Genetic Diversity and Population Structure of Jubaea chilensis, an Endemic and Monotype Gender from Chile, Based on SNP Markers
Abstract
:1. Introduction
2. Results
2.1. Statistical Analysis and Genetic Diversity
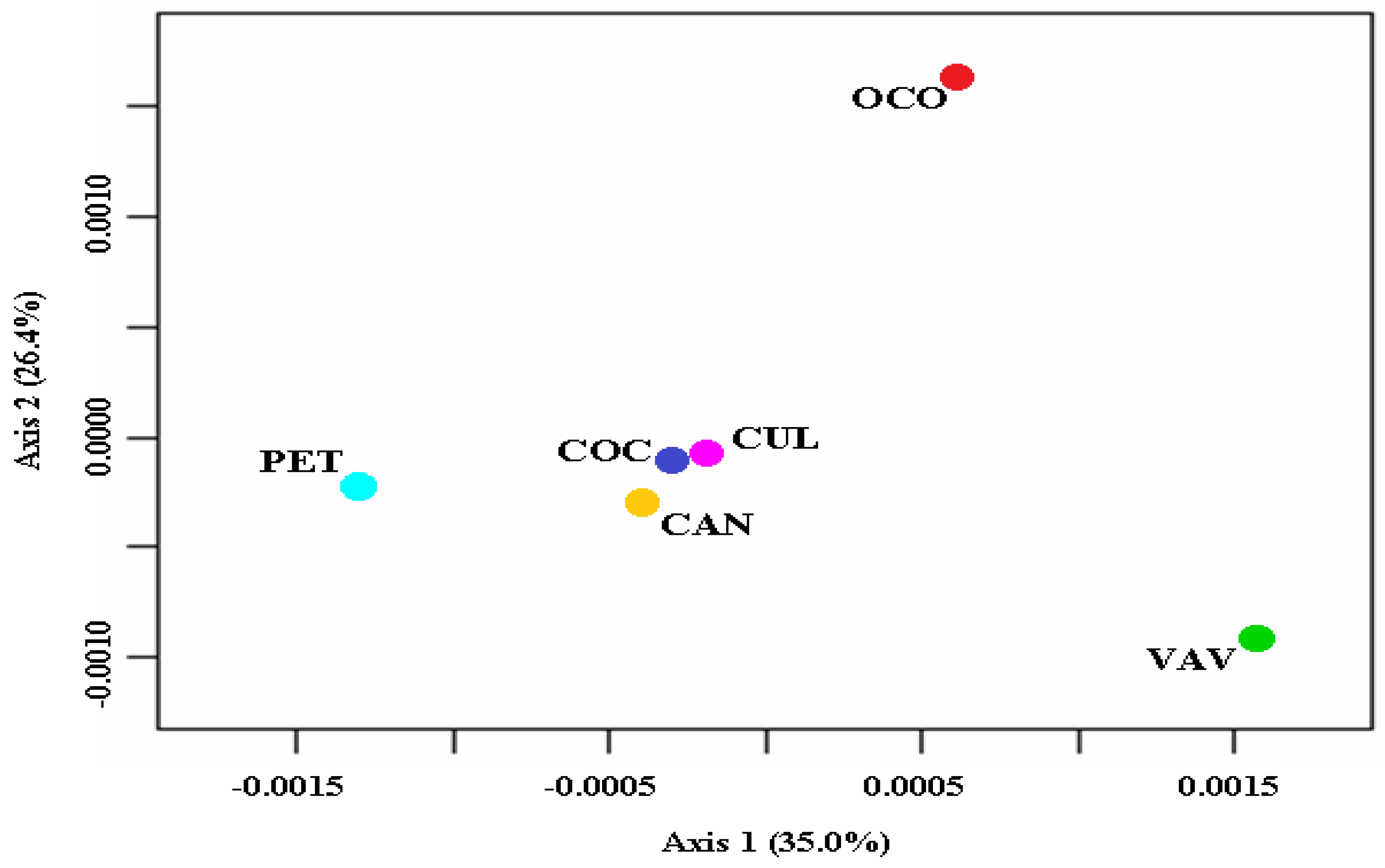
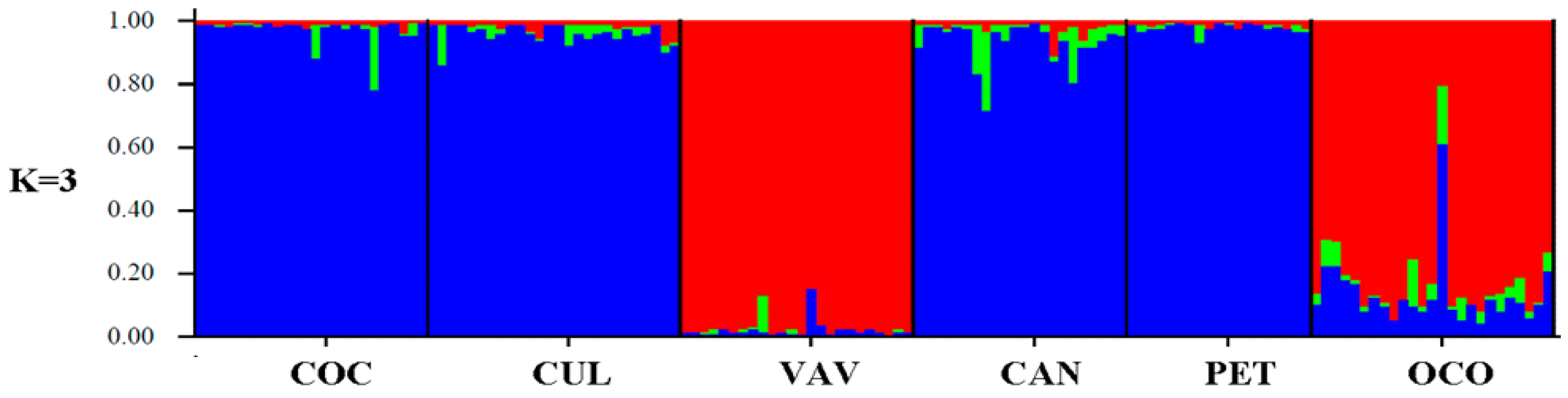
2.2. Genetic Structure and Admixture Levels
3. Discussion
4. Conclusions
5. Material and Methods
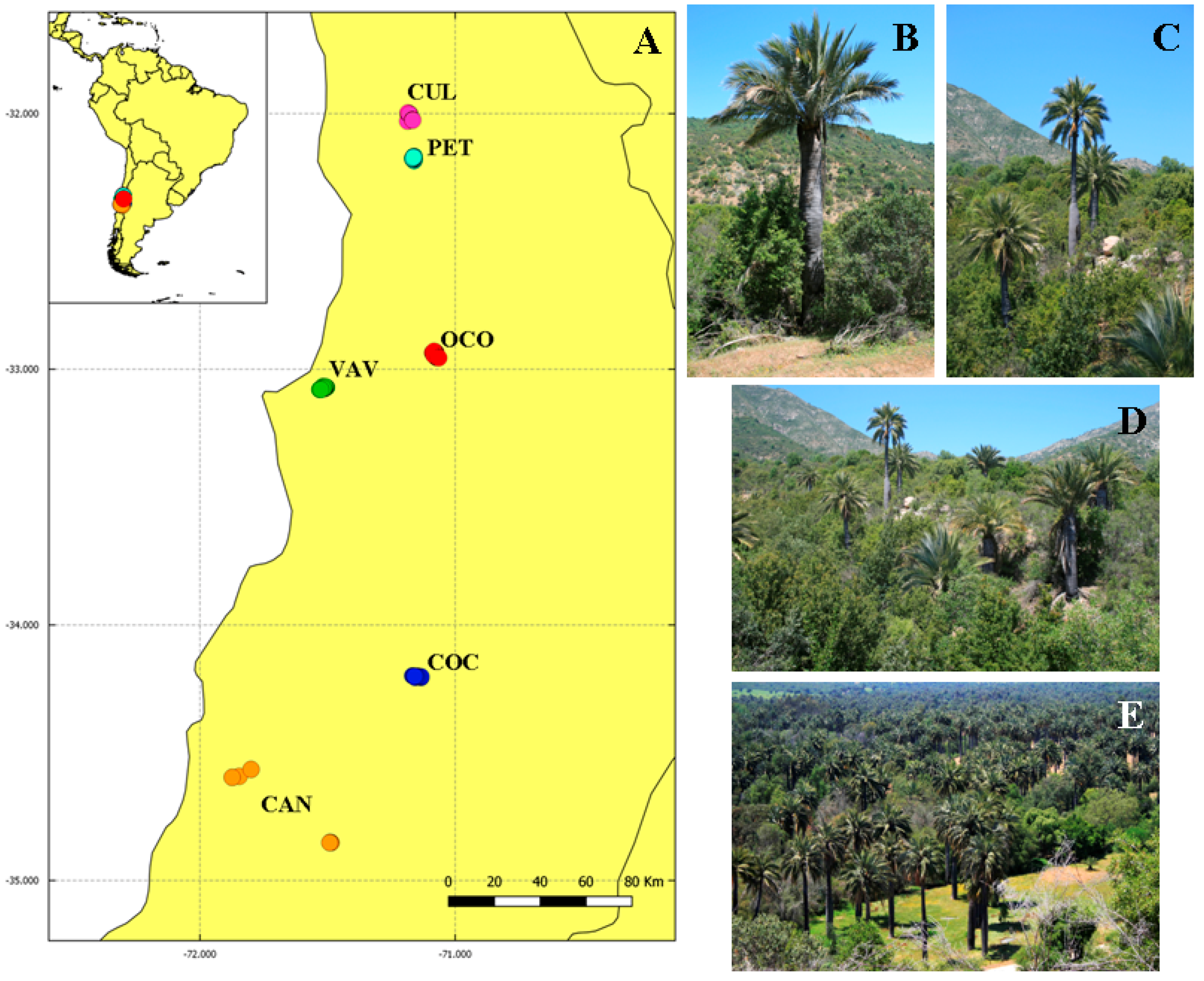
5.1. Study Organism
5.2. Study Area and Sampling
5.3. Genotyping-by-Sequencing Library and Single Nucleotide Polymorphisms’ Selection
5.4. Genetic Analysis
5.5. Genetic Structure and Admixture Levels
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef]
- Hughes, A.R.; Inouye, B.D.; Johnson, M.T.J.; Underwood, N.; Vellend, M. Ecological consequences of genetic diversity. Ecol. Lett. 2008, 11, 609–623. [Google Scholar] [CrossRef]
- Aguilar, R.; Quesada, M.; Ashworth, L.; Herrerias-Diego, Y.; Lobo, J. Genetic consequences of habitat fragmentation in plant populations: Susceptible signals in plant traits and methodological approaches. Mol. Ecol. 2008, 17, 5177–5188. [Google Scholar] [CrossRef]
- Fageria, M.S.; Rajora, O.P. Effects of harvesting of increasing intensities on genetic diversity and population structure of white spruce. Evol. Appl. 2013, 6, 778–794. [Google Scholar] [CrossRef]
- Dirzo, R.; Miranda, A. Altered patterns of herbivory and diversity in the forest understory: A case study of the possible consequences of contemporary defaunation. In Plant–Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions; Price, P.W., Ed.; Wiley: New York, NY, USA, 1991; pp. 273–287. [Google Scholar]
- Wright, S.J. The myriad consequences of hunting for vertebrates and plants in tropical forests. Perspect. Plant Ecol. Evol. Syst. 2003, 6, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, L.F.; Simonetti, J.A. Genetic structure of a mimosid tree deprived of it seed disperser, the spider monkey. Conserv. Biol. 2000, 14, 1766–1775. [Google Scholar] [CrossRef]
- Carvalho, C.S.; Galetti, M.; Colevatti, R.G.; Jordano, P. Defaunation leads tomicroevolutionary changes in a tropical palm. Sci. Rep. 2016, 6, 31957. [Google Scholar] [CrossRef] [Green Version]
- Giombini, M.I.; Bravo, S.P.; Sica, Y.V.; Tosto, D.S. Early genetic consequences of defaunation in a large-seeded vertebrate-dispersed palm (Syagrus romanzoffiana). Heredity 2017, 118, 568–577. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Méndez, N.; Jordano, P.; Valido, A. Persisting in defaunated landscapes: Reduced plant population connectivity after seed dispersal collapse. J. Ecol. 2018, 106, 936–947. [Google Scholar] [CrossRef]
- Moore, H.E. Endangerment at the specific and generic level in palms. Principes 1979, 23, 47–64. [Google Scholar]
- Henderson, A.; Galeano, G.; Bernal, R. Field Guide to the Palms of the Americas; Princeton University Press: Princeton, NJ, USA, 1995; ISBN 9780691656120. [Google Scholar]
- Zambrana, N.Y.P.; Byg, A.; Svenning, J.C.; Moraes, M.; Grandez, C.; Balslev, H. Diversity of palm uses in the western Amazon. Biodivers. Conserv. 2007, 16, 2771–2787. [Google Scholar] [CrossRef]
- Terborgh, J. Keystone plant resources in the tropical forest. In Conservation Biology; Soulé, M.E., Ed.; Sinauer: Sunderland, MA, USA, 1989. [Google Scholar]
- Kinnaird, M.F. Competition for a forest palm: Use of Phoenix reclinata by human and non human primates. Conserv. Biol. 1992, 6, 101–107. [Google Scholar] [CrossRef]
- Howe, H.F.; Westley, L.C. Ecological Relationships of Plants and Animals; Oxford University Press: New York, NY, USA, 1988; ISBN 100195063147. [Google Scholar]
- Polis, G.A.; Sears, A.L.W.; Huxel, G.X.; Strong, D.R.; Maron, J. When is a trophic cascade a trophic cascade? Trends Ecol. Evol. 2000, 15, 473–475. [Google Scholar] [CrossRef]
- Macía, M.J. Multiplicity in palm uses by the Huaorani of Amazonian Ecuador. Bot. J. Linn. 2004, 144, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Cayuela, L.; Golicher, D.J.; Benayas, J.M.R.; González-Espinoza, M.; Ramírez-Marcial, N. Fragmentation, disturbance and tree diversity in tropical montane forests. J. Appl. Ecol. 2006, 43, 1172–1181. [Google Scholar] [CrossRef]
- Zuidema, P.A.; De Kroon, H.; Werger, M.J. Testing sustainability by prospective and retrospective demographic analyses: Evaluation for palm leaf harvest. Ecol. Appl. 2007, 17, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Villagrán, C.M. Quaternary History of the Mediterranean Vegetation of Chile. In Ecology and Biogeography of Mediterranean Ecosystems in Chile, California, and Australia; Arroyo, M.T.K., Zedler, P.H., Fox, M.D., Eds.; Ecological Studies (Analysis and Synthesis); Springer: New York, NY, USA, 1995; Volume 108. [Google Scholar]
- Luebert, F.; Pliscoff, P. Sinopsis Bioclimática y Vegetacional de Chile; Editorial Universitaria: Santiago, Chile, 2006; ISBN 9789561125759. [Google Scholar]
- Moreno-Chacón, M.; Mardones, D.; Viveros, N.; Madriaza, K.; Carrasco-Urra, F.; Marticorena, A.; Baeza, B.; Rodríguez, R.; Saldaña, A. Flora vascular de un remanente de bosque esclerófilo mediterráneo costero: Estación de Biología Terrestre de Hualpén, Región del Biobío, Chile. Gayana Bot. 2018, 75, 466–481. [Google Scholar] [CrossRef]
- Zuloaga, F.O.; Morrone, O.; Belgrado, M.L. Catálogo de las Plantas Vasculares del Cono Sur (Argentina, Sur de Brasil, Chile, Paraguay y Uruguay); Missouri Botanical Garden Press: St. Louis, MO, USA, 2008; ISBN 1930723768. [Google Scholar]
- González, L.A.; Bustamante, R.O.; Navarro, R.M.; Herrera, M.A.; Ibáñez, M.T. Ecology and management of the Chilean Palm (Jubaea chilensis): History, current situation and perspectives. Palms 2009, 53, 68–74. Available online: http://repositorio.uchile.cl/handle/2250/120359 (accessed on 16 June 2022).
- González, L.A.; Toral, M.A.; Navarro, R. Palma chilena: Una Especie Emblemática que Chile Necesita Recuperar; 2017; ISBN 9789563161908. Available online: https://www.aminerals.cl/media/5322/palma-chilena-una-especie-emblematica-que-chile-necesita-recuperar.pdf (accessed on 16 June 2022).
- Edwards, A. Ejemplares gigantescos de la Palma chilena (Jubaea chilensis (Molina) Baill.). Rev. Chil. Hist. Nat. 1903, 7, 254. [Google Scholar]
- Rodríguez, R.; Matthei, O.; Quezada, M. Flora Arbórea de Chile. Editorial de la Universidad de Concepción, Chile; Editorial de la Universidad de Concepción: Concepción, Chile, 1983. [Google Scholar]
- Angulo, J. La Palma chilena: Interesante Recurso Natural Renovable; Sociedad Agrícola y Forestal Hacienda Las Palmas de Cocalán Ltd.a. Impreso en Origo China: Santiago, Chile, 1985; ISBN 9789563161908. [Google Scholar]
- Fleury, M.; Marcelo, W.; Vásquez, R.A.; González, L.A.; Bustamante, R.O. Recruitment Dynamics of the Relict Palm, Jubaea chilensis: Intricate and Pervasive Effects of Invasive Herbivores and Nurse Shrubs in Central Chile. PLoS ONE 2015, 10, e0133559. [Google Scholar] [CrossRef]
- Carrasco, G. Mamíferos Fósiles de Chile; Auto Ediciones: Talcahuano, Chile, 2013; ISBN 9789563321616. [Google Scholar]
- Perez, H.E.; Shiels, A.B.; Zaleski, H.M.; Drake, D.R. Germination after simulated rat damage in seeds of two endemic Hawaiian palm species. J. Trop. Ecol. 2008, 24, 555–558. [Google Scholar] [CrossRef]
- Maron, J.L.; Pearson, D.E. Vertebrate predators have minimal cascading effects on plant production or seed predation in an intact grassland ecosystem. Ecol. Lett. 2011, 14, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Cordero, S.; Gálvez, F.; Fontúrbel, F.E. Multiple anthropogenic pressures lead to seed dispersal collapse of the southernmost palm Jubaea chilensis. Front. Ecol. Evol. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Vander-Wall, S.B.; Longland, W.S. Diplochory: Are two seed dispersers better than one? Trends Ecol. Evol. 2004, 19, 155–161. [Google Scholar] [CrossRef]
- Sezen, U.U.; Chazdon, R.L.; Holsinger, K.E. Genetic consequences of tropical second-growth forest regeneration. Science 2005, 307, 891. [Google Scholar] [CrossRef] [Green Version]
- Montúfar, R.; Anthelme, F.; Pintaud, J.C.; Balslev, H. Disturbance and resilience in tropical american palm populations and communities. Bot. Rev. 2011, 77, 426–461. [Google Scholar] [CrossRef]
- Kremer, A.; Ronce, O.; Robledo-Arnuncio, J.J.; Guillaume, F.; Bohrer, G.; Nathan, R.; Bridle, J.R.; Gomulkiewicz, R.; Klein, E.K.; Ritland, K.; et al. Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecol. Lett. 2012, 15, 378–392. [Google Scholar] [CrossRef] [Green Version]
- White, G.M.; Boshier, D.H.; Powell, W. Genetic variation within a fragmented population of Swietenia humilis Zucc. Mol. Ecol. 1999, 8, 1899–1909. [Google Scholar] [CrossRef]
- Lowe, A.J.; Boshier, D.; Ward, D.; Bacles, C.F.E.; Navarro, C. Genetic resource impacts of habitat loss and degradation; reconciling empirical evidence and predicted theory for neotropical trees. Heredity 2005, 95, 255–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nora, S.; Albaladejo, R.; González-Martínez, S.; Robledo-Arnuncio, J.; Aparicio, A. Movimiento de genes (polen y semillas) en poblaciones fragmentadas de plantas. Rev. Ecosistemas. 2011, 20, 35–45. [Google Scholar] [CrossRef]
- Donoso, C. Monografía de la Palma chilena. Postítulo en Arquitectura y Manejo del Paisaje; Facultad de Arquitectura y Bellas Artes, Pontificia Universidad Católica de Chile: Santiago, Chile, 1992. [Google Scholar]
- Shapcott, A. Comparison of the population genetics and densities of five Pinanga palm species at Kuala Belalong, Brunei. Mol. Ecol. 2002, 8, 1641–1654. [Google Scholar] [CrossRef]
- Cibrián-Jaramillo, A.; Bacon, C.D.; Garwood, N.C.; Bateman, R.M.; Thomas, M.M.; Russell, S.; Bailey, D.; Hahn, W.J.; Bridgewater, S.G.M.; DeSalle, R. Population genetics of the understory fishtail palm Chamaedorea ernesti-augusti in Belize: High genetic connectivity with local differentiation. BMC Genet. 2009, 10, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, R.R.M.; Cavallari, M.M.; Pimenta, M.A.S.; Abreu, A.G.; Costa, M.R.; Guedes, M.L. Population genetic structure of Attalea vitrivir Zona (Arecaceae) in fragmented areas of southeast Brazil. Genet. Mol. 2014, 14, 6472–6481. [Google Scholar] [CrossRef]
- Lanes, E.C.M.; Motoike, S.Y.; Kuki, K.N.; Nick, C.; Freitas, R.D. Molecular Characterization and Population Structure of the Macaw Palm, Acrocomia aculeata (Arecaceae), Ex Situ Germplasm Collection Using Microsatellites Markers. J. Hered. 2015, 106, 102–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanes, E.C.M.; Motoike, S.Y.; Kuki, K.N.; Resende, M.D.V.; Caixeta, E.T. 2016 Mating System and Genetic Composition of the Macaw Palm (Acrocomia aculeata): Implications for Breeding and Genetic Conservation Programs. J. Hered. 2016, 107, 527–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mengistu, F.G.; Motoike, S.Y.; Cruz, C.D. Molecular Characterization and Genetic Diversity of the Macaw Palm Ex Situ Germplasm Collection Revealed by Microsatellite Markers. Diversity 2016, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, S.N.; Almeida, C. Genome survey and development of 15 microsatellite molecular markers in Syagrus coronata (Martius) Beccari (Arecaceae) by next-generation sequencing. Rev. Bras. Bot. 2019, 42, 195–200. [Google Scholar] [CrossRef]
- Rubinstein, A. Inventario y Estudio de Producción de un Rodal de Palma chilena, Jubaea chilensis (Mol.) Baillon. (Hacienda Ocoa, Provincia de Valparaíso). Tesis Ingeniería Forestal; Dpto. de Manejo de Recursos Forestales, Universidad de Chile. Fac. de Cs. Agrarias y Forestales: Santiago, Chile, 1969. [Google Scholar]
- Bascuñán, A. La palma, su cultivo y utilización en Chile. Bol. Soc. Nac. Agr. 1889, 20, 451–455, 489–492, 531–536, 570–574, 602–604. [Google Scholar]
- Crawley, M.J. Sex. In En Plant Ecology; Crawley, M.J., Ed.; Blackwell Science: Oxford, UK, 1997. [Google Scholar] [CrossRef]
- Young, A.G.; Clarke, G.M. Genetics, Demography and Viability of Fragmented Populations; Cambridge University Press: Cambridge, UK, 2000; ISBN 0521794218. [Google Scholar]
- Picó, F.; Quintana-Ascencio, P.F. Análisis de factores demográficos y genéticos para la conservación de poblaciones de plantas en un hábitat fragmentado. Ecosistemas 2005, 14, 108–115. [Google Scholar] [CrossRef]
- Clement, C.R.; Santos, R.P.; Desmouliere, S.J.M.; Ferreira, E.J.L.; Farias-Neto, J.T. Ecological Adaptation of Wild Peach Palm, Its In Situ Conservation and Deforestation-Mediated Extinction in Southern Brazilian Amazonia. PLoS ONE 2009, 4, e4564. [Google Scholar] [CrossRef] [Green Version]
- Galetti, M.; Donatti, C.I.; Pires, A.S.; Guimarães, P.R., Jr.; Jordano, P. Seed survival and dispersal of an endemic Atlantic forest palm: The combined effects of defaunation and forest fragmentation. Bot. J. Linn. 2006, 151, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, A.; Dirzo, R. Effects of fragmentation on pollinator abundance and fruit set of an abundant understory palm in a Mexican tropical forest. Biol. Conserv. 2008, 141, 375–384. [Google Scholar] [CrossRef]
- Borchsenius, F.; Borgtoft-Pedersen, H.; Balslev, H. Manual to the Palms of Ecuador; University of Aarhus: Aarhus, Denmark, 1998. [Google Scholar]
- Anthelme, F.; Lincango, J.; Gully, C.; Duarte, N.; Montúfar, R. How anthropogenic disturbances affect the resilience of a keystone palm tree in the threatened Andean cloud forest? Biol. Conserv. 2011, 144, 1059–1067. [Google Scholar] [CrossRef]
- Ben Abdallah, H.; Laajimi, A.; Guesmi, F.; Triki, T.; Ferchichi, A.; Hormaza, J.I.; Larranaga, N. Genetic diversity of endangered date palm (Phoenix dactylifera L.) in the oases of Nefzaoua, Tunisia, using SSR markers. Fruits 2020, 75, 84–91. [Google Scholar] [CrossRef]
- Zellmer, A.J.; Knowles, L.L. Disentangling the effects of historic vs contemporary landscape structure on population genetic divergence. Mol. Ecol. 2009, 18, 3593–3602. [Google Scholar] [CrossRef]
- Serra, M.T.; Gajardo, R.; Cabello, A. Jubaea chilensis (Mol.) Baillon. “Palma chilena” (Palmaceae). Especie Vulnerable. Santiago. Programa de Protección y Recuperación de la Flora Nativa de Chile; Depto. Silvicultura y Manejo. Ficha Técnica de Especies Amenazadas, Facultad de Ciencias Agrarias y Forestales, Corporación Nacional Forestal—Universidad de Chile: Santiago, Chile, 1986. [Google Scholar]
- Pires, M.M.; Guimarães, P.R.; Galetti, M.; Jordano, P. Pleistocene megafaunal extinctions and the functional loss of long-distance seed-dispersal services. Ecography 2018, 41, 153–163. [Google Scholar] [CrossRef] [Green Version]
- González, L.; Vita, A. Palma: Usos, regeneración y tratamientos. Rev. Campo. 1987, 11, 14–15. [Google Scholar]
- Infante, L. Estudio de la Germinación de la Palma chilena (Jubaea chilensis (Mol.) Baillón. Memoria de Esc; Ingeniería Forestal U. de Chile: Santiago, Chile, 1989. [Google Scholar]
- Saiz, F.; Antivilo, L.; Silva, I. Estudio sobra capacidad germinativa y viabilidad temporal de semillas de Jubaea chilensis. Bol. Cient. Mus. Hist. Nat. 1989, 21, 19–28. [Google Scholar]
- Cabello, A. Determinación del Contenido de Humedad y de la Germinación de Semillas de Jubaea chilensis en el Vivero y en el Laboratorio y de Embriones Extirpados Cultivados In Vitro. Notas del Centro Productor de Semillas de Árboles Forestales. 1999. Available online: www.uchile.cl/facultades/cs_forestales/publicaciones/cesaf/n11/2.html (accessed on 1 January 2021).
- Lewin, P. Ensayos de Fertilización para el Establecimiento de Palma chilena Jubaea chilensis (Mol.) Baillon. Tesis de Escuela de Ciencias Forestales; Universidad de Chile: Santiago, Chile, 2003. [Google Scholar]
- Flanagan, S.P.; Forester, F.R.; Latch, E.K.; Aitken, S.N.; Hoban, S. Guidelines for planning genomic assessment and monitoring of locally adaptive variation to inform species conservation. Evol. Appl. 2018, 11, 1035–1052. [Google Scholar] [CrossRef]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A Robust, Simple Genotyping-by-Sequencing (GBS) Approach for High Diversity Species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef] [Green Version]
- Eaton, D.A.R. PyRAD: Assembly of de novo RADseq loci for phylogenetic analyses. Bioinformatics 2014, 30, 1844–1849. [Google Scholar] [CrossRef]
- Foll, M.; Gaggiotti, G. A Genome-Scan Method to Identify Selected Loci Appropriate for Both Dominant and Codominant Markers: A Bayesian Perspective. Genetics 2008, 180, 977–993. [Google Scholar] [CrossRef] [Green Version]
- Antao, T.; Lopes, A.; Lopes, R.J.; Beja-Pereira, A.; Luikart, G. LOSITAN: A workbench to detect molecular adaptation based on a F(st)-outlier method. BMC Bioinform. 2008, 9, 323. [Google Scholar] [CrossRef] [Green Version]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. Group 1000 Genomes Project Analysis. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Xuereb, A.; Kimber, C.M.; Curtis, J.M.R.; Bernatchez, L.; Fortin, M.J. Putatively adaptive genetic variation in the giant California sea cucumber (Parastichopus californicus) as revealed by environmental association analysis of restriction-site associated DNA sequencing data. Mol. Ecol. 2018, 27, 5035–5048. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef]
- Gruber, B.; Unmack, P.J.; Berry, O.F.; Georges, A. DARTR: An r package to facilitate analysis of SNP data generated from reduced representation genome sequencing. Mol. Ecol. Resour. 2018, 18, 691–699. [Google Scholar] [CrossRef]
- Jombart, T. adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [Green Version]
- Jombart, T.; Ahmed, I. adegenet 1.3-1: New tools for the analysis of genome-wide SNP data. Bioinformatics 2011, 27, 3070–3071. [Google Scholar] [CrossRef] [Green Version]
- Hijmans, R.J. Geosphere: Spherical Trigonometry. R package version 1.5 10. 2009. Available online: https://CRAN.R-project.org/package=geosphere (accessed on 1 January 2021).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. Vegan: Community Ecology Package. R package version 2.5-6. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 January 2021).
- Nei, M. Genetic distance between populations. Am. Nat. 1972, 106, 283–292. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Dakin, E.; Avise, J. Microsatellite null alleles in parentage analysis. Heredity 2004, 93, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Mussmann, S.M.; Douglas, M.R.; Chafin, T.K.; Douglas, M.E. BA3-SNPs: Contemporary migration reconfigured in BayesAss for next-generation sequence data. Methods Ecol. Evol. 2019, 10, 1808–1813. [Google Scholar] [CrossRef] [Green Version]
- Meirmans, P.G. Nonconvergence in B ayesian estimation of migration rates. Mol. Ecol. Resour. 2014, 14, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Faubet, P.; Waples, R.S.; Gaggiotti, O.E. Evaluating the performance of a multilocus Bayesian method for the estimation of migration rates. Mol. Ecol. 2007, 6, 1149–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Dominant markers and null alleles. Mol. Ecol Notes 2007, 7, 574–578. [Google Scholar] [CrossRef]
- Earl, D.A.; Vonholdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudetet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
| Populational Grouping | Sample No | HO [95% CI] | HE [95% CI] | FIS [95% CI] |
|---|---|---|---|---|
| CUL | 26 | 0.036 [0.027–0.045] | 0.086 [0.086–0.087] | 0.586 [0.484–0.689] |
| PET | 19 | 0.108 [0.090–0.127] | 0.145 [0.144–0.145] | 0.251 [0.123–0.379] |
| OCO | 25 | 0.065 [0.058–0.073] | 0.101 [0.100–0.101] | 0.351 [0.276–0.425] |
| VAV | 24 | 0.107 [0.098–0.116] | 0.130 [0.130–0.131] | 0.177 [0.109–0.244] |
| COC | 24 | 0.061 [0.051–0.070] | 0.105 [0.104–0.106] | 0.420 [0.330–0.510] |
| CAN | 22 | 0.045 [0.039–0.051] | 0.107 [0.106–0.107] | 0.581 [0.528–0.633] |
| Overall | 140 | 0.014 [0.012–0.015] | 0.024 [0.023–0.024] | 0.424 [0.374–0.475] |
| CUL | PET | OCO | VAV | COC | CAN | |
|---|---|---|---|---|---|---|
| CUL | 0.0018 | 0.0020 | 0.0021 | 0.0015 | 0.0017 | |
| PET | 0.078 * | 0.0028 | 0.0030 | 0.0023 | 0.0025 | |
| OCO | 0.054 * | 0.080 * | 0.0027 | 0.0024 | 0.0026 | |
| VAV | 0.072 * | 0.113 * | 0.065 * | 0.0026 | 0.0027 | |
| COC | 0.063 * | 0.119 * | 0.074 * | 0.100 * | 0.0019 | |
| CAN | 0.049 * | 0.078 * | 0.059 * | 0.078 * | 0.068 * |
| Receiver/Source | CUL | PET | OCO | VAV | COC | CAN |
|---|---|---|---|---|---|---|
| CUL | 0.677 [0.657–0.696] | 0.010 [−0.009;0.030] | 0.010 [−0.009;0.031] | 0.010 [−0.009;0.030] | 0.280 [0.238;0.322] | 0.010 [−0.009;0.030] |
| PET | 0.013 [−0.011;0.037] | 0.680 [0.654–0.705] | 0.013 [−0.012;0.038] | 0.013 [−0.011;0.038] | 0.266 [0.215;0.317] | 0.013 [−0.011;0.038] |
| OCO | 0.010 [−0.009;0.031] | 0.010 [−0.009;0.030] | 0.688 [0.659–0.716] | 0.268 [0.222;0.314] | 0.010 [−0.009;0.029] | 0.010 [−0.009;0.030] |
| VAV | 0.011 [−0.009;0.031] | 0.011 [−0.009;0.032] | 0.011 [−0.009;0.031] | 0.932 [0.884–0.981] | 0.022 [−0.008;0.053] | 0.011 [−0.010;0.032] |
| COC | 0.011 [−0.010;0.032] | 0.011 [−0.009;0.032] | 0.011 [−0.010;0.032] | 0.010 [−0.009;0.031] | 0.944 [0.900–0.987] | 0.011 [−0.010;0.032] |
| CAN | 0.011 [−0.010;0.034] | 0.012 [−0.010;0.034] | 0.011 [−0.010;0.034] | 0.011 [−0.010;0.034] | 0.237 [0.181;0.293] | 0.714 [0.672–0.757] |
| Population Groups | Code | Lat/Long Coordinates | Climate | No. Specimens | No. Sampled Individuals |
|---|---|---|---|---|---|
| Culimo. Monte Aranda, Culimo, and El Naranjo | CUL | 32°00′–71°11′ | XO | 204 | 26 |
| Petorca. Túnel de Las Palmas and Las Palmas de Pedégua | PET | 32°09′–71°09′ | XO | 1300 | 19 |
| Ocoa. Parque La Campana, Hacienda Las Palmas del Ocoa, Oasis La Campana and Vichiculén-Llay Llay | OCO | 32°57′–71°04′ | PO | 70,308 | 25 |
| Viña del Mar/Valparaíso. Palmar Hacienda las Siete Hermanas and Subida Santos Ossas | VAV | 33°04′–71°31′ | PO | 7200 | 24 |
| Cocalán. Hacienda Las Palmas de Cocalán and La Palmería | COC | 34°12′–71°08′ | PO | 35,500 | 24 |
| Candelaria. Palmar de Candelaria | CAN | 34°51′–71°29′ | PO | 1900 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jara-Arancio, P.; da Silva Carvalho, C.; Carmona-Ortiz, M.R.; Bustamante, R.O.; Schmidt Villela, P.M.; da Silva Andrade, S.C.; Peña-Gómez, F.T.; González, L.A.; Fleury, M. Genetic Diversity and Population Structure of Jubaea chilensis, an Endemic and Monotype Gender from Chile, Based on SNP Markers. Plants 2022, 11, 1959. https://doi.org/10.3390/plants11151959
Jara-Arancio P, da Silva Carvalho C, Carmona-Ortiz MR, Bustamante RO, Schmidt Villela PM, da Silva Andrade SC, Peña-Gómez FT, González LA, Fleury M. Genetic Diversity and Population Structure of Jubaea chilensis, an Endemic and Monotype Gender from Chile, Based on SNP Markers. Plants. 2022; 11(15):1959. https://doi.org/10.3390/plants11151959
Chicago/Turabian StyleJara-Arancio, Paola, Carolina da Silva Carvalho, Martín R. Carmona-Ortiz, Ramiro O. Bustamante, Priscilla M. Schmidt Villela, Sónia C. da Silva Andrade, Francisco T. Peña-Gómez, Luís A. González, and Marina Fleury. 2022. "Genetic Diversity and Population Structure of Jubaea chilensis, an Endemic and Monotype Gender from Chile, Based on SNP Markers" Plants 11, no. 15: 1959. https://doi.org/10.3390/plants11151959
APA StyleJara-Arancio, P., da Silva Carvalho, C., Carmona-Ortiz, M. R., Bustamante, R. O., Schmidt Villela, P. M., da Silva Andrade, S. C., Peña-Gómez, F. T., González, L. A., & Fleury, M. (2022). Genetic Diversity and Population Structure of Jubaea chilensis, an Endemic and Monotype Gender from Chile, Based on SNP Markers. Plants, 11(15), 1959. https://doi.org/10.3390/plants11151959







