High Atmospheric CO2 Concentration Mitigates Drought Effects on Acanthostyles buniifolius an Important Grassland Weed in South America
Abstract
:1. Introduction
2. Material and Methods
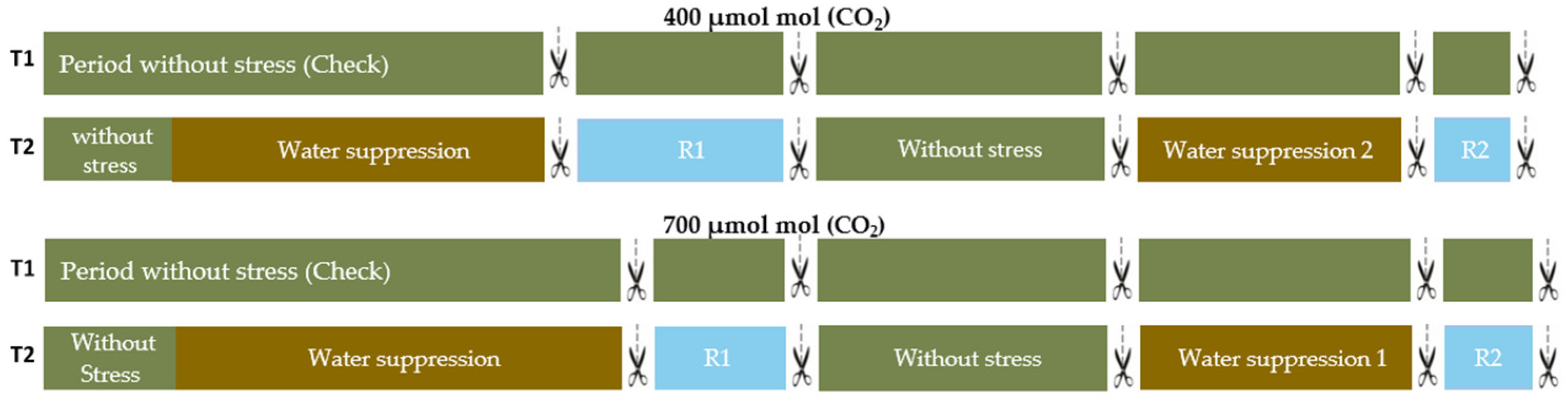
2.1. Plant Material and Growth Conditions
2.2. Biochemical Parameters
2.3. Estimation of Water Potential and Relative-Water Contents (RWC)
2.4. Biometric Parameters
2.5. Statistical Analysis
3. Results
3.1. Vegetative Stage
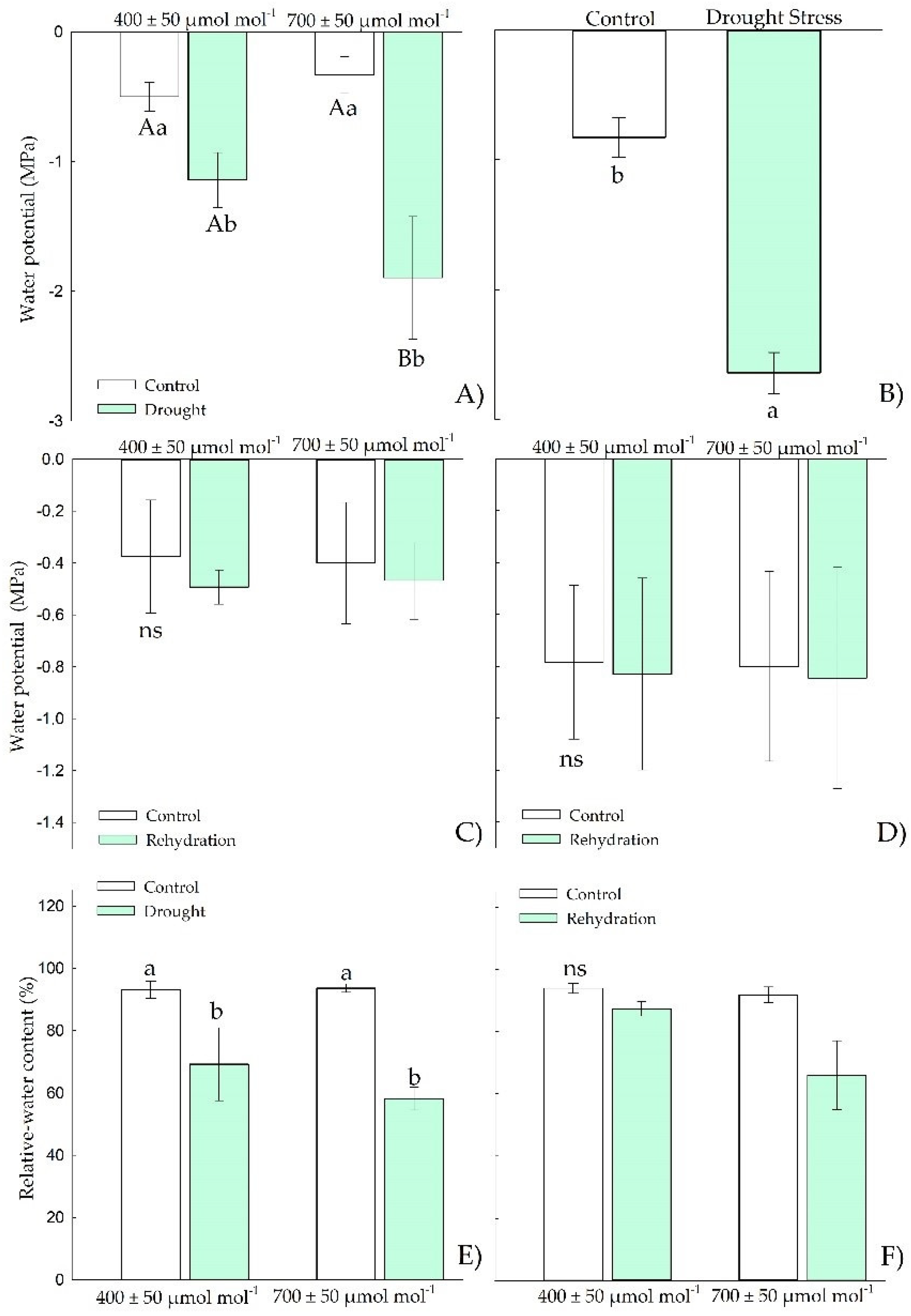
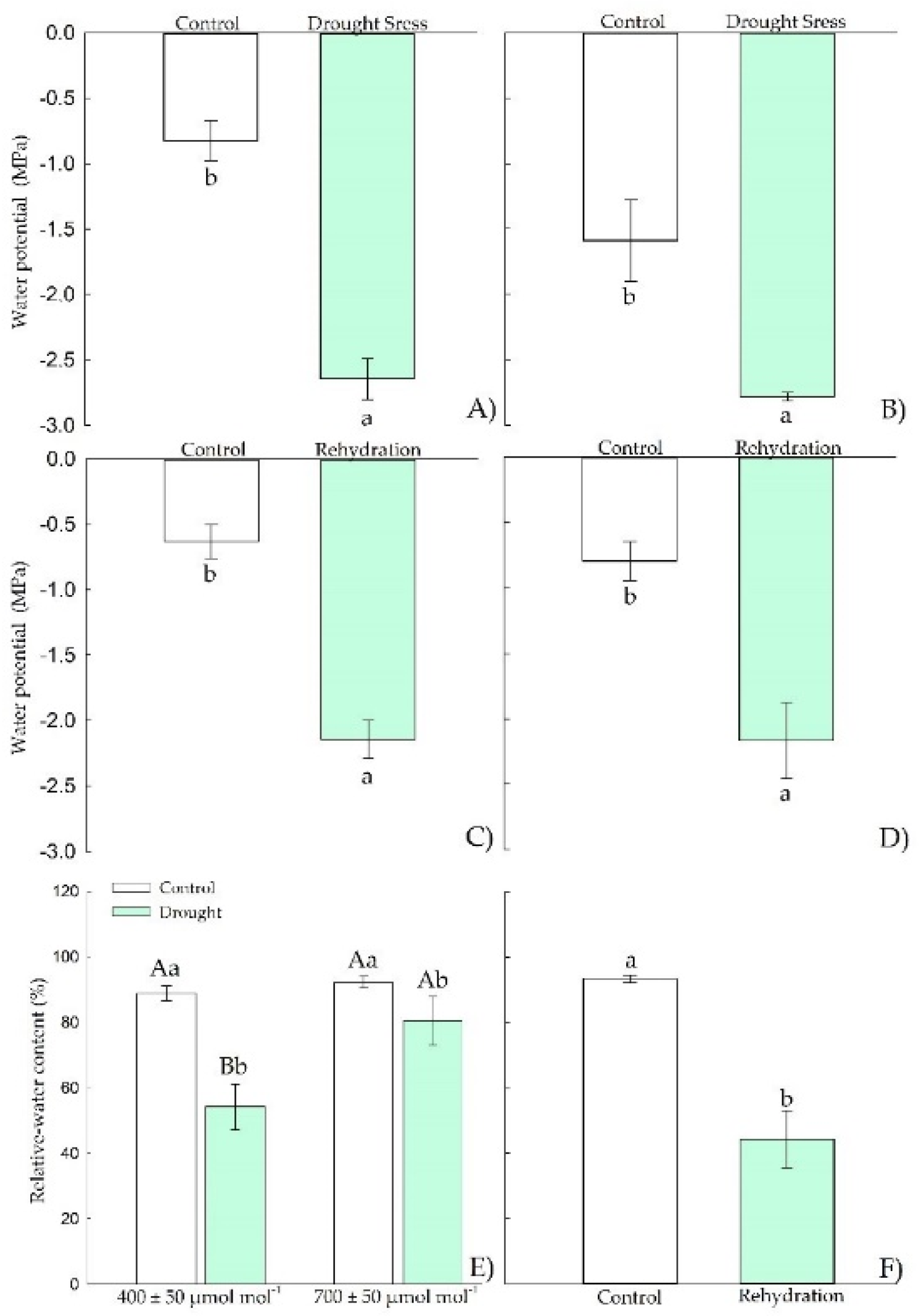
3.1.1. Water Relations
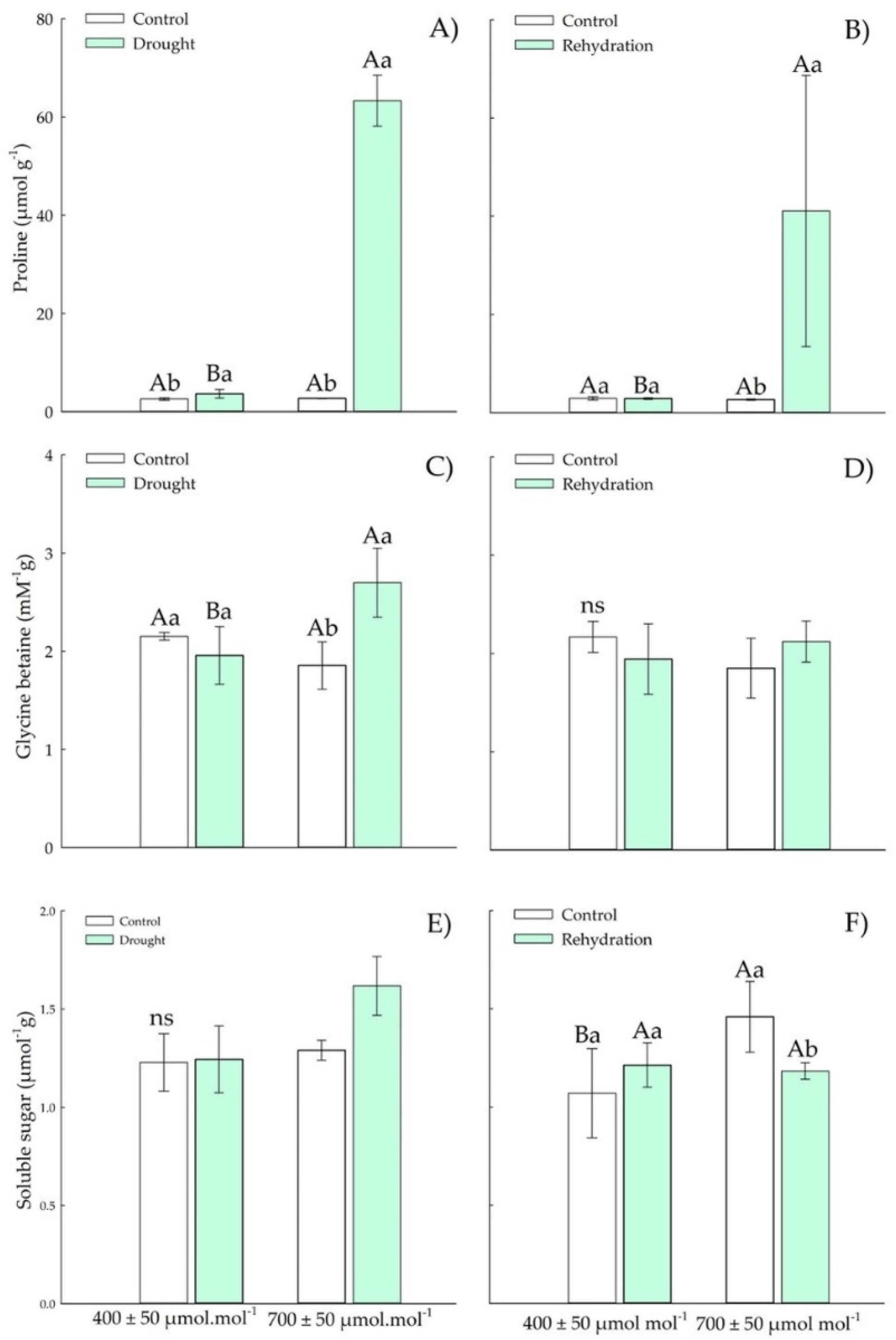
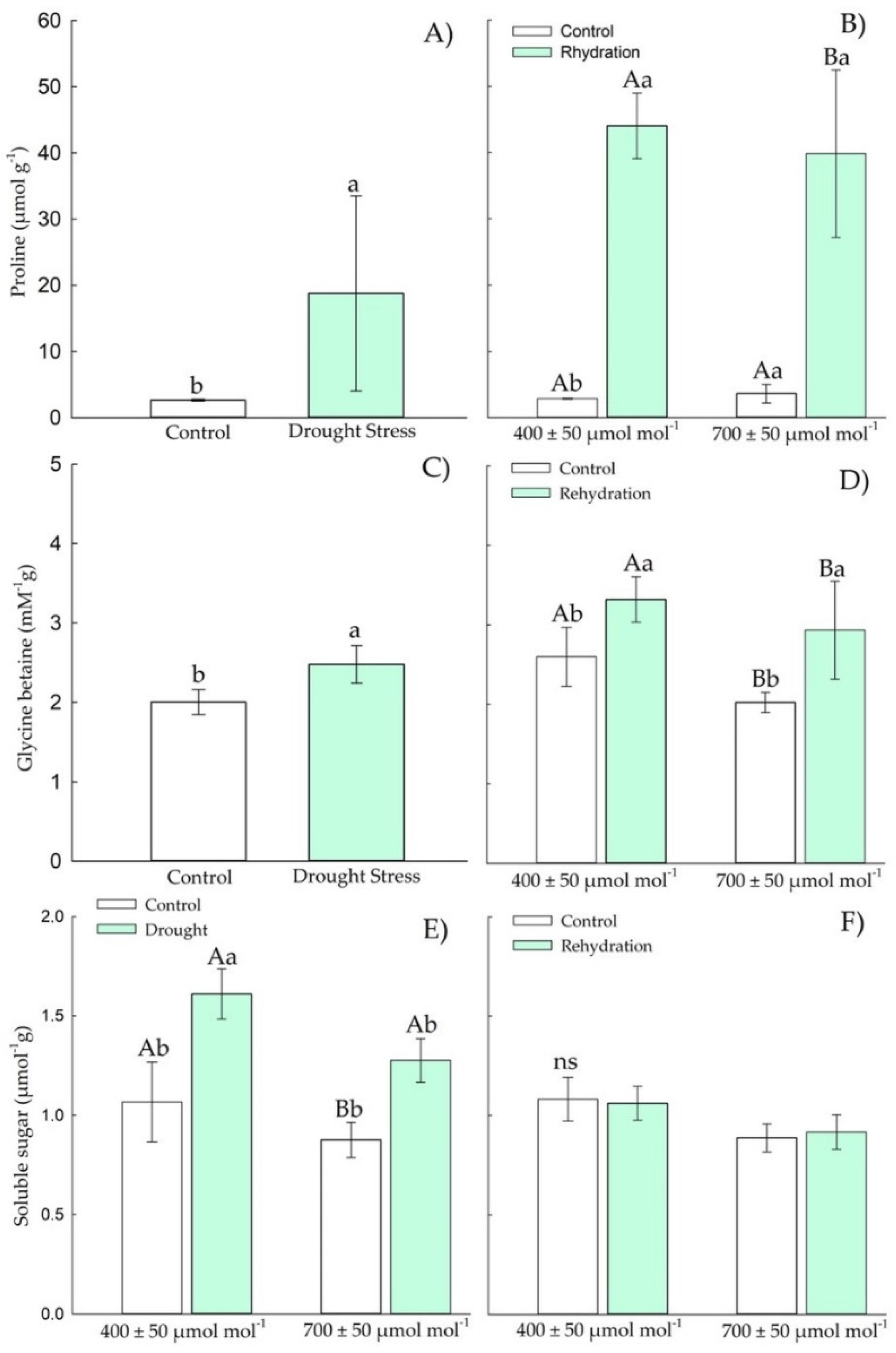
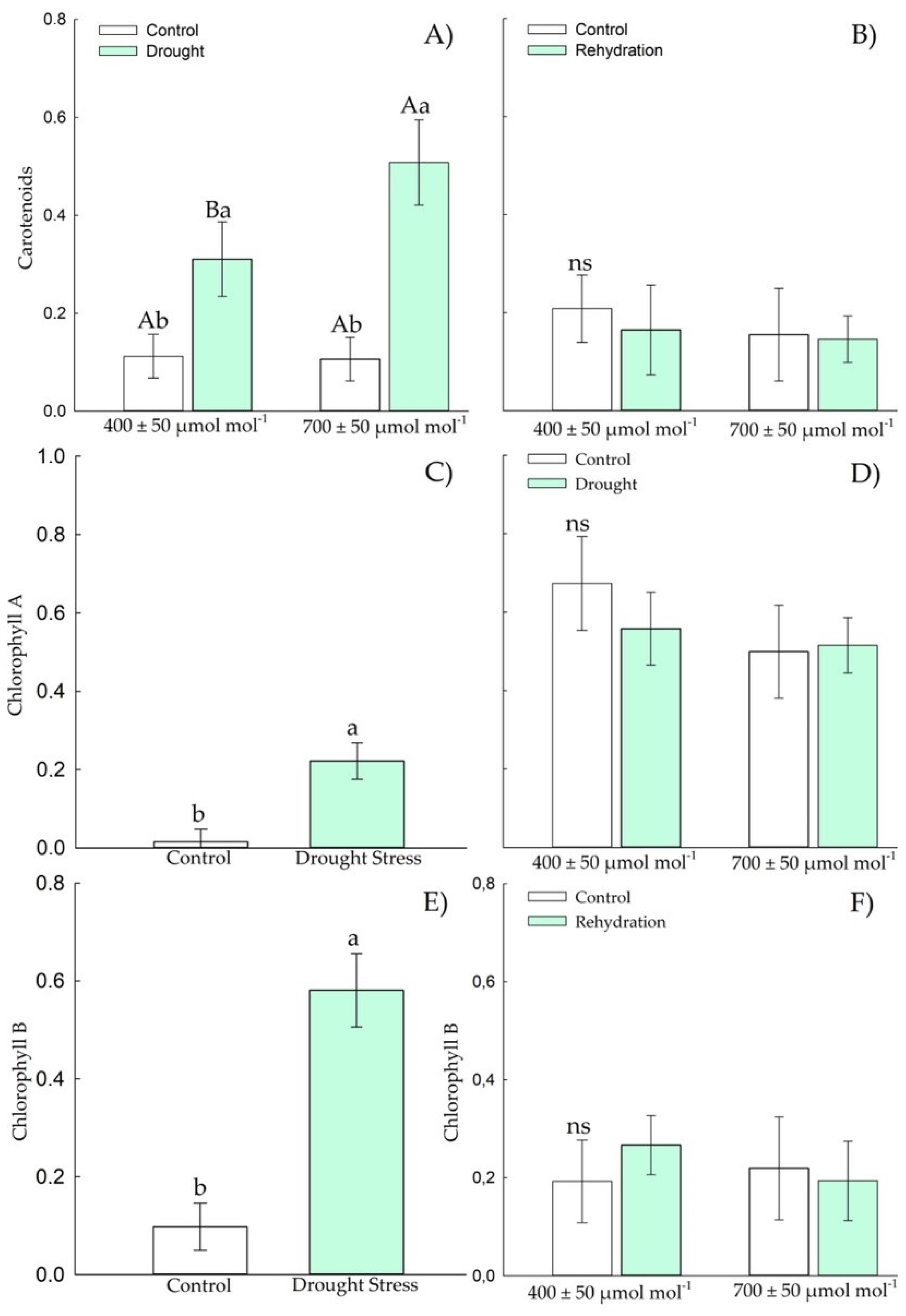
3.1.2. Osmotic Adjustment Components
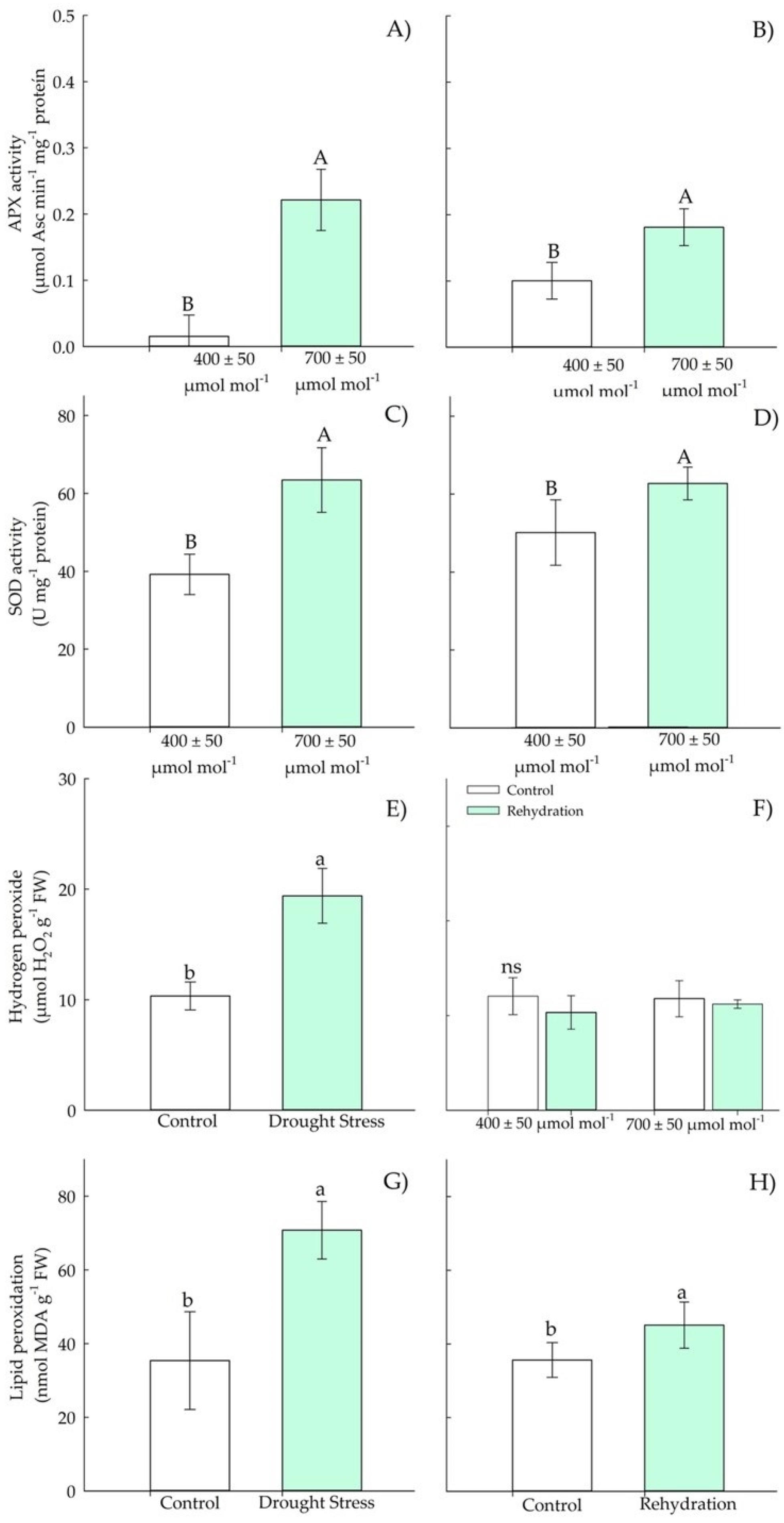
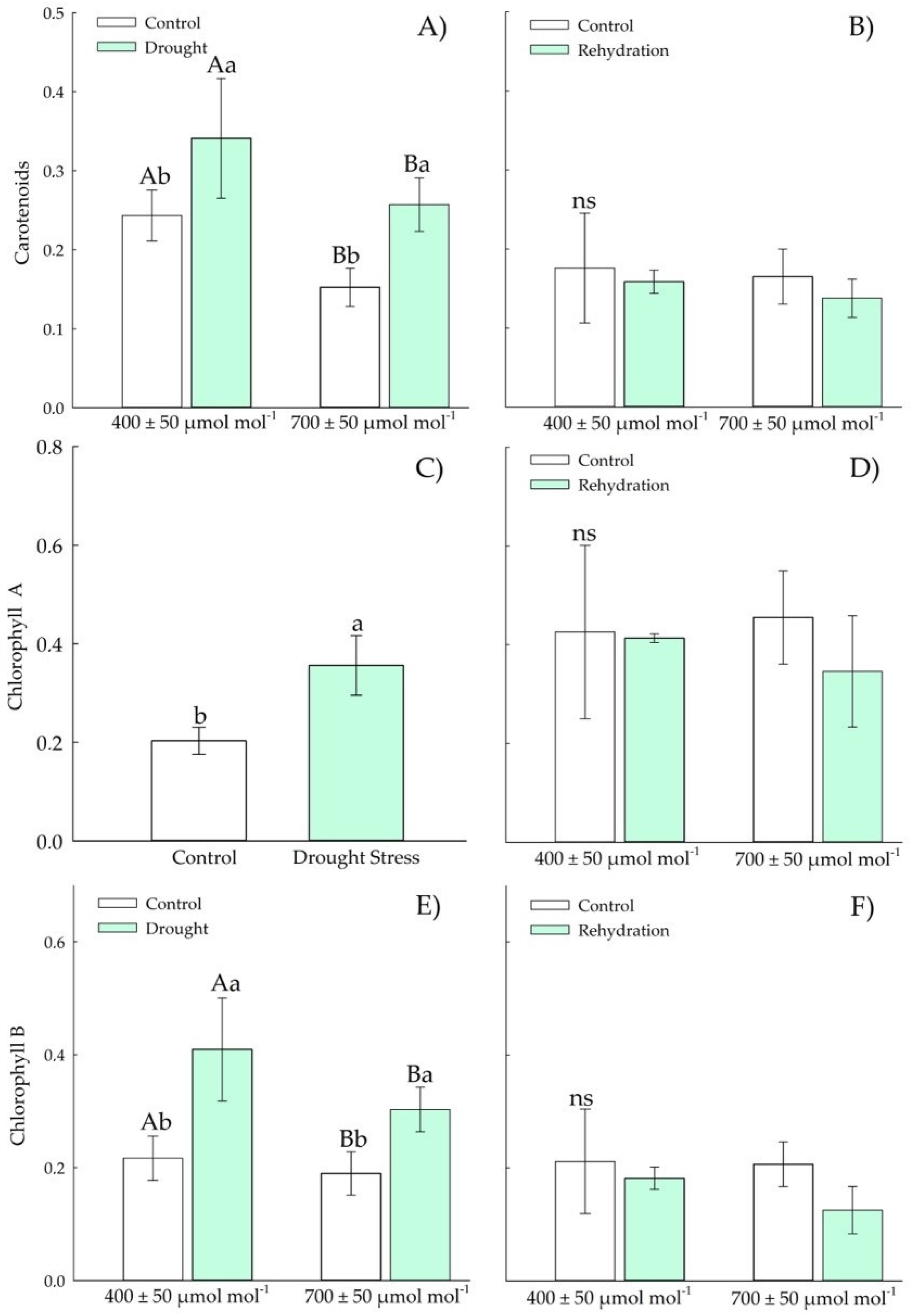
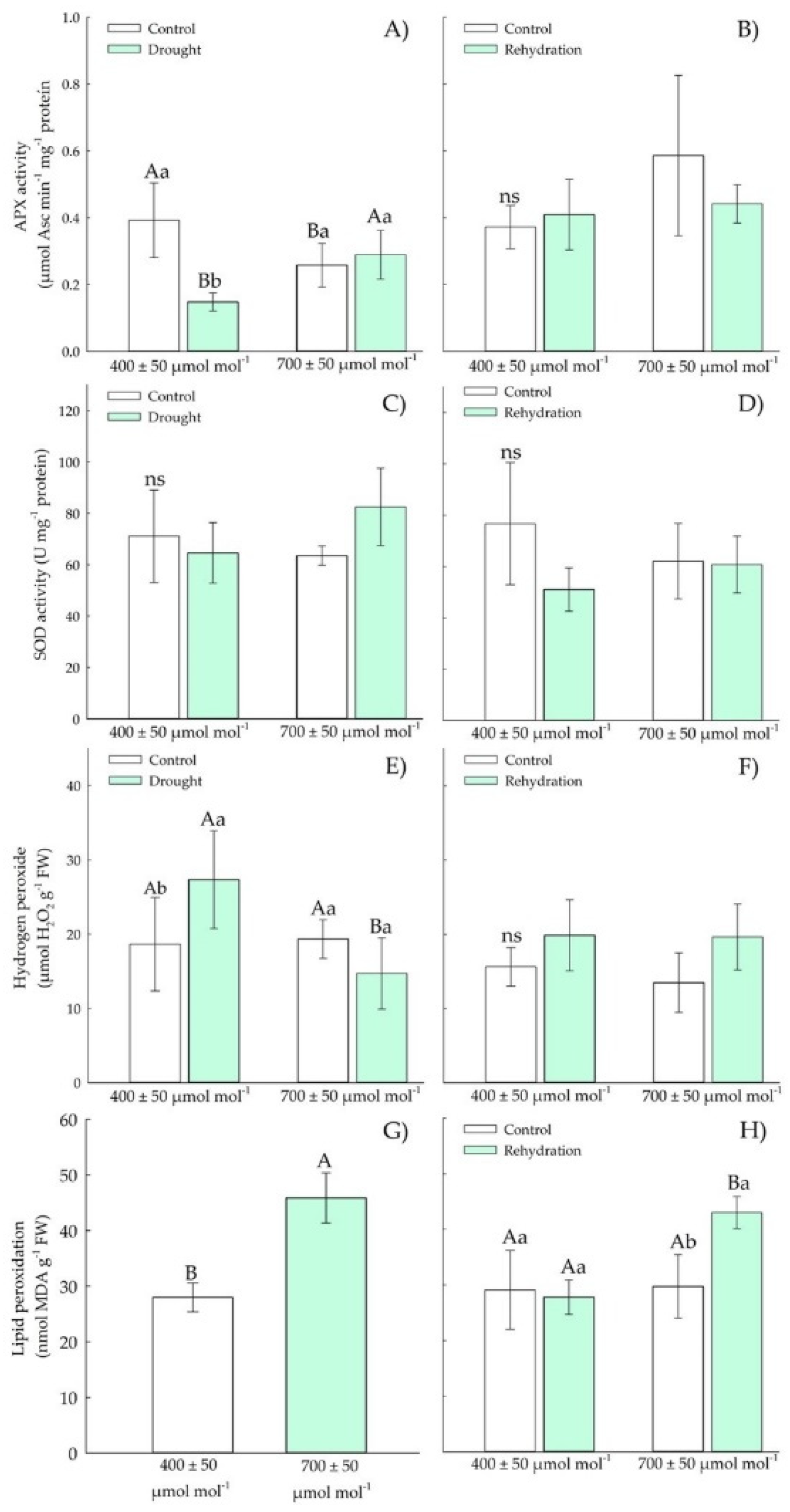
3.1.3. Biochemical Parameters—Antioxidants
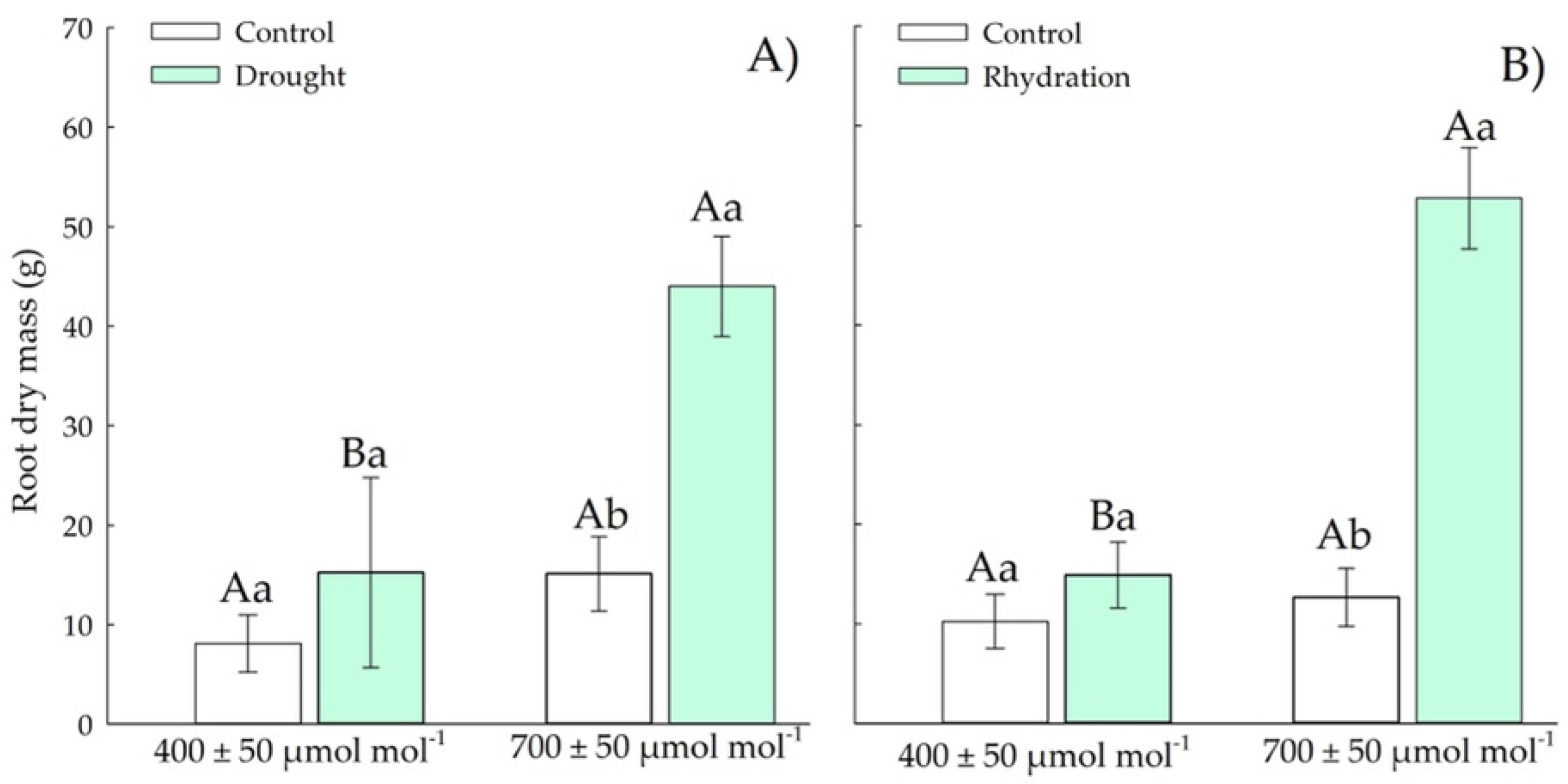
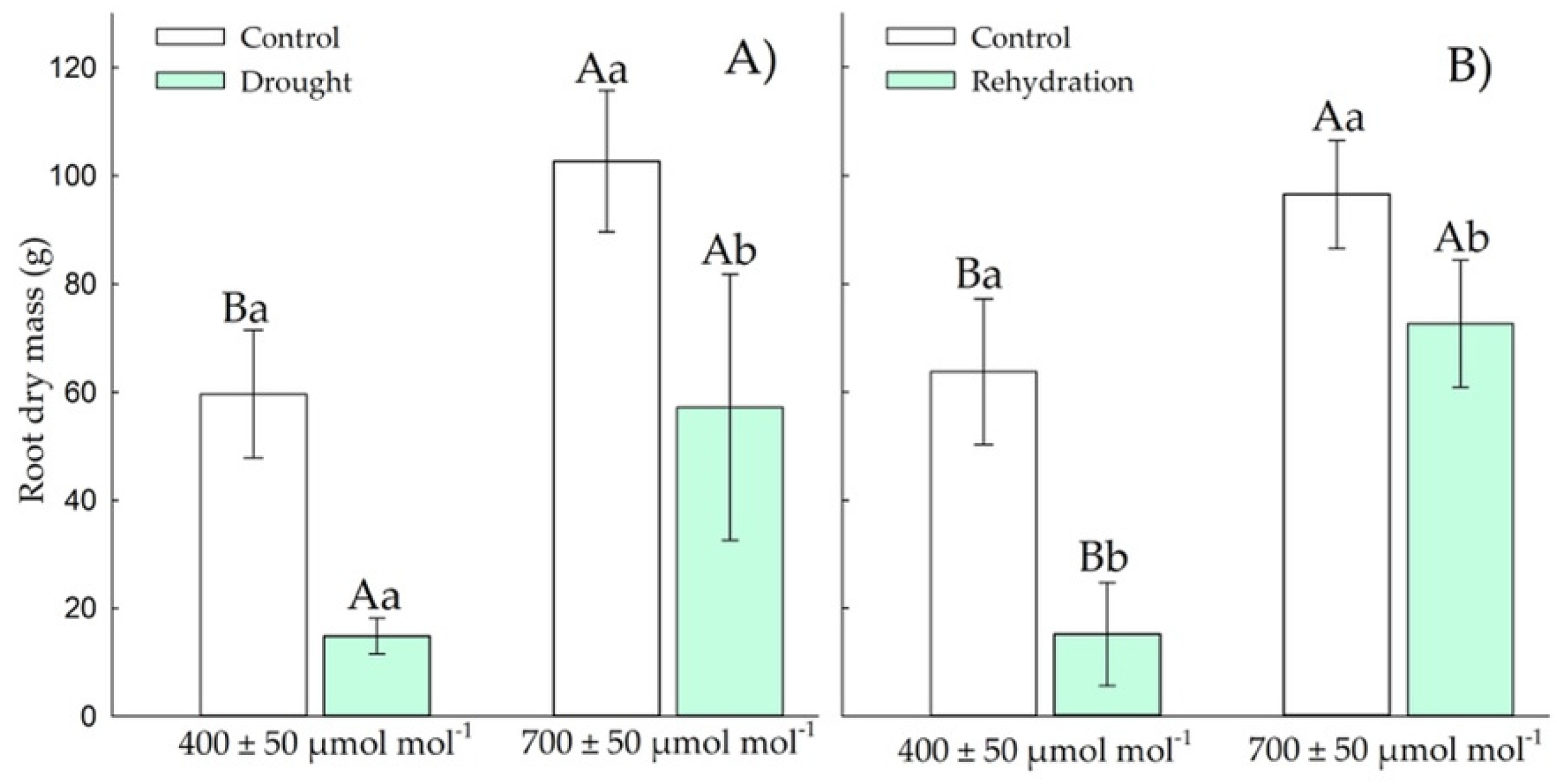
3.1.4. Root Dry Weight
3.2. Reproductive Stage
3.2.1. Water Relations
3.2.2. Osmotic Adjustment Components
3.2.3. Biochemical Parameters
3.2.4. Root Dry Mass
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yonaha, L.; Hasenack, H.; De Abelleyra, D.; Baeza, S. South American Pampa Has Lost 16.3% of Native Vegetation in 20 Years, Shows Unprecedented Mapping, Which Covers Brazil, Argentina and Uruguay. Available online: https://pampa.mapbiomas.org/collection-release (accessed on 10 July 2022).
- Severo, C.M.; Matte, A. Políticas públicas para a pecuária no Bioma Pampa: Análises para Brasil e Uruguai. Agricultura Familiar: Pesquisa, Formação e Desenvolvimento. RAF 2020, 14, 14–40. [Google Scholar] [CrossRef]
- Pillar, V.D.P.; Omara, L. (Eds.) Os Campos do Sul; Rede Campos Sulinos-UFRGS: Porto Alegre, Brazil, 2015; p. 192. ISBN 9788566106503. [Google Scholar]
- Pillar, V.D.P.; Vélez, E. Extinção dos Campos sulinos em unidade de conservação: Um fenômeno natural ou um problema ético. Nat. Conserv. 2010, 8, 84–86. [Google Scholar] [CrossRef]
- Marchi, M.M.; Barbieri, R.L.; Sallés, J.M.; Da Costa, F.A. Herbaceous and subshrubby flora associated with palm grove ecosystem in the Pampas Biome. Rodriguesia 2018, 69, 553–560. [Google Scholar] [CrossRef]
- Saberes, R.; Winckler, L.T. Por que e para que conservar o Pampa? In Anais do I Congresso sobre o Bioma Pampa; UFPel: Pelotas, Brazil, 2020. [Google Scholar] [CrossRef]
- Dias-Filho, M.B. Diagnóstico das Pastagens no Brasil, 1st ed.; Documentos 02; Embrapa Amazônia Oriental: Belém, Brazil, 2014; pp. 21–43. [Google Scholar]
- Gonçalves, J.O.N.; Girarde-Deiro, A.M.; Gonzaga, S.S. Evolução da Vegetação de um Campo Natural (Excluído e Pastejado) e Auto-ecologia de Plantas Indesejáveis; INFOTECA-E, 15; Embrapa-CNPO: Bagé, Brazil, 1990; p. 26. ISSN 0103-3743. [Google Scholar]
- Nabinger, C. Manejo e produtividade das pastagens nativas do subtrópico brasileiro. Simpósio Produção Anim. 2006, 1, 22–76. [Google Scholar]
- Roque, N.; Teles, A.M.; Nkajima, J.N. A família Asteraceae no Brasil: Classificação e diversidade. Salvador. EDUFBA 2017, 1–262. [Google Scholar] [CrossRef]
- Lorenzi, H. Plantas Daninhas do Brasil: Terrestres, Aquáticas, Parasitas e Tóxicas, 3rd ed.; Instituto Plantarum: Nova Odessa, Brazil, 2000. [Google Scholar]
- Kissmann, K.G.; Groth, D. Plantas Infestantes e Nocivas Tomo I, 1st ed.; BASF Brasileira S.A.: São Paulo, Brazil, 1992; Volume 3, p. 785. [Google Scholar]
- Gornish, E.S.; Tylianakis, J.M. Community shifts under climate change: Mechanisms at multiple scales. Am. J. Bot. 2013, 100, 1422–1434. [Google Scholar] [CrossRef]
- Mina, U.; Kumar, A.; Chaturvedi, A.K.; Kumar, P. Climate change and plant diversity: Threats and opportunities. In Climate change and the Microbiome; Springer: Cham, Switzerland, 2021; pp. 369–396. [Google Scholar]
- Madani, N.; Kimball, J.S.; Ballantyne, A.P.; Affleck, D.L.R.; Van Bodegom, P.M.; Reich, P.B.; Kattge, J.; Sala, A.; Nazeri, M.; Jones, M.O.; et al. Future global productivity will be affected by plant trait response to climate. Sci. Rep. 2018, 8, 2870. [Google Scholar] [CrossRef]
- Shahzad, A.; Ullah, S.; Dar, A.A.; Sardar, M.F.; Mehmood, T.; Tufail, M.A.; Shakoor, A.; Haris, M. Nexus on Climate Change: Agriculture and possible solution to cope future climate change stresses. Environ. Sci. Pollut. Res. 2021, 28, 14211–14232. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, A.; Trenberth, K.E. Climate change and drought: A perspective on drought indices. Curr. Clim. Chang. Rep. 2018, 4, 145–163. [Google Scholar] [CrossRef]
- Almazroui, M.; Ashfaq, M.; Islam, M.N.; Rashid, I.U.; Kamil, S.; Abid, M.A.; O’Brien, E.; Ismail, M.; Reboita, M.S.; Sörensson, A.A.; et al. Assessment of CMIP6 performance and projected temperature and precipitation changes over South America. Earth Syst. Environ. 2021, 5, 155–183. [Google Scholar] [CrossRef]
- Fathi, A.; Tari, D.B. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Heald, J.; Kingston-Smith, A.; Winters, A.; Rubiales, D.; Sanz, M.; Mur, L.A.J.; Prats, E. A Metabolomic study in oats (Avena Sativa) highlights a drought tolerance mechanism based upon salicylate signaling pathways and the modulation of carbon, antioxidant and photo-oxidative metabolism. Plant Cell Environ. 2015, 38, 1434–1452. [Google Scholar] [CrossRef]
- Gomes, F.P.; Prado, C.H.B.A. Ecophysiology of Coconut Palm under water stress. Braz. J. Plant Physiol. 2007, 19, 377–391. [Google Scholar] [CrossRef]
- Ahmad, P.; Sarwat, M.; Sharma, S. Reactive Oxygen Species, Antioxidants and Signaling in Plants. J. Plant Biol. 2008, 51, 167–173. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Marcos, F.C.C.; Silveira, N.M.; Mokochinski, J.B.; Sawaya, A.C.H.F.; Marchiori, P.E.R.; Machado, E.C.; Souza, G.M.; Landell, M.G.A.; Ribeiro, R.V. Drought tolerance of sugarcane is improved by previous exposure to water deficit. J. Plant Physiol. 2018, 223, 9–18. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Ma, X. Different response on drought tolerance and post-drought recovery between the small-leafed and the large-leafed white clover (Trifolium Repens L.) associated with antioxidative enzyme protection and lignin metabolism. Acta Physiol. Plant. 2013, 35, 213–222. [Google Scholar] [CrossRef]
- Peeva, V.; Cornic, G. Leaf photosynthesis of Haberlea rhodopensis before and during drought. Environ. Exp. Botany 2009, 65, 310–318. [Google Scholar] [CrossRef]
- Bertolli, S.C.; Mazzafera, P.; Souza, G.M. Why is it so difficult to identify a single indicator of water stress in plants? A proposal for a multivariate analysis to assess emergent properties. Plant Biol. 2014, 16, 578–585. [Google Scholar] [CrossRef]
- Posso, D.A.; Borella, J.; Reissig, G.N.; Guidorizi, K.A.; Bacarin, M.A. Short-term root flooding and recovery conditions—affected photosynthetic process of common bean plants. Theor. Exp. Plant Physiol. 2020, 32, 287–300. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 14, 307–313. [Google Scholar] [CrossRef]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; De Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive Maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Bielski, R.; Turner, N. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal. Biochem. 1966, 17, 278–293. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Springer Stable 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Graham, D.; Smydzuk, J. Use of anthrone in the quantitative determination of hexose phosphates. Anal. Biochem. 1965, 11, 246–255. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 413–428. [Google Scholar] [CrossRef]
- Kissmann, K.G.; Groth, D. Plantas Infestantes e Nocivas Tomo II. BASF 1997, 1, 978. [Google Scholar]
- Nabinger, C.; Pinto, C.E.; Boldrini, I.I. Os Campos Sulinos. In Forragicultura: Ciência, Tecnologia e Gestão dos Recursos Forrageiros; FUNEP: Jaboticabal, Brasil, 2013; pp. 157–172. [Google Scholar]
- Barnaby, J.Y.; Ziska, L.H. Plant Responses to elevated CO2. eLS 2012. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Zhou, G. Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Front. Plant Sci. 2015, 6, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, C.A.; de Oliveira, E.A.D.; Mello, T.R.P.; Alzate-Marin, A.L. Plants responses to increase in atmospheric carbon dioxide and temperature. Rev. Bras. Geogr. Física 2015, 8, 635–650. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol. Mol. Biol. Plants 2018, 24, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Kongsri, S.; Boonprakob, U.; Byrne, D.H. Assessment of morphological and physiological responses of Peach rootstocks under Drought and Aluminum Stress. Acta Hortic. 2014, 1059, 229–236. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated photosynthesis under. Regulation 2002, 44, 275–294. [Google Scholar]
- Benzarti, M.; Rejeb, K.B.; Messedi, D.; Mna, A.B.; Hessini, K.; Ksontini, M.; Abdelly, C.; Debez, A. Effect of high salinity on Atriplex portulacoides: Growth, leaf water relations and solute accumulation in relation with osmotic adjustment. S. Afr. J. Bot. 2014, 95, 70–77. [Google Scholar] [CrossRef]
- Molinari, H.B.C.; Marur, C.J.; Daros, E.; De Campos, M.K.F.; De Carvalho, J.F.R.P.; Filho, J.C.B.; Pereira, L.F.P.; Vieira, L.G.E. Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum Spp.): Osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol. Plant. 2007, 130, 218–229. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Sangam, S.; Amrutha, R.N.; Sri Laxmi, P.; Naidu, K.R.; Rao, K.R.S.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Ben Rejeb, K.; Abdelly, C.; Savouré, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar] [CrossRef]
- Jiménez, S.; Dridi, J.; Gutiérrez, D.; Moret, D.; Irigoyen, J.J.; Moreno, M.A.; Gogorcena, Y. Physiological, biochemical, and molecular responses in four prunus rootstocks submitted to drought stress. Tree Physiol. 2013, 33, 1061–1075. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, N.F.; de Brito do Nascimento, L.B.; de Carvalho Gonçalves, J.F. Respostas funcionais foliares de plantas jovens de Hevea Brasiliensis submetidas à deficiência hídrica e à reidratação. Ciência Florestal. 2019, 29, 1019. [Google Scholar] [CrossRef] [Green Version]
- Mona, G. Dawood influence of osmoreglators on plant tolerance to water stress. Sci. Agric. 2016, 13, 10–13. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Zhang, L.X.; Lai, J.H.; Liang, Z.S.; Ashraf, M. Interactive effects of sudden and gradual drought stress and foliar-applied glycine betaine on growth, water relations, osmolyte accumulation and antioxidant defense system in two Maize cultivars differing in drought tolerance. J. Agron. Crop Sci. 2014, 200, 425–433. [Google Scholar] [CrossRef]
- Anjum, S.A.; Farooq, M.; Wang, L.C.; Xue, L.L.; Wang, S.G.; Wang, L.; Zhang, S.; Chen, M. Gas exchange and chlorophyll synthesis of maize cultivars are enhanced by exogenously-applied glycine betaine under drought conditions. Plant Soil Environ. 2011, 57, 326–331. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Samphumphuang, T.; Tisarum, R.; Theerawitaya, C.; Cha-Um, S. Arbuscular Mycorrhizal Fungi (AMF) improved water deficit tolerance in two different sweet Potato genotypes involves osmotic adjustments via soluble sugar and free proline. Sci. Hortic. 2016, 198, 107–117. [Google Scholar] [CrossRef]
- Dos Santos, C.M.; Endres, L.; Da Silva, A.C.S.; Silva, J.V.; De Souza Barbosa, G.V.; Froehlich, A.; Teixeira, M.M. Water relations and osmolyte accumulation related to sugarcane yield under drought stress in a tropical climate. Int. J. Plant Prod. 2019, 13, 227–239. [Google Scholar] [CrossRef]
- White, L.M. Carbohydrate reserves of grasses: A review. J. Range Manag. 1973, 26, 13. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Vaca, C.E.; Wilhelm, J.; Harms-Ringdahl, M. Interaction of lipid peroxidation products with DNA. A review. Mutat. Res./Rev. Genet. Toxicol. 1988, 195, 137–149. [Google Scholar] [CrossRef]
- Barreiros, A.L.B.S.; David, J.M.; David, J.P. Oxidative Stress: Relations between the formation of reactive species and the organism’s defense. Quimica Nova. 2006, 29, 113–123. [Google Scholar] [CrossRef]
- Ren, J.; Sun, L.N.; Zhang, Q.Y.; Song, X.S. Drought tolerance is correlated with the activity of antioxidant enzymes in Cerasus humilis seedlings. BioMed Res. Int. 2016, 2016, 9851095. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.R.; de Araujo Silva, M.M.; Willadino, L.; Ulisses, C.; Camara, T.R. Geração e desintoxicação enzimática de espécies reativas de oxigênio em plantas. Ciência Rural. 2014, 44, 453–460. [Google Scholar] [CrossRef]
- Dalal, V.K.; Tripathy, B.C. Modulation of chlorophyll biosynthesis by water stress in rice seedlings during chloroplast biogenesis. Plant Cell Environ. 2012, 35, 1685–1703. [Google Scholar] [CrossRef]
- Santos, R.F.; Carlesso, R. Water deficit and morphologic and physiologic behavior of the plants. Rev. Bras. Eng. Agrícola E Ambient. 1998, 2, 287–294. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 5th ed.; Artmed: Porto Alegre, Brazil, 2014. [Google Scholar]
- Kumar, V.; Sharma, A.; Kumar Soni, J.; Pawar, N.; Vimal Kumar, C. Physiological response of C3, C4 and CAM plants in changeable climate. Pharma Innov. 2017, 6, 70–79. [Google Scholar]
- Hummel, I.; Pantin, F.; Sulpice, R.; Piques, M.; Rolland, G.; Dauzat, M.; Christophe, A.; Pervent, M.; Bouteillé, M.; Stitt, M.; et al. Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: An integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol. 2010, 154, 357–372. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential-are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heck, T.; Fipke, M.V.; Polito, R.A.; Souza, G.M.; Agostinetto, D.; Nunes, A.L.; Avila, L.A.d. High Atmospheric CO2 Concentration Mitigates Drought Effects on Acanthostyles buniifolius an Important Grassland Weed in South America. Plants 2022, 11, 2270. https://doi.org/10.3390/plants11172270
Heck T, Fipke MV, Polito RA, Souza GM, Agostinetto D, Nunes AL, Avila LAd. High Atmospheric CO2 Concentration Mitigates Drought Effects on Acanthostyles buniifolius an Important Grassland Weed in South America. Plants. 2022; 11(17):2270. https://doi.org/10.3390/plants11172270
Chicago/Turabian StyleHeck, Tamara, Marcus Vinícius Fipke, Rubens Antonio Polito, Gustavo Maia Souza, Dirceu Agostinetto, Anderson Luis Nunes, and Luis Antonio de Avila. 2022. "High Atmospheric CO2 Concentration Mitigates Drought Effects on Acanthostyles buniifolius an Important Grassland Weed in South America" Plants 11, no. 17: 2270. https://doi.org/10.3390/plants11172270
APA StyleHeck, T., Fipke, M. V., Polito, R. A., Souza, G. M., Agostinetto, D., Nunes, A. L., & Avila, L. A. d. (2022). High Atmospheric CO2 Concentration Mitigates Drought Effects on Acanthostyles buniifolius an Important Grassland Weed in South America. Plants, 11(17), 2270. https://doi.org/10.3390/plants11172270







