Physiological and Molecular Responses of Woody Plants Exposed to Future Atmospheric CO2 Levels under Abiotic Stresses
Abstract
1. Introduction
2. Woody Plant Growth and Development under Elevated CO2
2.1. Effect on Leaf Photosynthesis
2.2. Effect on Source–Sink Relationship and Nitrogen Metabolism
2.3. Growth and Developmental Stage-Dependent Regulation
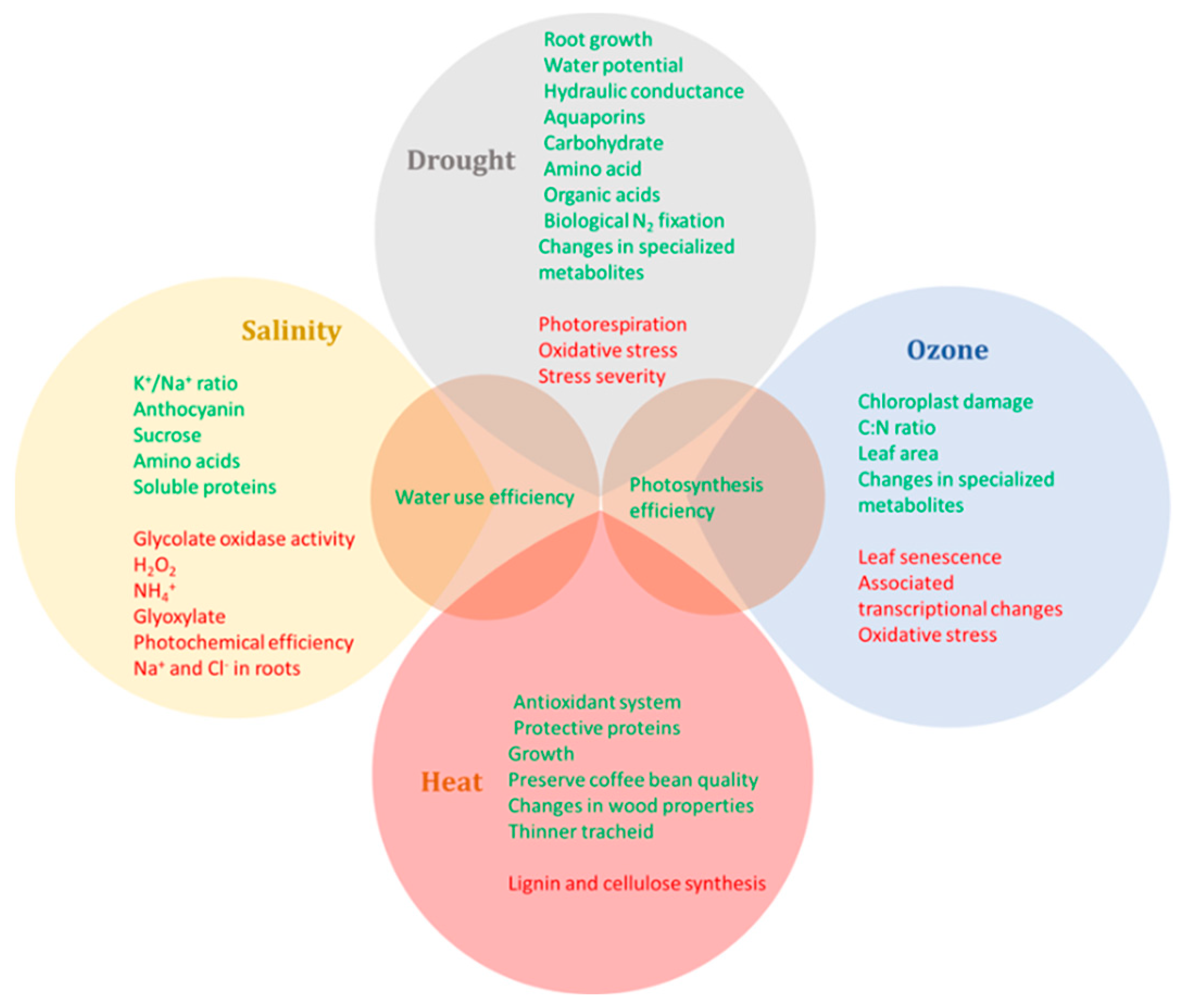
3. Integrative Responses of Woody Plants under Elevated CO2 and Other Abiotic Stresses
3.1. Effects of Elevated CO2 and Heat Stress
3.2. Effects of Elevated CO2 and Drought
3.3. Effects of Elevated CO2 and Salinity
3.4. Effects of Elevated CO2 and Ozone
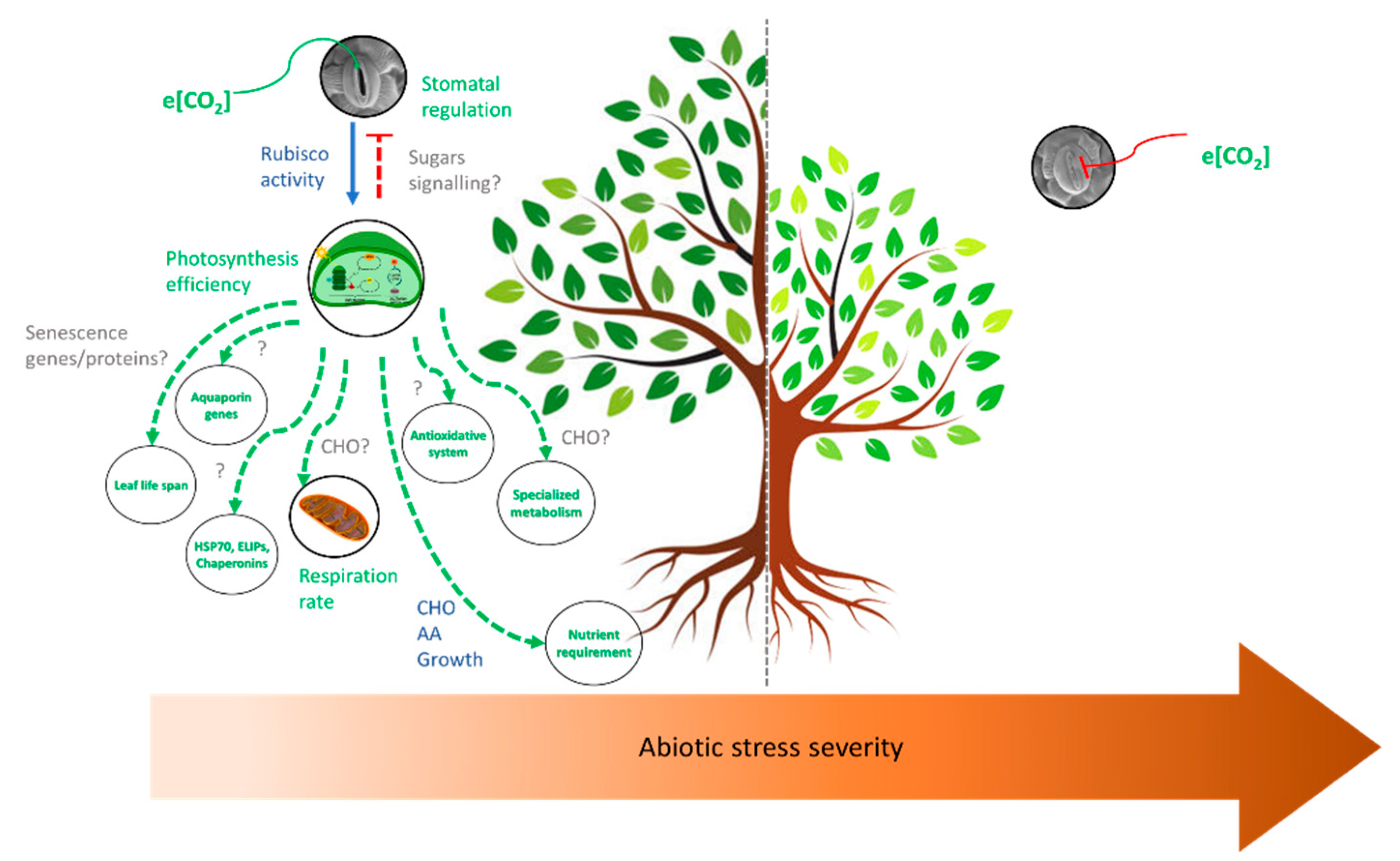
3.5. Effects of Elevated CO2 and Multiple Stresses
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Vergara, W.; Rios, A.R.; Trapido, P.; Malarín, H. Agriculture and Future Climate in Latin America and the Caribbean: Systemic Impacts and Potential Responses. Inter-Am. Dev. Bank 2014, No. IDB-DP, 1–20. [Google Scholar]
- Dlugokencky, E.J.; Hall, B.D.; Montzka, S.A.; Dutton, G.; Mühle, J.E.J.W. Long-lived greenhouse gases. In State of the Climate in 2017; American Meteorological Society: Boston, MA, USA, 2018; Volume 99, pp. S46–S49. [Google Scholar]
- Burton, D.A. Sea-Level Information. Available online: https://www.sealevel.info/co2_and_ch4.html (accessed on 1 July 2022).
- NOAA. National Oceanic and Atmospheric Administration. 2020. Available online: https://www.eea.europa.eu/data-and-maps/indicators/atmospheric-greenhouse-gas-concentrations-7/noaa-2020-national-oceanic-atmospheric-administration (accessed on 1 July 2022).
- Hartmann, D.L.; Tank, A.M.G.K.; Rusticucci, M.; Alexander, L.V.; Brönnimann, S.; Charabi, Y.; Dentener, F.J.; Dlugokencky, E.J.; Kaplan, A.; Soden, B.J.; et al. Observations: Atmosphere and surface. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 159–254. [Google Scholar]
- IPCC. Contribuição do Grupo de Trabalho II para o Quinto Relatório de Avaliação do Painel Intergovernamental Sobre Alterações Climáticas (IPCC); IPCC: Geneva, Switzerland, 2014; ISBN 978-972-9083-18-1. [Google Scholar]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Avila, R.; Cardoso, A.A.; Martins, S.C.V.; Ramalho, J.C. Physiological and Agronomic Performance of the Coffee Crop in the Context of Climate Change and Global Warming: A Review. J. Agric. Food Chem. 2018, 66, 5264–5274. [Google Scholar] [CrossRef]
- Hartmann, H.; Bastos, A.; Das, A.J.; Esquivel-Muelbert, A.; Hammond, W.M.; Martínez-Vilalta, J.; McDowell, N.G.; Powers, J.S.; Pugh, T.A.; Ruthrof, K.X.; et al. Climate Change Risks to Global Forest Health: Emergence of Unexpected Events of Elevated Tree Mortality Worldwide. Annu. Rev. Plant Biol. 2022, 73, 673–702. [Google Scholar] [CrossRef]
- Anderson, R.; Bayer, P.E.; Edwards, D. Climate change and the need for agricultural adaptation. Curr. Opin. Plant Biol. 2020, 56, 197–202. [Google Scholar] [CrossRef]
- Cataldo, E.C.; Salvi, L.S.; Paoli, F.P.; Fucile, M.F.; Masciandaro, G.M.; Manzi, D.M.; Mansini, C.M.; Mattii, G.B.M. Effects of natural clinoptilolite on physiology, water stress, sugar, and anthocyanin content in Sanforte (Vitis vinifera L.) young vineyard. J. Agric. Sci. 2021, 7–8, 488–499. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Mattii, G.B. Effects of Kaolin and Shading Net on the Ecophysiology and Berry Composition of Sauvignon Blanc Grapevines. Agriculture 2022, 4, 491. [Google Scholar] [CrossRef]
- Wong, S.C. Elevated atmospheric partial pressure of CO2 and plant growth-I. Interactions of nitrogen nutrition and photosynthetic capacity in C3 and C4 plants. Oecologia 1979, 44, 68–74. [Google Scholar] [CrossRef]
- Moore, B.D.; Palmquist, D.E.; Seemann, J.R. Influence of plant growth at high CO2 concentrations on leaf content of ribulose-1,5-bisphosphate carboxylase/oxygenase and intracellular distribution of soluble carbohydrates in tobacco, snapdragon, and parsley. Plant Physiol. 1997, 115, 241–248. [Google Scholar] [CrossRef]
- Taub, D.R.; Seemann, J.R.; Coleman, J.S. Growth in elevated CO2 protects photosynthesis against high-temperature damage. Plant Cell Environ. 2000, 23, 649–656. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Zhou, G. Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Front. Plant Sci. 2015, 6, 1–17. [Google Scholar] [CrossRef]
- Gamage, D.; Thompson, M.; Sutherland, M.; Hirotsu, N.; Makino, A.; Seneweera, S. New insights into the cellular mechanisms of plant growth at elevated atmospheric carbon dioxide concentrations. Plant Cell Environ. 2018, 41, 1233–1246. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Lemonnier, P.; Wedow, J.M. The influence of rising tropospheric carbon dioxide and ozone on plant productivity. Plant Biol. 2020, 22, 5–11. [Google Scholar] [CrossRef]
- Lewis, J.D.; Smith, R.A.; Ghannoum, O.; Logan, B.A.; Phillips, N.G.; Tissue, D.T. Industrial-age changes in atmospheric [CO2] and temperature differentially alter responses of faster- and slower-growing Eucalyptus seedlings to short-term drought. Tree Physiol. 2013, 33, 475–488. [Google Scholar] [CrossRef]
- Klein, T.; Ramon, U. Stomatal sensitivity to CO2 diverges between angiosperm and gymnosperm tree species. Funct. Ecol. 2019, 33, 1411–1424. [Google Scholar] [CrossRef]
- Wustman, B.A.; Oksanen, E.; Karnosky, D.F.; Noormets, A.; Isebrands, J.G.; Pregitzer, K.S.; Hendrey, G.R.; Sober, J.; Podila, G.K. Effects of elevated CO2 and O3 on aspen clones of varying O3 sensitivity. Dev. Environ. Sci. 2003, 3, 391–409. [Google Scholar] [CrossRef]
- Kgope, B.S.; Bond, W.J.; Midgley, G.F. Growth responses of African savanna trees implicate atmospheric [CO2] as a driver of past and current changes in savanna tree cover. Austral Ecol. 2010, 35, 451–463. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Beemster, G.T.S.; Janssens, I.A.; Asard, H. Future climate CO2 levels mitigate stress impact on plants: Increased defense or decreased challenge? Front. Plant Sci. 2016, 7, 556. [Google Scholar] [CrossRef]
- Damatta, F.M.; Godoy, A.G.; Menezes-Silva, P.E.; Martins, S.C.V.; Sanglard, L.M.V.P.; Morais, L.E.; Torre-Neto, A.; Ghini, R. Sustained enhancement of photosynthesis in coffee trees grown under free-air CO2 enrichment conditions: Disentangling the contributions of stomatal, mesophyll, and biochemical limitations. J. Exp. Bot. 2016, 67, 341–352. [Google Scholar] [CrossRef]
- Rakocevic, M.; Ribeiro, R.V.; Ribeiro Marchiori, P.E.; Filizola, H.F.; Batista, E.R. Structural and functional changes in coffee trees after 4 years under free air CO2 enrichment. Ann. Bot. 2018, 121, 1065–1078. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, M.; Gebrekirstos, A.; Braüning, A. Disentangling the effects of atmospheric CO2 and climate on intrinsic water-use efficiency in South Asian tropical moist forest trees. Tree Physiol. 2020, 40, 904–916. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Lobo, A.K.M.; de Oliveira Martins, M.; Lima Neto, M.C.; Machado, E.C.; Ribeiro, R.V.; Silveira, J.A.G. Exogenous sucrose supply changes sugar metabolism and reduces photosynthesis of sugarcane through the down-regulation of Rubisco abundance and activity. J. Plant Physiol. 2015, 179, 113–121. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- von Caemmerer, S. Biochemical models of leaf photosynthesis. In Techniques in Plant Science, No. 2; CSIRO Publishing: Clayton, Australia; Collingwood: Melbourne, Australia, 2000. [Google Scholar]
- Sharkey, T.D.; Stitt, M.; Heineke, D.; Gerhardt, R.; Raschke, K.; Heldt, H.W. Limitation of Photosynthesis by Carbon Metabolism: II. O₂- Insensitive CO₂ Uptake Results from Limitation of Triose Phosphate Utilization. Plant Physiol. 1986, 81, 1123–1129. [Google Scholar] [CrossRef]
- Gago, J.; De Menezes Daloso, D.; Figueroa, C.M.; Flexas, J.; Fernie, A.R.; Nikoloski, Z. Relationships of leaf net photosynthesis, stomatal conductance, and mesophyll conductance to primary metabolism: A multispecies meta-analysis approach. Plant Physiol. 2016, 171, 265–279. [Google Scholar] [CrossRef]
- Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Merilo, E.; Laanemets, K.; Waadt, R.; Pater, D.; Kollist, H.; Schroeder, J.I. Abscisic acid-independent stomatal CO2 signal transduction pathway and convergence of CO2 and ABA signaling downstream of OST1 kinase. Proc. Natl. Acad. Sci. USA 2018, 115, E9971–E9980. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Barton, C.V.M.; Broadmeadow, M.S.J.; Ceulemans, R.; De Angelis, P.; Forstreuter, M.; Freeman, M.; Jackson, S.B.; Kellomäki, S.; Laitat, E.; et al. Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: A synthesis. New Phytol. 2001, 149, 247–264. [Google Scholar] [CrossRef]
- Ellsworth, D.S. CO2 enrichment in a maturing pine forest: Are CO2 exchange and water status in the canopy affected? Plant Cell Environ. 1999, 22, 461–472. [Google Scholar] [CrossRef]
- Kogawara, S.; Norisada, M.; Tange, T.; Yagi, H.; Kojima, K. Elevated atmospheric CO2 concentration alters the effect of phosphate supply on growth of Japanese red pine (Pinus densiflora) seedlings. Tree Physiol. 2006, 26, 25–33. [Google Scholar] [CrossRef][Green Version]
- Purcell, C.; Batke, S.P.; Yiotis, C.; Caballero, R.; Soh, W.K.; Murray, M.; McElwain, J.C. Increasing stomatal conductance in response to rising atmospheric CO2. Ann. Bot. 2018, 121, 1137–1149. [Google Scholar] [CrossRef]
- Konrad, W.; Roth-Nebelsick, A.; Grein, M. Modelling of stomatal density response to atmospheric CO2. J. Theor. Biol. 2008, 253, 638–658. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Rodrigues, A.P.; Semedo, J.N.; Pais, I.P.; Martins, L.D.; Simões-Costa, M.C.; Leitão, A.E.; Fortunato, A.S.; Batista-Santos, P.; Palos, I.M.; et al. Sustained photosynthetic performance of Coffea spp. under long-term enhanced [CO2]. PLoS ONE 2013, 8, e82712. [Google Scholar] [CrossRef]
- Koike, T.; Watanabe, M.; Watanabe, Y.; Agathokleous, E.; Eguchi, N.; Takagi, K.; Satoh, F.; Kitaoka, S.; Funada, R. Ecophysiology of deciduous trees native to Northeast Asia grown under FACE Free Air CO2 Enrichment. J. Agric. Meteorol. 2015, 71, 174–184. [Google Scholar] [CrossRef]
- Lawson, T.; Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef]
- Sage, R.F.; Sharkey, T.D.; Seemann, J.R. Acclimation of Photosynthesis to Elevated CO2 in Five C3 Species. Plant Physiol. 1989, 89, 590–596. [Google Scholar] [CrossRef]
- Tausz-Posch, S.; Tausz, M.; Bourgault, M. Elevated [CO2] effects on crops: Advances in understanding acclimation, nitrogen dynamics and interactions with drought and other organisms. Plant Biol. 2020, 22, 38–51. [Google Scholar] [CrossRef]
- Moore, B.D.; Cheng, S.H.; Sims, D.; Seemann, J.R. The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant Cell Environ. 1999, 22, 567–582. [Google Scholar] [CrossRef]
- Macabuhay, A.; Houshmandfar, A.; Nuttall, J.; Fitzgerald, G.J.; Tausz, M.; Tausz-Posch, S. Can elevated CO2 buffer the effects of heat waves on wheat in a dryland cropping system? Environ. Exp. Bot. 2018, 155, 578–588. [Google Scholar] [CrossRef]
- Ruiz-Vera, U.M.; De Souza, A.P.; Ament, M.R.; Gleadow, R.M.; Ort, D.R. High sink strength prevents photosynthetic down-regulation in cassava grown at elevated CO2 concentration. J. Exp. Bot. 2021, 72, 542–560. [Google Scholar] [CrossRef]
- Ulfat, A.; Shokat, S.; Li, X.; Fang, L.; Großkinsky, D.K.; Majid, S.A.; Roitsch, T.; Liu, F. Elevated carbon dioxide alleviates the negative impact of drought on wheat by modulating plant metabolism and physiology. Agric. Water Manag. 2021, 250, 1–10. [Google Scholar] [CrossRef]
- Avila, R.T.; de Almeida, W.L.; Costa, L.C.; Machado, K.L.G.; Barbosa, M.L.; de Souza, R.P.B.; Martino, P.B.; Juárez, M.A.T.; Marçal, D.M.S.; Martins, S.C.V.; et al. Elevated air [CO2] improves photosynthetic performance and alters biomass accumulation and partitioning in drought-stressed coffee plants. Environ. Exp. Bot. 2020, 177, 104137. [Google Scholar] [CrossRef]
- Dier, M.; Sickora, J.; Erbs, M.; Weigel, H.J.; Zörb, C.; Manderscheid, R. Decreased wheat grain yield stimulation by free air CO2 enrichment under N deficiency is strongly related to decreased radiation use efficiency enhancement. Eur. J. Agron. 2018, 101, 38–48. [Google Scholar] [CrossRef]
- Crous, K.Y.; Wujeska-Klause, A.; Jiang, M.; Medlyn, B.E.; Ellsworth, D.S. Nitrogen and phosphorus retranslocation of leaves and stemwood in a mature Eucalyptus forest exposed to 5 years of elevated CO2. Front. Plant Sci. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Shi, S.; Xu, X.; Dong, X.; Xu, C.; Qiu, Y.; He, X. Photosynthetic Acclimation and Growth Responses to Elevated CO2 Associate with Leaf Nitrogen and Phosphorus Concentrations in Mulberry (Morus multicaulis Perr.). Forests 2021, 12, 660. [Google Scholar] [CrossRef]
- Feng, Z.; Rütting, T.; Pleijel, H.; Wallin, G.; Reich, P.B.; Kammann, C.I.; Newton, P.C.D.; Kobayashi, K.; Luo, Y.; Uddling, J. Constraints to nitrogen acquisition of terrestrial plants under elevated CO2. Glob. Chang. Biol. 2015, 21, 3152–3168. [Google Scholar] [CrossRef]
- Krämer, K.; Kepp, G.; Brock, J.; Stutz, S.; Heyer, A.G. Acclimation to elevated CO2 affects the C/N balance by reducing de novo N-assimilation. Physiol. Plant. 2022, 174, 1–13. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A.; Nelson, R.; Long, S.P. Testing the “source-sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric. For. Meteorol. 2004, 122, 85–94. [Google Scholar] [CrossRef]
- Eguchi, N.; Karatsu, K.; Ueda, T.; Funada, R.; Takagi, K.; Hiura, T.; Sasa, K.; Koike, T. Photosynthetic responses of birch and alder saplings grown in a free air CO2 enrichment system in northern Japan. Trees Struct. Funct. 2008, 22, 437–447. [Google Scholar] [CrossRef]
- Watanabe, Y.; Satomura, T.; Sasa, K.; Funada, R.; Koike, T. Differential anatomical responses to elevated CO2 in saplings of four hardwood species. Plant Cell Environ. 2010, 33, 1101–1111. [Google Scholar] [CrossRef]
- Grombone-Guaratini, M.T.; Gaspar, M.; Oliveira, V.F.; Torres, M.A.M.G.; Do Nascimento, A.; Aidar, M.P.M. Atmospheric CO2 enrichment markedly increases photosynthesis and growth in a woody tropical bamboo from the Brazilian atlantic forest. New Zealand J. Bot. 2013, 51, 275–285. [Google Scholar] [CrossRef]
- Eichelmann, H.; Oja, V.; Rasulov, B.; Padu, E.; Bichele, I.; Pettai, H.; Möls, T.; Kasparova, I.; Vapaavuori, E.; Laisk, A. Photosynthetic parameters of birch (Betula pendula Roth) leaves growing in normal and in CO2- and O3-enriched atmospheres. Plant Cell Environ. 2004, 27, 479–495. [Google Scholar] [CrossRef]
- Riikonen, J.; Holopainen, T.; Oksanen, E.; Vapaavuori, E. Leaf photosynthetic characteristics of silver birch during three years of exposure to elevated concentrations of CO2 and O3 in the field. Tree Physiol. 2005, 25, 621–632. [Google Scholar] [CrossRef][Green Version]
- Taylor, G.; Street, N.R.; Tricker, P.J.; Sjödin, A.; Graham, L.; Skogström, O.; Calfapietra, C.; Scarascia-Mugnozza, G.; Jansson, S. The transcriptome of Populus in elevated CO2. New Phytol. 2005, 167, 143–154. [Google Scholar] [CrossRef]
- Cseke, L.J.; Tsai, C.J.; Rogers, A.; Nelsen, M.P.; White, H.L.; Karnosky, D.F.; Podila, G.K. Transcriptomic comparison in the leaves of two aspen genotypes having similar carbon assimilation rates but different partitioning patterns under elevated [CO2]. New Phytol. 2009, 182, 891–911. [Google Scholar] [CrossRef]
- Amthor, J.S. The McCree-de Wit-Penning de Vries-Thornley respiration paradigms: 30 years later. Ann. Bot. 2000, 86, 1–20. [Google Scholar] [CrossRef]
- Way, D.A.; Oren, R.; Kroner, Y. The space-time continuum: The effects of elevated CO2 and temperature on trees and the importance of scaling. Plant Cell Environ. 2015, 38, 991–1007. [Google Scholar] [CrossRef]
- Crous, K.Y.; Zaragoza-Castells, J.; Löw, M.; Ellsworth, D.S.; Tissue, D.T.; Tjoelker, M.G.; Barton, C.V.M.; Gimeno, T.E.; Atkin, O.K. Seasonal acclimation of leaf respiration in Eucalyptus saligna trees: Impacts of elevated atmospheric CO2 and summer drought. Glob. Chang. Biol. 2011, 17, 1560–1576. [Google Scholar] [CrossRef]
- Tissue, D.T.; Lewis, J.D.; Wullschleger, S.D.; Amthor, J.S.; Griffin, K.L.; Anderson, O.R. Leaf respiration at different canopy positions in sweetgum (Liquidambar styraciflua) grown in ambient and elevated concentrations of carbon dioxide in the field. Tree Physiol. 2002, 22, 1157–1166. [Google Scholar] [CrossRef]
- Wu, F.; Sun, X.; Zou, B.; Zhu, P.; Lin, N.; Lin, J.; Ji, K. Transcriptional analysis of masson pine (Pinus massoniana) under high CO2 stress. Genes 2019, 10, 804. [Google Scholar] [CrossRef]
- Sanches, R.F.E.; da Cruz Centeno, D.; Braga, M.R.; da Silva, E.A. Impact of high atmospheric CO2 concentrations on the seasonality of water-related processes, gas exchange, and carbohydrate metabolism in coffee trees under field conditions. Clim. Chang. 2020, 162, 1231–1248. [Google Scholar] [CrossRef]
- Markelz, R.J.C.; Vosseller, L.N.; Leakey, A.D.B. Developmental stage specificity of transcriptional, biochemical and CO2 efflux responses of leaf dark respiration to growth of Arabidopsis thaliana at elevated [CO2]. Plant Cell Environ. 2014, 37, 2542–2552. [Google Scholar] [CrossRef]
- Jiang, M.; Medlyn, B.E.; Drake, J.E.; Duursma, R.A.; Anderson, I.C.; Barton, C.V.M.; Boer, M.M.; Carrillo, Y.; Castañeda-Gómez, L.; Collins, L.; et al. The fate of carbon in a mature forest under carbon dioxide enrichment. Nature 2020, 580, 227–231. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Sun, B.; Zhang, S.; Zhang, Y.; Liao, Y.; Zhou, Y.; Xia, X.; Shi, K.; Yu, J. Stimulated leaf dark respiration in tomato in an elevated carbon dioxide atmosphere. Sci. Rep. 2013, 3, 2–9. [Google Scholar] [CrossRef]
- Gupta, P.; Duplessis, S.; White, H.; Karnosky, D.F.; Martin, F.; Podila, G.K. Gene expression patterns of trembling aspen trees following long-term exposure to interacting elevated CO2 and tropospheric O3. New Phytol. 2005, 167, 129–142. [Google Scholar] [CrossRef]
- Taylor, G.; Tallis, M.J.; Giardina, C.P.; Percy, K.E.; Miglietta, F.; Gupta, P.S.; Gioli, B.; Calfapietra, C.; Gielen, B.; Kubiske, M.E.; et al. Future atmospheric CO2 leads to delayed autumnal senescence. Glob. Chang. Biol. 2008, 14, 264–275. [Google Scholar] [CrossRef]
- Druart, N.; Rodríguez-Buey, M.; Barron-Gafford, G.; Sjödin, A.; Bhalerao, R.; Hurry, V. Molecular targets of elevated [CO2] in leaves and stems of Populus deltoides: Implications for future tree growth and carbon sequestration. Funct. Plant Biol. 2006, 33, 121. [Google Scholar] [CrossRef]
- Tallis, M.J.; Lin, Y.; Rogers, A.; Zhang, J.; Street, N.R.; Miglietta, F.; Karnosky, D.F.; De Angelis, P.; Calfapietra, C.; Taylor, G. The transcriptome of Populus in elevated CO2 reveals increased anthocyanin biosynthesis during delayed autumnal senescence. New Phytol. 2010, 186, 415–428. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; He, C.; Duan, A. Genes responsive to elevated CO2 concentrations in triploid white poplar and integrated gene network analysis. PLoS ONE 2014, 9, e98300. [Google Scholar] [CrossRef]
- Atwell, B.J.; Henery, M.L.; Whitehead, D. Sapwood development in Pinus radiata trees grown for three years at ambient and elevated carbon dioxide partial pressures. Tree Physiol. 2003, 23, 13–21. [Google Scholar] [CrossRef]
- Kilpeläinen, A.; Peltola, H.; Ryyppö, A.; Sauvala, K.; Laitinen, K.; Kellomäki, S. Wood properties of Scots pines (Pinus sylvestris) grown at elevated temperature and carbon dioxide concentration. Tree Physiol. 2003, 23, 889–897. [Google Scholar] [CrossRef]
- Kaplan, F.; Zhao, W.; Richards, J.T.; Wheeler, R.M.; Guy, C.L.; Levine, L.H. Transcriptional and metabolic insights into the differential physiological responses of arabidopsis to optimal and supraoptimal atmospheric CO2. PLoS ONE 2012, 7, e43583. [Google Scholar] [CrossRef]
- Kontunen-Soppela, S.; Parviainen, J.; Ruhanen, H.; Brosché, M.; Keinänen, M.; Thakur, R.C.; Kolehmainen, M.; Kangasjärvi, J.; Oksanen, E.; Karnosky, D.F.; et al. Gene expression responses of paper birch (Betula papyrifera) to elevated CO2 and O3 during leaf maturation and senescence. Environ. Pollut. 2010, 158, 959–968. [Google Scholar] [CrossRef]
- Taylor, G.; Tricker, P.J.; Zhang, F.Z.; Alston, V.J.; Miglietta, F.; Kuzminsky, E. Spatial and temporal effects of free-air CO2 enrichment (POPFACE) on leaf growth, cell expansion, and cell production in a closed canopy of poplar. Plant Physiol. 2003, 131, 177–185. [Google Scholar] [CrossRef]
- Taylor, G.; Ranasinghe, S.; Bosac, C.; Gardner, S.D.L.; Ferris, R. Elevated CO2 and plant growth: Cellular mechanisms and responses of whole plants. J. Exp. Bot. 1994, 45, 1761–1774. [Google Scholar] [CrossRef]
- Cheng, S.H.; Willmann, M.R.; Chen, H.C.; Sheen, J. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002, 129, 469–485. [Google Scholar] [CrossRef]
- Dhankher, O.P.; Foyer, C.H. Climate resilient crops for improving global food security and safety. Plant Cell Environ. 2018, 41, 877–884. [Google Scholar] [CrossRef]
- Carmo-Silva, A.E.; Gore, M.A.; Andrade-Sanchez, P.; French, A.N.; Hunsaker, D.J.; Salvucci, M.E. Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ. Exp. Bot. 2012, 83, 1–11. [Google Scholar] [CrossRef]
- Meehl, G.A.; Tebaldi, C.; Tilmes, S.; Lamarque, J.-F.; Bates, S.; Pendergrass, A.; Lombardozzi, D. Future heat waves and surface ozone. Environ. Res. Lett. 2018, 13, 064004. [Google Scholar] [CrossRef]
- IPCC. Summary for policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; IPCC: Geneva, Switzerland, 2013. [Google Scholar]
- Grace, J. Climatic Tolerance and the Distribution of Plants. New Phytol. 1987, 106, 113–130. [Google Scholar] [CrossRef]
- Wise, R.R.; Olson, A.J.; Schrader, S.M.; Sharkey, T.D. Electron transport is the functional limitation of photosynthesis in field-grown Pima cotton plants at high temperature. Plant Cell Environ. 2004, 27, 717–724. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Grandis, A.; Arenque, B.C.; Buckeridge, M.S. Impacts of climate changes on crop physiology and food quality. Food Res. Int. 2010, 43, 1814–1823. [Google Scholar] [CrossRef]
- Vu, J.C.V.; Allen, L.H. Stem juice production of the C4 sugarcane (Saccharum officinarum) is enhanced by growth at double-ambient CO2 and high temperature. J. Plant Physiol. 2009, 166, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, O.; Phillips, N.G.; Conroy, J.P.; Smith, R.A.; Attard, R.D.; Woodfield, R.; Logan, B.A.; Lewis, J.D.; Tissue, D.T. Exposure to preindustrial, current and future atmospheric CO2 and temperature differentially affects growth and photosynthesis in Eucalyptus. Glob. Chang. Biol. 2010, 16, 303–319. [Google Scholar] [CrossRef]
- Madan, P.; Jagadish, S.V.K.; Craufurd, P.Q.; Fitzgerald, M.; Lafarge, T.; Wheeler, T.R. Effect of elevated CO2 and high temperature on seed-set and grain quality of rice. J. Exp. Bot. 2012, 63, 3843–3852. [Google Scholar] [CrossRef]
- Yu, J.; Du, H.; Xu, M.; Huang, B. Metabolic responses to heat stress under elevated atmospheric CO2 concentration in a cool-season grass species. J. Am. Soc. Hortic. Sci. 2012, 137, 221–228. [Google Scholar] [CrossRef]
- Rodrigues, W.P.; Martins, M.Q.; Fortunato, A.S.; Rodrigues, A.P.; Semedo, J.N.; Simões-Costa, M.C.; Pais, I.P.; Leitão, A.E.; Colwell, F.; Goulao, L.; et al. Long-term elevated air [CO2] strengthens photosynthetic functioning and mitigates the impact of supra-optimal temperatures in tropical Coffea arabica and C. canephora species. Glob. Chang. Biol. 2016, 22, 415–431. [Google Scholar] [CrossRef]
- Ghannoum, O.; Phillips, N.G.; Sears, M.A.; Logan, B.A.; Lewis, J.D.; Conroy, J.P.; Tissue, D.T. Photosynthetic responses of two eucalypts to industrial-age changes in atmospheric [CO2] and temperature. Plant Cell Environ. 2010, 33, 1671–1681. [Google Scholar] [CrossRef]
- Martins, M.Q.; Rodrigues, W.P.; Fortunato, A.S.; Leitão, A.E.; Rodrigues, A.P.; Pais, I.P.; Martins, L.D.; Silva, M.J.; Reboredo, F.H.; Partelli, F.L.; et al. Protective response mechanisms to heat stress in interaction with high [CO2] conditions in Coffea spp. Front. Plant Sci. 2016, 7, 947. [Google Scholar] [CrossRef]
- Marques, I.; Fernandes, I.; Paulo, O.S.; Lidon, F.C.; Damatta, F.M.; Ramalho, J.C.; Ribeiro-barros, A.I. A transcriptomic approach to understanding the combined impacts of supra-optimal temperatures and CO2 revealed different responses in the polyploid coffea arabica and its diploid progenitor c. Canephora. Int. J. Mol. Sci. 2021, 22, 3125. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.C.; Pais, I.P.; Leitão, A.E.; Guerra, M.; Reboredo, F.H.; Máguas, C.M.; Carvalho, M.L.; Scotti-Campos, P.; Ribeiro-Barros, A.I.; Lidon, F.J.C.; et al. Can elevated air [CO2] conditions mitigate the predicted warming impact on the quality of coffee bean? Front. Plant Sci. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.J.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef]
- Rigling, A.; Bigler, C.; Eilmann, B.; Feldmeyer-Christe, E.; Gimmi, U.; Ginzler, C.; Graf, U.; Mayer, P.; Vacchiano, G.; Weber, P.; et al. Driving factors of a vegetation shift from Scots pine to pubescent oak in dry Alpine forests. Glob. Chang. Biol. 2013, 19, 229–240. [Google Scholar] [CrossRef]
- Cochrane, J.A.; Hoyle, G.L.; Yates, C.J.; Wood, J.; Nicotra, A.B. Climate warming delays and decreases seedling emergence in a Mediterranean ecosystem. Oikos 2015, 124, 150–160. [Google Scholar] [CrossRef]
- Creek, D.; Blackman, C.J.; Brodribb, T.J.; Choat, B.; Tissue, D.T. Coordination between leaf, stem, and root hydraulics and gas exchange in three arid-zone angiosperms during severe drought and recovery. Plant Cell Environ. 2018, 41, 2869–2881. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Bobich, E.G.; Barron-Gafford, G.A.; Rascher, K.G.; Murthy, R. Effects of drought and changes in vapour pressure deficit on water relations of Populus deltoides growing in ambient and elevated CO2. Tree Physiol. 2010, 30, 866–875. [Google Scholar] [CrossRef]
- Warren, J.M.; Norby, R.J.; Wullschleger, S.D.; Oren, R. Elevated CO2 enhances leaf senescence during extreme drought in a temperate forest. Tree Physiol. 2011, 31, 117–130. [Google Scholar] [CrossRef]
- Bachofen, C.; Moser, B.; Hoch, G.; Ghazoul, J.; Wohlgemuth, T. No carbon “bet hedging” in pine seedlings under prolonged summer drought and elevated CO2. J. Ecol. 2018, 106, 31–46. [Google Scholar] [CrossRef]
- Birami, B.; Nägele, T.; Gattmann, M.; Preisler, Y.; Gast, A.; Arneth, A.; Ruehr, N.K. Hot drought reduces the effects of elevated CO2 on tree water-use efficiency and carbon metabolism. New Phytol. 2020, 226, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Sanches, R.F.E.; Catarino, I.C.A.; Braga, M.R.; Silva, E.A. da Influência da alta concentração atmosférica de CO2(↑[CO2]atm) × disponibilidade hídrica nas relações hídricas, trocas gasosas e acúmulo de carboidratos em Coffea arabica L. Hoehnea 2017, 44, 635–643. [Google Scholar] [CrossRef]
- Avila, R.T.; Cardoso, A.A.; de Almeida, W.L.; Costa, L.C.; Machado, K.L.G.; Barbosa, M.L.; de Souza, R.P.B.; Oliveira, L.A.; Batista, D.S.; Martins, S.C.V.; et al. Coffee plants respond to drought and elevated [CO2] through changes in stomatal function, plant hydraulic conductance, and aquaporin expression. Environ. Exp. Bot. 2020, 177, 104148. [Google Scholar] [CrossRef]
- Catarino, I.C.A.; Monteiro, G.B.; Ferreira, M.J.P.; Torres, L.M.B.; Domingues, D.S.; Centeno, D.C.; Lobo, A.K.M.; Silva, E.A. Elevated [CO2] Mitigates Drought Effects and Increases Leaf 5-O-Caffeoylquinic Acid and Caffeine Concentrations During the Early Growth of Coffea Arabica Plants. Front. Sustain. Food Syst. 2021, 5, 1–12. [Google Scholar] [CrossRef]
- Semedo, J.N.; Rodrigues, A.P.; Lidon, F.C.; Pais, I.P.; Marques, I.; Gouveia, D.; Armengaud, J.; Silva, M.J.; Martins, S.; Semedo, M.C.; et al. Intrinsic non-stomatal resilience to drought of the photosynthetic apparatus in Coffea spp. is strengthened by elevated air [CO2]. Tree Physiol. 2021, 41, 708–727. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Jorge, T.; Osorio, S.; Pott, D.M.; Lidon, F.C.; Damatta, F.M.; Marques, I.; Ribeiro-Barros, A.I.; Ramalho, J.C.; António, C. Primary metabolite profile changes in Coffea spp. Promoted by single and combined exposure to drought and elevated CO2 concentration. Metabolites 2021, 11, 427. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? Tansley insight Salinity tolerance of crops—What is the cost ? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Koyro, H.W.; Geissler, N.; Hussin, S.; Debez, A.; Huchzermeyer, B.S. Survival at extreme locations: Life strategies of halophytes—The long way from system ecology, whole plant physiology, cell biochemistry and molecular aspects back to sustainable utilization at field sites. In Biosaline Agriculture and High Salinity Tolerance; Springer London: London, UK, 2008; pp. 1–20. [Google Scholar]
- Athar, H.R.; Ashraf, M. The Church in the World: A Half Century of Ecumenism in Hungary. Theol. Today 1994, 51, 289–290. [Google Scholar] [CrossRef]
- Young, I.; Renault, S.; Markham, J. Low levels organic amendments improve fertility and plant cover on non-acid generating gold mine tailings. Ecol. Eng. 2015, 74, 250–257. [Google Scholar] [CrossRef]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- FAO. Land and Plant Nutrition Management Service. Available online: http://www.fao.org/ag/agl/agll/spush (accessed on 1 July 2022).
- Shao, J.; Markham, J.; Renault, S. Nitrogen fixation symbiosis and salt tolerance of the boreal woody species Elaeagnus commutata. Acta Physiol. Plant. 2020, 42, 1–9. [Google Scholar] [CrossRef]
- Vicente, O.; Boscaiu, M.; Naranjo, M.Á.; Estrelles, E.; Bellés, J.M.; Soriano, P. Responses to salt stress in the halophyte Plantago crassifolia (Plantaginaceae). J. Arid Environ. 2004, 58, 463–481. [Google Scholar] [CrossRef]
- Bhaskar, G.; Bingru, H. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 19. [Google Scholar]
- Melgar, J.C.; Syvertsen, J.P.; García-Sánchez, F. Can elevated CO2 improve salt tolerance in olive trees? J. Plant Physiol. 2008, 165, 631–640. [Google Scholar] [CrossRef]
- Souza, N.C.S.; Silveira, J.A.G.; Silva, E.N.; Lima Neto, M.C.; Lima, C.S.; Aragão, R.M.; Ferreira-Silva, S.L. High CO2 favors ionic homeostasis, photoprotection, and lower photorespiration in salt-stressed cashew plants. Acta Physiol. Plant. 2019, 41, 1–14. [Google Scholar] [CrossRef]
- Pérez-López, U.; Robredo, A.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. The impact of salt stress on the water status of barley plants is partially mitigated by elevated CO2. Environ. Exp. Bot. 2009, 66, 463–470. [Google Scholar] [CrossRef]
- Pérez-López, U.; Miranda-Apodaca, J.; Muñoz-Rueda, A.; Mena-Petite, A. Lettuce production and antioxidant capacity are differentially modified by salt stress and light intensity under ambient and elevated CO2. J. Plant Physiol. 2013, 170, 1517–1525. [Google Scholar] [CrossRef]
- Geissler, N.; Hussin, S.; El-Far, M.M.M.; Koyro, H.W. Elevated atmospheric CO2 concentration leads to different salt resistance mechanisms in a C3 (Chenopodium quinoa) and a C4 (Atriplex nummularia) halophyte. Environ. Exp. Bot. 2015, 118, 67–77. [Google Scholar] [CrossRef]
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fugle-Stvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and natural radiative forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2013. [Google Scholar]
- Oliver, R.J.; Mercado, L.M.; Sitch, S.; Simpson, D.; Medlyn, B.E.; Lin, Y.S.; Folberth, G.A. Large but decreasing effect of ozone on the European carbon sink. Biogeosciences 2018, 15, 4245–4269. [Google Scholar] [CrossRef]
- Yue, X.; Unger, N.; Harper, K.; Xia, X.; Liao, H.; Zhu, T.; Xiao, J.; Feng, Z.; Li, J. Ozone and haze pollution weakens net primary productivity in China. Atmos. Chem. Phys. 2017, 17, 6073–6089. [Google Scholar] [CrossRef]
- McGrath, J.M.; Betzelberger, A.M.; Wang, S.; Shook, E.; Zhu, X.G.; Long, S.P.; Ainsworth, E.A. An analysis of ozone damage to historical maize and soybean yields in the United States. Proc. Natl. Acad. Sci. USA 2015, 112, 14390–14395. [Google Scholar] [CrossRef] [PubMed]
- Riikonen, J.; Lindsberg, M.M.; Holopainen, T.; Oksanen, E.; Lappi, J.; Peltonen, P.; Vapaavuori, E. Silver birch and climate change: Variable growth and carbon allocation responses to elevated concentrations of carbon dioxide and ozone. Tree Physiol. 2004, 24, 1227–1237. [Google Scholar] [CrossRef][Green Version]
- Kontunen-Soppela, S.; Riikonen, J.; Ruhanen, H.; Brosché, M.; Somervuo, P.; Peltonen, P.; Kangasjärvi, J.; Auvinen, P.; Paulin, L.; Keinänen, M.; et al. Differential gene expression in senescing leaves of two silver birch genotypes in response to elevated CO2 and tropospheric ozone. Plant Cell Environ. 2010, 33, 1016–1028. [Google Scholar] [CrossRef]
- Riikonen, J.; Syrjälä, L.; Tulva, I.; Mänd, P.; Oksanen, E.; Poteri, M.; Vapaavuori, E. Stomatal characteristics and infection biology of Pyrenopeziza betulicola in Betula pendula trees grown under elevated CO2 and O3. Environ. Pollut. 2008, 156, 536–543. [Google Scholar] [CrossRef]
- Tobita, H.; Uemura, A.; Kitao, M.; Kitaoka, S.; Utsugi, H. Interactive effects of elevated CO2, phosphorus deficiency, and soil drought on nodulation and nitrogenase activity in alnus hirsuta and alnus maximowiczii. Symbiosis 2010, 50, 59–69. [Google Scholar] [CrossRef]
- Duan, H.; Ontedhu, J.; Milham, P.; Lewis, J.D.; Tissue, D.T. Effects of elevated carbon dioxide and elevated temperature on morphological, physiological and anatomical responses of Eucalyptus tereticornis along a soil phosphorus gradient. Tree Physiol. 2019, 39, 1821–1837. [Google Scholar] [CrossRef]
- Bauweraerts, I.; Wertin, T.M.; Ameye, M.; Mcguire, M.A.; Teskey, R.O.; Steppe, K. The effect of heat waves, elevated [CO2] and low soil water availability on northern red oak (Quercus rubra L.) seedlings. Glob. Chang. Biol. 2013, 19, 517–528. [Google Scholar] [CrossRef]
- Kumar, A.; Anju, T.; Kumar, S.; Chhapekar, S.S.; Sreedharan, S.; Singh, S.; Choi, S.R.; Ramchiary, N.; Lim, Y.P. Integrating omics and gene editing tools for rapid improvement of traditional food plants for diversified and sustainable food security. Int. J. Mol. Sci. 2021, 22, 8093. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobo, A.K.M.; Catarino, I.C.A.; Silva, E.A.; Centeno, D.C.; Domingues, D.S. Physiological and Molecular Responses of Woody Plants Exposed to Future Atmospheric CO2 Levels under Abiotic Stresses. Plants 2022, 11, 1880. https://doi.org/10.3390/plants11141880
Lobo AKM, Catarino ICA, Silva EA, Centeno DC, Domingues DS. Physiological and Molecular Responses of Woody Plants Exposed to Future Atmospheric CO2 Levels under Abiotic Stresses. Plants. 2022; 11(14):1880. https://doi.org/10.3390/plants11141880
Chicago/Turabian StyleLobo, Ana Karla M., Ingrid C. A. Catarino, Emerson A. Silva, Danilo C. Centeno, and Douglas S. Domingues. 2022. "Physiological and Molecular Responses of Woody Plants Exposed to Future Atmospheric CO2 Levels under Abiotic Stresses" Plants 11, no. 14: 1880. https://doi.org/10.3390/plants11141880
APA StyleLobo, A. K. M., Catarino, I. C. A., Silva, E. A., Centeno, D. C., & Domingues, D. S. (2022). Physiological and Molecular Responses of Woody Plants Exposed to Future Atmospheric CO2 Levels under Abiotic Stresses. Plants, 11(14), 1880. https://doi.org/10.3390/plants11141880





