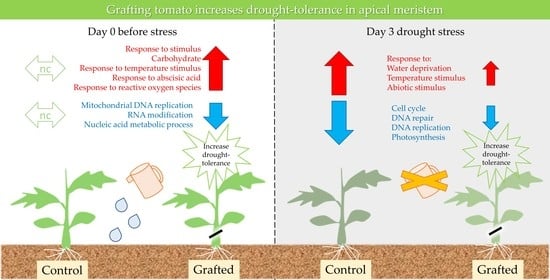
Comparative Transcriptome Analysis of Grafted Tomato with Drought Tolerance
Abstract
:1. Introduction
2. Results
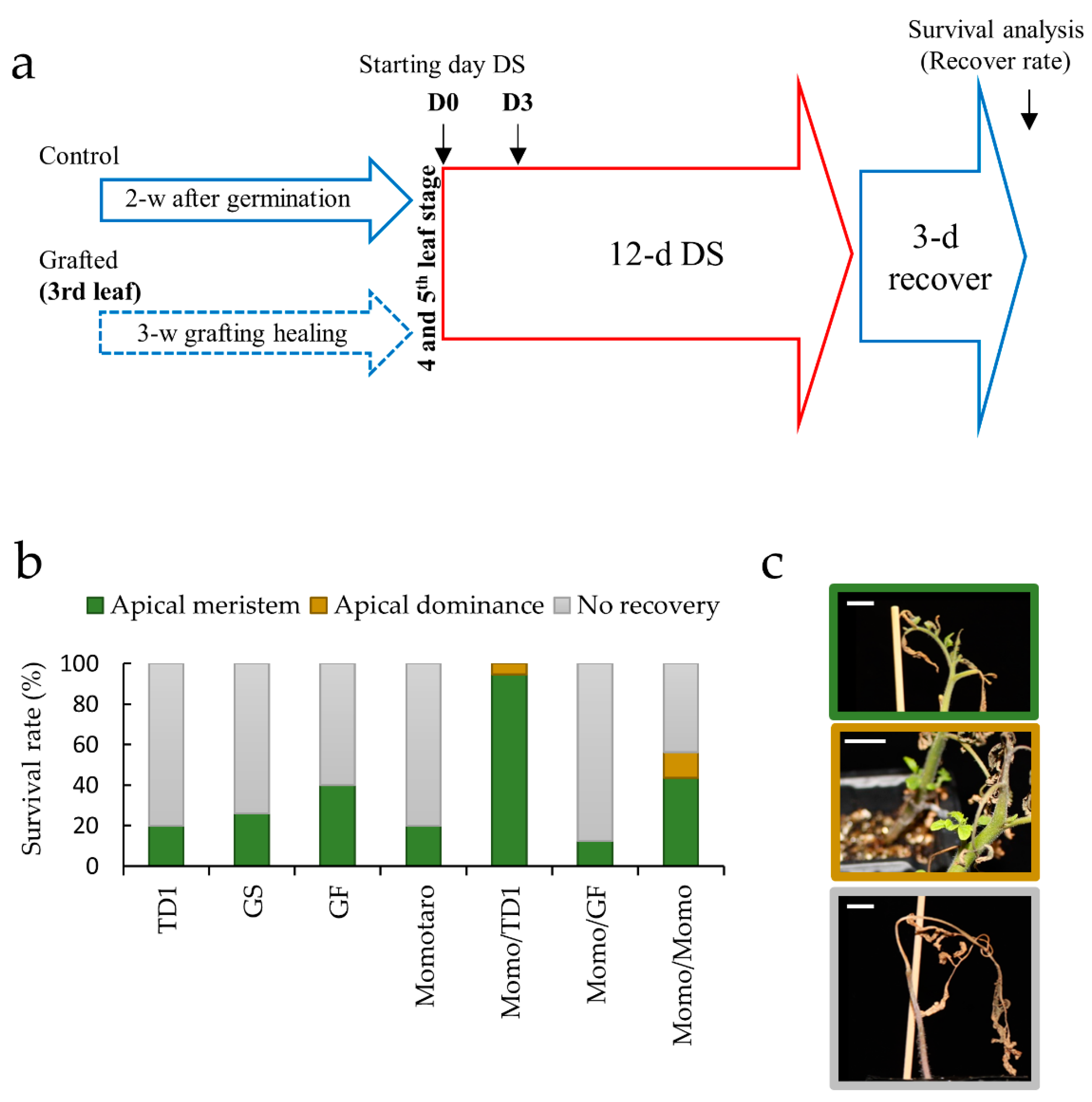
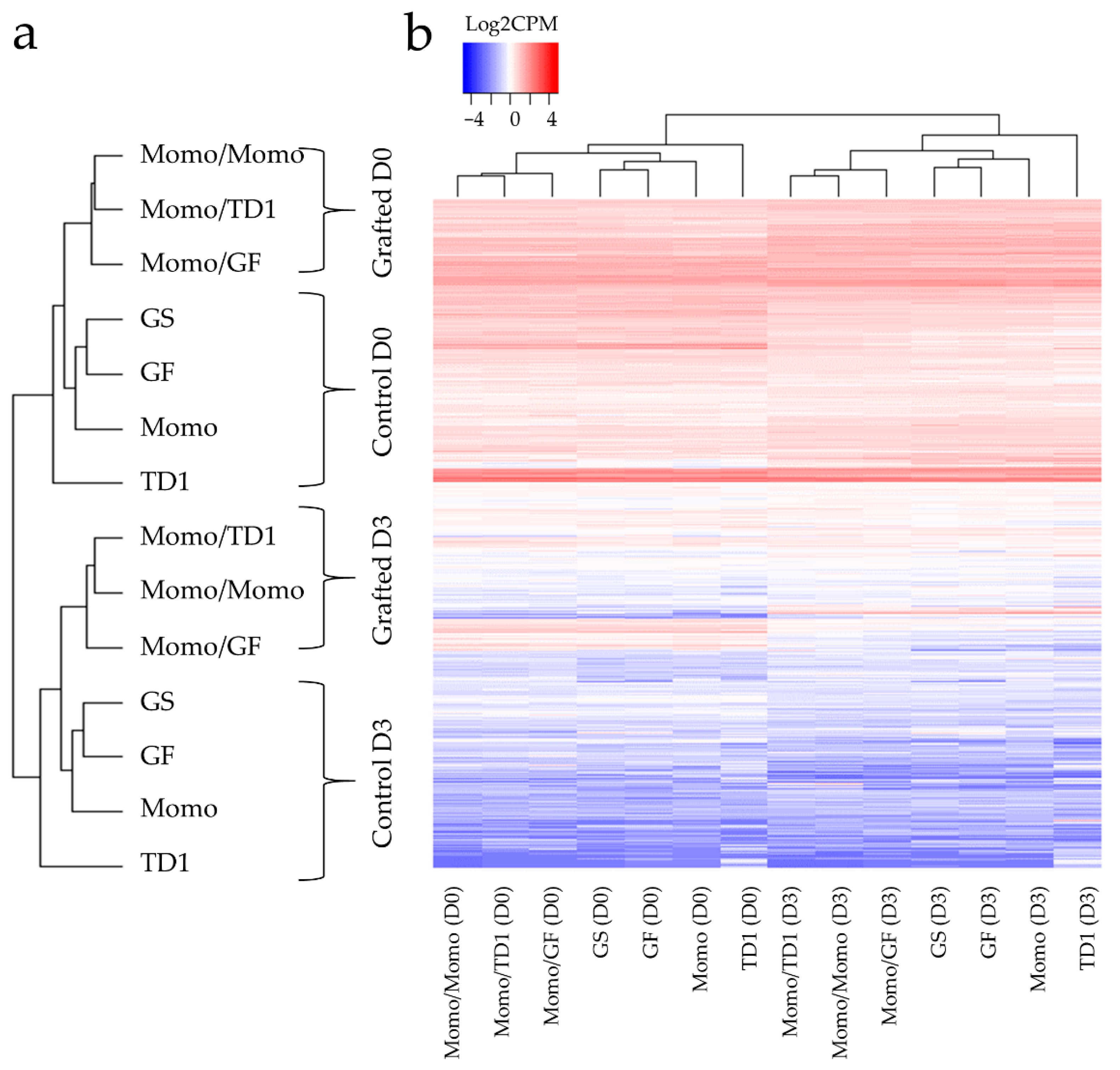
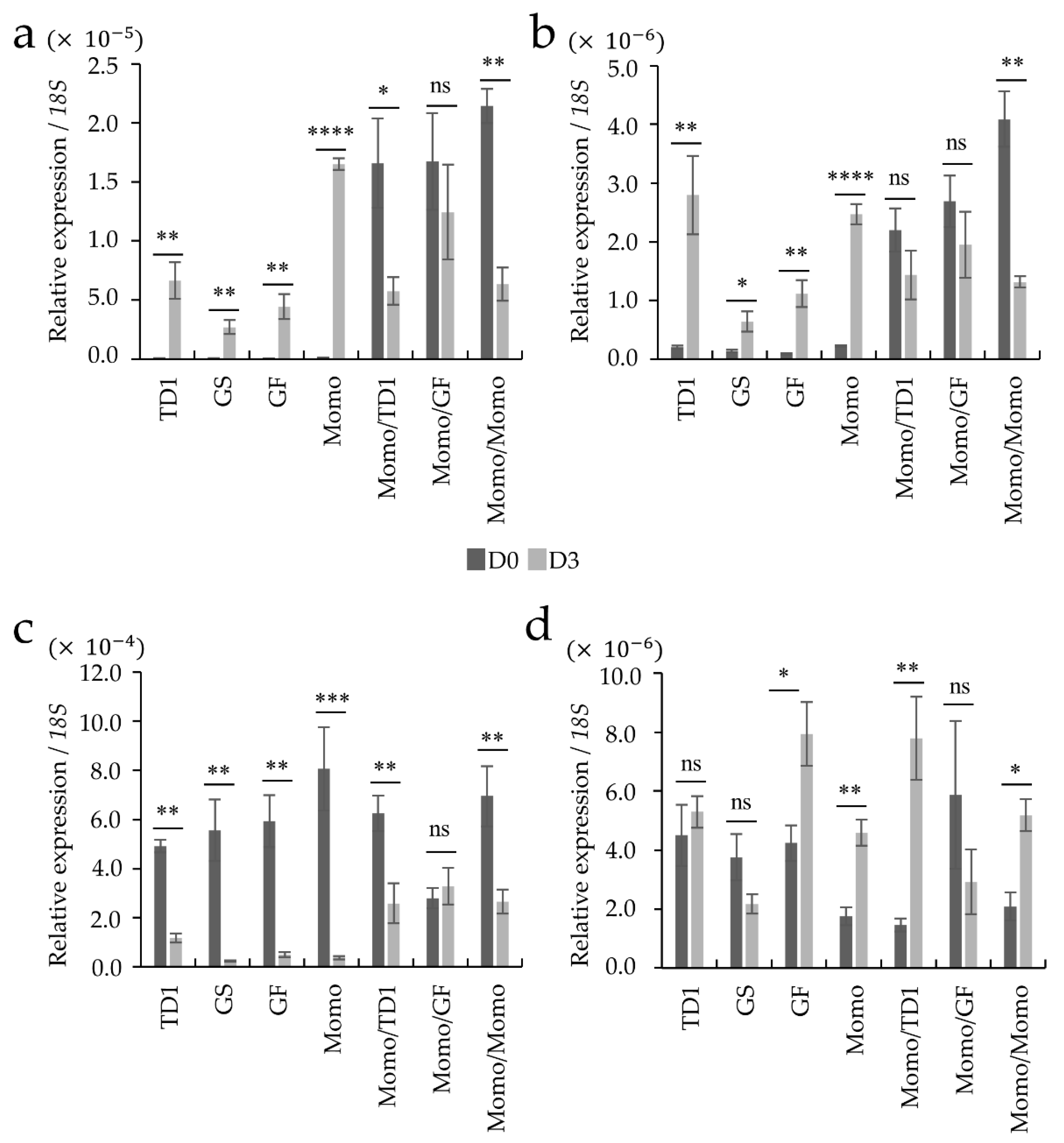
2.1. Identification of Tissue Types Involved in the Recovery of Drought-Tolerant Grafted Lines
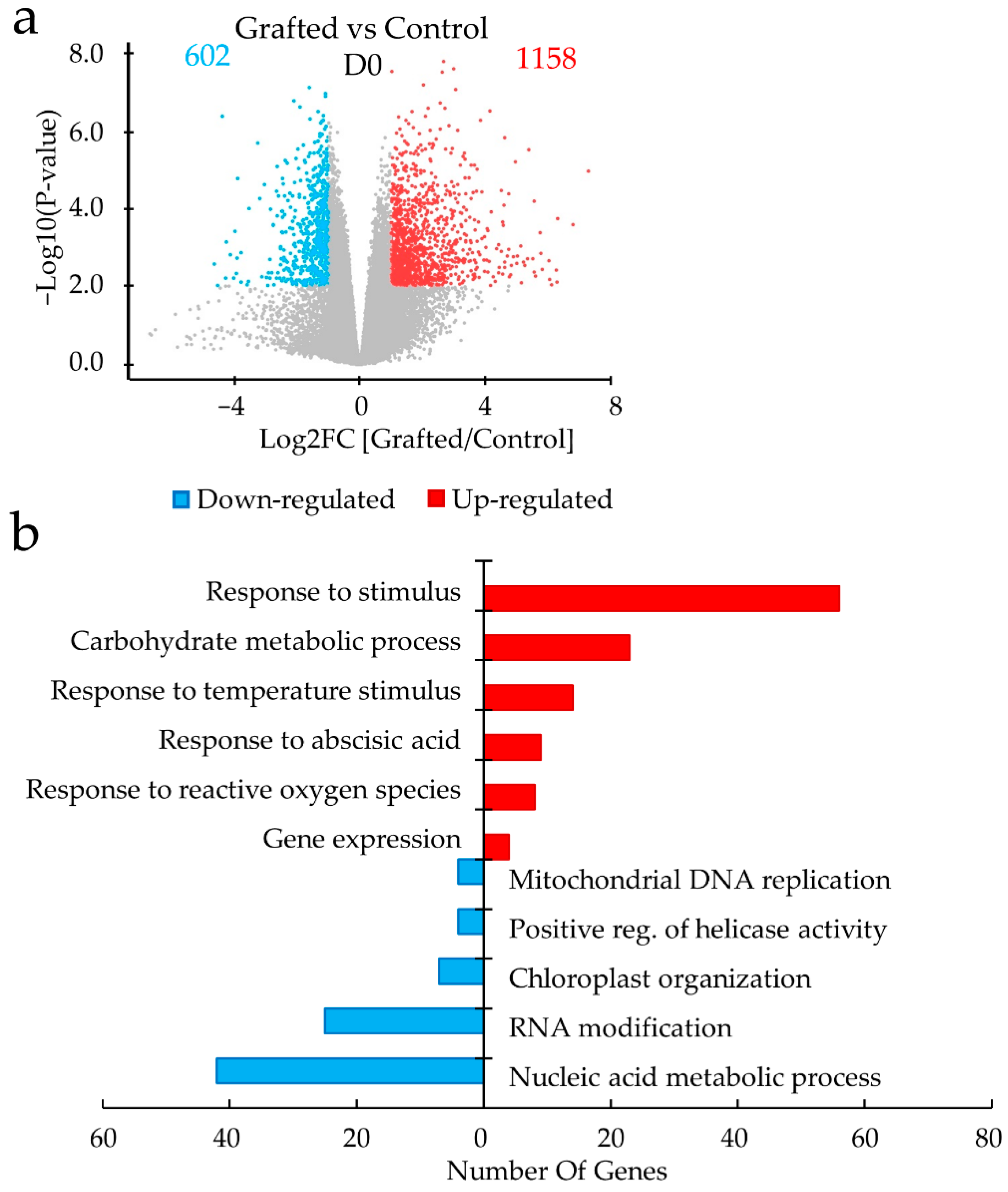
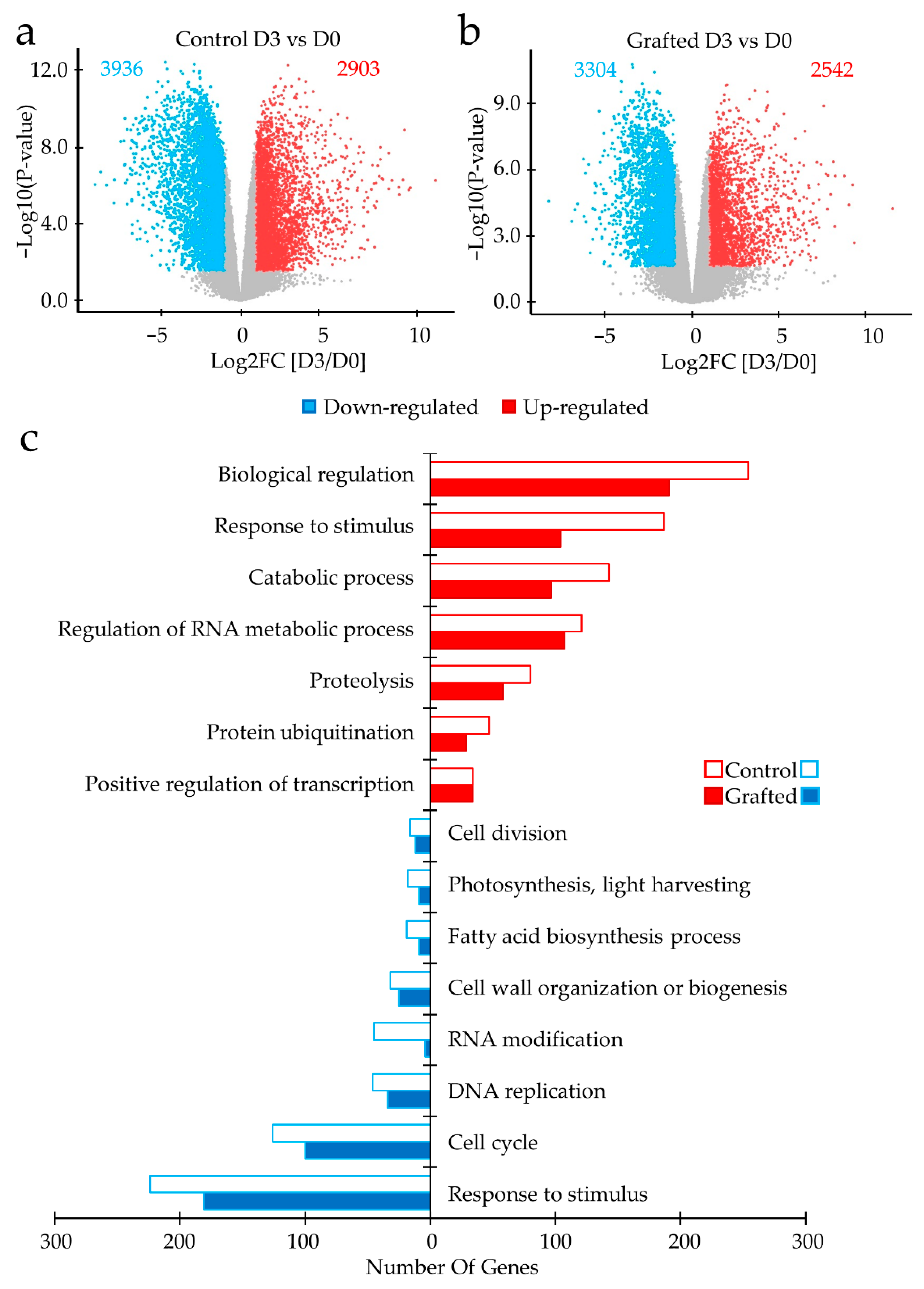
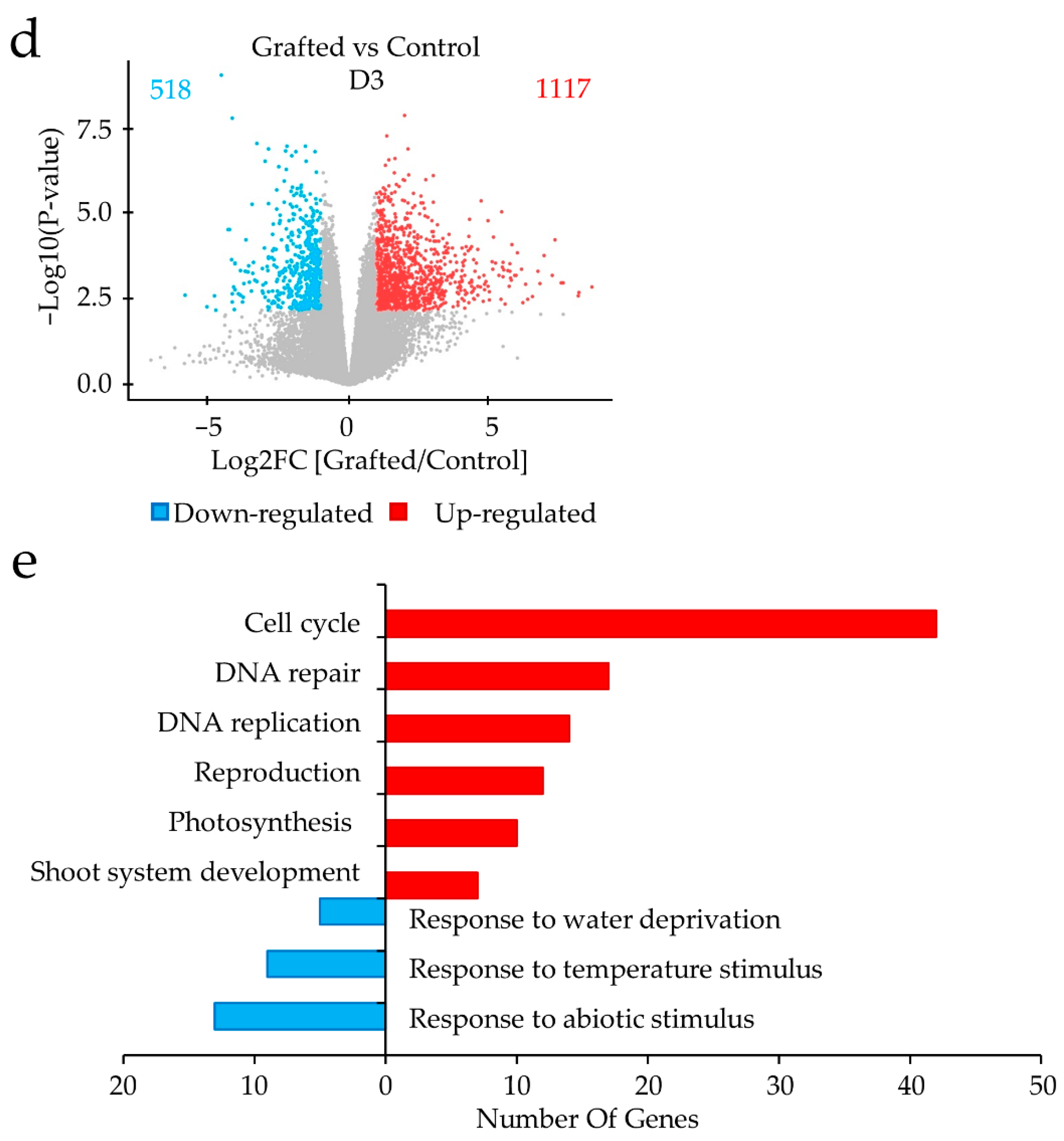
2.2. Differences in Gene Expression between Grafted and Non-Grafted Plants before and during Drought Stress Treatment
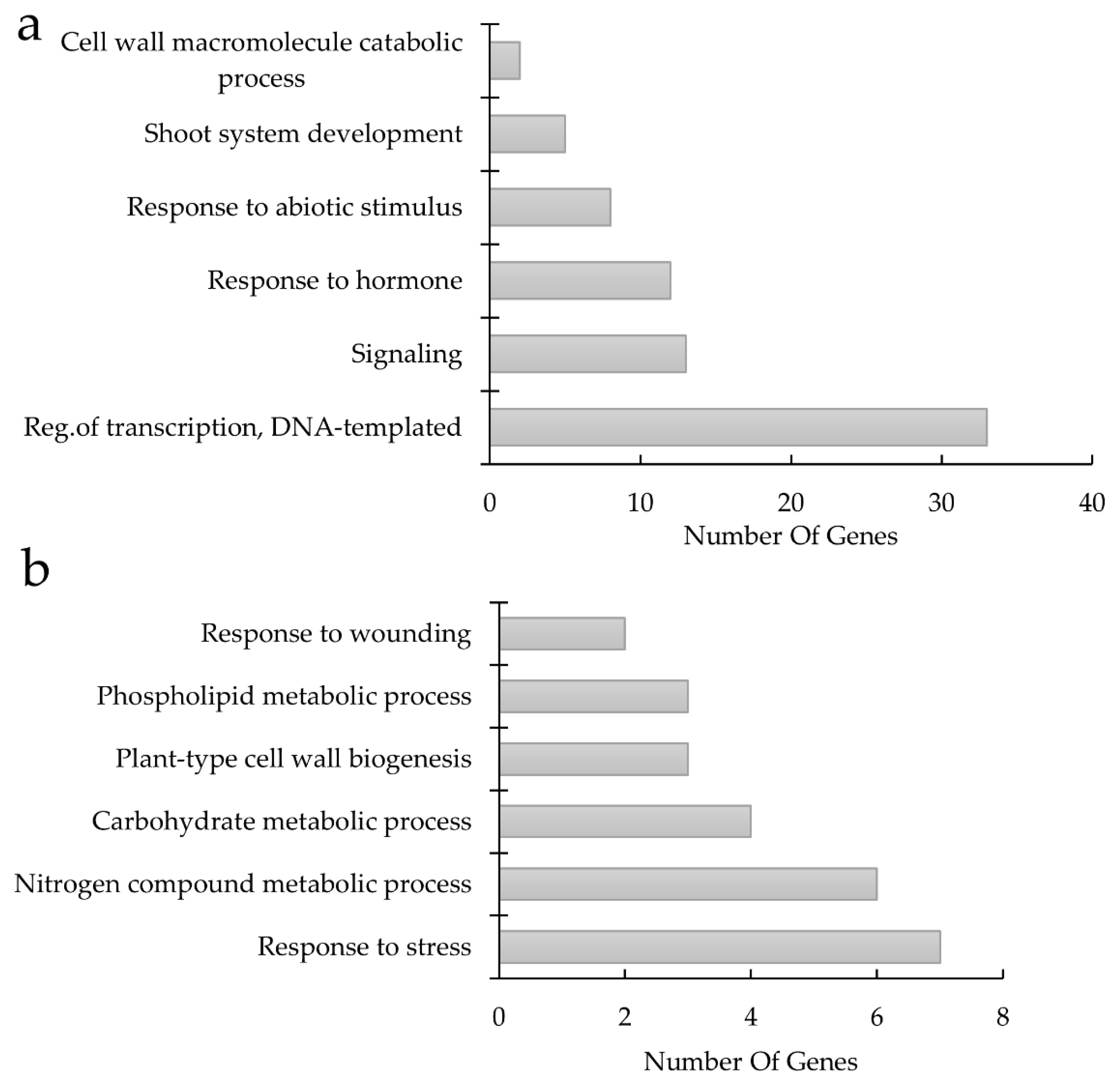
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Grafting
4.2. Drought Stress Treatment and Sample Collection
4.3. RNA Extraction and RNA Sequencing and Data Analysis
4.4. Real-Time Quantitative PCR (RT-PCR) in Stress-Responsive Genes
4.5. Gene Ontology Categorization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oda, M. New Grafting Methods for Fruit-Bearing Vegetables in Japan. Jpn. Agric. Res. Q. 1995, 29, 187–194. [Google Scholar]
- Albacete, A.; Martínez-Andújar, C.; Martínez-Pérez, A.; Thompson, A.J.; Dodd, I.C.; Pérez-Alfocea, F. Unravelling Rootstock×scion Interactions to Improve Food Security. J. Exp. Bot. 2015, 66, 2211–2226. [Google Scholar] [CrossRef] [Green Version]
- Goldschmidt, E.E. Plant Grafting: New Mechanisms, Evolutionary Implications. Front. Plant. Sci. 2014, 5, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melnyk, C.W. Plant Grafting: Insights into Tissue Regeneration. Regeneration 2017, 4, 3–14. [Google Scholar] [CrossRef]
- Melnyk, C.W.; Schuster, C.; Leyser, O.; Meyerowitz, E.M. A Developmental Framework for Graft Formation and Vascular Reconnection in Arabidopsis Thaliana. Curr. Biol. 2015, 25, 1306–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuoka, K.; Sugawara, E.; Aoki, R.; Takuma, K.; Terao-Morita, M.; Satoh, S.; Asahina, M. Differential Cellular Control by Cotyledon-Derived Phytohormones Involved in Graft Reunion of Arabidopsis Hypocotyls. Plant. Cell Physiol. 2016, 57, 2620–2631. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, D.; Rouphael, Y.; Colla, G.; Venema, J.H. Grafting as a Tool to Improve Tolerance of Vegetables to Abiotic Stresses: Thermal Stress, Water Stress and Organic Pollutants. Sci. Hortic. 2010, 127, 162–171. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Colla, G.; Zrenner, R.; Schwarz, D. Vegetable Grafting: The Implications of a Growing Agronomic Imperative for Vegetable Fruit Quality and Nutritive Value. Front. Plant. Sci. 2017, 8, 741. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Vegetable Grafting as a Tool to Improve Drought Resistance and Water Use Efficiency. Front. Plant. Sci. 2017, 8, 1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osakabe, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. ABA Control of Plant Macroelement Membrane Transport Systems in Response to Water Deficit and High Salinity. New Phytol. 2014, 202, 35–49. [Google Scholar] [CrossRef]
- Medyouni, I.; Zouaoui, R.; Rubio, E.; Serino, S.; Ahmed, H.B.; Bertin, N. Effects of Water Deficit on Leaves and Fruit Quality during the Development Period in Tomato Plant. Food Sci. Nutr. 2021, 9, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of Plants to Water Stress. Front. Plant. Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-a Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant. Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Cruz De Carvalho, M.H. Drought Stress and Reactive Oxygen Species: Production, Scavenging and Signaling. Plant. Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashide, T.; Heuvelink, E. Physiological and Morphological Changes Over the Past 50 Years in Yield Components in Tomato. J. Am. Soc. Hortic. Sci. 2009, 134, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Higashide, T.; Nakano, A.; Yasuba, K.I. Yield and Dry Matter Production of a Japanese Tomato “Momotaro York” Are Improved by Grafting onto a Dutch Rootstock “Maxifort”. J. Jpn. Soc. Hortic. Sci. 2014, 83, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Ikeuchi, M.; Iwase, A.; Rymen, B.; Lambolez, A.; Kojima, M.; Takebayashi, Y.; Heyman, J.; Watanabe, S.; Seo, M.; de Veylder, L.; et al. Wounding Triggers Callus Formation via Dynamic Hormonal and Transcriptional Changes. Plant. Physiol. 2017, 175, 1158–1174. [Google Scholar] [CrossRef] [Green Version]
- Vega-Muñoz, I.; Duran-Flores, D.; Fernández-Fernández, Á.D.; Heyman, J.; Ritter, A.; Stael, S. Breaking Bad News: Dynamic Molecular Mechanisms of Wound Response in Plants. Front. Plant. Sci. 2020, 11, 610445. [Google Scholar] [CrossRef]
- Tsaballa, A.; Xanthopoulou, A.; Madesis, P.; Tsaftaris, A.; Nianiou-Obeidat, I. Vegetable Grafting From a Molecular Point of View: The Involvement of Epigenetics in Rootstock-Scion Interactions. Front. Plant. Sci. 2021, 11, 621999. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, P.; Zhu, W.; Wang, F. De Novo Comparative Transcriptome Analysis of Genes Differentially Expressed in the Scion of Homografted and Heterografted Tomato Seedlings. Sci. Rep. 2019, 9, 20240. [Google Scholar] [CrossRef]
- Sato, Y.; Yokoya, S. Enhanced Tolerance to Drought Stress in Transgenic Rice Plants Overexpressing a Small Heat-Shock Protein, SHSP17.7. Plant. Cell Rep. 2008, 27, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Chen, X.; Hu, W.; Xiang, Y.; Yan, M.; Wang, J. Overexpressing Heat-Shock Protein OsHSP50.2 Improves Drought Tolerance in Rice. Plant. Cell Rep. 2018, 37, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Badawi, G.H.; Kawano, N.; Yamauchi, Y.; Shimada, E.; Sasaki, R.; Kubo, A.; Tanaka, K. Over-Expression of Ascorbate Peroxidase in Tobacco Chloroplasts Enhances the Tolerance to Salt Stress and Water Deficit. Physiol. Plant. 2004, 121, 231–238. [Google Scholar] [CrossRef]
- Faize, M.; Burgos, L.; Faize, L.; Piqueras, A.; Nicolas, E.; Barba-Espin, G.; Clemente-Moreno, M.J.; Alcobendas, R.; Artlip, T.; Hernandez, J.A. Involvement of Cytosolic Ascorbate Peroxidase and Cu/Zn-Superoxide Dismutase for Improved Tolerance against Drought Stress. J. Exp. Bot. 2011, 62, 2599–2613. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Effect of Abscisic Acid on Active Oxygen Species, Antioxidative Defence System and Oxidative Damage in Leaves of Maize Seedlings. Plant. Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Cho, E.K.; Hong, C.B. Over-Expression of Tobacco NtHSP70-1 Contributes to Drought-Stress Tolerance in Plants. Plant. Cell Rep. 2006, 25, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, M.; Chen, J.; Lou, Q. Genome-Wide Identification and Expression Analysis of Heat Shock Protein 70 (HSP70) Gene Family in Pumpkin (Cucurbita Moschata) Rootstock under Drought Stress Suggested the Potential Role of These Chaperones in Stress Tolerance. Int. J. Mol. Sci. 2022, 23, 1918. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, S.S.; Yi, C.Y.; Wang, F.; Zhou, J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Hydrogen Peroxide Mediates Abscisic Acid-Induced HSP70 Accumulation and Heat Tolerance in Grafted Cucumber Plants. Plant. Cell Environ. 2014, 37, 2768–2780. [Google Scholar] [CrossRef]
- Ikegami, K.; Okamoto, M.; Seo, M.; Koshiba, T. Activation of Abscisic Acid Biosynthesis in the Leaves of Arabidopsis Thaliana in Response to Water Deficit. J. Plant. Res. 2009, 122, 235–243. [Google Scholar] [CrossRef]
- Stortenbeker, N.; Bemer, M. The SAUR Gene Family: The Plant’s Toolbox for Adaptation of Growth and Development. J. Exp. Bot. 2019, 70, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Markakis, M.N.; Boron, A.K.; van Loock, B.; Saini, K.; Cirera, S.; Verbelen, J.P.; Vissenberg, K. Characterization of a Small Auxin-up RNA (SAUR)-like Gene Involved in Arabidopsis Thaliana Development. PLoS ONE 2013, 8, e82596. [Google Scholar] [CrossRef] [Green Version]
- van Mourik, H.; van Dijk, A.D.J.; Stortenbeker, N.; Angenent, G.C.; Bemer, M. Divergent Regulation of Arabidopsis SAUR Genes: A Focus on the SAUR10-Clade. BMC Plant. Biol. 2017, 17, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Sun, N.; Yang, J.; Deng, Z.; Lan, J.; Qin, G.; He, H.; Deng, X.W.; Irish, V.F.; Chen, H.; et al. The Transcription Factors Tcp4 and Pif3 Antagonistically Regulate Organ-Specific Light Induction of Saur Genes to Modulate Cotyledon Opening during de-Etiolation in Arabidopsis. Plant. Cell 2019, 31, 1155–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, N.; Wang, J.; Gao, Z.; Dong, J.; He, H.; Terzaghi, W.; Wei, N.; Wang, X.; Chen, H. Arabidopsis Saurs Are Critical for Differential Light Regulation of the Development of Various Organs. Proc. Natl. Acad. Sci. USA 2016, 113, 6071–6076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Z.; Mei, Y.; Zhou, J.; Cui, Y.; Wang, D.; Wang, N.N. SAUR49 Can Positively Regulate Leaf Senescence by Suppressing SSPP in Arabidopsis. Plant. Cell Physiol. 2020, 61, 644–658. [Google Scholar] [CrossRef]
- Rae, G.M.; David, K.; Wood, M. The Dormancy Marker DRM1/ARP Associated with Dormancy but a Broader Role In Planta. Dev. Biol. J. 2013, 2013, 632524 . [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Saxena, S.; Sinha, A.; Nandi, A.K. DORMANCY/AUXIN ASSOCIATED FAMILY PROTEIN 2 of Arabidopsis Thaliana Is a Negative Regulator of Local and Systemic Acquired Resistance. J. Plant. Res. 2020, 133, 409–417. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant Hormone-Mediated Regulation of Stress Responses. BMC Plant. Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Gupta, S.; Rashotte, A.M. Solanum lycopersicum Cytokinin Response Factor (SlCRF) Genes: Characterization of CRF Domain-Containing ERF Genes in Tomato. J. Exp. Bot. 2012, 63, 973–982. [Google Scholar] [CrossRef] [Green Version]
- Nanda, A.K.; Melnyk, C.W. The Role of Plant Hormones during Grafting. J. Plant. Res. 2018, 131, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Zhai, L.; Wang, X.; Tang, D.; Qi, Q.; Yer, H.; Jiang, X.; Han, Z.; McAvoy, R.; Li, W.; Li, Y. Molecular and Physiological Characterization of the Effects of Auxin-Enriched Rootstock on Grafting. Hortic. Res. 2021, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, X. Expression Pattern of GASA, Downstream Genes of DELLA, in Arabidopsis. Sci. Bull. 2008, 53, 3839–3846. [Google Scholar] [CrossRef] [Green Version]
- Nahirnak, V.; Almasia, N.I.; Hopp, H.E.; Vazquez-Rovere, C. Snakin/GASA Protein Involvement in Hormone Crosstalk and Redox Homeostasis. Plant. Signal. Behav. 2012, 7, 1004–1008. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, M.; Taji, A.; Backhouse, D.; Oda, M. Anatomy and Physiology of Graft Incompatibility in Solanaceous Plants. J. Hortic. Sci. Biotechnol. 2008, 83, 581–588. [Google Scholar] [CrossRef]
- Oda, M.; Maruyama, M.; Mori, G. Water Transfer at Graft Union of Tomato Plants Grafted. J. Jpn. Soc. Hortic. Sci. 2005, 74, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Kurotani, K.I.; Notaguchi, M. Cell-to-Cell Connection in Plant Grafting—Molecular Insights into Symplasmic Reconstruction. Plant. Cell Physiol. 2021, 62, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Heddad, M.; Adamska, I. Light Stress-Regulated Two-Helix Proteins in Arabidopsis Thaliana Related to the Chlorophyll Ab-Binding Gene Family PLANT BIOLOGY. Proc. Natl. Acad. Sci. USA 2000, 28, 3741–3746. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-Induced Responses of Photosynthesis and Antioxidant Metabolism in Higher Plants. J. Plant. Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Irisarri, P.; Binczycki, P.; Errea, P.; Martens, H.J.; Pina, A. Oxidative Stress Associated with Rootstock-Scion Interactions in Pear/Quince Combinations during Early Stages of Graft Development. J. Plant. Physiol. 2015, 176, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Durbin, R. Fast and Accurate Long-Read Alignment with Burrows–Wheeler Transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, A.R.; Hall, I.M. BEDTools: A Flexible Suite of Utilities for Comparing Genomic Features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Tomato Genome Consortium. The Tomato Genome Sequence Provides Insights into Fleshy Fruit Evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2018 Update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [Green Version]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. Voom: Precision Weights Unlock Linear Model Analysis Tools for RNA-Seq Read Counts. Genome Biol. 2014, 15, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mi, H.; Huang, X.; Muruganujan, A.; Tang, H.; Mills, C.; Kang, D.; Thomas, P. PANTHER Version 14: More Genomes, a New PANTHER GO-Slim and Improvements in Enrichment Analysis Tools. Nucleic Acids Res. 2019, 47, 419–426. [Google Scholar] [CrossRef] [PubMed]
| Gene ID | Annotation | Log2FC * (Grafted /Control) | Adj. p-Value |
|---|---|---|---|
| Abscisic Acid-related genes | |||
| Solyc03g095780 | Abscisic acid receptor PYL4-like | 4.2 | 4.2 × 10−3 |
| Solyc09g015380 | Abscisic acid receptor PYL5 | 3.2 | 4.2 × 10−3 |
| Solyc06g050500 | Abscisic acid receptor PYL4-like | 3.2 | 9.6 × 10−3 |
| Solyc10g076410 | Abscisic acid receptor PYL6 | 3.0 | 2.0 × 10−2 |
| Solyc10g085310 | Abscisic acid receptor PYL5 | 2.4 | 5.5 × 10−3 |
| Solyc12g055990 | Abscisic acid receptor PYL10 | 1.2 | 4.4 × 10−3 |
| Auxin-related genes | |||
| Solyc07g042490 | Auxin-responsive SAUR protein | 3.7 | 2.5 × 10−3 |
| Solyc02g037550 | Auxin efflux carrier | 2.6 | 4.8 × 10−3 |
| Solyc01g110580 | Small auxin up-regulated RNA5 | 1.4 | 5.2 × 10−3 |
| Solyc02g077560 | Auxin response factor 3 | 1.3 | 4.7 × 10−4 |
| Solyc01g110920 | Small auxin up-regulated RNA26 | 1.3 | 2.9 × 10−2 |
| Solyc09g007810 | Auxin Response Factor 16A | 1.0 | 2.0 × 10−3 |
| Solyc05g046320 | Small auxin up-regulated RNA55 | −1.1 | 1.3 × 10−2 |
| Solyc06g075150 | Auxin Response Factor 10B | −1.5 | 4.3 × 10−3 |
| Solyc01g099840 | Dormancy/auxin associated protein | −1.6 | 1.6 × 10−2 |
| Solyc02g077880 | Auxin-repressed protein | −2.6 | 1.2 × 10−3 |
| Cytokinin-related genes | |||
| Solyc06g075090 | Cytokinin riboside 5-monophosphate phosphoribohydrolase | 1.7 | 3.7 × 10−3 |
| Solyc04g080820 | Cytokinin oxidase 4 | 1.4 | 4.6 × 10−2 |
| Solyc10g082020 | Cytokinin riboside 5-monophosphate phosphoribohydrolase | 1.2 | 2.4 × 10−2 |
| Solyc03g007460 | Solanum lycopersicum Cytokinin Response Factor 4 | −1.5 | 1.6 × 10−2 |
| Ethylene-related genes | |||
| Solyc04g011440 | Ethylene-responsive heat shock protein cognate 70 | 5.4 | 8.6 × 10−4 |
| Solyc09g059390 | Ethylene-responsive transcription factor | 4.9 | 1.8 × 10−2 |
| Solyc12g056590 | Ethylene Response Factor D.2 | 3.5 | 1.4 × 10−2 |
| Solyc01g090300 | Ethylene-responsive transcription factor 2 | 2.4 | 3.3 × 10−2 |
| Solyc06g065820 | Ethylene Response Factor H.1 | 2.0 | 4.5 × 10−2 |
| Solyc01g106820 | Ethylene-dependent gravitropism-deficient and yellow-green-like 3 | 1.9 | 9.6 × 10−3 |
| Solyc08g066660 | Ethylene-responsive transcription factor TINY | 1.8 | 3.8 × 10−2 |
| Solyc04g012050 | Ethylene-responsive transcription factor | 1.7 | 2.6 × 10−2 |
| Solyc03g118190 | Ethylene-responsive transcription factor | 1.7 | 4.4 × 10−2 |
| Solyc03g093610 | Ethylene response factor A.2 | 1.6 | 3.0 × 10−3 |
| Solyc01g006650 | ETHYLENE INSENSITIVE 3-like 3 protein | 1.5 | 1.0 × 10−2 |
| Solyc04g078640 | Ethylene-responsive transcription factor RAP2-1 | −1.3 | 3.9 × 10−2 |
| Solyc07g054220 | Ethylene-responsive transcription factor | −1.5 | 2.4 × 10−2 |
| Solyc06g019200 | Ethylene-dependent gravitropism-deficient and yellow-green-like 2 | −1.7 | 1.8 × 10−2 |
| Solyc08g007230 | Ethylene-responsive transcription factor 2 | −1.8 | 4.9 × 10−2 |
| Solyc02g088310 | Ethylene-responsive transcription factor | −2.0 | 1.1 × 10−2 |
| Gibberellin-related genes | |||
| Solyc02g083880 | Gibberellin-regulated protein 3 | 4.2 | 4.3 × 10−4 |
| Solyc01g150176 | Gibberellin-regulated protein | 3.9 | 4.8 × 10−4 |
| Solyc03g113910 | Gibberellin-regulated protein 14 | 2.9 | 2.7 × 10−3 |
| Solyc12g042500 | Gibberellin-regulated family protein | 1.8 | 3.3 × 10−2 |
| Solyc09g074270 | Gibberellin receptor | 1.5 | 7.6 × 10−3 |
| Jasmonic acid-related genes | |||
| Solyc01g103595 | Jasmonate ZIM domain protein l | 2.0 | 4.2 × 10−2 |
| Gene ID | Annotation | Log2FC * (Compatible vs. Incompatible) | |
|---|---|---|---|
| Momo/TD1 vs. Momo/GF | Momo/Momo vs. Momo/GF | ||
| GO:0006355 Regulation of transcription, DNA-templated | |||
| Solyc04g079930 | Histone deacetylase complex subunit | 1.0 | 1.3 |
| Solyc07g008540 | Zinc finger protein CONSTANS-LIKE 2 | 1.2 | 1.8 |
| GO:0009725 Response to hormone | |||
| Solyc05g047590 | Pectinesterase | 1.3 | 1.8 |
| Solyc06g050500 | Abscisic acid receptor PYL4-like | 1.2 | 1.6 |
| GO:0009628 Response to abiotic stimulus | |||
| Solyc08g061130 | Transcription factor HY5 | 1.1 | 1.1 |
| Solyc09g065660 | Heat shock transcription factor | 1.6 | 3.6 |
| GO:0006950 Response to stress -> GO:0042594 Response to starvation | |||
| Solyc02g088240 | Phosphate transporter PHO1-like protein 5 | −2.6 | −2.9 |
| Solyc08g068240 | Phosphate transporter PHO1-like protein | −1.2 | −1.7 |
| GO:0005975 Carbohydrate metabolic process -> GO:0009832 Plant-type cell wall biogenesis | |||
| Solyc01g103860 | COBRA-like protein | −1.2 | −1.0 |
| Solyc03g114900 | COBRA-like protein | −2.1 | −3.0 |
| GO:0006950 Response to stress -> GO:0009611 Response to wounding | |||
| Solyc08g036660 | Jasmonate ZIM domain protein f | −4.7 | −3.6 |
| Solyc08g036640 | Jasmonate ZIM domain protein f | −3.1 | −2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes-Merlos, M.I.; Bamba, M.; Sato, S.; Higashitani, A. Comparative Transcriptome Analysis of Grafted Tomato with Drought Tolerance. Plants 2022, 11, 1947. https://doi.org/10.3390/plants11151947
Fuentes-Merlos MI, Bamba M, Sato S, Higashitani A. Comparative Transcriptome Analysis of Grafted Tomato with Drought Tolerance. Plants. 2022; 11(15):1947. https://doi.org/10.3390/plants11151947
Chicago/Turabian StyleFuentes-Merlos, Maria Isabel, Masaru Bamba, Shusei Sato, and Atsushi Higashitani. 2022. "Comparative Transcriptome Analysis of Grafted Tomato with Drought Tolerance" Plants 11, no. 15: 1947. https://doi.org/10.3390/plants11151947
APA StyleFuentes-Merlos, M. I., Bamba, M., Sato, S., & Higashitani, A. (2022). Comparative Transcriptome Analysis of Grafted Tomato with Drought Tolerance. Plants, 11(15), 1947. https://doi.org/10.3390/plants11151947