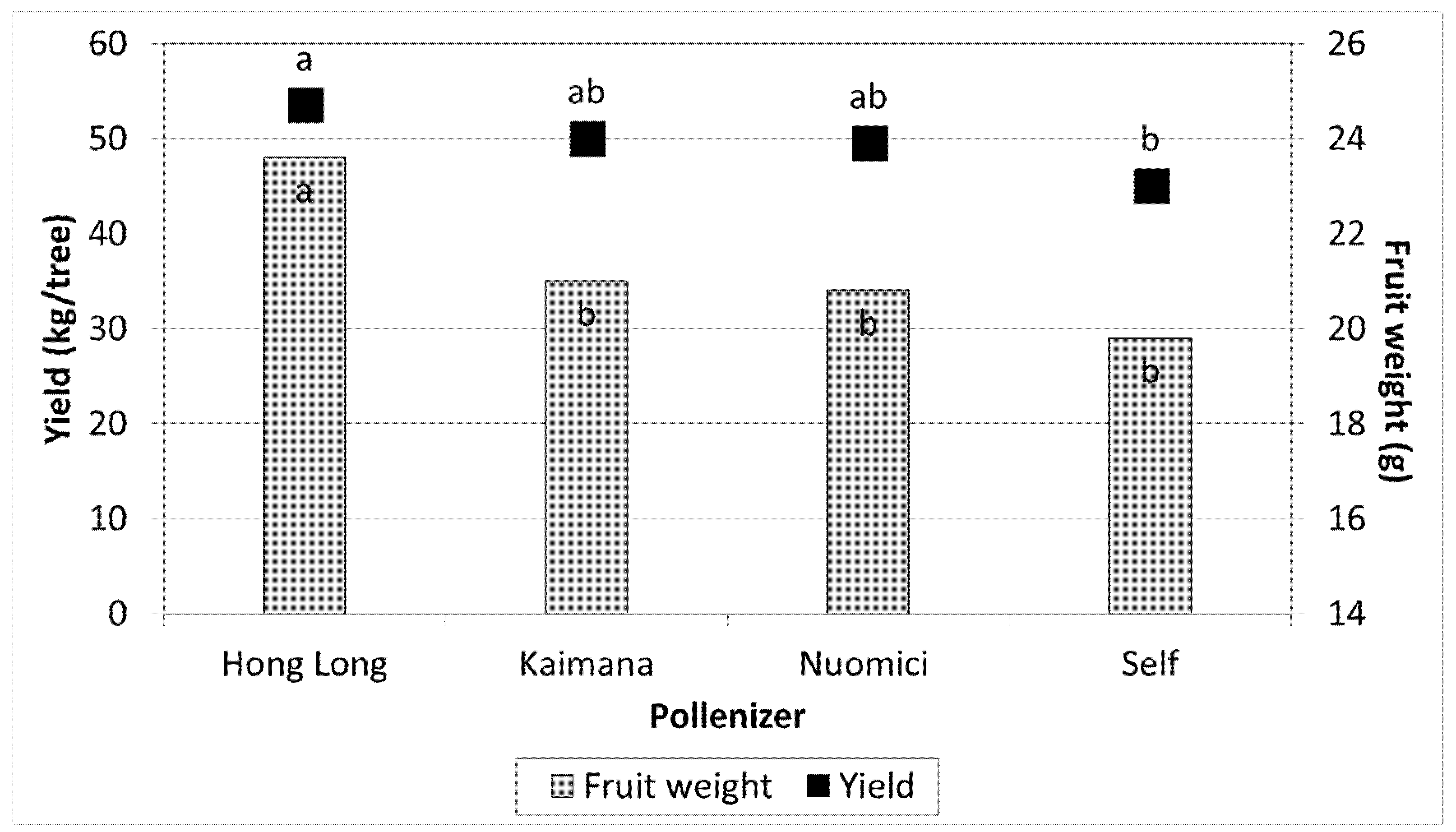
3.1. Effect of Different Pollinizers on the Yield of MA
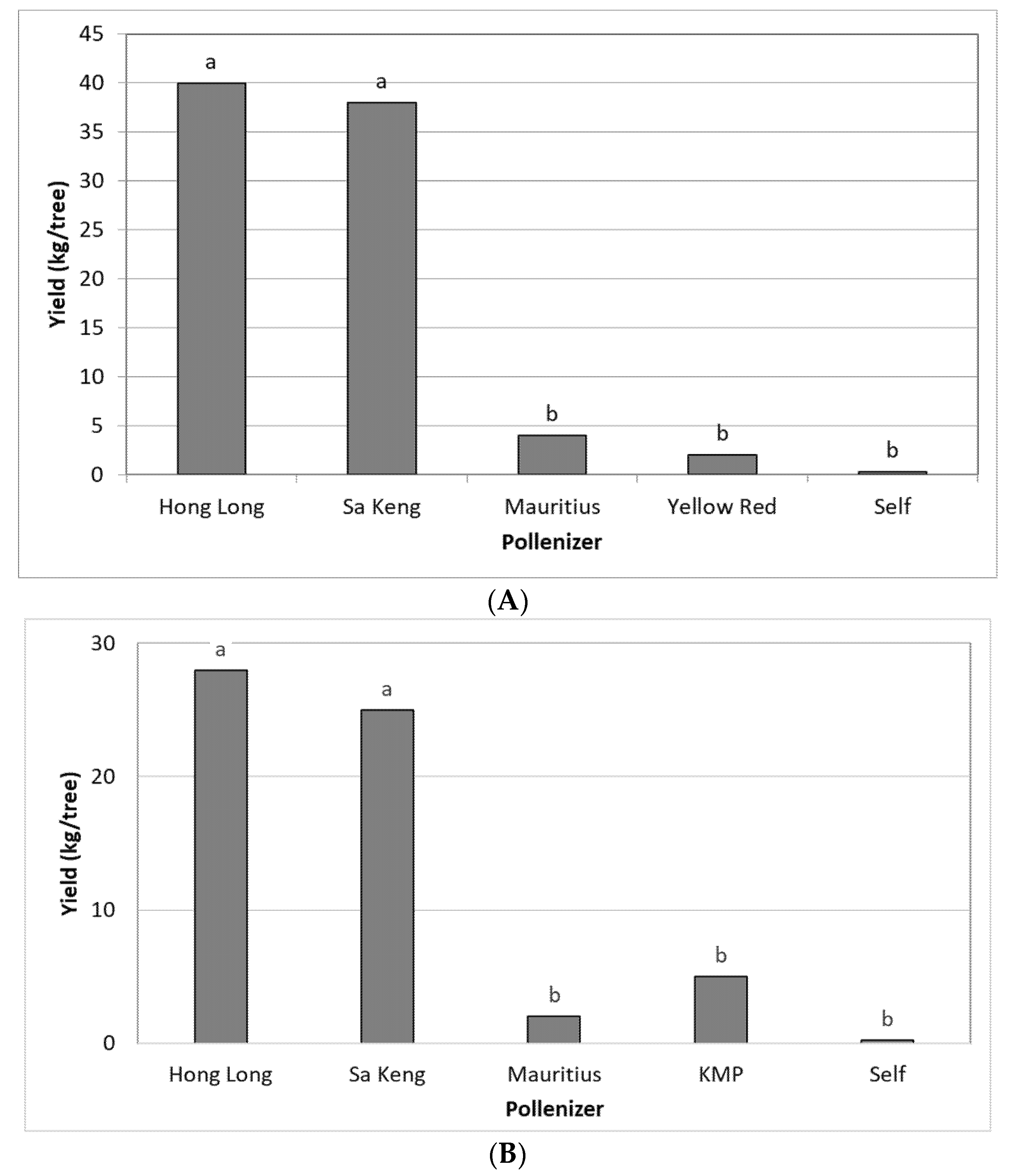
3.2. Effect of Different Pollinizers on the Yield of FZX
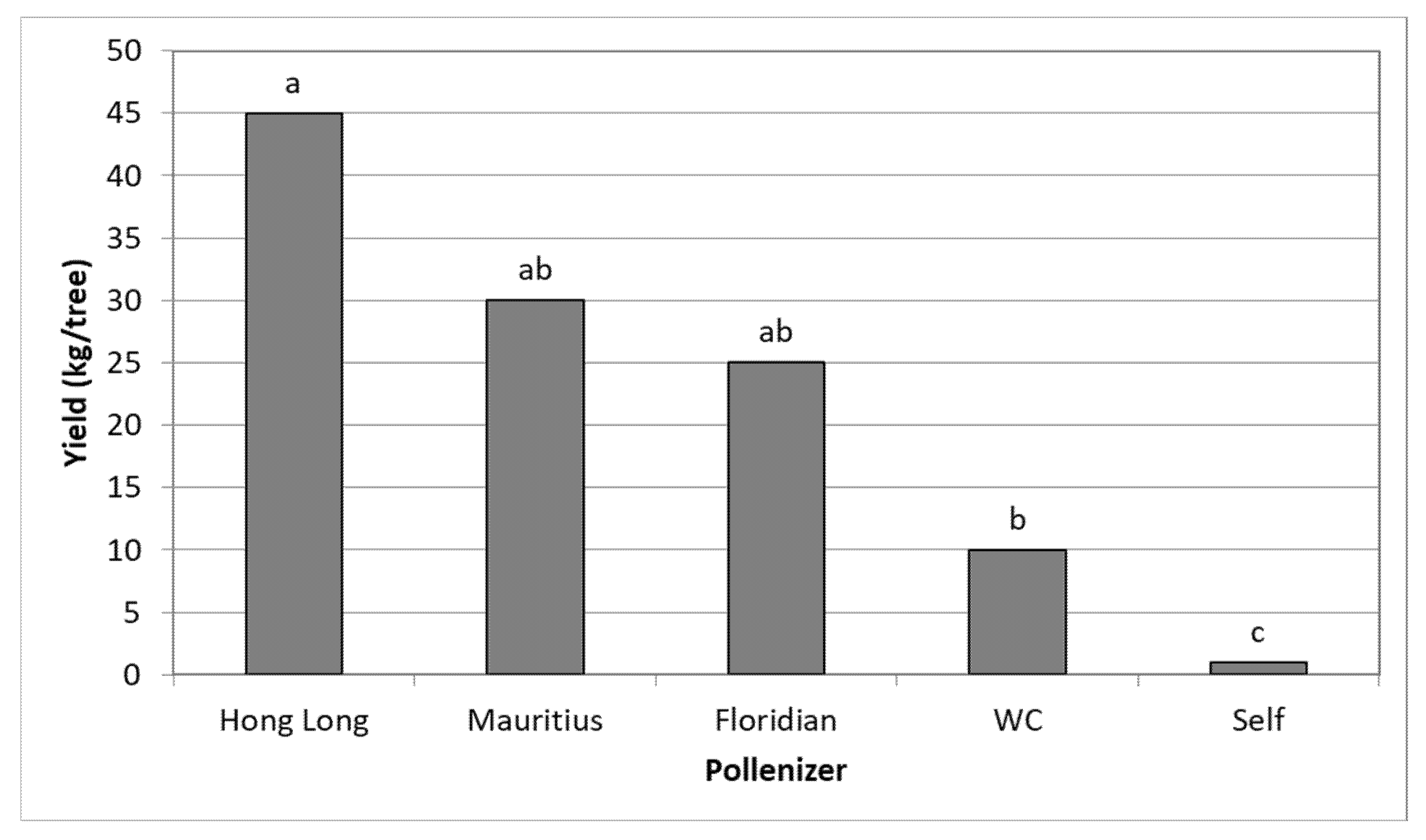
3.3. Effect of Different Pollinators on the Yield of TA
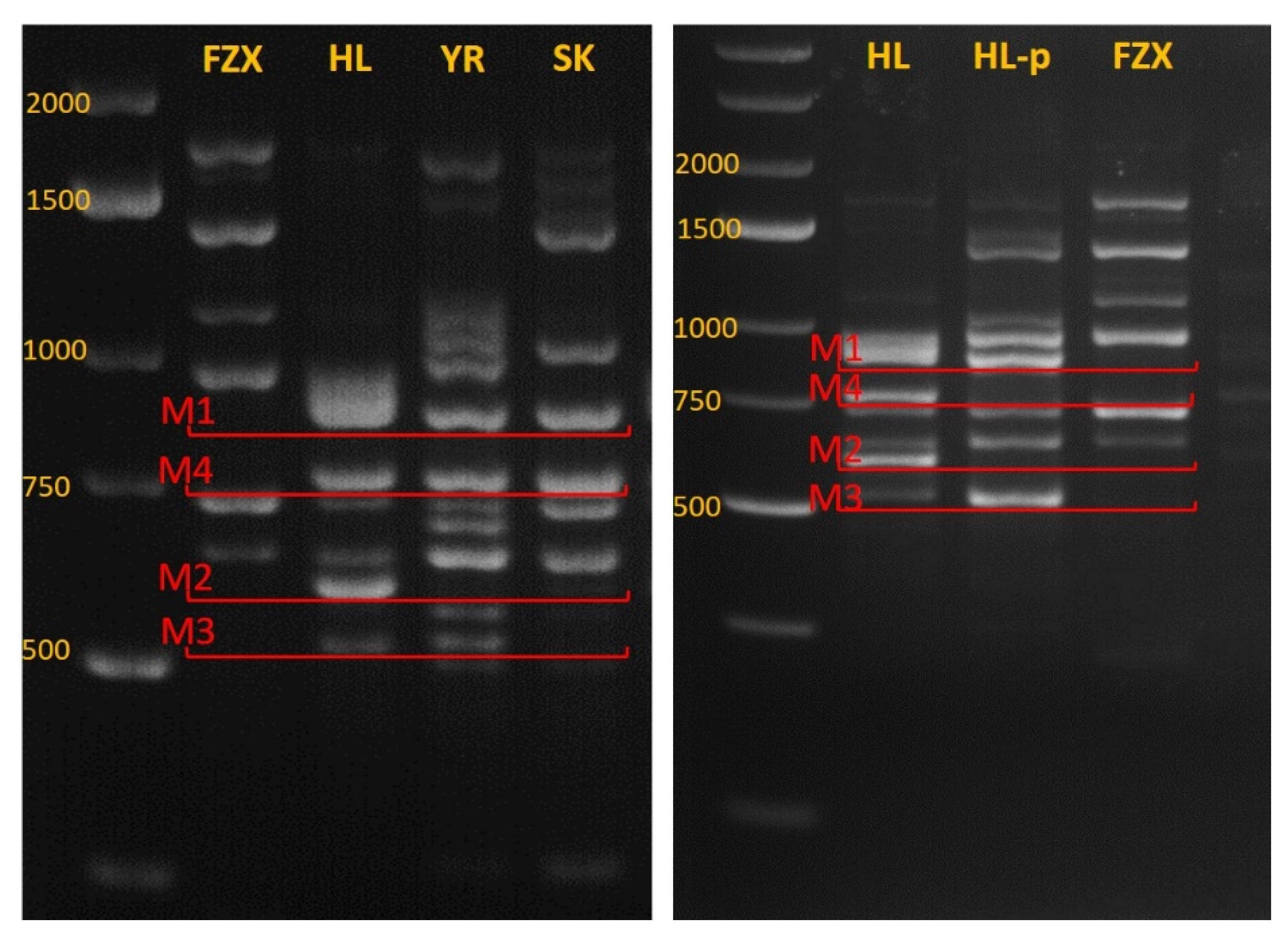
3.4. Identifying Genetic Markers for Paternal Identification
3.5. Genetic Analysis of FZX Fertilization by HL
3.6. Fruitlet Genetic Analysis and Yield of FZX Fertilization by SK
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Subhadrabandhu, S.; Mitra, S.K.; Ben-Arie, R.; Stern, R.A. Origin, History, Production and Processing. In Litchi and Longan: Botany, Cultivation and Uses; Menzel, C.M., Wait, G.K., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 1–23. [Google Scholar]
- Huang, X. Achieving Sustainable Cultivation of Litchi. In Achieving Sustainable Cultivation of Tropical Fruit; Yahia, E.M., Ed.; Burleigh Dodds Science Publishing Limited: Cambridge UK, 2019; pp. 457–469. [Google Scholar]
- Stern, R.A.; Gazit, S. The Reproductive Biology of the Lychee. In Horticultural Reviews; Janick, J., Ed.; John Wiley and Sons, Inc. Publishing: Hoboken, NJ, USA, 2003; pp. 393–453. [Google Scholar]
- Davenport, T.; Stern, R.A. Flowering. In Litchi and Longan: Botany, Cultivation and Uses; Menzel, C.M., Waite, G.K., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 87–113. [Google Scholar]
- Joubert, A.J. Litchi. In Handbook of Fruit Set and Development; Monselise, S.P., Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 233–246. [Google Scholar]
- Stern, R.A.; Gazit, S. Lychee Pollination by the Honeybee. J. Am. Soc. Hortic. Sci. 1996, 121, 152–157. [Google Scholar] [CrossRef] [Green Version]
- Osuna, E.T.; Valenzuela, R.G.; Muy, R.D.; Gardea, B.A.; Villarreal, R.M. Sex Expression and Flower Anatomy of Litchi (Litchi chinensis Sonn.). Rev. Fitotec. Mex. 2008, 31, 51–56. [Google Scholar]
- Stern, R.A.; Kigel, J.; Tomer, E.; Gazit, S. ‘Mauritius’ Lychee Fruit Development and Reduced Abscission after Treatment with the Auxin 2,4,5-TP. J. Am. Soc. Hortic. Sci. 1995, 120, 65–70. [Google Scholar] [CrossRef] [Green Version]
- McConchie, C.A.; Batten, D.J. Floral Biology and Fruit Set in Lychee. In Proceedings of the 2nd National Lychee Symposium, Cairnes, Australia, 21–23 September 1989; pp. 71–74. [Google Scholar]
- Batten, D.J.; McConchie, C.A. Pollination in Lychee. In Proceedings of the Third National Lychee Seminar, Bundaberg, Australia, 25–27 September 1992; pp. 23–28. [Google Scholar]
- Stern, R.A.; Gazit, S.; El Batsri, R.; Degani, C. Pollen Parent Effect on Outcrossing Rate, Yield, and Fruit Characteristics of ‘Floridian’ and ‘Mauritius’ Lychee. J. Am. Soc. Hortic. Sci. 1993, 118, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Degani, C.; Stern, R.A.; El-Batsri, R.; Gazit, S. Pollen Parent Effect on the Selective Abscission of ‘Mauritius’ and ‘Floridian’ Lychee Fruitlets. J. Am. Soc. Hortic. Sci. 1995, 120, 523–526. [Google Scholar] [CrossRef]
- Froneman, I.J.; Bijzet, Z.; Sippel, A.D.; Bower, J.P. Effect of Different Pollen Parents on Fruit Retention and Fruit Characteristics in ‘Wai Chee’ Litchi. Acta Hortic. 2012, 932, 51–58. [Google Scholar] [CrossRef]
- Chu, Y.C.; Lin, T.S.; Chang, J.C. Pollen Effects on Fruit Set, Seed Weight, and Shriveling of ‘73-S-20’ Litchi-with Special Reference to Artificial Induction of Parthenocarpy. HortScience 2015, 50, 369–373. [Google Scholar] [CrossRef] [Green Version]
- Goren, M. The Advantage of Growing New Litchi Varieties. Alon Hanotea 2013, 67, 42–44. [Google Scholar]
- Sapir, G.; Goldway, M.; Stern, R.A. Supplementing Bumblebees to ‘Mauritius’ Lychee Improves Yield. Sci. Hortic. 2019, 251, 162–166. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Kalendar, R.; Antonius, K.; Smýkal, P.; Schulman, A.H. IPBS: A Universal Method for DNA Fingerprinting and Retrotransposon Isolation. Theor. Appl. Genet. 2010, 121, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Feng, J.; Xiang, X.; Wang, J.; Salojärvi, J.; Liu, C.; Wu, Z.; Zhang, J.; Liang, X.; Jiang, Z.; et al. Two Divergent Haplotypes from a Highly Heterozygous Lychee Genome Suggest Independent Domestication Events for Early and Late-Maturing Cultivars. Nat. Genet. 2022, 54, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.A.; Gazit, S. Pollen Viability in Lychee. J. Am. Soc. Hortic. Sci. 1998, 123, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Gazit, S.; Gafni, E. Effect of Hand Pollination with Different Pollen Donors on Initial Fruit-Set in Avocado. Isr. Agresearch 1986, 1, 3–17. [Google Scholar]
- Degani, C.; Goldring, A.; Gazit, S. Pollen Parent Effect on Outcrossing Rate in ‘Hass’ and ‘Fuerte’ Avocado Plots during Fruit Development. J. Amer. Soc. Hort. Sci 1989, 114, 106–111. [Google Scholar]
- Gazit, S.; Degani, C. Reproductive Biology. In The Avocado: Botany, Production, and Uses; Whiley, A.W., Schaffer, B., Wolstenholme, B.N., Eds.; CABI Pub: New York, NY, USA, 2002; p. 416. ISBN 0851993575. [Google Scholar]
- Stahl, P.; Lev Mirom, Y.; Stern, R.A.; Goldway, M. Comparing ‘Iriet’ and ‘Ettinger’ Avocado Cultivars as Pollinators of ‘Hass’ Using SNPs for Paternal Identification. Sci. Hortic. 2019, 248, 50–57. [Google Scholar] [CrossRef]
- McConchie, C.A.; Batten, D.J.; Vivian-Smith, A. Pollination in Lychee. Yearb. Aust. Lychee Grow. Assoc. 1991, 1, 93–96. [Google Scholar]
- Xie, D.-R.; Ma, X.-S.; Rahman, M.Z.; Yang, M.-C.; Huang, X.-M.; Li, J.-G.; Wang, H.-C. Thermo-Sensitive Sterility and Self-Sterility Underlie the Partial Seed Abortion Phenotype of Litchi Chinensis. Sci. Hortic. 2019, 247, 156–164. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Xu, H.-Y.; Wang, H.-C.; Hu, G.; Li, J.-G.; Chen, H.-B.; Huang, X. A Comparison of the Costs of Flowering in ‘Feizixiao’ and ‘Baitangying’ Litchi. Sci. Hortic. 2012, 148, 118–125. [Google Scholar] [CrossRef]
- Sedgley, M.; Griffin, A.R. Sexual Reproduction of Tree Crops; Academic Press: London, UK, 1989. [Google Scholar]
- Wang, J.; Chen, J.; Huang, S.; Han, D.; Li, J.; Guo, D. Investigating the Mechanism of Unilateral Cross Incompatibility in Longan (Dimocarpus longan Lour.) Cultivars (Yiduo × Shixia). Front. Plant Sci. 2022, 12, 821147. [Google Scholar] [CrossRef] [PubMed]
- Cuveas, J.; Polito, V.S. Compatibility Relationships in ‘Manzanillo’ Olive. Hortic. Res. 1997, 32, 1056–1058. [Google Scholar] [CrossRef] [Green Version]
- Sapir, G.; Stern, R.A.; Shafir, S.; Goldway, M. S-RNase Based S-Genotyping of Japanese Plum (Prunus salicina Lindl.) and its Implication on the Assortment of Cultivar-Couples in the Orchard. Sci. Hortic. 2008, 118, 8–13. [Google Scholar] [CrossRef]
- Goldway, M.; Stern, R.A.; Zisovich, A.H.; Shafir, S. Application of S -RNase Allele Molecular Analysis as a Means for Elucidating Low Yields in the Pear Orchard. Acta Hortic. 2005, 671, 137–142. [Google Scholar] [CrossRef]
- Zisovich, A.H.; Stern, R.A.; Shafir, S.; Goldway, M. Identification of Seven S-Alleles from the European Pear (Pyrus communis)and the Determination of Compatibility among Cultivars. J. Hortic. Sci. Biotechnol. 2004, 79, 101–106. [Google Scholar] [CrossRef]
- Schneider, D.; Stern, R.A.; Goldway, M. A Comparison between Semi- and Fully Compatible Apple Pollinators Grown under Suboptimal Pollination Conditions. HortScience 2005, 40, 1280–1282. [Google Scholar] [CrossRef] [Green Version]
- Hajari, E.; Nonyane, D.; Cronje, R. Sequence-Related Amplified Polymorphism Markers—A Tool for Litchi Breeders in Africa. S. Afr. J. Sci. 2020, 116, 1–6. [Google Scholar] [CrossRef]
- Pathak, A.K.; Singh, S.P.; Tuli, R. Amplified Fragment Length Polymorphism Fingerprinting to Identify Genetic Relatedness among Lychee Cultivars and Markers Associated with Small-Seeded Cultivars. J. Am. Soc. Hortic. Sci. 2014, 139, 657–668. [Google Scholar] [CrossRef] [Green Version]
- Tongpamnak, P.; Patanatara, A.; Srinives, P.; Babprasert, C. Determination of Genetic Diversity and Relationships among Thai Litchi Accessions by RAPD and AFLP Markers. Kasetsart J. Nat. Sci. 2002, 36, 370–380. [Google Scholar]
| Year | Orchard | Altitude (m) | Female cv. Tested | Pollinizer | Type of Pollination |
|---|---|---|---|---|---|
| 2016 | Almagor | −200 | MA | HL | OP |
| 2017 | Almagor | −200 | MA | HL | OP |
| Ravid | +200 | MA | HL | OP | |
| Lavi | +300 | MA | HL, KA, NMC | OP | |
| 2018–2020 | Orchard Farm | +100 | MA | HL | Greenhouse |
| 2020 | Moran | +150 | FZX | HL, SK, MA, YR | Net |
| Ravid | +200 | TA | HL, MA, WC, FL | Net | |
| 2021 | Moran | +150 | FZX | HL, MA, SK, KMP | Net |
| Ravid | +200 | TA | HL, MA | OP |
| MA Row (no.) | Distance from HL (m) | 2016 | 2017 | ||
|---|---|---|---|---|---|
| Fruit Set (%) | Yield (kg/Tree) | Fruit Set (%) | Yield (kg/Tree) | ||
| 1 | 6 | 10.3 a | 52 a | 12.5 a | 62 a |
| 2 | 12 | 7.0 b | 36 b | 9.3 ab | 30 b |
| 3 | 18 | 4.5 b | 24 c | 5.0 b | 21 c |
| 4 | 24 | 4.7 b | 28 c | - | - |
| 5 | 30 | 3.8 b | 22 c | - | - |
| 6 | 36 | 4.4 b | 20 c | - | - |
| Greenhouse | HL Pollinator (+/−) | MA Yield | |||||
|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | |||||
| Yield (kg/tree) | Fruit Weight (g) | Yield (kg/tree) | Fruit Weight (g) | Yield (kg/tree) | Fruit Weight (g) | ||
| 1 | − | 25 b | 21.8 b | 20 b | 22.4 a | 23 b | 22.1 b |
| 2 | + | 38 a | 24.6 a | 35 a | 24.5 a | 32 a | 23.9 a |
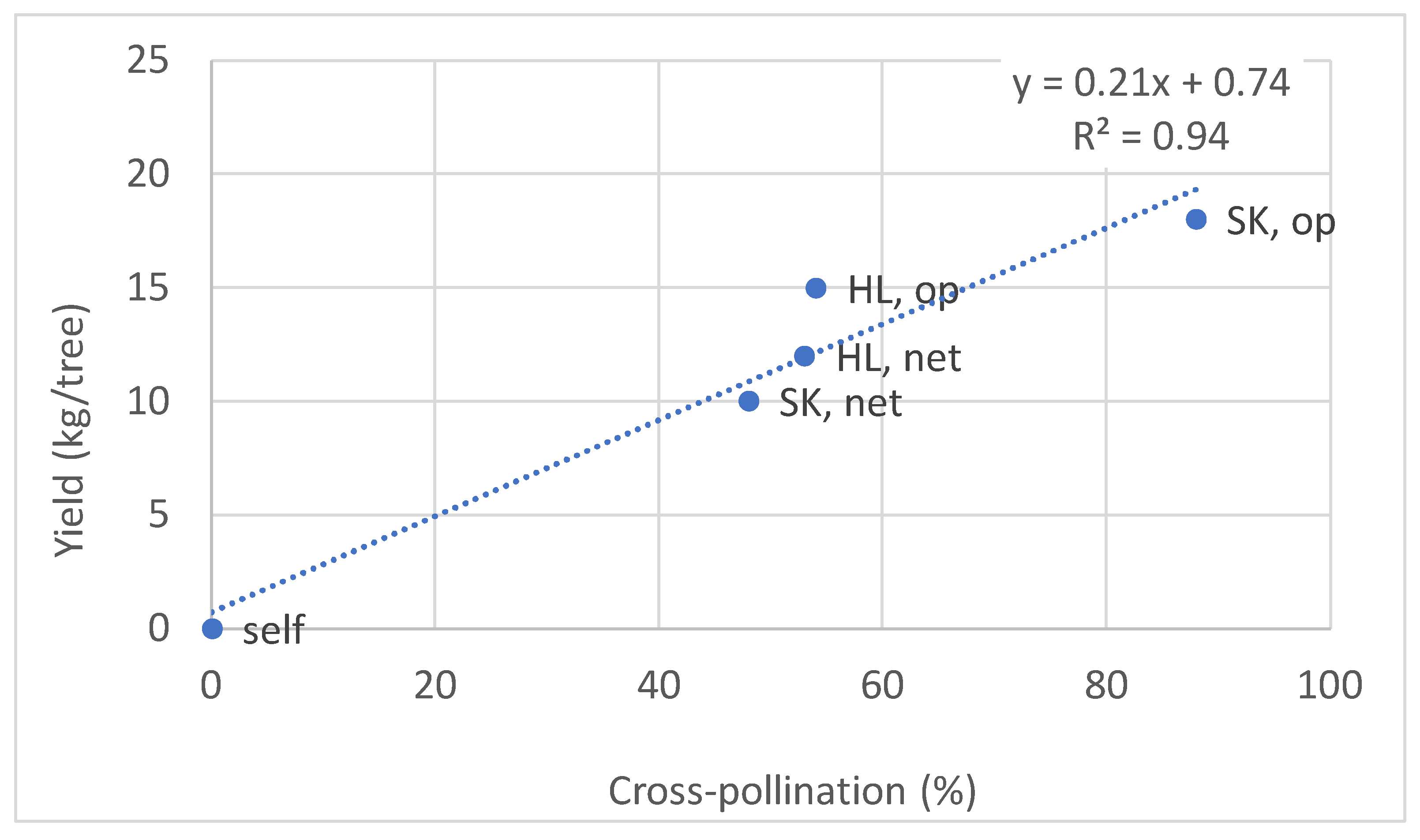
| Orchard Plot | Pollinizers | Cross-Fertilized Progenies (%) | Self-Fertilized Progenies (%) | Yield (kg/Tree) |
|---|---|---|---|---|
| East | FZX, self | 0 | 100 | 0 |
| East | HL, net | 53 | 47 | 12 |
| East | HL, op | 54 | 46 | 15 |
| West | SK, net | 48 | 52 | 10 |
| West | SK, op | 88 | 12 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raz, A.; Goldway, M.; Sapir, G.; Stern, R.A. “Hong Long” Lychee (Litchi chinensis Sonn.) Is the Optimal Pollinizer for the Main Lychee Cultivars in Israel. Plants 2022, 11, 1996. https://doi.org/10.3390/plants11151996
Raz A, Goldway M, Sapir G, Stern RA. “Hong Long” Lychee (Litchi chinensis Sonn.) Is the Optimal Pollinizer for the Main Lychee Cultivars in Israel. Plants. 2022; 11(15):1996. https://doi.org/10.3390/plants11151996
Chicago/Turabian StyleRaz, Amir, Martin Goldway, Gal Sapir, and Raphael A. Stern. 2022. "“Hong Long” Lychee (Litchi chinensis Sonn.) Is the Optimal Pollinizer for the Main Lychee Cultivars in Israel" Plants 11, no. 15: 1996. https://doi.org/10.3390/plants11151996
APA StyleRaz, A., Goldway, M., Sapir, G., & Stern, R. A. (2022). “Hong Long” Lychee (Litchi chinensis Sonn.) Is the Optimal Pollinizer for the Main Lychee Cultivars in Israel. Plants, 11(15), 1996. https://doi.org/10.3390/plants11151996





