Abstract
Stilbenes are plant defense compounds known to rapidly accumulate in grapevine and some other plant species in response to microbial infection and several abiotic stresses. Stilbenes have attracted considerable attention due to valuable biological effects with multi-spectrum therapeutic application. However, there is a lack of information on natural signaling pathways and transcription factors regulating stilbene biosynthesis. It has been previously shown that MYB R2R3 transcription factor genes VaMyb40 and VaMyb60 were up-regulated in cell cultures of wild-growing grapevine Vitis amurensis Rupr. in response to UV irradiation. In this study, the effects of VaMyb40 or VaMyb60 overexpression in cell cultures of V. amurensis on their capability to produce stilbenes were investigated. Overexpression of the VaMyb60 gene led to a considerable increase in the content of stilbenes in three independently transformed transgenic lines in 5.9–13.9 times, while overexpression of the VaMyb40 gene also increased the content of stilbenes, although to a lesser extent (in 3.4–4.0 times) in comparison with stilbene levels in the empty vector-transformed calli. Stilbene content and stilbene production in the VaMyb60-transgenic calli reached 18.8 mg/g of dry weight (DW) and 150.8 mg/L, respectively. Using HPLC analysis, we detected eight individual stilbenes: t-resveratrol diglucoside, t-piceid, t-resveratrol, ε-viniferin, δ-viniferin, cis-resveratrol, cis-piceid, t-piceatannol. T-resveratrol prevailed over other stilbenoid compounds (53.1–89.5% of all stilbenes) in the VaMyb-overexpressing cell cultures. Moreover, the VaMyb40- and VaMyb60-transformed calli were capable of producing anthocyanins up to 0.035 mg/g DW, while the control calli did not produce anthocyanins. These findings show that the VaMyb40 and VaMyb60 genes positively regulate the stilbene biosynthesis as strong positive transcription regulators and can be used in biotechnological applications for stilbene production or high-quality viticulture and winemaking.
1. Introduction
Stilbenes are plant natural phytoalexins with antifungal and insecticide activities whose synthesis is induced upon pathogen attack and other environmental stresses in grapevine and other plant species from a number of unrelated plant families [1,2,3]. Plant stilbenes have become a focus of multiple studies in medicine as promising agents with diverse biological activities, such as antitumor, cardioprotective, antiangiogenic and immunomodulatory properties [4,5,6,7]. Trans-resveratrol or t-resveratrol (3,5,4′-trihydroxystilbene) is the most prominent and well-studied stilbene with a great variety of pharmacological effects that are perspective for applications in pharmaceutical and cosmetic industries [8,9].
The stilbene biosynthetic pathway diverges from the phenylpropanoid/polymalonate pathway, and the last step of this pathway is being catalyzed by stilbene synthases [3,10]. Stilbene synthases (STS; EC 2.3.1.95) are members of the type III polyketide synthases family and catalyze synthesis of the parent monomeric stilbenes, such as resveratrol or pinosylvin, from coenzyme A-esters of cinnamic acid derivatives and three malonyl-CoA units in a single reaction [10]. The monomeric stilbenes may undergo oxidative dimerization to form stilbene oligomers (viniferins, ampelopsin, or hopeaphenol), glycosylation to form glycosylated stilbenes (piceid or astringin), methoxylation to form O-methylated stilbenes (pterostilbene or pinosylvin-3-O-methyl ether), or isoprenylation to form isoprenylated stilbenes (arachidin-3) [11,12,13]. A number of studies show that stilbene production is relatively low under natural conditions in most plant species and strongly depend on stage of development and environmental conditions [3]. Various strategies have been developed to enhance stilbene production levels and to control the composition of produced stilbenes, e.g., plant cell culture elicitation or genetic engineering approaches [3]. Studying the molecular and genetic mechanisms of stilbene biosynthesis control is a prerequisite for further development of biotechnological approaches of commercial stilbene production as well as new plant protection strategies.
Current literature reveals that stilbene biosynthesis is regulated at the biochemical level by plant stress hormone signaling [14,15,16,17,18,19], ROS production [14,16,19], calcium signaling [14,15,20,21,22,23,24], and MAP kinase cascade [25]. After an external stimulus is perceived, activated receptors are proposed to convey the signal by activation of the MAPK cascade and calcium influx induction, leading to the activation of calcium sensor proteins [3]. Activated MAPK cascade and calcium sensor proteins could then induce ROS production and stimulate plant hormone signaling. These signaling events eventually lead to the activation of specific transcription factors (TFs) responsible for the induction of stilbene synthases (STS) genes and other genes responsible for stilbene biosynthesis [17,26,27]. However, at present, there is scarce information on the transcriptional regulation of stilbene biosynthesis.
It is known that biosynthesis of secondary metabolites in plants is regulated at the transcriptional level by multiple TFs, including MYC, MYB, WRKY and AP2/ERF TF families [28,29,30]. These TFs integrate internal and external cues and bind to certain cis-elements in gene promoter regions to induce or repress expression of the genes encoding enzymes responsible for biosynthesis of plant secondary metabolites [28,29,30]. The current literature presents multiple investigations that provide compelling evidence on the important roles of V-myb myeloblastosis viral oncogene homolog (MYB) TF family in the regulation of phenylpropanoid-derived secondary metabolites in plants (e.g., flavonoids, anthocyanines, and others), reviewed in [29]. Bearing in mind the contingency of biosynthesis of flavonoids and stilbenes, it is most probable that genes regulating the stilbene biosynthesis, such as STS genes, are also regulated by MYB transcription factors.
The first accession of the grapevine genome (×8.4) allowed the identification of 108 genes in the Vitis vinifera R2R3-MYB family [31,32]. According to the most recent data, the MYB R2R3 TF subfamily includes 134 annotated genes in V. vinifera [33]. The first findings on TF involvement in stilbene biosynthesis regulation revealed a positive role of Myb14 and Myb15 genes in the regulation of resveratrol biosynthesis in grapevine V. vinifera [26,27]. The results by Holl et al. (2013) [26] indicated that MYB14 and MYB15 TFs are involved in the induction of STS29 and STS41 transcription in V. vinifera. Using a one-hybrid yeast assay, it has been shown that MYB14 directly interacts with the STS promoter (Box-L5 motif) in vitro. A different study [27] confirmed that a transient overexpression of MYB14 induced STS expression in grapevine leaves. Since the STS gene family comprises more than 30 functional STS genes [34], it is possible that stilbene biosynthesis in grapevine is regulated by more than two MYB TFs. Besides STSs, stilbene biosynthesis and modification pathway depend on other enzymes (glucosyltransferases, polyphenol oxidases, methyltransferases) whose expression could also involve regulation by MYB TFs. Recently, we have shown that, in addition to the VaMyb14 and VaMyb15 genes, the expression of VaMYB9, 40, 60, and 107 MYB TF genes was up-regulated in the tissues of wild grape Vitis amurensis Rupr. producing high stilbene amounts in response to UV or genetic transformation with a calcium sensor protein gene [35].
In this study, we show that the VaMyb40 and VaMyb60 genes function as potential positive regulators of stilbene biosynthesis in the grapevine by overexpressing the Myb40 or Myb60 genes in cell cultures of V. amurensis. The data provided evidence for the involvement of the VaMyb40 and VaMyb60 genes in positive regulation of stilbene biosynthetic processes in V. amurensis.
2. Results and Discussion
2.1. Genetic Transformation and Selection of the VaMyb-Transgenic Cell Lines
To establish cell cultures of Vitis amurensis Rupr. overexpressing the full-length VaMyb40 and VaCML60 genes, the V7 suspension culture of V. amurensis was incubated with A. tumefaciens strains bearing the pZP-RCS2-VaMyb40/60-nptII constructs for generation of the VaMyb-transgenic cells or the pZP-RCS2-nptII construct—for the control KA0 cell line, which contained only the kanamycin (Km) resistance gene nptII. All transgenes in the obtained constructs were under the control of the double cauliflower mosaic virus (CaMV 35 S) promoters. Then, we selected transgenic callus cell aggregates in the presence of 10–15 mg/L of Km for four months and established several Km-resistant independently obtained callus cell lines as described [23]. The selected transformed calli represented friable vigorously growing homogenous tissues, which did not undergo differentiation on the WB/A medium supplemented with 6-benzylaminopurine (BAP) and α-naphthaleneacetic acid (NAA) in the dark. For further analysis, we used KA0 control transgenic cell line and six transgenic cell lines transformed with the VaMyb40 and VaMyb60 genes: three VaMyb40-transformed cell lines (40-1, 40-2, 40-3) and three VaMyb60-transformed cell lines (60-1, 60-2, 60-3) (Figure 1). After 2–3 months, the appearance of colored red areas was observed in the transgenic lines (Supplementary Figure S1). We found that these red tissue zone contained anthocyanins, which are described in detail below.

Figure 1.
Quantification the transgene (a,b), endogenous (c,d), and total (e,f) mRNAs of the VaMyb40 and VaMyb60 genes in the transgenic callus cell lines of Vitis amurensis performed by quantitative RT-PCR. KA0—the control KA0 cell line of V. amurensis transformed with the vector harboring only the nptII selective marker; 40-1, 40-2, and 40-3—cell lines of V. amurensis transformed with the VaMyb40 gene; 60-1, 60-2, and 60-3—cell lines of V. amurensis transformed with the VaMyb60 gene. The data are presented as mean ± SE (two independent experiments with eight technical replicates). *, **—significantly different from the values of Myb expression in the control KA0 cell line at p ≤ 0.05 and 0.01 according to the Student’s t-test.
The VaMyb-transgenic callus cell lines were confirmed by qRT-PCR for expression of the VaMyb40 and VaMyb60 transgenes (Figure 1). All of the VaMyb-transformed cell lines actively expressed the transgenes (Figure 1a,b). The expression analysis of the endogenous VaMyb40 and VaMyb60 genes revealed that expression of the endogenous VaMyb40 and VaMyb60 was not affected in the 40-1, 40-2, 60-1, 60-2 cell lines in comparison with that in the control KA0 cell line (Figure 1c,d). However, the endogenous VaMyb40 and VaMyb60 expression was considerably increased in 1.9 and 2.1 times in the 40-3 and 60-3 lines. The mechanism of this increase is unclear, since a more common effect after transformation in such cases is a decrease in expression of endogenous gene counterpart observed due to gene silencing [36]. Perhaps, this increase was due to the fact that there was the highest value of total transgene and endogenous VaMyb40 and VaMyb60 in the 40-3 and 60-3 lines, and primers could have an additional glow, since one primer in a pair was the same. Then, we performed the analysis of the total transgene and endogenous VaMyb40 and VaMyb60 expression levels (Figure 1e,f). The total expression of VaMyb40 and VaMyb60 in all of the VaMyb-transformed calli considerably exceeded that in the KA0 control calli (Figure 1e,f). The total expression of the Myb40 in the VaMyb40-transformed calli was elevated in 4.0–7.3 times (Figure 1e), and the total expression of the Myb60 in the VaMyb60-transformed calli—in 2.5–6.2 times (Figure 1f), compared with KA0.
2.2. Stilbene Content and Biomass Accumulation in the Grapevine VaMyb-Transgenic Cell Lines
V. amurensis cell culture samples were collected from the 35-day-old calli for stilbene extraction and biomass analysis, because it has been shown that the highest content of stilbenes in the callus cell cultures was typical for the 35th day of cultivation [37]. In the Table 1, we presented fresh and dry biomass accumulation in the control KA0 and VaMyb-transformed callus cell lines of V. amurensis. Transformation of the V. amurensis calli with both the VaMyb40 and VaMyb60 genes degreased fresh weight (FW) accumulation in two cell lines out of three obtained, i.e., in 1.1–1.4 times and 1.1–1.2 times in the 40-1, 40-2, 60-2, and 60-3 lines, respectively, in comparison with FW of the KA0 cells (Table 1). However, the dry weight (DW) biomass levels of the VaMyb-transformed calli did not considerably differ from the DW levels of the KA0 cells (Table 1).

Table 1.
Biomass accumulation and total stilbene production in the cell lines of Vitis amurensis overexpressing the VaMyb40 or VaMyb60 genes.
Using HPLC, we determined the content and composition of stilbenes in the obtained VaMyb-transgenic cell lines. Overexpression of the VaMyb60 gene led to a considerable increase in the content of stilbenes in all obtained transgenic lines in 5.9–13.9 times (Figure 2). The content of stilbenes was significantly increased in the cell lines of V. amurensis overexpressing the VaMyb40 gene, although to a lesser extent than in the VaMyb60-overexpressing cell lines, i.e., in 3.4–4.0 times in comparison with stilbene content in KA0 (Figure 2).
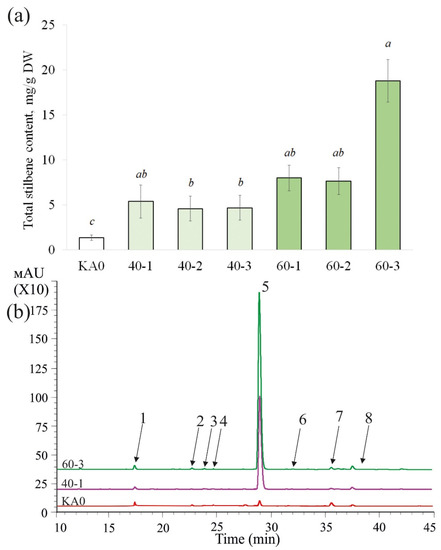
Figure 2.
Total stilbene content ((a), mg per g of the dry weight (DW)) and a representative HPLC-UV profile ((b), 310 nm) of the callus cell lines of Vitis amurensis transformed with the VaMyb40 or VaMyb60 genes. KA0—control cell line transformed with the vector harboring only the nptII selective marker; 40-1, 40-2, 40-3—cell lines of Vitis amurensis transformed with the VaMyb40 gene; 60-1, 2, 3—cell lines of V. amurensis transformed with the VaMyb60 gene. T-resveratrol diglucoside (1), t-piceid (2), t-piceatannol (3), cis-piceid (4), t-resveratrol (5), cis-resveratrol (6), t-ε-viniferin (7), t-delta-viniferin (8). Means followed by the same letter were not different using one-way analysis of variance (ANOVA), followed by the Tukey HSD multiple comparison test (three independent experiments with four technical replicates). p < 0.05 was considered statistically significant.
The highest stilbene content and stilbene production were observed in the 60-3 VaMyb60-transgenic cell line and reached 18.8 mg/g DW and 148.7 mg/L, respectively (Figure 2; Table 1). To the best of our knowledge, this is one of the highest values of stilbene levels produced by plant cell cultures reported in the literature [3,38]. Stilbene content and production level in the 60-3 was in 13.2 times higher than stilbene production by the control cell culture KA0 (Table 1). It was also 4.2 times higher than stilbene production in transgenic grapevine cell lines transformed with the VaCPK20 gene encoding a CDPK (up to 35 mg/L, [23]) and 1.1 times higher than stilbene production in grapevine cell lines transformed with the VaCML65 gene encoding a calmodulin-like protein (up to 135.7 mg/L, [39]), but on 2.3% lower than that in the rolB-transgenic cell culture of V. amurensis (152 mg/L, [40]).
Overexpression of the VaMyb40 and VaMyb60 genes did not change the spectrum of detected individual stilbenes (Table 2). As in previously published works [39,41], we detected presence of eight stilbenes. Five stilbenes in all lines were well detectable (more than 0.06 mg/g DW): t-resveratrol diglucoside (1), t-piceid (2), t-resveratrol (3), ε-viniferin (4), δ-viniferin (5), and three stilbenes were usually detected in trace amounts (no more than 0.01 mg/g DW): cis-resveratrol (6), cis-piceid (7), t-piceatannol (8). The data revealed that the increase in the total content of stilbenes in the VaMyb40-transgenic cell lines was primarily due to a strong elevation in the content of t-piceid (in 2.5–5.4 times) and t-resveratrol (9.9–17.7 times) (Table 2). While the total content of stilbenes in the VaMyb60-transgenic cell lines was increased primarily due to a significant elevation in the content t-resveratrol (25.6–67.2 times), ε-viniferin (3.0–6.7 times), and δ-viniferin (2.4–4.6 times) (Table 2). T-resveratrol prevailed over other stilbenoid compounds (53.1–89.5% of all stilbenes) in both the VaMyb40- and VaMyb60-overexpressing cell cultures. The increase or decrease in the content of other stilbenes was not considerable (Table 2). Thus, our data indicate that overexpression of the VaMyb40 and VaMyb60 genes led to a marked increase in the content of stilbenes via a strong activation of t-resveratrol biosynthesis (Table 2).

Table 2.
The content of individual stilbenes (mg per g of the dry weight (DW)) in the transgenic cell lines of Vitis amurensis transformed with VaMyb40 or VaMyb60 genes.
Then, it was important to verify whether the enhanced production of stilbenes in the grapevine cell cultures overexpressing the VaMyb40 and VaMyb60 genes was due to the activation of stilbene biosynthesis or to a reduction in the degradation of these compounds. For this purpose, we analyzed the expression of several important stilbene biosynthesis genes, including five phenylalanine ammonia-lyase (PAL) genes, one chalcone synthase (CHS), and ten stilbene synthase (STS) genes (Figure 3), which are known as important enzymes in stilbene biosynthesis [3].
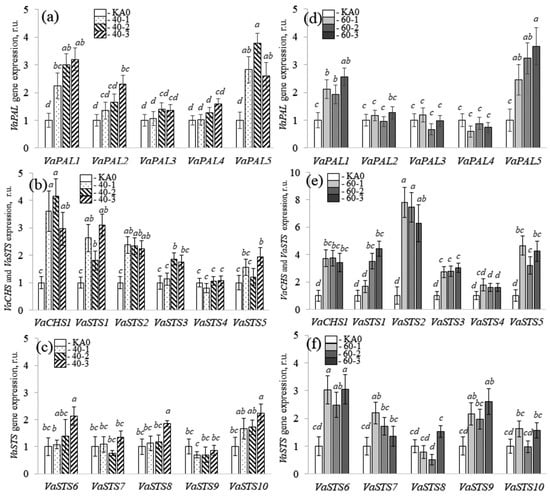
Figure 3.
Quantification the VaPAL1-5, VaCHS1, and VaSTS1-10 gene expression in the VaMyb40- (a–c) and VaMyb60-transgenic (d–f) cell lines of Vitis amurensis performed by quantitative RT-PCR. Means on each figure followed by the same letter were not different using one-way analysis of variance (ANOVA), followed by the Tukey HSD multiple comparison test (two independent experiments with eight technical replicates). p < 0.05 was considered to be statistically significant.
The data obtained revealed that overexpression of the VaMyb40 gene led to a considerable increase in the mRNA levels of the VaPAL1 and VaPAL5 (Figure 3a), VaCHS1 (Figure 3b), and VaSTS1 and VaSTS2 genes (Figure 3b,c) in the VaMyb40-transgenic cell lines. Overexpression the VaMyb60 gene led to a considerable increase in the mRNA levels of the VaPAL1 and VaPAL5 (Figure 3d), VaCHS1 (Figure 3e), and VaSTS1, 2, 3, 5, 6, and 9 genes (Figure 3e,f) in the VaMyb60-transgenic cell lines. These results indicate that the enhanced content of stilbenes in the obtained VaMyb40/60-transgenic grape cells was associated with an activation of stilbene biosynthesis via a considerable increase in the expression of certain PAL and STS genes (Figure 3).
2.3. Anthocyanin Content in the Grapevine VaMyb-Transgenic Cell Lines
In red callus zones of the Myb-transformed V. amurensis cell lines, we detected the presence of five anthocyanins that were not present in the control KA0 cell line: cyanidin-3,5-o-diglucoside (1), delphinidin-3-o-glucoside (2), malvidin-3,5-o-diglucoside (3), cyanidin-3-o-glucoside (4), petunidin-3-galactoside (5) (supplementary Figure S2; Table S1). The total anthocyanin content in the VaMyb40-overexpressing lines was 0.035 ± 0.012 mg/g FW (40-1); 0.014 ± 0.003 mg/g FW (40-2); 0.013 ± 0.002 mg/g FW (40-3); and in the Myb60-overexpressing lines—0.009 ± 0.004 mg/g FW (60-1); 0.008 ± 0.004 mg/g FW (60-2); 0.008 ± 0.005 mg/g FW (60-3), respectively. Notably, we did not detect anthocyanins in in the control KA0 cell line. As can be seen from Table 1, the tissues of transgenic cultures dry out by about 22 times, thus, the content of anthocyanins in VaMyb40-overexpressing lines reached about 0.77 mg/g DW, and in VaMyb60-overexpressing lines—0.2 mg/g DW.
3. Materials and Methods
3.1. Plant Material and Cell Cultures
The V7 callus culture were initiated in 2017 from young stems of the wild-growing mature V. amurensis vines collected near Vladivostok as described [42]. The plant transgenic cell lines were established by Agrobacterium-mediated transformation as described [23].
3.2. Isolation and Sequencing of VaMyb40 and VaMyb60 Genes
To obtain the full-length cDNA of the VaMyb40 and VaMyb60 genes, we used primers (Table S2) designed to the beginning and to the end (from start to end codons) of the protein coding sequences based on the known VvMyb40 and VrMyb60 genes sequences in V. vinifera and V. riparia, respectively (GenBank accession number XM_019224778, XM_034837254). We were able to use primers designed for other grapevines, because we showed that the sequences of the Myb genes were very similar (supplementary Table S3). The generated PCR products of VaMyb40 and VaMyb60 genes were subcloned into a pJET1.2 using CloneJET PCR Cloninig Kit (ThermoFisher Scientific, Waltham, MA, USA) and sequenced using an ABI 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) according to the manuphacturer’s instructions.
3.3. Overexpression of VaMyb40 and VaMyb60 in V. Amurensis Cell Cultures
Using VaMyb40 and VaMyb60 genes in pJET1.2 we performed PCR with the forward primer that contained a BglII and the reverse primer that contained a Sal I restriction site, which are underlined (Supplementary Table S2). The full-length cDNA of Mybs was cloned into the pSAT1 vector under the control of the double CaMV 35 S promoter [43] by the BglII and Sal I sites. Then, the expression cassette from pSAT1 with the Myb genes was cloned into the pZP-RCS2-nptII vector [43] using the PalAI (AscI) sites. The pZP-RCS2-nptII construction also included the nptII gene under the control of the double CaMV 35 S promoter. The restriction enzymes were obtained from SibEnzyme (Novosibirsk, Russia). The independently transformed VaMyb-transgenic callus cell lines of V. amurensis, designated as 40-1, 40-2, 40-3 (VaMyb40 gene) and 60-1, 60-2, 60-3 (VaMyb60 gene), were obtained in 2021 by transformation of the V7 cell suspension with A. tumefaciens strain GV3101::pMP90 containing pZP-RCS2-VaMybs-nptII as described [23,38,44].
Transcript level of the nptII gene was analyzed using semiquantitative RT-PCR with the primes and PCR conditions described [45]. The absence of A. tumefaciens was confirmed by RT-PCR of the VirB2 gene using primers listed in the Table S2 [45]. All transgenic cell lines were cultivated in 100 mL flasks with 20 mL of the solid Murashige and Skoog modified WB/A medium [46] supplemented with 0.5 mg/L BAP, 2 mg/L NAA, and 8 g/L agar in the dark. For stilbene and anthocyanin analysis, the V. amurensis calli were cultivated at 35-day subculture intervals in the dark at 24–25 °C in test tubes (height 150 mm, internal diameter 14 mm) with 7–8 mL of the WB/A medium.
3.4. Anthocyanin and Stilbene Analysis by High Performance Liquid Chromatography (HPLC) and Mass Spectrometry
Anthocyanin and stilbene content analysis was performed by the method HPLC-MS as described [45,47,48]. Briefly, for anthocyanins 100 mg fresh cells tissue were subsequently homogenized using a mortar and a pestle in 1 mL of 1% (v/v) hydrochloric acid in methanol. Then, shredded tissue was extracted for 1 d at 4 °C. For stilbenes 100 mg of the dried shredded cells tissue were extracted for 2 h at 60 °C in 3 mL of methanol. Then, anthocyanin and stilbene extracts were filtered through a 0.25-um nylon membrane for further analysis. Next, samples were separated on Shim-pack GIST C18 column (150 mm, 2.1-nm i.d., 3-_m part size; Shimadzu, Japan) the on HPLC LC-20AD XR analytical system (Shimadzu, Japan), equipped with an SPD-M20A photodiode array detector. Liquid chromatography-high-resolution mass spectrometry for qualification of all components was performed using a 1260 Infinity analytical system (Agilent Technologies, Santa Clara, CA, USA) as described [24,44].
The commercial standard cyanidin chloride, petunidin chloride, delphinidin chloride, malvidin chloride, t-resveratrol, t-piceid, t-piceatannol, ε-viniferin were obtained from Sigma-Aldrich (St. Louis, MO, USA) and used as the control.
3.5. RNA Isolation, Reverse Transcription and Quantitative Real-Time PCR (qRT-PCR)
Total RNA extraction was performed using the cetyltrimethylammonium bromide-based extraction as described [49]. Complementary DNAs were synthesized using the MMLV Reverse transcription PCR Kit with oligo(dT)15 (RT-PCR, Evrogen, Moscow, Russia) as described [50]. qRT-PCRs were performed using the real-time PCR kit (Evrogen) and SybrGreen I Real-time PCR dye (Evrogen) using total cDNAs as described [49,50].
The expression was calculated by the 2−ΔΔCT method with two internal controls VaGAPDH and VaActin1, as described [51]. The qRT-PCR primers are listed in the Table S2. We used different primer sets for analyzing expression of the exogenous (transgene) and endogenous VaMyb genes (Supplementary Figure S3; Table S2).
3.6. Statistical Analysis
For quantification the VaMyb, VaPAL, and VaSTS genes expression, we used two independent experiments with ten technical replicates (five qPCR reactions normalized to VaGAPDH and five qPCR reactions—to VaActin gene in each independent experiment). We used three independent experiments with ten technical replicates in each experiment for callus tissue weight calculations and three independent experiments with two technical replicates for total stilbene measurement.
4. Conclusions
Previously, we found that two newly identified VaMyb40 and VaMyb60 genes were highly up-regulated in the wild-growing grapevine V. amurensis in response to UV irradiation and were suggested as promising candidates for playing important roles in stilbene biosynthesis [37]. Moreover, expression of the VaMyb40 and VaMyb60 genes was significantly increased in grapevine cell lines with elevated stilbene content as a result of overexpressing the VaCPK20 and the calmodulin-like VaCML65 genes [39]. In this paper, we investigated the effect of overexpressing VaMyb40 and VaMyb60 genes in callus cell cultures of V. amurensis on stilbene levels and composition. Both VaMyb60 and VaMyb40 gene overexpression activated stilbene biosynthesis and promoted stilbene accumulation, though effect of VaMyb40 was lower. Overexpression of both VaMyb40 and VaMyb60 genes induced biosynthesis of t-resveratrol to a greater extent than biosynthesis of other individual stilbenes. Furthermore, we found that expression of stilbene biosynthesis genes, including PAL and STS, was considerably increased in the VaMyb-transgenic lines with elevated stilbene levels. Taken together, this indicates that these transcription factors primarily activate expression of stilbene biosynthesis-related genes and not the genes responsible for further stilbene metabolism.
The proposed model of the signaling pathway leading to stilbene biosynthesis induction with the involvement of VaMyb40 or VaMyb60 in this process was presented on the Figure S4. Briefly, after signal perception, calcium influx is induced, which then leads to the activation of calcium sensor proteins. Then, the signal is transferred via activation of mitogen-activated protein kinases (MAPK) cascade, CDPK, CML, hormone signaling, and some TFs, e.g., VaMyb40 or VaMyb06, which in turn lead to the transcriptional activation of the stilbene biosynthesis-related genes, such as PAL or STS.
In conclusion, the results are important for understanding the signaling pathways and mechanisms regulating biosynthesis of stilbenes and other phenolic metabolites and might be in demand for plant biotechnology and agriculture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11151916/s1, Table S1: List of the stilbenes and anthocyanins derivatives identified in the methanol extracts of Vitis amurensis cells. Table S2: Primers used for amplification of Vitis amurensis cDNAs in PCR. Table S3: Comparison of the nucleotide sequences (BLAST) Vitis amurensis VaMyb40 and VaMyb60 genes with known VvMyb40 and VrMyb60 genes from Vitis vinifera and Vitis riparia respectively. The Genbank accession numbers are given in parentheses. Figure S1: Vitis amurensis cell lines used in the experiments. Figure S2: HPLC chromatograms of anthocyanins in Vitis amurensis in control KA0 cell culture (A) and cell line 40-1 overexpressed VaMyb40 gene (detected at 530 nm). Figure S3: VaMyb40, VaMyb60, VvMyb40, and VvMyb60 genes sequences and primer design for PCR analysis. The colors of the primers match the primers shown in the supplementary table S1. Figure S4: Proposed model of the signaling pathway leading to stilbene biosynthesis induction and VaMyb40 and VaMyb60 functions in this process in the grapevine cells.
Author Contributions
A.S.D. and K.V.K. performed research design, interpretation and paper preparation. A.A.A. and O.A.A. performed experiments with cell cultures, RNA isolations, data analysis. A.R.S. performed HPLC analysis. A.A.A., Z.V.O. and N.N.N. performed qRT-PCRs and participated in data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grant 22-16-00078 from the Russian Science Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Langcake, P.; Pryce, R.J. A new class of phytoalexins from grapevines. Experientia 1977, 33, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, K.V. Perspectives for production and application of resveratrol. Appl. Microbiol. Biotechnol. 2011, 90, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Dubrovina, A.S.; Kiselev, K.V. Regulation of stilbene biosynthesis in plants. Planta 2017, 346, 597–623. [Google Scholar] [CrossRef]
- Colica, C.; Milanovic, M.; Milic, N.; Aiello, V.; De Lorenzo, A.; Abenavoli, L. A systematic review on natural antioxidant properties of resveratrol. Nat. Prod. Commun. 2018, 13, 1195–1203. [Google Scholar] [CrossRef] [Green Version]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikuten, I.; Stambuk, P.; Andabaka, Z.; Tomaz, I.; Markovic, Z.; Stupic, D.; Maletic, E.; Kontic, J.K.; Preiner, D. Grapevine as a rich source of polyphenolic compounds. Molecules 2020, 25, 5604. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clément, C.; Courot, E. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. Biofactors 2010, 36, 331–341. [Google Scholar] [CrossRef]
- Riccio, B.V.F.; Fonseca-Santos, B.; Ferrari, P.C.; Chorilli, M. Characteristics, biological properties and analytical methods of trans-resveratrol: A review. Crit. Rev. Anal. Chem. 2020, 50, 339–358. [Google Scholar] [CrossRef]
- Chong, J.L.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Austin, M.B.; Bowman, M.E.; Ferrer, J.L.; Schröder, J.; Noel, J.P. An aldol switch discovered in stilbene synthases mediates cyclization specificity of type III polyketide synthases. Chem. Biol. 2004, 11, 1179–1194. [Google Scholar] [CrossRef] [Green Version]
- Schmidlin, L.; Poutaraud, A.; Claudel, P.; Mestre, P.; Prado, E.; Santos-Rosa, M.; Wiedemann-Merdinoglu, S.; Karst, F.; Merdinoglu, D.; Hugueney, P. A stress-inducible resveratrol O-methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol. 2008, 148, 1630–1639. [Google Scholar] [CrossRef] [Green Version]
- Hall, D.; De Luca, V. Mesocarp localization of a bi-functional resveratrol/hydroxycinnamic acid glucosyltransferase of Concord grape (Vitis labrusca). Plant J. 2007, 49, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Dry, I.B.; Robinson, S.P. Molecular cloning and characterisation of grape berry polyphenol oxidase. Plant Mol. Biol. 1994, 26, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Faurie, B.; Cluzet, S.; Mérillon, J.M. Implication of signaling pathways involving calcium, phosphorylation and active oxygen species in methyl jasmonate-induced defense responses in grapevine cell cultures. J. Plant Physiol. 2009, 166, 1863–1877. [Google Scholar] [CrossRef] [PubMed]
- Vandelle, E.; Vannozzi, A.; Wong, D.; Danzi, D.; Digby, A.M.; Dal Santo, S.; Astegno, A. Identification, characterization, and expression analysis of calmodulin and calmodulin-like genes in grapevine (Vitis vinifera) reveal likely roles in stress responses. Plant Physiol. Biochem. 2018, 129, 221–237. [Google Scholar] [CrossRef]
- Belchi-Navarro, S.; Almagro, L.; Sabater-Jara, A.B.; Fernandez-Perez, F.; Bru, R.; Pedreno, M.A. (2013) Induction of trans-resveratrol and extracellular pathogenesis-related proteins in elicited suspension cultured cells of Vitis vinifera cv Monastrell. J. Plant Physiol. 2013, 170, 258–264. [Google Scholar] [CrossRef]
- Nicolas, P.; Lecourieux, D.; Kappel, C.; Cluzet, S.; Cramer, G.; Delrot, S.; Lecourieux, F. The basic leucine zipper transcription factor ABSCISIC ACID RESPONSE ELEMENT-BINDING FACTOR2 is an important transcriptional regulator of abscisic acid-dependent grape berry ripening processes. Plant Physiol. 2014, 164, 365–383. [Google Scholar] [CrossRef] [Green Version]
- Xu, A.; Zhan, J.C.; Huang, W.D. Effects of ultraviolet C, methyl jasmonate and salicylic acid, alone or in combination, on stilbene biosynthesis in cell suspension cultures of Vitis vinifera L. cv. Cabernet Sauvignon. Plant Cell Tissue Organ Cult. 2015, 122, 197–211. [Google Scholar] [CrossRef]
- Degu, A.; Ayenew, B.; Cramer, G.R.; Fait, A. Polyphenolic responses of grapevine berries to light, temperature, oxidative stress, abscisic acid and jasmonic acid show specific developmental-dependent degrees of metabolic resilience to perturbation. Food Chem. 2016, 212, 828–836. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V.; Veselova, M.V.; Isaeva, G.A.; Fedoreyev, S.A.; Zhuravlev, Y.N. Enhanced resveratrol accumulation in rolB transgenic cultures of Vitis amurensis correlates with unusual changes in CDPK gene expression. J. Plant Physiol. 2009, 166, 1194–1206. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Shumakova, O.A.; Manyakhin, A.Y.; Mazeika, A.N. Influence of calcium influx induced by the calcium ionophore, A23187, on resveratrol content and the expression of CDPK and STS genes in the cell cultures of Vitis amurensis. Plant Growth Regul. 2012, 68, 371–381. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Shumakova, O.A.; Manyakhin, A.Y. Effects of the calmodulin antagonist W7 on resveratrol biosynthesis in Vitis amurensis Rupr. Plant Mol. Biol. Rep. 2013, 31, 1569–1575. [Google Scholar] [CrossRef]
- Aleynova-Shumakova, O.A.; Dubrovina, A.S.; Manyakhin, A.Y.; Karetin, Y.A.; Kiselev, K.V. VaCPK20 gene overexpression significantly increased resveratrol content and expression of stilbene synthase genes in cell cultures of Vitis amurensis Rupr. Appl. Microbiol. Biotechnol. 2014, 98, 5541–5549. [Google Scholar] [CrossRef] [PubMed]
- Aleynova, O.A.; Dubrovina, A.S.; Manyakhin, A.Y.; Karetin, Y.A.; Kiselev, K.V. Regulation of resveratrol production in Vitis amurensis cell cultures by calcium-dependent protein kinases. Appl. Biochem. Biotechnol. 2015, 175, 1460–1476. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wang, D.; Wang, L.; Jiang, C.; Wang, Y. VqMAPKKK38 is essential for stilbene accumulation in grapevine. Hortic. Res. 2017, 4, 17058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holl, J.; Vannozzi, A.; Czemmel, S.; D’Onofrio, C.; Walker, A.R.; Rausch, T.; Lucchin, M.; Boss, P.K.; Dry, I.B.; Bogs, J. The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera. Plant Cell 2013, 25, 4135–4149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Hou, Y.; Wang, L.; Xin, H.; Wang, N.; Li, S. Myb14, a direct activator of STS, is associated with resveratrol content variation in berry skin in two grape cultivars. Plant Cell Rep. 2014, 33, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Huang, J.J.; Luo, Z.Y. JA-mediated transcriptional regulation of secondary metabolism in medicinal plants. Sci. Bull. 2015, 60, 1062–1072. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Osbourn, A.; Ma, P. MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol. Plant. 2015, 8, 689–708. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Li, K.; Li, Y.; Zhao, X.; Wang, L. MYB transcription factors as regulators of secondary metabolism in plants. Biology 2020, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Matus, J.T.; Aquea, F.; Arce-Johnson, P. Analysis of the grape MYB R2R3 subfamily reveals expanded wine quality-related clades and conserved gene structure organization across Vitis and Arabidopsis genomes. BMC Plant Biol. 2008, I, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czemmel, S.; Heppel, S.C.; Bogs, J. R2R3 MYB transcription factors: Key regulators of the flavonoid biosynthetic pathway in grapevine. Protoplasma 2012, 249, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.C.J.; Schlechter, R.; Vannozzi, A.; Höll, J.; Hmmam, I.; Bogs, J.; Tornielli, G.B.; Castellarin, S.D.; Matus, J.T. A systems-oriented analysis of the grapevine R2R3-MYB transcription factor family uncovers new insights into the regulation of stilbene accumulation. DNA Res. 2016, 23, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Parage, C.; Tavares, R.; Réty, S.; Baltenweck-Guyot, R.; Poutaraud, A.; Renault, L.; Heintz, D.; Lugan, R.; Marais, G.A.B.; Aubourg, S.; et al. Structural, functional, and evolutionary analysis of the unusually large stilbene synthase gene family in grapevine. Plant Physiol. 2012, 160, 1407–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiselev, K.V.; Aleynova, O.A.; Tyunin, A.P. Expression of the R2R3 MYB transcription factors in Vitis amurensis Rupr. plants and cell cultures with different resveratrol content. Russ. J. Genet. 2017, 53, 465–471. [Google Scholar] [CrossRef]
- Aleynova, O.A.; Dubrovina, A.S.; Kiselev, K.V. Activation of stilbene synthesis in cell cultures of Vitis amurensis by calcium-dependent protein kinases VaCPK1 and VaCPK26. Plant Cell Tiss. Organ Cult. 2017, 130, 141–152. [Google Scholar] [CrossRef]
- Suprun, A.R.; Ogneva, Z.V.; Dubrovina, A.S.; Kiselev, K.V. Effect of spruce PjSTS1a, PjSTS2, or PjSTS3 gene overexpression on stilbene biosynthesis in callus cultures of Vitis amurensis Rupr. Biotechnol. Appl. Biochem. 2020, 67, 234–239. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Dubrovina, A.S. Overexpression of stilbene synthase genes to modulate the properties of plants and plant cell cultures. Biotechnol. Appl. Biochem. 2021, 68, 13–19. [Google Scholar] [CrossRef]
- Aleynova, O.A.; Suprun, A.R.; Ananev, A.A.; Nityagovsky, N.N.; Ogneva, Z.V.; Dubrovina, A.S.; Kiselev, K.V. Effect of calmodulin-like gene (CML) overexpression on stilbene biosynthesis in cell cultures of Vitis amurensis Rupr. Plants 2022, 11, 171. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Dubrovina, A.S.; Veselova, M.V.; Bulgakov, V.P.; Fedoreyev, S.A.; Zhuravlev, Y.N. The rolB gene-induced overproduction of resveratrol in Vitis amurensis transformed cells. J. Biotechnol. 2007, 128, 681–692. [Google Scholar] [CrossRef]
- Aleynova, O.A.; Suprun, A.R.; Nityagovsky, N.N.; Dubrovina, A.S.; Kiselev, K.V. The influence of the grapevine bacterial and fungal endophytes on biomass accumulation and stilbene production by the in vitro cultivated cells of Vitis amurensis Rupr. Plants 2021, 10, 1276. [Google Scholar] [CrossRef] [PubMed]
- Tyunin, A.P.; Suprun, A.R.; Nityagovsky, N.N.; Manyakhin, A.Y.; Karetin, Y.A.; Dubrovina, A.S.; Kiselev, K.V. The effect of explant origin and collection season on stilbene biosynthesis in cell cultures of Vitis amurensis Rupr. Plant Cell Tiss. Organ Cult. 2019, 136, 189–196. [Google Scholar] [CrossRef]
- Tzfira, T.; Tian, G.W.; Lacroix, B.; Vyas, S.; Li, J.; Leitner-Dagan, Y.; Krichevsky, A.; Taylor, T.; Vainstein, A.; Citovsky, V. pSAT vectors: A modular series of plasmids for autofluorescent protein tagging and expression of multiple genes in plants. Plant Mol. Biol. 2005, 57, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Aleynova, O.A.; Grigorchuk, V.P.; Dubrovina, A.S.; Rybin, V.G.; Kiselev, K.V. Stilbene accumulation in cell cultures of Vitis amurensis Rupr. overexpressing VaSTS1, VaSTS2, and VaSTS7 genes. Plant Cell Tiss. Organ Cult. 2016, 125, 329–339. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Aleynova, O.A.; Grigorchuk, V.P.; Dubrovina, A.S. Stilbene accumulation and expression of stilbene biosynthesis pathway genes in wild grapevine Vitis amurensis Rupr. Planta 2017, 245, 151–159. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Manyakhin, A.Y.; Zhuravlev, Y.N.; Kiselev, K.V. Resveratrol content and expression of phenylalanine ammonia-lyase and stilbene synthase genes in rolC transgenic cell cultures of Vitis amurensis. Appl. Microbiol. Biotechnol. 2010, 88, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, K.V.; Aleynova, O.A.; Ogneva, Z.V.; Suprun, A.R.; Ananev, A.A.; Nityagovsky, N.N.; Dubrovina, A.S. The effect of stress hormones, UV-C, and stilbene precursors on calmodulin (CaM) and calmodulin-like gene (CML) expression in Vitis amurensis Rupr. Plant Cell Tiss. Organ Cult. 2021, 146, 59–68. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Suprun, A.R.; Aleynova, O.A.; Ogneva, Z.V.; Kalachev, A.V.; Dubrovina, A.S. External dsRNA downregulates anthocyanin biosynthesis-related genes and affects anthocyanin accumulation in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 6749. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Ogneva, Z.V.; Suprun, A.R.; Grigorchuk, V.P.; Dubrovina, A.S. Action of ultraviolet-C radiation and p-coumaric acid on stilbene accumulation and expression of stilbene biosynthesis-related genes in the grapevine Vitis amurensis Rupr. Acta Physiol. Plant 2019, 41, 28. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Dubrovina, A.S.; Shumakova, O.A.; Karetin, Y.A.; Manyakhin, A.Y. Structure and expression profiling of a novel calcium-dependent protein kinase gene, CDPK3a, in leaves, stems, grapes, and cell cultures of wild-growing grapevine Vitis amurensis Rupr. Plant Cell Rep. 2013, 32, 431–442. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).