Source Identification and Genome-Wide Association Analysis of Crown Rot Resistance in Wheat
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Analyses
2.2. Evaluation of Resistance to CR in Wheat Cultivars
2.3. SNPs Linked to CR Resistance Identified by GWAS
3. Discussion
4. Materials and Methods
4.1. Pathogen and Wheat Materials
4.2. Seedling Resistance Assessment
4.3. Genotyping Analysis
4.4. Population Structure, Linkage Disequilibrium, and Genome-Wide Association Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Burgess, L.W.; Backhouse, D.; Summerell, B.A.; Swan, L.J. Crown rot of wheat. In Fusarium Paul E. Nelson Memorial Symposium; Summerell, B.A., Leslie, J.F., Backhouse, D., Bryden, W.L., Burgess, L.W., Eds.; APS Press: St. Paul, MN, USA, 2001; Chapter 20. [Google Scholar]
- Nicol, J.M.; Bolat, N.; Bagci, A.; Trethowan, R.T.; William, M.; Hekimhan, H.; Yildirim, A.F.; Sahin, E.; Elekcioglu, H.; Toktay, H. The International Breeding Strategy for the Incorporation of Resistance in Bread Wheat against the Soil Borne Pathogens (Dryland Root Rot and Cyst and Lesion Cereal Nematodes) Using Conventional and Molecular Tools. Dev. Plant Breed. 2007, 12, 125–137. [Google Scholar] [CrossRef]
- Smiley, R.W.; Gourlie, J.A.; Easley, S.A.; Patterson, L.M.; Whittaker, R.G. Crop Damage Estimates for Crown Rot of Wheat and Barley in the Pacific Northwest. Plant Dis. 2007, 89, 595–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.X.; Sun, H.Y.; Shen, C.M.; Li, W.; Chen, H.G. Survey of Fusarium spp. causing wheat crown rot in major winter wheat growing regions of China. Plant Dis. 2015, 99, 1610–1615. [Google Scholar] [CrossRef] [Green Version]
- Kazan, K.; Gardiner, D.M. Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: Recent progress and future prospects. Mol. Plant Pathol. 2018, 19, 1547–1562. [Google Scholar] [CrossRef] [Green Version]
- Li, H.L.; Yuan, H.X.; Fu, B.; Xing, X.P.; Tang, W.H. First Report of Fusarium pseudograminearum Causing Crown Rot of Wheat in Henan, China. Plant Dis. 2012, 96, 1065. [Google Scholar] [CrossRef]
- Yang, X.; Pan, Y.; Singh, P.K.; He, X.; Ren, Y. Investigation and genome-wide association study for Fusarium crown rot resistance in Chinese common wheat. BMC Plant Biol. 2019, 153, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.F.; Yang, Y.; Niu, Y.J.; Yuan, H.X.; Li, H.L. Occurrence and Control Methods of Crown Rot of Wheat. JAST 2014, 43, 114–117. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Q. The cause reason and comprehensive control of wheat crown rot in Xinxiang China. J. Plant Prot. 2016, 36, 40–42. [Google Scholar]
- Burgess, L.W. 2011 McAlpine Memorial Lecture—A Love Affair with Fusarium. Australas. Plant Pathol. 2014, 43, 359–368. Available online: https://kns.cnki.net/kns/detail/detail.aspx?QueryID=2&CurRec=1&DbCode=CJFD&dbname=CJFDLAST2016&filename=ZBJS201607008&urlid=&yx= (accessed on 1 June 2022). [CrossRef]
- Chakraborty, S.; Liu, C.J.; Mitter, V.; Scott, J.B.; Simpfendorfer, S. Pathogen population structure and epidemiology are keys to wheat crown rot and Fusarium head blight management. Australas. Plant Pathol. 2006, 35, 643–655. [Google Scholar] [CrossRef]
- Dodman, R.L.; Wildermuth, G.B. Inoculation methods for assessing resistance in wheat to crown rot caused by Fusarium graminearum group 1. Aust. J. Agric. Res. 1987, 38, 473–486. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Voigt, W.G.J.; Lamprecht, S.C.; Knox-Davies, P.S. Crown rot and head blight of wheat caused by Fusarium graminearum Groups 1 and 2 in the southern Cape Province. Phytophylactica 1988, 20, 385–389. [Google Scholar]
- Wallwork, H.; Butt, M.; Cheong, J.P.E.; Williams, K.J. Resistance to crown rot in wheat identified through an improved method for screening adult plants. Australas. Plant Pathol. 2004, 33, 1–7. [Google Scholar] [CrossRef]
- Wildermuth, G.B.; Mcnamara, R.B.; Quick, J.S. Crown depth and susceptibility to crown rot in wheat. Euphytica 2001, 122, 397–405. [Google Scholar] [CrossRef]
- Liu, C.J.; Ma, J.; Li, H.B.; Liu, Y.X.; Chakraborty, S. The homoeologous regions on long arms of group 3 chromosomes in wheat and barley harbour major crown rot resistance loci. Czech J. Genet. Plant Breed. 2011, 47, S109–S114. [Google Scholar] [CrossRef] [Green Version]
- Mitter, V.; Zhang, M.C.; Liu, C.J.; Ghosh, R.; Ghosh, M.; Chakraborty, S. A high-throughput glasshouse bioassay to detect crown rot resistance in wheat germplasm. Plant Pathol. 2010, 55, 433–441. [Google Scholar] [CrossRef]
- Alahmad, S.; Kang, Y.; Dinglasan, E.; Mazzucotelli, E.; Hickey, L.T. Adaptive traits to improve durum wheat yield in drought and Crown Rot environments. Int. J. Mol. Sci. 2020, 21, 5260. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, J.; Pu, L.; Sun, D.; Hu, X. Identification of New Sources of Resistance to Crown Rot and Fusarium Head Blight in Wheat. Plant Dis. 2020, 104, 1979–1985. [Google Scholar] [CrossRef]
- Jin, J.; Duan, S.; Qi, Y.; Yan, S.; Ma, J. Identification of a novel genomic region associated with resistance to Fusarium crown rot in wheat. Theor. Appl. Genet. 2020, 133, 2063–2073. [Google Scholar] [CrossRef]
- Pariyar, S.R.; Said, D.; Benedict, O.C.; Léon, J.; Grundler, F. Dissecting the genetic complexity of Fusarium Crown Rot resistance in wheat. Sci. Rep. 2020, 10, 3200. [Google Scholar] [CrossRef] [Green Version]
- Jun, S.; Zhao, J.; Zhao, S.; Li, M.; Wang, X. Genetics of resistance to Common Root Rot (Spot Blotch), Fusarium Crown Rot, and Sharp Eyespot in wheat. Front. Genet. 2021, 12, 1008. [Google Scholar] [CrossRef]
- Martin, A.; Bovill, W.D.; Percy, C.D.; Herde, D.; Fletcher, S.; Kelly, A.; Neate, S.M.; Sutherland, M.W. Markers for seedling and adult plant crown rot resistance in four partially resistant bread wheat sources. Theor. Appl. Genet. 2015, 128, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Poole, G.J.; Smiley, R.W.; Paulitz, T.C.; Walker, C.A.; Carter, A.H.; See, D.R.; Garland-Campbell, K. Identification of quantitative trait loci (QTL) for resistance to Fusarium crown rot (Fusarium pseudograminearum) in multiple assay environments in the Pacific Northwestern US. Theor. Appl. Genet. 2012, 125, 91–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z. A systematic study of Fusarium crown rot resistance in hexaploid wheat. Ph.D. Dissertation, University of Western Australia, Nedlands, Australia, 2015. [Google Scholar]
- Flint-Garcia, S.A. Genetics and Consequences of Crop Domestication. J. Agric. Food Chem. 2013, 61, 8267–8276. [Google Scholar] [CrossRef]
- Tommasini, L.; Schnurbusch, T.; Fossati, D.; Mascher, F.; Keller, B. Association mapping of Stagonospora nodorum blotch resistance in modern European winter wheat varieties. Theor. Appl. Genet. 2007, 115, 697–708. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.H.; Bai, Y.; Haley, S.D.; Lapitan, N. Microsatellite-based molecular diversity of bread wheat germplasm and association mapping of wheat resistance to the Russian wheat aphid. Genetica 2009, 135, 95–122. [Google Scholar] [CrossRef]
- Yao, F.J.; Zhang, X.M.; Ye, X.L.; Li, J.; Long, L.; Yu, C.; Li, J.; Wang, Y.Q.; Wu, Y.; Wang, J.R. Characterization of molecular diversity and genome-wide association study of stripe rust resistance at the adult plant stage in Northern Chinese wheat landraces. BMC Genet. 2019, 20, 38. [Google Scholar] [CrossRef] [Green Version]
- Pu, L.F.; Ren, H.; Ou, Y.C.; Ren, H.L.; Zeng, Q.D.; Hu, X.P.; Li, C.L.; Han, D.J. The resistant germplasms screening and associated SNPs for Crown rot and Fusarium Head Blight in wheat. J. Triticeae Crops 2020, 7, 780–788. [Google Scholar] [CrossRef]
- Yang, X.; Zhong, S.; Zhang, Q.; Ren, Y.; Sun, C.; Chen, F. A loss-of-function of the dirigent gene TaDIR-B1 improves resistance to Fusarium crown rot in wheat. Plant Biotechnol. J. 2021, 19, 866–868. [Google Scholar] [CrossRef]
- Akgül, D.S.; Erkiliç, A. Effect of wheat cultivars, fertilizers, and fungicides on Fusarium foot rot disease of wheat. Turk. J. Agric. For. 2016, 40, 101–108. [Google Scholar] [CrossRef]
- Al-Fahdawi, M.S.; Able, J.A.; Evans, M.; Able, A.J. Response of durum wheat to different levels of zinc and Fusarium pseudograminearum. Crop Pasture Sci. 2014, 65, 61–73. [Google Scholar] [CrossRef]
- Chen, C.; Payne, W.A.; Smiley, R.W.; Stoltz, M.A. Yield and water-use efficiency of eight wheat cultivars planted on seven dates in Northeastern oregon. Agron. J. 2003, 95, 836–843. [Google Scholar] [CrossRef]
- Evans, M.L.; Hollaway, G.J.; Dennis, J.I.; Correll, R.; Wallwork, H. Crop sequence as a tool for managing populations of Fusarium pseudograminearum and F. culmorum in south-eastern Australia. Australas. Plant Path. 2010, 39, 376–382. [Google Scholar] [CrossRef]
- Paulitz, T.C. Low input No-till cereal production in the Pacific Northwest of the U.S.: The challenges of root diseases. Eur. J. Plant Pathol. 2006, 115, 271–281. [Google Scholar] [CrossRef]
- Yang, Y.; He, X.L.; Hu, Y.F.; Hou, Y.; Niu, Y.; Dai, J.; Yuan, H.; Li, H.L. Resistance of Wheat Cultivars in Huang-Huai Region of China to Crown Rot Caused by Fusarium pseudograminearum. J. Triticeae Crops 2015, 35, 339–345. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Wei, Y.; Yan, G.; Zhou, M.; Wei, Y.; Zheng, Y.; Liu, C. Different tolerance in bread wheat, durum wheat and barley to Fusarium Crown Rot disease caused by Fusarium pseudograminearum. J. Phytopathol. 2012, 160, 412–417. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.J.; Donnelly, P.J. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.; Bender, D.; Maller, J.; Sklar, P.; Bakker, P.; Daly, M.J. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
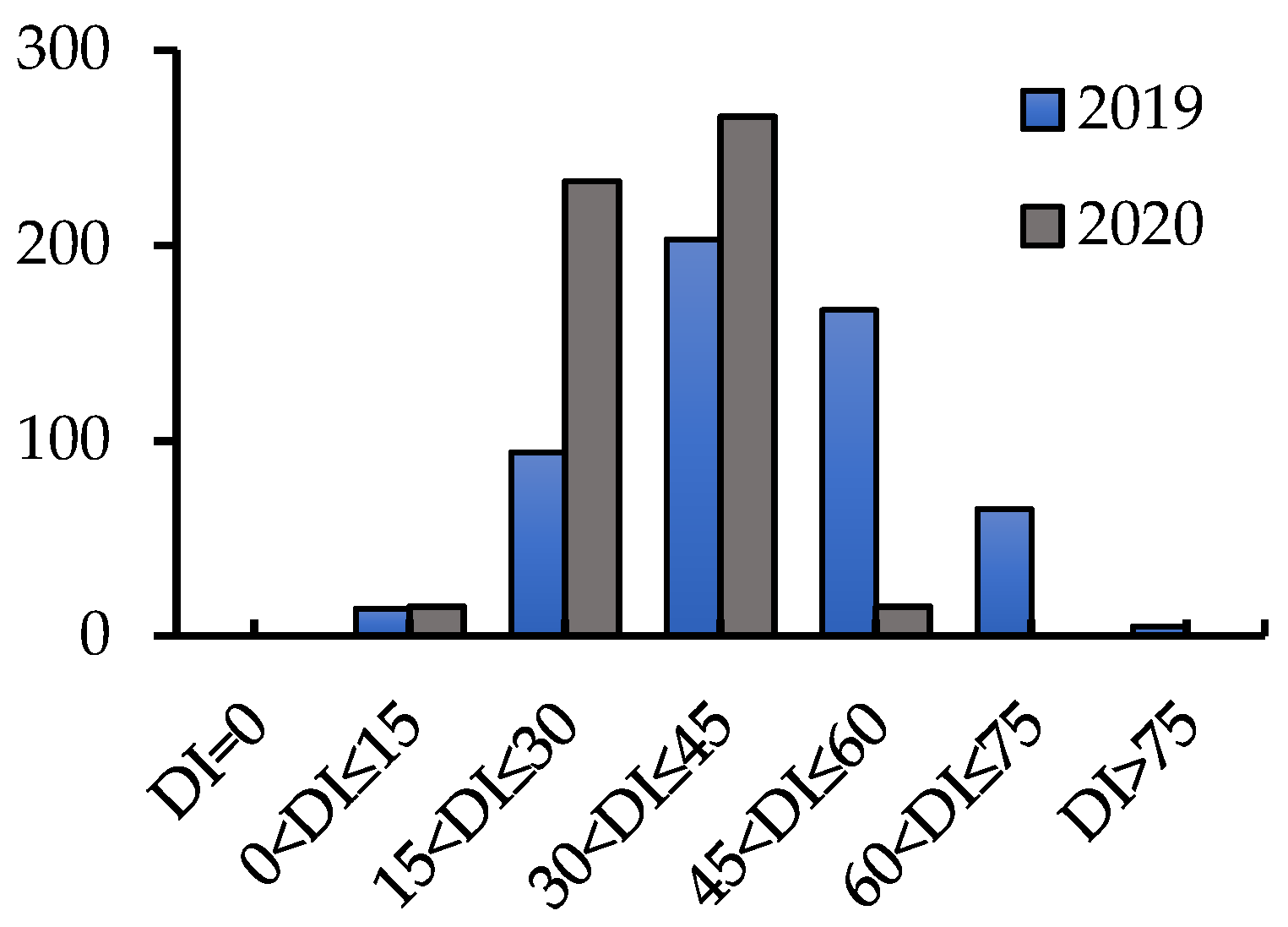

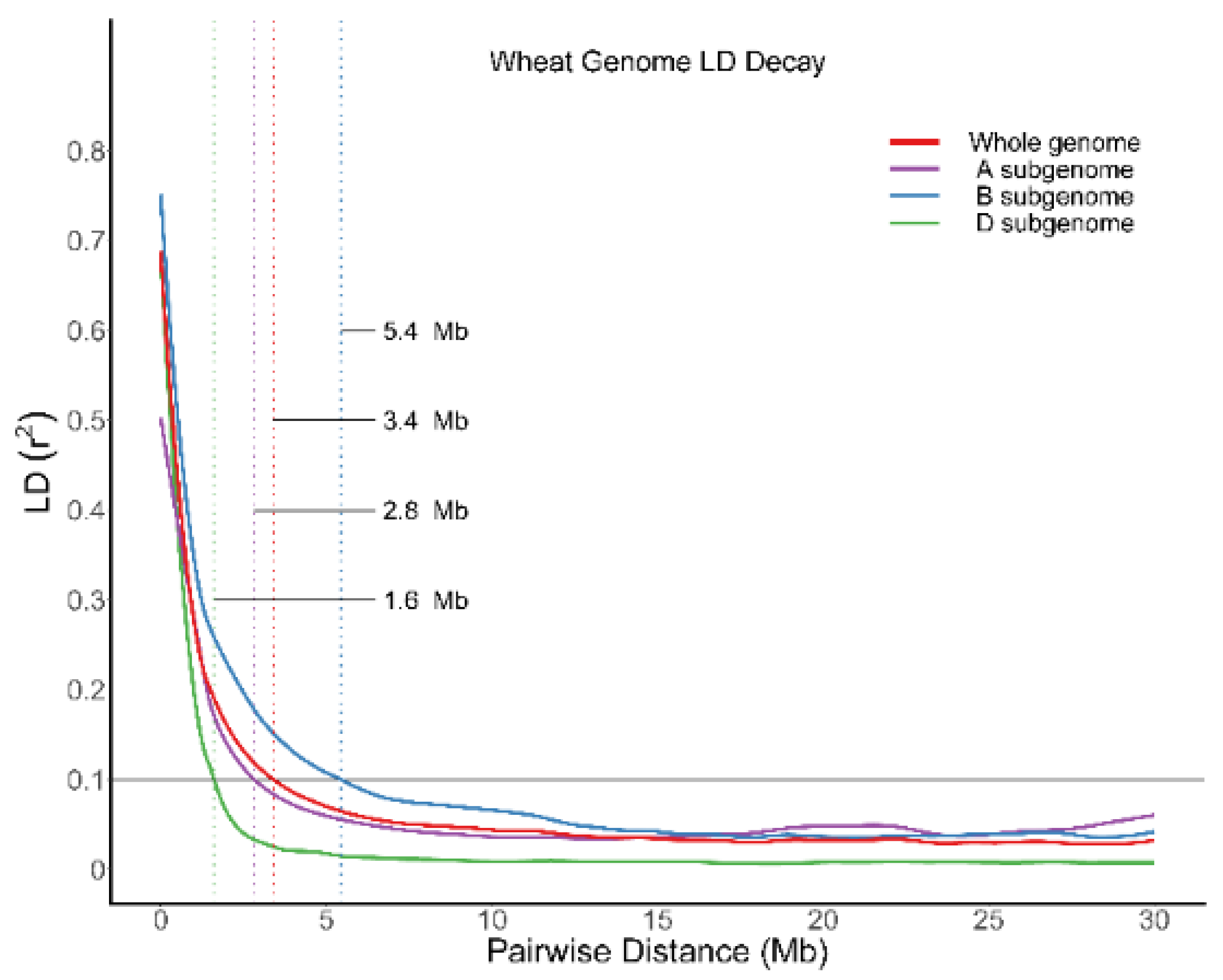
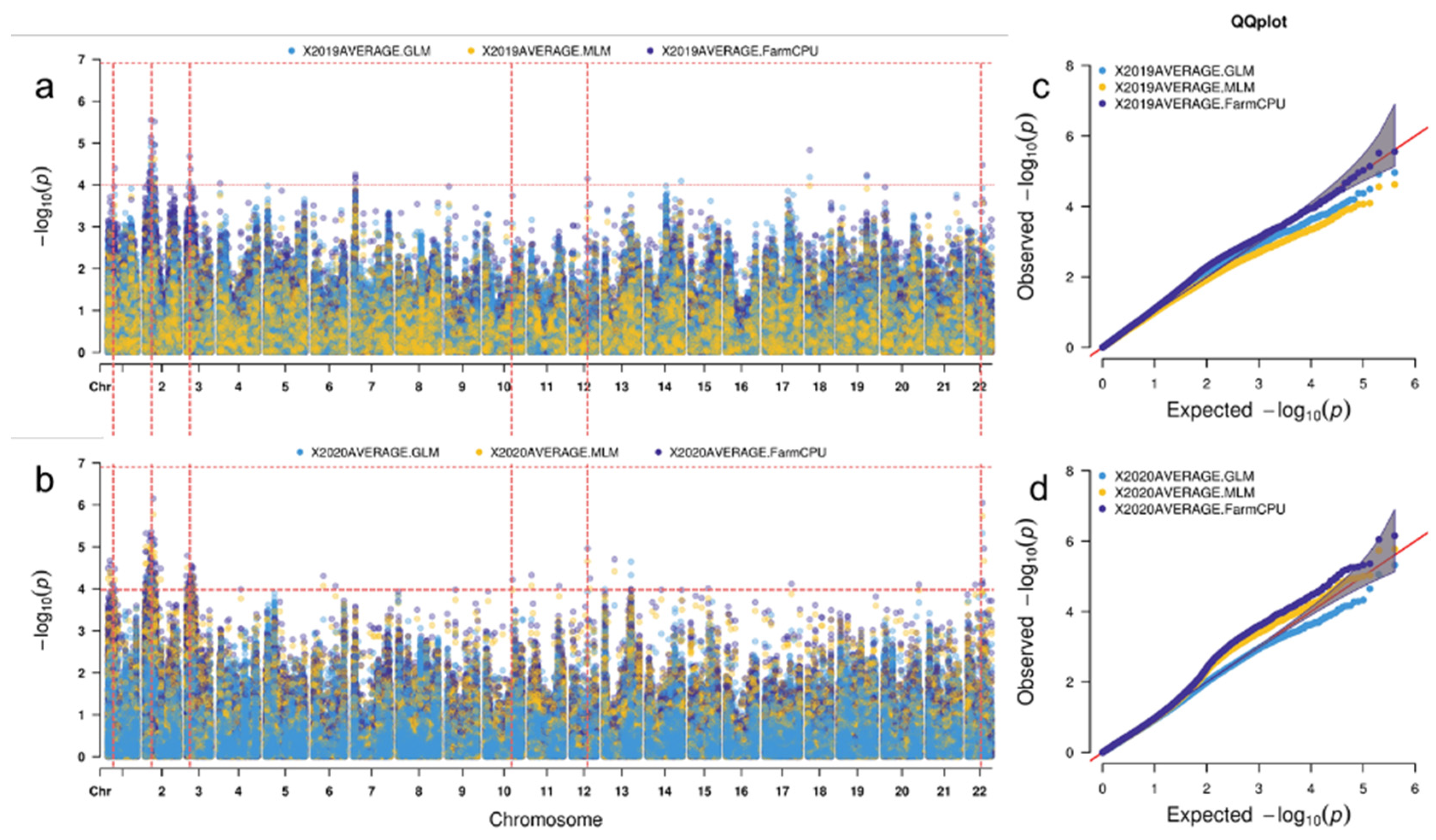
| Years | SS | DF | MS | F Value | p-Value | |
|---|---|---|---|---|---|---|
| 2019 | Cultivar | 195,492.43 | 521 | 375.23 | 11.109 | <0.0001 |
| Replication | 38.72 | 1 | 38.72 | 0.18 | 0.67 | |
| Error | 15,672.93 | 464 | 33.78 | |||
| Total | 211,204.08 | 986 | ||||
| 2020 | Cultivar | 93,754.55 | 521 | 179.95 | 14.5 | <0.0001 |
| Replication | 180.53 | 1 | 180.53 | 1.88 | 0.17 | |
| Error | 6478.14 | 522 | 12.41 | |||
| Total | 100,413.22 | 1044 | ||||
| Years | 2019 | 2020 | ||
|---|---|---|---|---|
| Varieties | DI | Resistance | DI | Resistance |
| Zimai12 | 6.7 | R | 15 | R |
| Huximai | 10.8 | R | 10.4 | R |
| SIRMIONE | 11.7 | R | 16.7 | MR |
| Xinong509 | 11.8 | R | 13.9 | R |
| Sifangmai | 13 | R | 12.5 | R |
| Dawson | 13 | R | 14.2 | R |
| Mazhamai | 13.5 | R | 11.1 | R |
| ROMANIAN | 14.8 | R | 14.6 | R |
| Zhengmai9405 | 14.9 | R | 19.4 | MR |
| Yuqiumai | 15.2 | MR | 11.5 | R |
| OPATA | 15.2 | MR | 20 | MR |
| Xibei612 | 15.2 | MR | 17.4 | MR |
| Liaomai16 | 15.5 | MR | 18.3 | MR |
| Owens | 16.7 | MR | 18.1 | MR |
| Aguilal | 18.2 | MR | 16.4 | MR |
| Heng6632 | 18.5 | MR | 18.3 | MR |
| Ron 2-Fnd×CMH74A.630 | 18.6 | MR | 15.8 | MR |
| Mianyang26 | 19.2 | MR | 16.7 | MR |
| Zhenmai168 | 19.4 | MR | 12.9 | R |
| VAIOLET | 19.8 | MR | 18.1 | MR |
| Zhongyu12 | 20.1 | MR | 17.4 | MR |
| Ailiduo | 20.8 | MR | 16.8 | MR |
| Jichun1016 | 22.5 | MR | 18.1 | MR |
| Snps | Chromosomes | Position | Allele | P-2019 | P-2020 | |
|---|---|---|---|---|---|---|
| AX-94897165 | 1A | 149510383 | G | C | 3.99 × 10−5 | 3.36 × 10−5 |
| AX-95121113 | 1A | 130909578 | C | G | 1.09 × 10−4 | 9.73 × 10−5 |
| AX-109317481 | 1B | 130743321 | A | G | 2.81 × 10−6 | 1.97 × 10−5 |
| AX-111771935 | 1B | 192551773 | A | G | 3.07 × 10−6 | 8.73 × 10−6 |
| AX-110134543 | 1B | 131236241 | T | C | 7.28 × 10−6 | 1.11 × 10−4 |
| AX-109373335 | 1B | 134968473 | A | C | 9.47 × 10−6 | 1.06 × 10−4 |
| AX-110379190 | 1B | 196926881 | A | C | 1.11 × 10−5 | 4.13 × 10−5 |
| AX-94871733 | 1B | 172946932 | T | C | 1.67 × 10−5 | 7.04 × 10−7 |
| AX-111634558 | 1B | 176745047 | T | C | 3.23 × 10−5 | 4.87 × 10−5 |
| AX-94548073 | 1B | 135938256 | A | C | 3.48 × 10−5 | 4.40 × 10−6 |
| AX-111818830 | 1B | 93459336 | T | G | 5.06 × 10−5 | 7.24 × 10−6 |
| AX-94843699 | 1B | 120884018 | A | G | 5.58 × 10−5 | 5.65 × 10−6 |
| AX-94428697 | 1B | 113516152 | C | T | 8.54 × 10−5 | 2.00 × 10−5 |
| AX-111040346 | 1B | 162264806 | G | A | 1.08 × 10−4 | 9.07 × 10−6 |
| AX-111533020 | 1B | 104346417 | C | A | 1.11 × 10−4 | 5.38 × 10−5 |
| AX-110436287 | 1B | 6798183 | T | C | 1.15 × 10−4 | 1.30 × 10−4 |
| AX-94860874 | 1B | 123352247 | G | C | 1.19 × 10−4 | 5.38 × 10−6 |
| AX-108876206 | 1B | 207814115 | G | A | 1.25 × 10−4 | 1.40 × 10−4 |
| AX-111186969 | 1B | 187708395 | T | C | 1.26 × 10−4 | 6.66 × 10−6 |
| AX-95201485 | 1B | 120884153 | G | T | 1.31 × 10−4 | 1.84 × 10−5 |
| AX-94850477 | 1B | 120883952 | T | G | 1.34 × 10−4 | 9.92 × 10−5 |
| AX-111081045 | 1B | 93564840 | A | C | 1.39 × 10−4 | 6.15 × 10−5 |
| AX-95083508 | 1B | 120884060 | T | C | 1.45 × 10−4 | 5.65 × 10−6 |
| AX-109621209 | 1B | 217554339 | A | C | 1.72 × 10−4 | 2.70 × 10−5 |
| AX-109981169 | 1B | 113652092 | T | C | 1.89 × 10−4 | 6.49 × 10−5 |
| AX-94799254 | 1D | 90633472 | T | C | 1.14 × 10−4 | 2.74 × 10−5 |
| AX-95232541 | 1D | 40579315 | A | G | 1.14 × 10−4 | 9.13 × 10−5 |
| AX-94445466 | 1D | 123109328 | A | G | 1.44 × 10−4 | 2.99 × 10−5 |
| AX-94818806 | 1D | 73729963 | G | A | 1.48 × 10−4 | 5.20 × 10−5 |
| AX-94692276 | 1D | 130308958 | A | C | 1.52 × 10−4 | 9.80 × 10−5 |
| AX-110415885 | 1D | 580926 | C | T | 1.79 × 10−4 | 4.88 × 10−5 |
| AX-109468542 | 4A | 539162489 | T | C | 1.82 × 10−4 | 6.06 × 10−5 |
| AX-95120025 | 4D | 329008071 | A | G | 7.05 × 10−5 | 1.10 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, L.; Goher, F.; Zeng, M.; Wu, D.; Zeng, Q.; Han, D.; Li, C. Source Identification and Genome-Wide Association Analysis of Crown Rot Resistance in Wheat. Plants 2022, 11, 1912. https://doi.org/10.3390/plants11151912
Pu L, Goher F, Zeng M, Wu D, Zeng Q, Han D, Li C. Source Identification and Genome-Wide Association Analysis of Crown Rot Resistance in Wheat. Plants. 2022; 11(15):1912. https://doi.org/10.3390/plants11151912
Chicago/Turabian StylePu, Lefan, Farhan Goher, Mengke Zeng, Dongsheng Wu, Qingdong Zeng, Dejun Han, and Chunlian Li. 2022. "Source Identification and Genome-Wide Association Analysis of Crown Rot Resistance in Wheat" Plants 11, no. 15: 1912. https://doi.org/10.3390/plants11151912
APA StylePu, L., Goher, F., Zeng, M., Wu, D., Zeng, Q., Han, D., & Li, C. (2022). Source Identification and Genome-Wide Association Analysis of Crown Rot Resistance in Wheat. Plants, 11(15), 1912. https://doi.org/10.3390/plants11151912







