Rhizospheric Actinomycetes Revealed Antifungal and Plant-Growth-Promoting Activities under Controlled Environment
Abstract
1. Introduction
2. Results
2.1. Isolation and Preliminary Screening
2.2. Molecular Identification
2.3. Extracellular Hydrolytic Enzymes
2.4. In Vivo Growth Promoting and Disease Control
2.4.1. Eco-Physiological Parameters
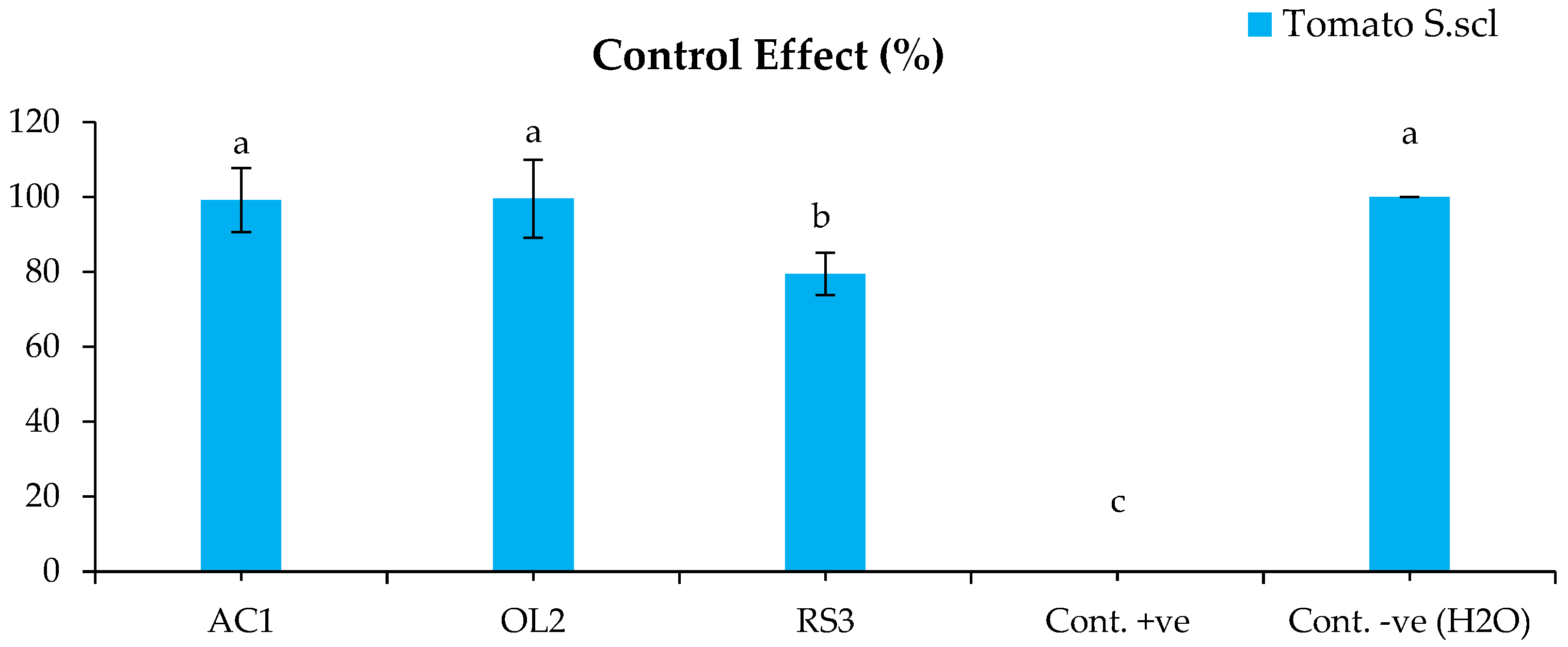
2.4.2. Disease Control
3. Discussion
4. Materials and Methods
4.1. Soil Sampling and Isolation
4.2. Antagonistic Activity
4.3. Molecular Identification
4.4. Extracellular Hydrolytic Enzymes
4.5. In Vivo Growth Promoting Effect and Disease Control
4.6. Statistical Analysis
| Primers | Sequences | Target | Amplified Fragment (kb) | Gene | Reference |
|---|---|---|---|---|---|
| Y1 | 5′-TGGCTCAGAACGAACGCTGGCGGC-3′ | Bacteria | 0.43 | 16S rDNA | Darrasse et al. [53] |
| Y2 | 5′-CCCACTGCTGCCTCCCGTAGGAGT-3′ |
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Urban, C.; Segal-Maurer, S.; Rahal, J.J. Considerations in control and treatment of nosocomial infections due to multidrug resistant Acinetobacter baumannii. Clin. Infect. Dis. 2003, 36, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L.; Ko, W.C.; Von Gottberg, A.; Mohapatra, S.; Casellas, J.M.; Goossens, H.; Mulazimoglu, L.; Trenholme, G.; Klugman, K.P.; Bonomo, R.A.; et al. International prospective study of Klebsiella pneumoniae bacteremia: Implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann. Inter. Med. 2004, 140, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest. Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Sakr, S.; Bufo, S.A.; Camele, I. An attempt of biocontrol the tomato-wilt disease caused by Verticillium dahliae using Burkholderia gladioli pv. agaricicola and its bioactive secondary metabolites. Int. J. Plant Biol. 2017, 8, 57–60. [Google Scholar]
- Della Pepa, T.; Elshafie, H.S.; Capasso, R.; De Feo, V.; Camele, I.; Nazzaro, F.; Scognamiglio, M.R.; Caputo, L. Antimicrobial and phytotoxic activity of Origanum heracleoticum and O. majorana essential oils growing in Cilento (Southern Italy). Molecules 2019, 24, 2576. [Google Scholar] [CrossRef]
- Gruľová, D.; Caputo, L.; Elshafie, H.S.; Baranová, B.; De Martino, L.; Sedlák, V.; Camele, I.; De Feo, V. Thymol chemotype Origanum vulgare L. essential oil as a potential selective bio-based herbicide on monocot plant species. Molecules 2020, 25, 595. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Sofo, A.; Mazzone, G.; Caivano, M.; Masi, S.; Caniani, D. Mycoremediation effect of Trichoderma harzianum strain T22 combined with ozonation in diesel-contaminated sand. Chemosphere 2020, 252, 126597. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Viggiani, L.; Mostafa, M.S.; El-Hashash, M.A.; Bufo, S.A.; Camele, I. Biological activity and chemical identification of ornithine lipid produced by Burkholderia gladioli pv. agaricicola ICMP 11096 using LC-MS and NMR analyses. J. Biol. Res. 2017, 90, 96–103. [Google Scholar]
- Vitti, A.; Elshafie, H.S.; Logozzo, G.; Marzario, S.; Scopa, A.; Camele, I.; Nuzzaci, M. Physico-chemical Characterization and biological activities of a digestate and a more stabilized digestate-derived compost from agro-waste. Plants 2021, 10, 386. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Caputo, L.; De Martino, L.; Sakr, S.H.; De Feo, V.; Camele, I. Study of bio-pharmaceutical and antimicrobial properties of pomegranate (Punica granatum L.) Leathery Exocarp Extract. Plants 2021, 10, 153. [Google Scholar] [CrossRef]
- Camele, I.; Elshafie, H.S.; Caputo, L.; Sakr, S.H.; De Feo, V. Bacillus mojavensis: Biofilm formation and biochemical investigation of its bioactive metabolites. J. Biol. Res. 2019, 92, 39–45. [Google Scholar] [CrossRef]
- Sofo, A.; Elshafie, H.S.; Camele, I. Structural and functional organization of the root system: A comparative study on five plant species. Plants 2020, 9, 1338. [Google Scholar] [CrossRef] [PubMed]
- D’Ippolito, I.; Mang, S.M.; Elshafie, H.S.; Camele, I.; Scillitani, G.; Mastrodonato, M.; Sofo, A.; Mininni, A.N.; Xylogiannis, E. Morpho-anatomical and microbiological analysis of kiwifruit roots with KVDS symptoms. Acta Hortic. 2022, 1332, 131–136. [Google Scholar] [CrossRef]
- El-Sayed, W.S.; Akhkha, A.; El-Naggar, M.Y.; Elbadry, M. In vitro antagonistic activity, plant growth promoting traits and phylogenetic affiliation of rhizobacteria associated with wild plants grown in arid soil. Front. Microbiol. 2014, 5, 651. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Saini, K.C.; Bast, F.; Mehariya, S.; Bhatia, S.K.; Lavecchia, R.; Zuorro, A. Microorganisms: A Potential Source of Bioactive Molecules for Antioxidant Applications. Molecules 2021, 26, 1142. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, L.S.H.; Norzaimawati, A.N.; Rosnah, H. Prescreening of bioactivities from actinomycetes isolated from forest peat soil in Sarawak. J. Trp. Agric. Fd. Sci. 2011, 39, 245–254. [Google Scholar]
- Manivasagan, P.; Kang, K.H.; Sivakumar, K.; Li-Chan, E.C.; Oh, H.M.; Kim, S.K. Marine Actinobacteria: An important source of bioactive natural products. Environ. Toxicol. Pharmacol. 2014, 38, 172–188. [Google Scholar] [CrossRef]
- Vikineswary, S.; Nadaraj, P.; Wong, W.H.; Balabaskaran, S. Actinomycetes from a tropical mangrove ecosystem—Antifungal activity of selected strains. Asia Pac. J. Mol. Biol. Biotechnol. 1997, 5, 81–86. [Google Scholar]
- Pepper, I.L.; Gentry, T.J. Chapter 4—“Earth Environments”. In Environmental Microbiology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 59–88. [Google Scholar]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.A.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Williams and Wilkins: Baltimore, MD, USA, 1994. [Google Scholar]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Pharmaceutically active secondary metabolites of marine Actinobacteria. Microbiol. Res. 2014, 169, 262–278. [Google Scholar] [CrossRef]
- Marcolefas, E.; Leung, T.; Okshevsky, M.; McKay, G.; Hignett, E.; Hamel, J.; Aguirre, G.; Blenner-Hassett, O.; Boyle, B.; Lévesque, R.C.; et al. Culture-Dependent bioprospecting of bacterial isolates from the canadian high arctic displaying antibacterial activity. Front. Microbiol. 2019, 10, 1836. [Google Scholar] [CrossRef]
- Girão, M.; Ribeiro, I.; Ribeiro, T.; Azevedo, I.C.; Pereira, F.; Urbatzka, R.; Leão, P.N.; Carvalho, M.F. Actinobacteria Isolated from Laminaria ochroleuca: A source of new bioactive compounds. Front. Microbiol. 2019, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Solecka, J.; Zajko, J.; Postek, M.; Rajnisz, A. Biologically active secondary metabolites from Actinomycetes. Cent. Eur. J. Biol. 2012, 7, 373–390. [Google Scholar] [CrossRef]
- Berdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1. [Google Scholar] [CrossRef] [PubMed]
- Vurukonda, S.S.; Giovanardi, D.; Stefani, E. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef] [PubMed]
- Rajan, B.M.; Kannabiran, K. Extraction and identification of antibacterial secondary metabolites from marine Streptomyces sp. VITBRK2. Int. J. Mol. Cell Med. 2014, 3, 130–137. [Google Scholar]
- Khan, A.; Halo, B.A.; Elyassi, A.; Ali, S.; Al-Hosni, K.; Hussain, J.; Al-Harrasi, A.; Lee, I. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Electron. J. Biotechnol. 2016, 21, 58–64. [Google Scholar] [CrossRef]
- Moon, Y.S.; Ali, S. Isolation and identification of multi-trait plant growth–promoting rhizobacteria from coastal sand dune plant species of Pohang beach. Folia Microbiol. 2022, 67, 523–533. [Google Scholar] [CrossRef]
- Ali, S.; Moon, Y.S.; Hamayun, M.; Khan, M.A.; Bibi, K.; Lee, I. Pragmatic role of microbial plant biostimulants in abiotic stress relief in crop plants. J. Plant Interact. 2022, 17, 705–718. [Google Scholar] [CrossRef]
- Sousa, J.A.A.; Olivares, F.L. Plant growth promotion by Streptomycetes: Ecophysiology, mechanisms and applications. Chem. Biol. Technol. Agric. 2016, 3, 24. [Google Scholar] [CrossRef]
- Manulis, S.; Epstein, E.; Shafrir, H.; Lichter, A.; Barash, I. Biosynthesis of indole-3-acetic acid via the indole-3-acetamide pathway in Streptomyces spp. Microbiology 1994, 140, 1045–1050. [Google Scholar] [CrossRef]
- Dochhil, H.; Dkhar, M.S.; Barman, D. Seed germination enhancing activity of endophytic Streptomyces isolated from indigenous ethno-medicinal plant Centella asiatica. Int. J. Pharm. Biol. Sci. 2013, 4, 256–262. [Google Scholar]
- Sacramento, D.R.; Coelho, R.R.; Wigg, M.D.; Linhares, L.F.; Santos, M.G.; Semedo, L.T.; Silca, A.J. Antimicrobial and antiviral activities of an actinomycetes (Streptomyces sp.) isolated from a Brazilian tropical forest soil. World J. Microbiol. Biotechnol. 2004, 20, 225–229. [Google Scholar] [CrossRef]
- Prapagdee, B.; Kuekulvong, C.; Mongkolsuk, S. Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. Int. J. Biol. Sci. 2008, 4, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Suwan, N.; Boonying, W.; Nalumpang, S. Antifungal activity of soil actinomycetes to control chilli anthracnose caused by Colletotrichum gloeosporioides. J. Agric. Technol. 2012, 8, 725–737. [Google Scholar]
- Soltanzadeh, M.; Nejad, M.S.; Bonjar, G.H. Application of soil-borne actinomycetes for biological control against fusarium wilt of chickpea (Cicer arietinum) caused by Fusarium solani f. sp. Pisi. J. Phytopathol. 2016, 164, 967–978. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Racioppi, R.; Scrano, L.; Iacobellis, N.S.; Bufo, S.A. In vitro antifungal activity of Burkholderia gladioli pv. agaricicola against some phytopathogenic fungi. Int. J. Mol. Sci. 2012, 13, 16291–16302. [Google Scholar]
- Djebaili, R.; Pellegrini, M.; Bernardi, M.; Smati, M.; Kitouni, M.; Del Gallo, M. Biocontrol activity of actinomycetes strains against fungal and bacterial pathogens of Solanum lycopersicum L. and Daucus carota L.: In vitro and in planta antagonistic activity. Biol. Life Sci. Forum 2021, 4, 27. [Google Scholar]
- Ordentlich, A.; Elad, Y.; Chet, I. The role of chitinase of Serratia marcescens in biocontrol Sclerotium rolfsii. Phytopathology 1988, 78, 84–87. [Google Scholar]
- Saligkarias, I.D.; Gravanis, F.T.; Harry, A.S. Biological control of Botrytis cinerea on tomato plants by the use of epiphytic yeasts Candida guilliermondii strains 101 and US 7 and Candida oleophila strain I-182: II. A study on mode of action. Biol. Control 2002, 25, 151–161. [Google Scholar] [CrossRef]
- Odumosu, B.T.; Buraimoh, O.M.; Okeke, C.J.; Ogah, J.O.; Michel, F.C., Jr. Antimicrobial activities of the Streptomyces ceolicolor strain AOBKF977550 isolated from a tropical estuary. J. Taibah Uni. Sci. 2017, 11, 836–841. [Google Scholar] [CrossRef][Green Version]
- Chaudhary, H.S.; Yadav, J.; Shrivastava, A.R.; Singh, S.; Singh, A.K.; Gopalan, N. Antibacterial activity of actinomycetes isolated from different soil samples of Sheopur (A city of central India). J Adv. Pharm. Technol. Res. 2013, 4, 118–123. [Google Scholar] [CrossRef]
- Lacret, R.; Oves-Costales, D.; Gómez, C.; Díaz, C.; de la Cruz, M.; Pérez-Victoria, I.; Vicente, F.; Genilloud, O.; Reyes, F. New ikarugamycin derivatives with antifungal and antibacterial properties from Streptomyces zhaozhouensis. Mar. Drugs 2014, 13, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Islam, M.Z.; Islam, M.A. Antibacterial activities of Actinomycete isolates collected from soils of Rajshahi, Bangladesh. Biotechnol. Res. Int. 2011, 2011, 857925. [Google Scholar] [CrossRef] [PubMed]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSIBLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Tahtamouni, M.E.W.; Hameed, K.M.; Saadoun, I.M. Biological control of Sclerotinia sclerotiorum using indigenous chitolytic actinomycetes in Jordan. J. Plant Pathol. 2006, 22, 107–114. [Google Scholar] [CrossRef]
- Teather, R.M.; Wood, P.J. Use of Congo red-polysacchatide interaction in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl. Environ. Microbiol. 1998, 43, 777–780. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Garg, N. Exploitation of Micro-Organisms for Isolation and Screening of Pectinase from Environment. In Proceedings of the 8th International Conference, Making Innovation Work for Society: Linking, Leveraging and Learning, Kuala Lumpur, Malaysia, 1–3 November 2010. [Google Scholar]
- Evmert, M.K.; Gabriele, B.G.; Piechulla, B. Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch. Microbiol. 2007, 187, 351–360. [Google Scholar]
- Lee, K.J.; Kamala-Kannan, S.; Sub, H.S.; Seong, C.K.; Lee, G.W. Biological control of Phytophthora blight in red pepper (Capsicum annuum L.) using Bacillus subtilis. World J. Microb. Biotecnol. 2008, 24, 1139–1145. [Google Scholar] [CrossRef]
- Darrasse, A.; Priou, S.; Kotoujansky, A.; Bertheau, Y. PCR and Restriction Fragment Length Polymorphism of a pel Gene as a Tool to Identify Erwinia carotovora in Relation to Potato Diseases. Appl. Environ. Microbiol. 1994, 60, 1437–1443. [Google Scholar] [CrossRef]

| Isolates | Antagonistic Activity | |
|---|---|---|
| E. coli | B. megaterium | |
| AC1 * | +++ | +++ |
| AC2 | - | - |
| AC3 | + | + |
| RS1 | + | - |
| RS2 | - | - |
| RS3 * | +++ | +++ |
| FG1 | + | ++ |
| FG2 | + | - |
| OL1 | + | - |
| OL2 * | +++ | ++ |
| Enzyme | Substrates | Staining | Diameter of Hydrolysis Area (mm) | ||
|---|---|---|---|---|---|
| AC1 Streptomyces sp. | RS3 S. atratus | OL2 A. humicola | |||
| Chitinase | Chitin azure (1%) | Congo red (0.03%) | 23.0 ± 2.3 b | 0.0 ± 0.0 c | 31.5 ± 1.7 a |
| Chitin crab shells (1%) | Congo red (0.03%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Amylase | Soluble starch (1%) | Lugol solution (a) | 37.5 ± 2.9 a | 42.0 ± 1.2 a | 28.0 ± 3.5 b |
| Glucanase | Lichenan (0.2%) | Congo red (0.03%) | 22.0 ± 2.3 b | 0.0 ± 0.0 c | 36.0 ± 1.2 a |
| Pectinase | Pectin (0.5%) | CTAB (b) (2%) | 14.0 ± 1.2 a | 10.5 ± 1.7 a | 0.0 ± 0.00 b |
| Protease | Skim milk (1%) | - | 14.5 ± 2.9 b | 12.5 ± 2.9 b | 21.5 ± 1.7 a |
| Polygalacturanase | Polygalacturonic acid (1%) | Ruthenium red (0.1%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Actinomycetes Isolates | Eco-Physiological Parameters | ||||
|---|---|---|---|---|---|
| TN (n) | SL (cm) | LN (n) | SFW (g) | SDW (g) | |
| Cont. -ve | 8 ± 1.4 a | 36.05 ± 3.2 ab | 116 ± 6.9 b | 150.02 ± 4.1 b | 15.33 ± 1.4 b |
| AC1: Streptomyces sp. | 8 ± 0.9 a | 39.01 ± 4.1 a | 195 ± 11.8 a | 204.00 ± 13.4 a | 33.02 ± 4.5 a |
| RS3: Streptomyces atratus | 6 ± 1.2 a | 38.25 ± 7.1 a | 123 ± 13.6 b | 119.33 ± 8.0 c | 15.78 ± 1.9 b |
| OL2: Arthrobacter humicola | 7 ± 1.0 a | 46.00 ± 3.2 a | 151 ± 7.2 a | 184.01 ± 7.9 a | 24.76 ± 2.7 a |
| Actinomycetes Isolates | Eco-Physiological Parameters | ||||
|---|---|---|---|---|---|
| TW (n) | SL (cm) | LN (n) | SFW (g) | SDW (g) | |
| Cont. -ve | 5 ± 0.2 b | 42.32 ± 0.3 a | 161 ± 1.0 bc | 142.33 ± 1.0 c | 24.04 ± 0.2 ab |
| AC1: Streptomyces sp. | 8 ± 0.1 a | 54.31 ± 0.4 a | 333 ± 2.3 a | 240.12 ± 2.5 a | 30.67 ± 0.3 a |
| RS3: Streptomyces atratus | 5 ± 0.1 b | 44.34 ± 0.8 a | 210 ± 1.8 b | 214.67 ± 1.9 ab | 23.00 ± 1.0 ab |
| OL2: Arthrobacter humicola | 8 ± 0.1 a | 51.76 ± 0.2 a | 477 ± 3.7 a | 304.65 ± 0.8 a | 29.67 ± 0.6 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshafie, H.S.; Camele, I. Rhizospheric Actinomycetes Revealed Antifungal and Plant-Growth-Promoting Activities under Controlled Environment. Plants 2022, 11, 1872. https://doi.org/10.3390/plants11141872
Elshafie HS, Camele I. Rhizospheric Actinomycetes Revealed Antifungal and Plant-Growth-Promoting Activities under Controlled Environment. Plants. 2022; 11(14):1872. https://doi.org/10.3390/plants11141872
Chicago/Turabian StyleElshafie, Hazem S., and Ippolito Camele. 2022. "Rhizospheric Actinomycetes Revealed Antifungal and Plant-Growth-Promoting Activities under Controlled Environment" Plants 11, no. 14: 1872. https://doi.org/10.3390/plants11141872
APA StyleElshafie, H. S., & Camele, I. (2022). Rhizospheric Actinomycetes Revealed Antifungal and Plant-Growth-Promoting Activities under Controlled Environment. Plants, 11(14), 1872. https://doi.org/10.3390/plants11141872







