4-Hydroxybenzoic Acid as an Antiviral Product from Alkaline Autoxidation of Catechinic Acid: A Fact to Be Reviewed
Abstract
:1. Introduction
2. Results and Discussion
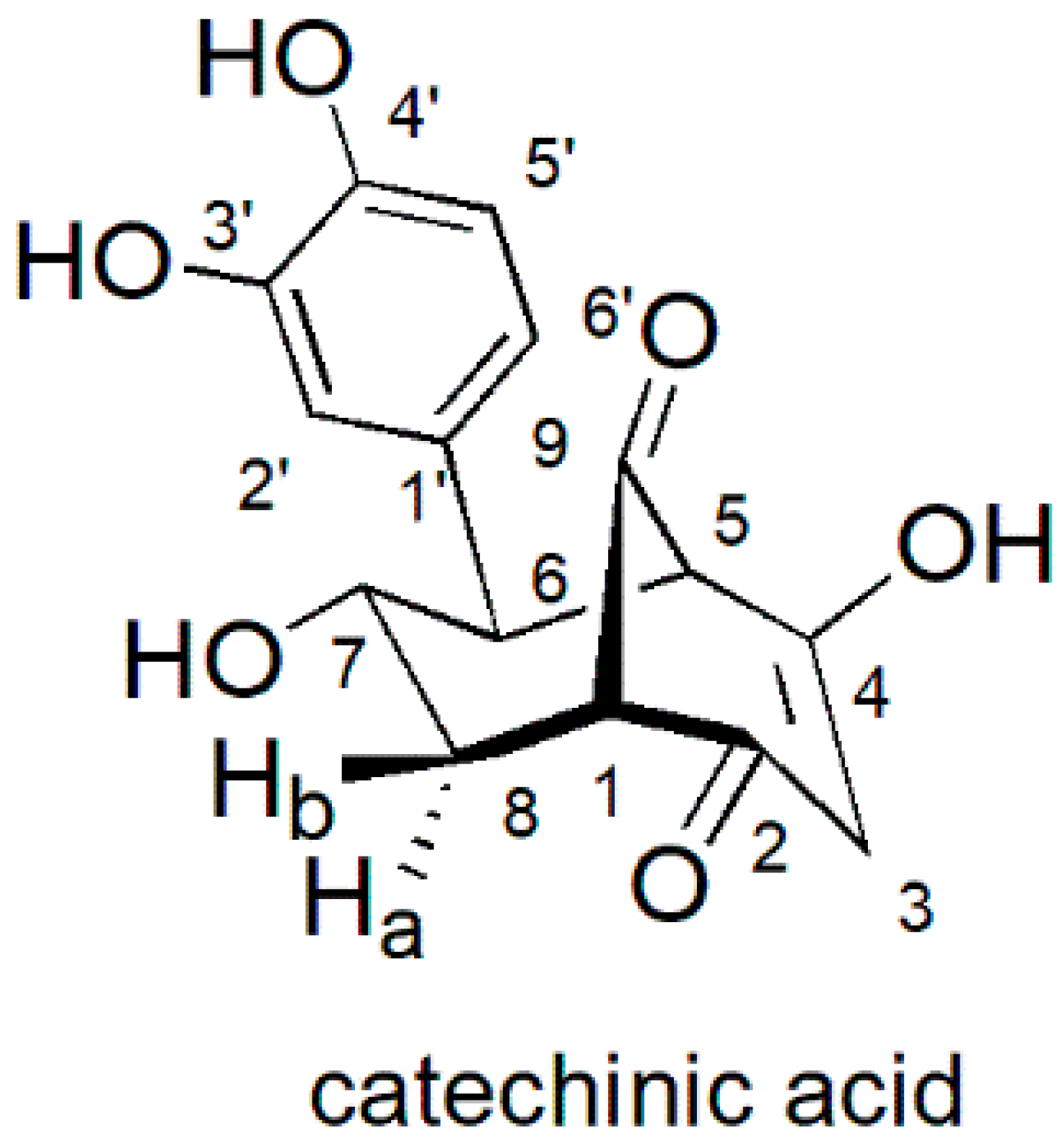
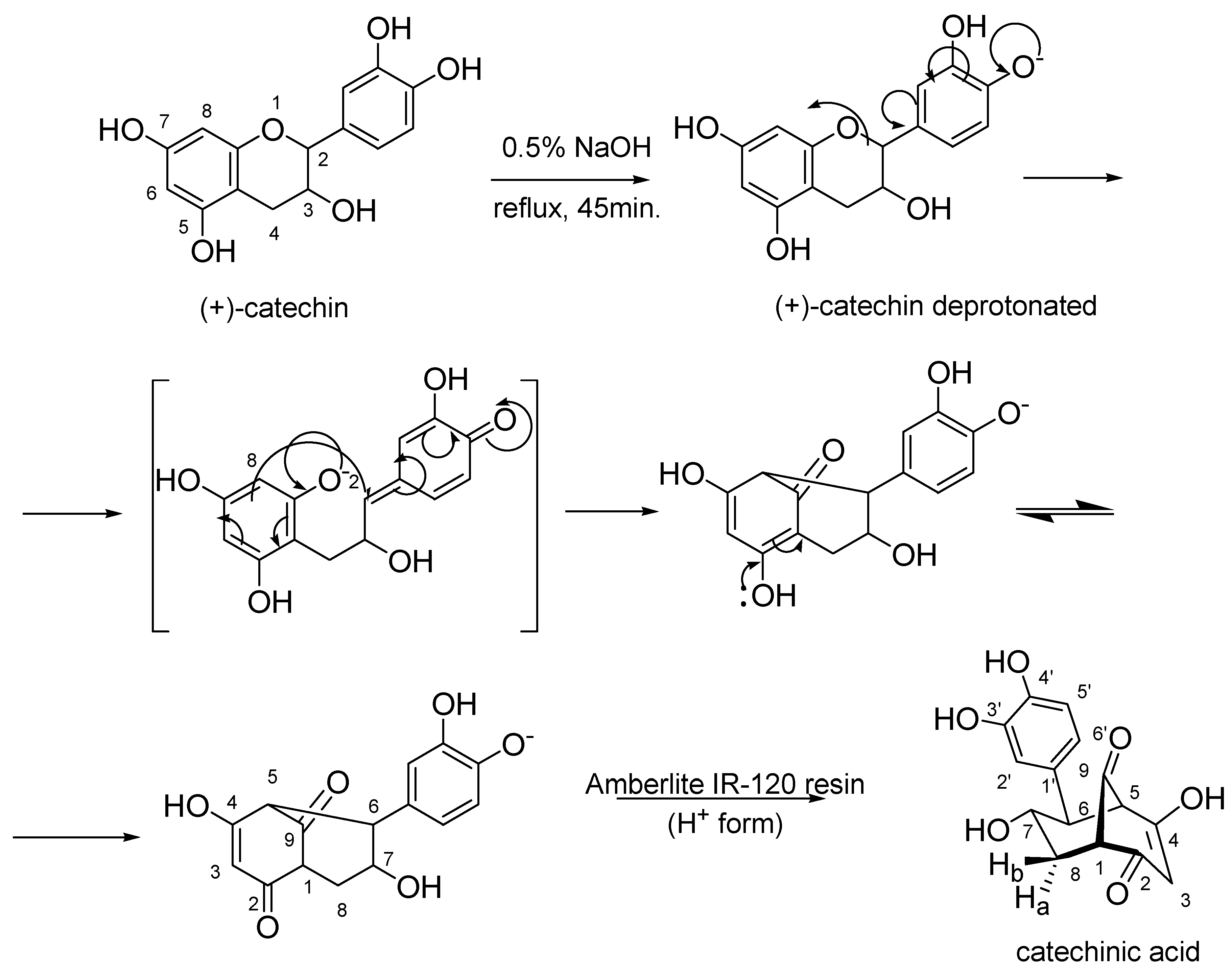
2.1. Preparation of Catechinic Acid (CA)
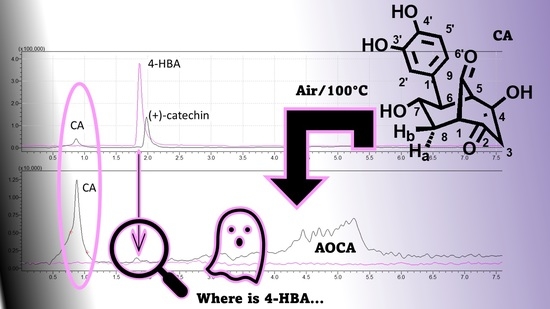
2.2. Preparation of AOCA and Search for the Presence of 4-HBA
2.2.1. TLC Analyses of AOCA and 4-HBA
2.2.2. UV–Vis Analyses of AOCA and 4-HBA
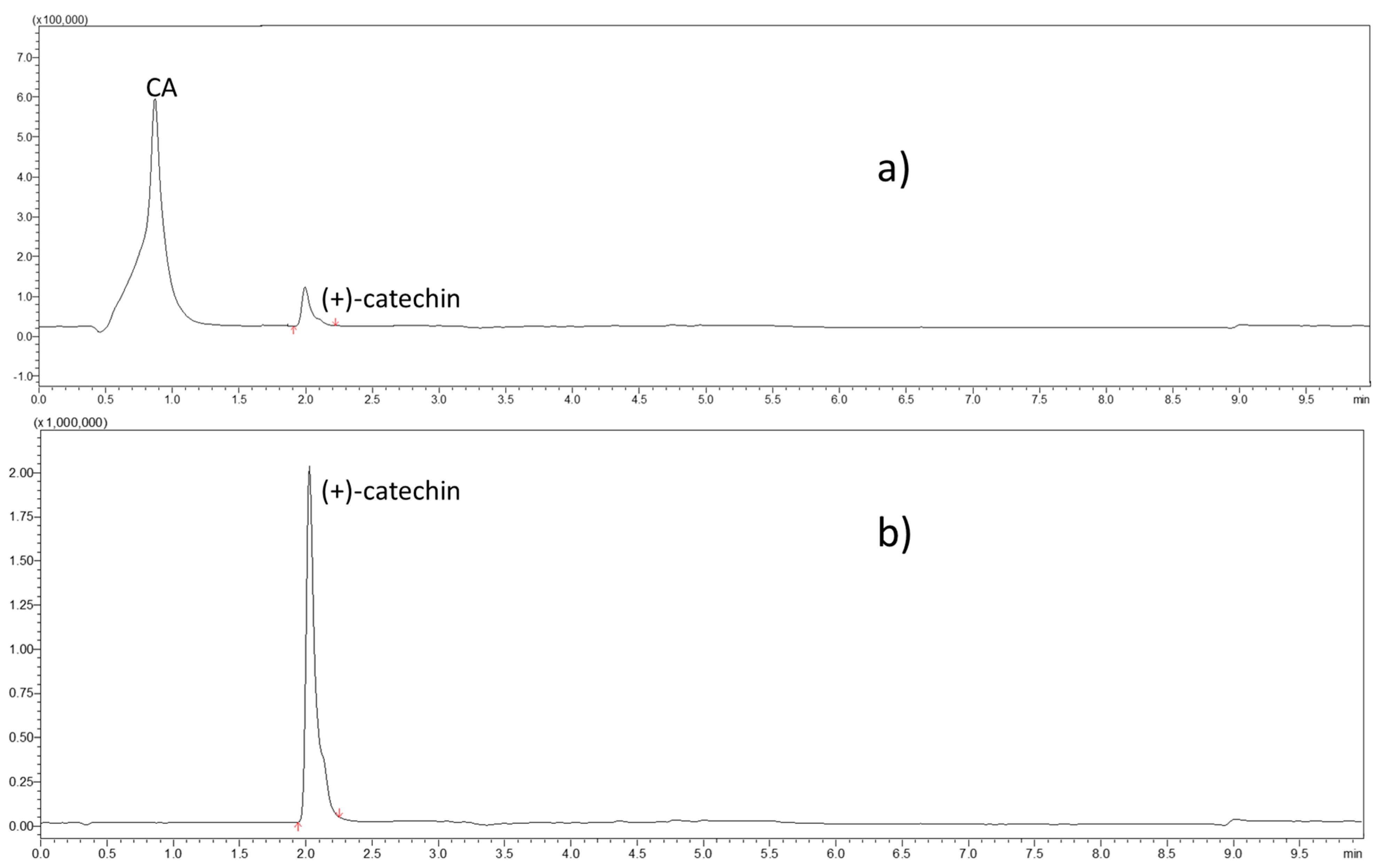
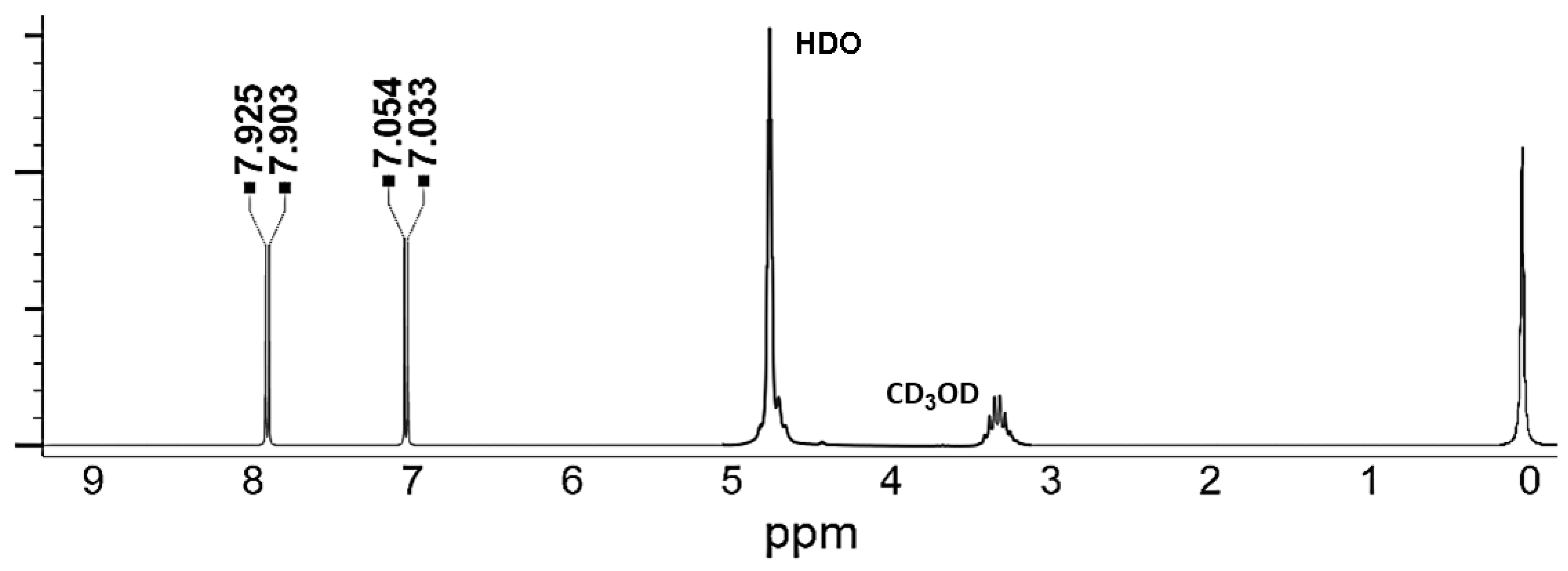
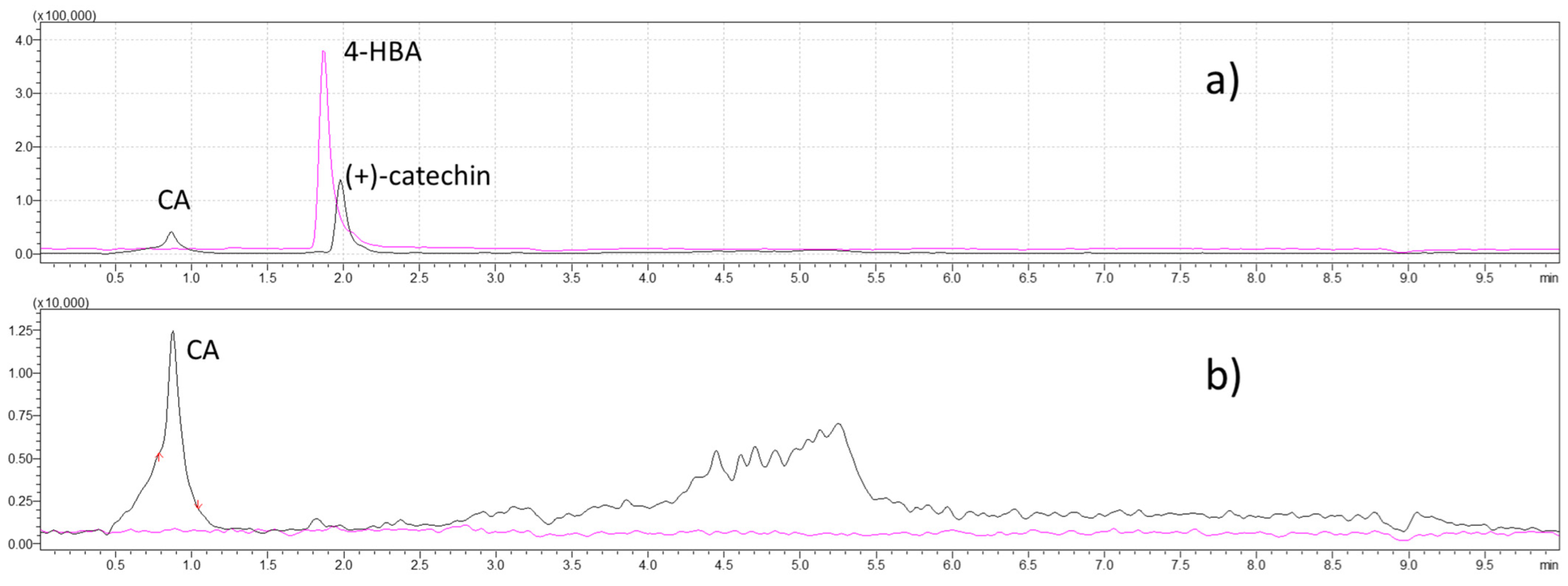
2.2.3. UHPLC–MS/MS Analyses of (+)-Catechin, CA, AOCA, and 4-HBA
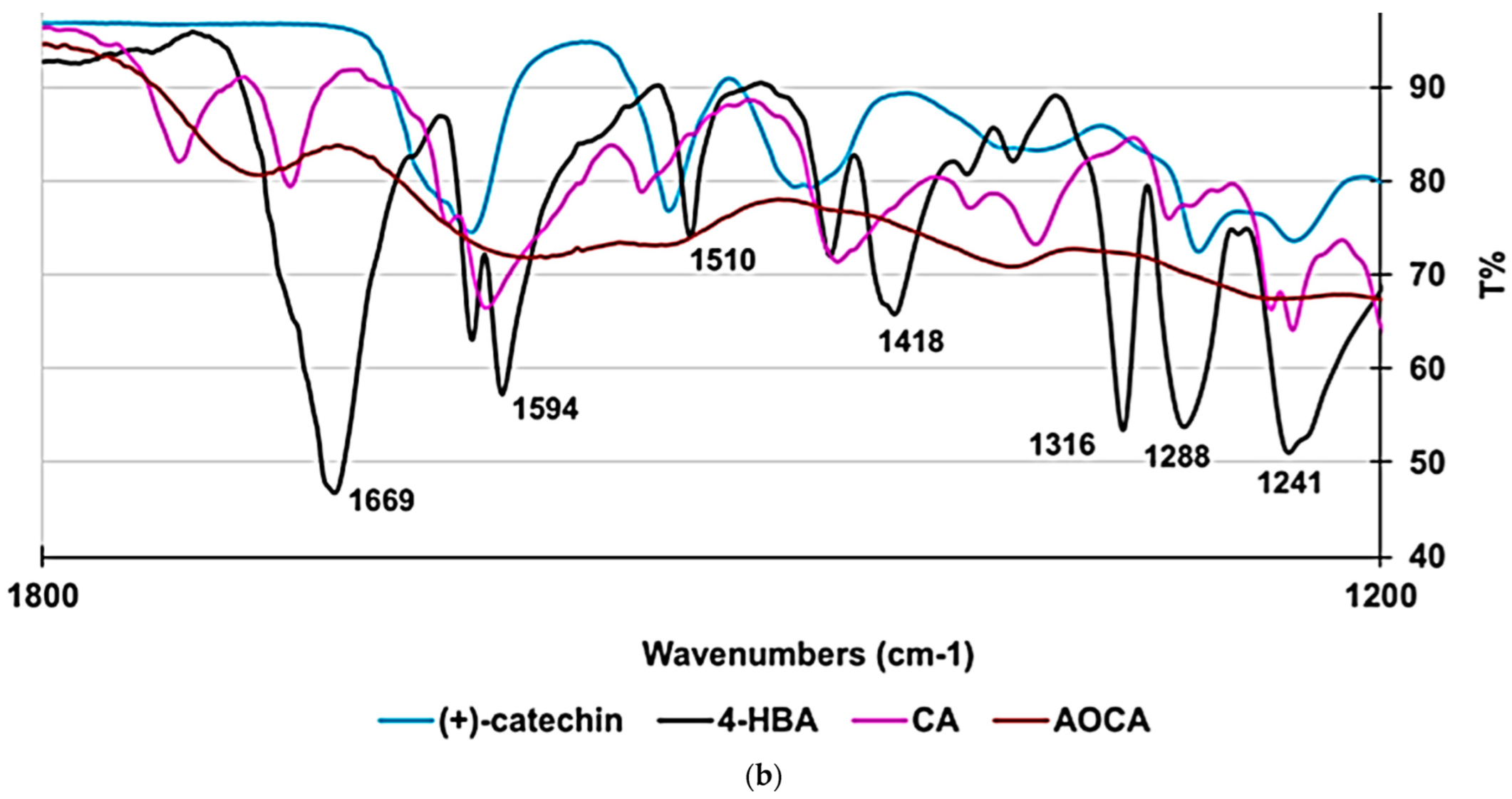
2.2.4. Chemometric-Assisted ATR–FTIR Analyses of (+)-Catechin, CA, AOCA, and 4-HBA
2.3. Evaluation of the Antiherpetic Effects of AOCA on HSV-2 In Vitro
3. Materials and Methods
3.1. Chemical Compounds and Instruments
3.2. Preparation of Catechinic Acid (CA)
3.3. Alkaline Autoxidation of CA
3.4. TLC Analyses of (+)-Catechin, CA, AOCA, and 4-HBA
3.5. UV–Vis Analyses of (+)-Catechin, CA, AOCA, and 4-HBA
3.6. UHPLC–MS/MS Analyses of (+)-Catechin, CA, AOCA, and 4-HBA Standard Solutions
3.7. Chemometric-Assisted ATR–FTIR Analyses of (+)-Catechin, CA, AOCA, and 4-HBA
3.8. Antiherpetic Effects of 4-HBA and AOCA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welch, C.; Zhen, J.; Bassène, E.; Raskin, I.; Simon, J.E.; Wu, Q. Bioactive polyphenols in kinkéliba tea (Combretum micranthum) and their glucose-lowering activities. J. Food Drug Anal. 2018, 26, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Welch, C.; Guo, Y.; Bassène, E.; Raskin, I.; Simon, J.E.; Wu, Q. Novel skeleton flavan-alkaloids from African herb tea kinkéliba: Isolation, characterization, semisynthesis, and bioactivities. In African Natural Plant Products, Volume III: Discoveries and Innovations in Chemistry, Bioactivity, and Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2020; Volume 1361, pp. 297–312. [Google Scholar]
- D’Agostino, M.; Biagi, C.; De Feo, V.; Zollo, F.; Pizza, C. Flavonoids of Combretum micranthum. Fitoterapia 1990, 61, 477. [Google Scholar]
- Tine, D.; Fall, A.D.; Dieng, S.I.; Sarr, A.; Bassene, E. Total polyphenol, tannin and flavonoid contents of Combretum micranthum leaves harvested in three regions of Senegal: Diass, Sandiara and Essyl. Int. J. Biol. Chem. Sci. 2019, 13, 1817–1820. [Google Scholar] [CrossRef]
- Ferrea, G.; Canessa, A.; Sampietro, F.; Cruciani, M.; Romussi, G.; Bassetti, D. In vitro activity of a Combretum micranthum extract against Herpes simplex virus types 1 and 2. Antivir. Res. 1993, 21, 317–325. [Google Scholar] [CrossRef]
- El Sayed, K.A. Natural products as antiviral agents. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 24, pp. 473–572. [Google Scholar]
- Sears, K.D.; Casebier, R.L.; Hergert, H.L.; Stout, G.H.; McCandlish, L.E. Structure of catechinic acid. Base rearrangement product of catechin. J. Org. Chem. 1974, 39, 3244–3247. [Google Scholar] [CrossRef]
- Iobbi, V.; Lanteri, A.P.; Minuto, A.; Santoro, V.; Ferrea, G.; Fossa, P.; Bisio, A. Autoxidation Products of the Methanolic Extract of the Leaves of Combretum micranthum Exert Antiviral Activity against Tomato Brown Rugose Fruit Virus (ToBRFV). Molecules 2022, 27, 760. [Google Scholar] [CrossRef]
- Germann, D.; Stark, T.D.; Hofmann, T. Formation and characterization of polyphenol-derived red chromophores. Enhancing the color of processed cocoa powders: Part 1. J. Agric. Food Chem. 2019, 67, 4632–4642. [Google Scholar] [CrossRef]
- Laks, P.E.; Hemingway, R.W.; Conner, A.H. Condensed tannins. Base-catalysed reactions of polymeric procyanidins with phloroglucinol: Intramolecular rearrangements. J. Chem. Soc. Perkin Trans. 1987, 1, 1875–1881. [Google Scholar] [CrossRef]
- Ohara, S.; Hemingway, R.W. Condensed tannins: The formation of a diarylpropanol-catechinic acid dimer from base-catalyzed reactions of (+)-catechin. J. Wood Chem. Technol. 1991, 11, 195–208. [Google Scholar] [CrossRef]
- Hashida, K.; Ohara, S. Formation of a novel catechinic acid stereoisomer from base-catalyzed reactions of (+)-catechin. J. Wood Chem. Technol. 2002, 22, 11–23. [Google Scholar] [CrossRef]
- Hashida, K.; Ohara, S.; Makino, R. Base-Catalyzed Reactions of (−)-Epicatechin: Formation of Enantiomers of Base-Catalyzed Reaction Products from (+)-Catechin. J. Wood Chem. Technol. 2003, 23, 227–232. [Google Scholar] [CrossRef]
- Newhall, W.F.; Ting, S.V. Degradation of hesperetin and naringenin to phloroglucinol. J. Agric. Food Chem. 1967, 15, 776–777. [Google Scholar] [CrossRef]
- Niklas, K.J.; Giannasi, D.E. Geochemistry and thermolysis of flavonoids. Science 1977, 197, 767–769. [Google Scholar] [CrossRef]
- Jensen, O.N.; Pedersen, J.A. The oxidative transformations of (+)catechin and (-)epicatechin as studied by ESR. Tetrahedron 1983, 39, 1609–1615. [Google Scholar] [CrossRef]
- Navarrete, P.; Pizzi, A.; Bertaud, F.; Rigolet, S. Condensed tannin reactivity inhibition by internal rearrangements: Detection by CP-MAS 13C NMR. Maderas. Cienc. Tecnol. 2011, 13, 59–68. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Munro, M.H.G.; Powell, H.K.J.; Poter, L.J.; Foo, L.Y. The protonation reactions of catechin, epicatechin and related compounds. Aust. J. Chem. 1984, 37, 885–892. [Google Scholar] [CrossRef]
- Kol’tsov, A.I.; Elkin, A.A.; Zheglova, D.K. 13C NMR spectra and enol—Enol tautomerism in crystalline keto—Enols. J. Mol. Struct. 1990, 221, 309–313. [Google Scholar] [CrossRef]
- Chen, W.; Wang, X.; Chen, C. Characterization of nine traditional Chinese plant extracts with specific acid dissociation constants by UV-Vis spectrophotometry. Anal. Methods 2014, 6, 581–588. [Google Scholar] [CrossRef]
- Nagarajan, S.; Nagarajan, R.; Braunhut, S.J.; Bruno, F.; McIntosh, D.; Samuelson, L.; Kumar, J. Biocatalytically Oligomerized Epicatechin with Potent and Specific Anti-proliferative Activity for Human Breast Cancer Cells. Molecules 2008, 13, 2704–2716. [Google Scholar] [CrossRef]
- Alfei, S.; Brullo, C.; Caviglia, D.; Zuccari, G. Preparation and Physicochemical Characterization of Water-Soluble Pyrazole-Based Nanoparticles by Dendrimer Encapsulation of an Insoluble Bioactive Pyrazole Derivative. Nanomaterials 2021, 11, 2662. [Google Scholar] [CrossRef]
- Alfei, S.; Zuccari, G.; Schito, A.M. Considerable Improvement of Ursolic Acid Water Solubility by its Encapsulation in Dendrimer Nanoparticles: Design, Synthesis and Physicochemical Characterization. Nanomaterials 2021, 11, 2196. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Spallarossa, A.; Lusardi, M.; Zuccari, G. Successful Dendrimer and Liposome-Based Strategies to Solubilize an Antiproliferative Pyrazole Otherwise Not Clinically Applicable. Nanomaterials 2022, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Oliveri, P.; Malegori, C. Assessment of the Efficiency of a Nanospherical Gallic Acid Dendrimer for Long-Term Preservation of Essential Oils: An Integrated Chemometric-Assisted FTIR Study. Chem. Sel. 2019, 4, 8891–8901. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Domenicotti, C. Development of a fast, low cost, conservative and eco-friendly method for quantifying gallic acid in polymeric formulations by FTIR spectroscopy in solution. Chem. Sel. 2020, 5, 4381–4388. [Google Scholar]

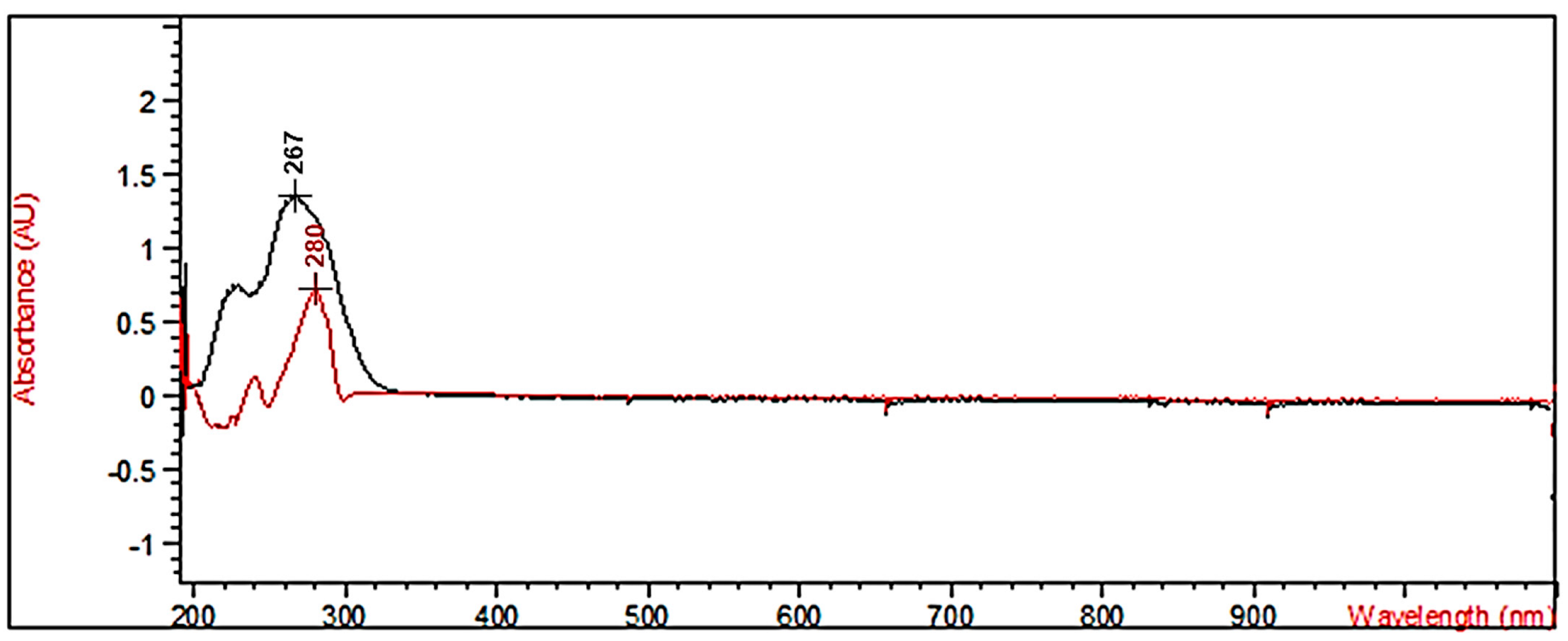




| Sample | (mg/mL) | λmax (nm) | Abs (AU) |
|---|---|---|---|
| (+)-catechin | 0.00482 | 289.0 | 0.72128 |
| CA | 0.00876 | 267.0 | 1.35380 |
| AOCA | 0.00462 | 268.0 | 0.64020 |
| 4-HBA | 0.00653 | 257.0 | 0.93378 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfei, S.; Caviglia, D.; Penco, S.; Zuccari, G.; Gosetti, F. 4-Hydroxybenzoic Acid as an Antiviral Product from Alkaline Autoxidation of Catechinic Acid: A Fact to Be Reviewed. Plants 2022, 11, 1822. https://doi.org/10.3390/plants11141822
Alfei S, Caviglia D, Penco S, Zuccari G, Gosetti F. 4-Hydroxybenzoic Acid as an Antiviral Product from Alkaline Autoxidation of Catechinic Acid: A Fact to Be Reviewed. Plants. 2022; 11(14):1822. https://doi.org/10.3390/plants11141822
Chicago/Turabian StyleAlfei, Silvana, Debora Caviglia, Susanna Penco, Guendalina Zuccari, and Fabio Gosetti. 2022. "4-Hydroxybenzoic Acid as an Antiviral Product from Alkaline Autoxidation of Catechinic Acid: A Fact to Be Reviewed" Plants 11, no. 14: 1822. https://doi.org/10.3390/plants11141822
APA StyleAlfei, S., Caviglia, D., Penco, S., Zuccari, G., & Gosetti, F. (2022). 4-Hydroxybenzoic Acid as an Antiviral Product from Alkaline Autoxidation of Catechinic Acid: A Fact to Be Reviewed. Plants, 11(14), 1822. https://doi.org/10.3390/plants11141822