Improving Yield Components and Desirable Eating Quality of Two Wheat Genotypes Using Si and NanoSi Particles under Heat Stress
Abstract
:1. Introduction
2. Results
2.1. Changes in Growth Parameters
2.2. Changes in Nutritional and Antinutritional Values of Wheat Grains
2.3. Changes in Straw Yield and Quality
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Methods
4.2.1. Stress Intensity Index (SII)
4.2.2. Harvest Index (HI)
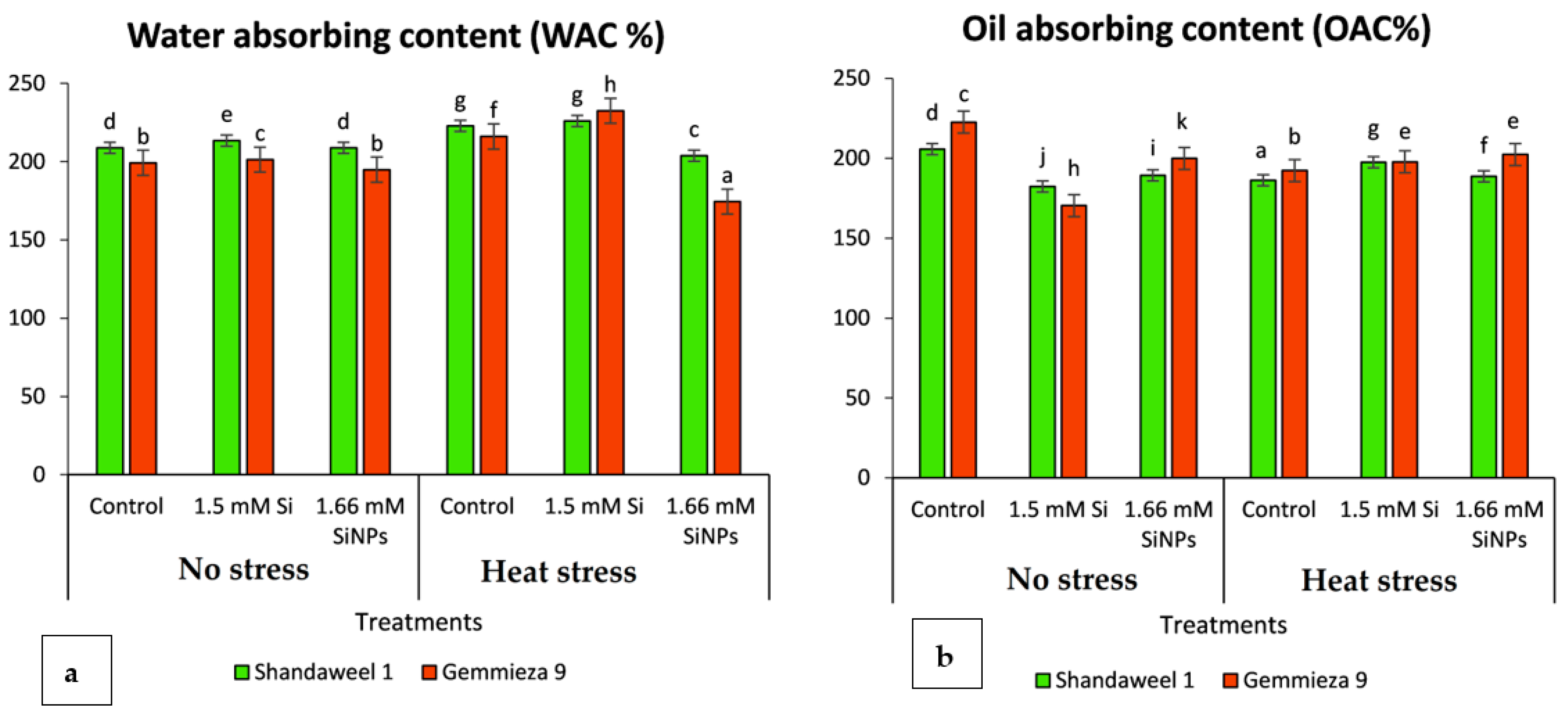
4.2.3. Water Absorption Capacity
4.2.4. Oil Absorption Capacity
4.2.5. Determination of Carbohydrates
4.2.6. Assay of α-Amylase Activity
4.2.7. Determination of Nitrogenous Constituents
4.2.8. Determination of Flavonoids
4.2.9. Determination of Total Phenols
4.2.10. Determination of Total Antioxidant Capacity (TAC)
4.2.11. Determination of Certain Minerals
4.2.12. Determination of Phytate Phosphorous and Phytic Acid Contents
4.2.13. Determination of Oxalate
4.2.14. Determination of Lignin, Cellulose and Pectin
4.2.15. Glutenin Profile
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sedri, M.H.; Roohi, E.; Niazian, M.; Niedbała, G. Interactive Effects of Nitrogen and Potassium Fertilizers on Quantitative-Qualitative Traits and Drought Tolerance Indices of Rainfed Wheat Cultivar. Agronomy 2022, 12, 30. [Google Scholar] [CrossRef]
- Elkelish, A.; El-Mogy, M.M.; Niedbała, G.; Piekutowska, M.; Atia, M.A.M.; Hamada, M.M.A.; Shahin, M.; Mukherjee, S.; El-Yazied, A.A.; Shebl, M.; et al. Roles of Exogenous α-Lipoic Acid and Cysteine in Mitigation of Drought Stress and Restoration of Grain Quality in Wheat. Plants 2021, 10, 2318. [Google Scholar] [CrossRef] [PubMed]
- Barron, C.; Holopainen-Mantila, U.; Sahlstrom, S.; Hotekjolen, K.; Lullien-Pellerin, V. Assessment of biochemical markers identified in wheat for monitoring barley grain tissue. J. Cereal. Sci. 2017, 74, 11–18. [Google Scholar] [CrossRef]
- Sramkova, Z.; Gregova, E.; Sturdik, E. Chemical composition and nutrition quality of wheat grain. Acta Chim. Slov. 2009, 2, 115–138. [Google Scholar]
- Barrera, G.N.; Tadini, C.C.; Leon, A.E.; Ribotta, P.D. Use of alpha-amylase and amyloglucosidase combinations to minimize the bread quality problems caused by high levels of damaged starch. J. Food Sci. Technol. 2016, 53, 3675–3684. [Google Scholar] [CrossRef]
- Singh, U. Functional properties of grain legume flours. J. Food Sci. Technol. 2001, 38, 191–199. [Google Scholar]
- Banigo, E.B.; Mepba, H.D. Certain functional properties of wheat-breadfruit composite flours. In Proceedings of the 29th Annual Conference of Nigerian Institute of Food Science and Tech. (NIFST), Owerri, Nigeria, 4–8 November 2005; pp. 49b–49c. [Google Scholar]
- Chaudhary, N.; Dangi, P.; Khatkar, B.S. Assessment of molecular weight distribution of wheat gluten proteins for chapatti quality. Food Chem. 2016, 199, 28–35. [Google Scholar] [CrossRef]
- Spiertz, J.H.J.; Hamer, R.J.; Xu, H.Y.; Primomartin, C.; Don, C.; Van Deputten, P.E.L. Heat stress in wheat; effects on grain weight and quality within genotypes. J. Eur. Agron. 2006, 25, 89–95. [Google Scholar] [CrossRef]
- He, H.; Hoseney, R.C. Gas retention of different cereal flours. Cereal Chem. 1991, 68, 334–336. [Google Scholar]
- Patel, M.J.; Ng, J.H.Y.; Hawkins, W.E.; Pitts, K.F.; ChakrabartiBell, S. Effects of fungal alpha-amylase on chemically leavened wheat flour doughs. J. Cereal Sci. 2012, 56, 644–651. [Google Scholar] [CrossRef]
- Hotz, C.; Gibson, R.S. Traditional food-processing and preparation practices to enhance the bioavailability of micronutrients in plant-based diets. J. Nutr. 2007, 137, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, A.K.; Kaur, N. Influence of drought and sowing time on protein composition, antinutrients, and mineral contents of wheat. Sci. World J. 2012, 9, 2344–2345. [Google Scholar] [CrossRef] [PubMed]
- Sarkiyayi, S.; Agar, T.M. Comparative analysis on the nutritional and anti-nutritional contents of the sweet and bitter cassava varieties. Adv. J. Food Sci. Technol. 2010, 2, 328–334. [Google Scholar]
- Dolan, L.C.; Matulka, R.A.; George, A.B. Naturally occurring food toxins. Toxins 2010, 2, 2289–2332. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.O.; Bracarense, A.P.F.R.L. Phytic Acid: From Antinutritional to Multiple Protection Factor of Organic Systems. J. Food Sci. 2016, 81, R1357–R1362. [Google Scholar] [CrossRef]
- Graf, E.; Eaton, J.W. Antioxidant functions of phytic acid. Free Rad. Biol. Med. 1990, 8, 61–69. [Google Scholar] [CrossRef]
- Shamsuddin, A.M.; Vucenik, I.; Cole, K.H. IP6: A novel anticancer agent. Life Sci. 1997, 61, 343–354. [Google Scholar] [CrossRef]
- Pekkarinen, S.S.; Stöckmann, H.; Schwarz, K.; Heinonen, I.M.; Hopia, A.I. Antioxidant activity and partitioning of phenolic acids in bulk and emulsified methyl linoleate. J. Agric. Food Chem. 1999, 47, 3036–3043. [Google Scholar] [CrossRef]
- Roohi, E.; Mohammadi, R.; Niane, A.A.; Niazian, M.; Niedbała, G. Agronomic Performance of Rainfed Barley Genotypes under Different Tillage Systems in Highland Areas of Dryland Conditions. Agronomy 2022, 12, 1070. [Google Scholar] [CrossRef]
- Panthapulakkal, S.; Zereshkian, A.; Sain, M. Preparation and characterization of wheat straw fibers for reinforcing application in injection molded thermoplastic composites. Bioresour. Technol. 2006, 97, 265–272. [Google Scholar] [CrossRef]
- Brown, R.H. Agronomic implications of C4 photosynthetis. In C4 Plant Biology; Sage, R.F., Monson, R.K., Eds.; Academic: San Diego, CA, USA, 1999; pp. 473–509. [Google Scholar]
- Abdelaal, K.; Attia, K.A.; Niedbała, G.; Wojciechowski, T.; Hafez, Y.; Alamery, S.; Alateeq, T.K.; Arafa, S.A. Mitigation of Drought Damages by Exogenous Chitosan and Yeast Extract with Modulating the Photosynthetic Pigments, Antioxidant Defense System and Improving the Productivity of Garlic Plants. Horticulturae 2021, 7, 510. [Google Scholar] [CrossRef]
- Panozzo, J.F.; Walker, C.K.; Partington, D.L.; Neumann, N.C.; Tausz, M.; Seneweera, S.; Fitzgerald, G.J. Elevated carbon dioxide changes grain protein concentration and composition and compromises baking quality. A FACE study. J. Cereal Sci. 2014, 60, 461–470. [Google Scholar] [CrossRef]
- Fernando, N.; Panozzo, J.; Tausz, M.; Norton, R.M.; Fitzgerald, G.J.; Myers, S.; Walker, C.; Stangoulis, J.; Seneweera, S. Wheat grain quality under increasing atmospheric CO2 concentrations in a semi-arid cropping system. J. Cereal Sci. 2012, 56, 684–690. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hossain, A.; Hakim, M.A.; Kabir, M.R.; Shah, M.M.R. Performance of wheat genotypes under optimum and late sowing condition. Int. J. Sustain. Crop Prod. 2009, 4, 34–39. [Google Scholar]
- Hossain, M.A.; Araki, H.; Takahashi, T. Poor grain filling induced by waterlogging is similar to that in abnormal early ripening in wheat in Western Japan. Field Crops Res. 2011, 123, 100–108. [Google Scholar] [CrossRef]
- Abdullah, M.; Aziz-Ur-Rehman; Ahmad, N.; Rasul, I. Planting time effect on grain and quality characteristics of wheat. Pak. J. Agri. Sci. 2007, 44, 200–202. [Google Scholar]
- Modarresi, M.; Mohammadi, V.; Zali, A.; Mardi, M. Response of wheat yield and yield related traits to high temperature. Cereal Res. Commun. 2010, 38, 23–31. [Google Scholar] [CrossRef]
- Peleg, Z.; Saranga, Y.; Suprunova, T.; Ronin, Y.; Röder, M.S.; Kilian, A.; Korol, A.B.; Fahima, T. High-density genetic map of durum wheat £ wild emmer wheat based on SSR and DArT markers. Theor. Appl. Genet. 2008, 117, 105–115. [Google Scholar] [CrossRef]
- Emam, M.M.; Khattab, H.E.; Helal, N.M.; Deraz, A. Effect of selenium and silicon on yield quality of rice plant grown under drought stress. Aust. J. Crop Sci. 2014, 8, 596–605. [Google Scholar]
- Khattab, H.E.; Emam, M.A.; Emam, M.M.; Helal, N.M.; Mohamed, M.R. Effect of selenium and silicon on transcription factors NAC5 and DREB2A involved in drought-responsive gene expression in rice. Biol. Plant 2014, 58, 265–273. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Yin, L.; Deng, X. How does silicon mediate plant water uptake and loss under water deficiency? Front. Plant Sci. 2018, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Sayed, E.G.; Mahmoud, A.W.M.; El-Mogy, M.M.; Ali, M.A.A.; Fahmy, M.A.M.; Tawfic, G.A. The Effective Role of Nano-Silicon Application in Improving the Productivity and Quality of Grafted Tomato Grown under Salinity Stress. Horticulturae 2022, 8, 293. [Google Scholar] [CrossRef]
- Saber, N.E.; Abdel-Rahman, M.M.; Mabrouk, M.E.; Eldebawy, E.M.; Ismail, G.S. Silicon Alleviates Cadmium Toxicity in Triticum aestivum L. Plants by Modulating Antioxidants, Nutrient Uptake and Gene Expression. Egypt. J. Bot. 2021, 62, 319–336. [Google Scholar]
- Fu, P.P. Introduction to the special issue: Nanomaterials—Toxicology and medical applications. J. Food Drug Anal. 2014, 22, 1–2. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Faisal, M.; Al Sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 33, 2429–2437. [Google Scholar] [CrossRef]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of nanotechnology in food Science: Perception and overview. Front. Microbiol. 2017, 8, e1501. [Google Scholar] [CrossRef]
- Baranwal, A.; Srivastava, A.; Kumar, P.; Bajpai, V.K.; Maurya, P.K.; Chandra, P. Prospects of nanostructure materials and their composites as antimicrobial agents. Front. Microbiol. 2018, 9, e422. [Google Scholar] [CrossRef]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Byrt, C.S. Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy 2019, 9, 246. [Google Scholar] [CrossRef]
- Slomberg, D.L.; Schoenfisch, M.H. Silica nanoparticle phytotoxicity to Arabidopsis thaliana. Environ. Sci. Technol. 2012, 46, 10247–10254. [Google Scholar] [CrossRef]
- Carson, G.R.; Edwards, N.M. Criteria of wheat and flour quality. In Wheat: Chemistry and Technology, 4th ed.; Khan, K.P., Shewry, R., Eds.; AACC International: St. Paul, MN, USA, 2009; pp. 97–118. [Google Scholar]
- Zhang, X.; Zhan, B.; Wu, H.; Lu, C.; Lu, G.; Liu, D.; Li, M.; Jiang, W.; Song, G.; Gao, D. Effect of high-molecular-weight glutenin subunit deletion on soft wheat quality properties and sugar-snap cookie quality estimated through near-isogenic lines. J. Integr. Agric. 2018, 17, 1066–1073. [Google Scholar] [CrossRef]
- Elsahookie, M.M.; Cheyed, S.H.; Dawood, A.A. Characteristics of Whole Wheat Grain Bread Quality. Systematic Reviews in Pharmacy 2021, 12, 593–597. [Google Scholar]
- Lambourne, J.; Tosi, P.; Marsh, J.; Bhandari, D.; Green, R.; Frazier, R.; Shewry, P.R. Characterisation of an s-type low molecular weight glutenin subunit of wheat and its proline and glutamine-rich repetitive domain. J. Cereal Sci. 2010, 51, 96–104. [Google Scholar] [CrossRef]
- Jiang, D.; Yue, H.; Wollenweber, B.; Tan, W.; Mu, H.; Bo, Y.; Dai, T.; Jing, Q.; Cao, W. Effects of post-anthesis drought and waterlogging on accumulation of high-molecular-weight glutenin subunits and glutenin macropolymers content in wheat grain. J. Agron. Crop Sci. 2009, 195, 89–97. [Google Scholar] [CrossRef]
- Khan, Z.R.; Midega, C.A.M.; Bruce, T.J.A.; Hooper, A.M.; Pickett, J.A. Exploiting phytochemicals for developing a ‘push–pull’ crop protection strategy for cereal farmers in Africa. J. Exp. Bot. 2010, 61, 4185–4196. [Google Scholar] [CrossRef] [PubMed]
- Regmi, A.P.; Ladha, J.K.; Pathak, H.; Pashuquin, H.E.; Bueno, C.; Dawe, D.; Hobbs, P.R.; Joshy, D.; Maskey, S.L.; Pandey, S.P. Yield and soil fertility trends in a 20-year rice-rice wheat experiment in Nepal. Soil Sci. Soc. Am. J. 2002, 66, 857–867. [Google Scholar] [CrossRef]
- El-Sabagh, A.; Hossain, A.; Barutcular, C.; Gormus, O.; Ahmed, Z.; Hussain, S.; Islam, M.S.; Alharby, H.; Bamagoos, A.; Kumar, N.; et al. Effects of drought stress on the quality of major oil seed crops: Implications and possible mitigation strategies—A review. Appl. Ecol. Environ. Res. 2019, 17, 4019–4043. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Dai, C.C.; Chen, L.Q. Regulation and accumulation of secondary metabolites in plant-fungus symbiotic system. Afr. J. Biotechnol. 2007, 6, 1266–1271. [Google Scholar]
- Maulana, F.; Anderson, J.D.; Butler, T.J.; Ma, X.F. Improving dual-purpose winter wheat in the southern great plains of the united states. In Recent Advances in Grain Crops Research; Intech Open: London, UK, 2019; pp. 1–16. [Google Scholar]
- Spink, J.H.; Semere, T.; Sparkes, D.L.; Whaley, J.M.; Foulkers, M.J.; Clare, R.W.; Scott, R.K. Effect of sowing date on plant density of winter wheat. Ann. Appl. Biol. 2000, 137, 179–188. [Google Scholar] [CrossRef]
- Singh, S.; Paul, M. Growth, yield and penological responses of wheat cultivars to delayed sowing. Ind. J. Plant Physiol. 2003, 8, 277–286. [Google Scholar]
- Ouda, S.A.; El-Marsafawy, S.M.; El-Kholy, M.A.; Gaballah, M.S. Simulating the effect of water stress and different sowing dates on wheat production in South Delta. J. Appl. Sci. Res. 2005, 1, 268–276. [Google Scholar]
- Barnabas, B.; Jager, K.; Feher, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Hamam, K.A.; Khaled, A.G.A. Stability of wheat genotypes under different environments and their evaluation under sowing dates and nitrogen fertilizer levels. Aust. J. Basic Appl. Sci. 2009, 3, 206–217. [Google Scholar]
- Mostafa, H.A.M.; Hassanein, R.A.; Khalil, S.I.; El-Khawas, S.A.; El-Bassiouny, H.M.S.; Abd El-Monem, A.A. Effect of arginine or putrescine on growth, yield and yield components of late sowing wheat. J. Appl. Sci. Res. 2009, 54, 177–183. [Google Scholar]
- Saini, H.S.; Aspinal, D. Sterility in wheat (Triticum aestivum L.) induced by water deficit or high temperature: Possible mediation by abscisic acid. Aust. J. Plant Physiol. 1982, 9, 529–537. [Google Scholar] [CrossRef]
- Arduini, I.; Pellegrino, E.; Ercoli, L. Contribution of main culm and tillers to grain yield of durum wheat: Influence of sowing date and plant traits. Ital. J. Agron. 2018, 13, 235–247. [Google Scholar] [CrossRef]
- Sanghera, A.K.; Thind, K.S. Screening of wheat genotypes at seedling stage for their potential to withstand heat stress on the basis of membrane thermosensitivity stress. Int. J. Agric. Crop Sci. 2014, 7, 640–645. [Google Scholar]
- Peng, J.H.; Zadeh, H.; Lazo, G.R.; Gustafson, J.P.; Chao, S. Chromosome bin map of expressed sequence tags in homoeologous group 1 of hexaploid wheat and homoeology with rice and Arabidopsis. Genetics 2004, 168, 609–623. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.M. Physiological and Biochemical Responses to Heat Stress on Barley Seedlings and Their Impact on Growth and Yield. Egypt. J. Bot. 2016, 56, 319–334. [Google Scholar]
- Monjardino, P.; Smith, A.G.; Jones, R.J. Heat stress effects on protein accumulation of maize endosperm. Crop Sci. 2005, 45, 1203–1210. [Google Scholar] [CrossRef]
- Boehlein, S.K.; Liu, P.; Webster, A.; Ribeiro, C.; Suzuki, M.; Wu, S.; Guan, J.C.; Stewart, J.D.; Tracy, W.F.; Settles, A.M.; et al. Effects of long-term exposure to elevated temperature on Zea mays endosperm development during grain fill. Plant J. 2019, 99, 23–40. [Google Scholar] [CrossRef]
- Johkan, M.; Oda, M.; Maruo, T.; Shinohara, Y. Crop Production and Global Warming. In Global Warming Impacts-Case Studies on the Economy, Human Health, and on Urban and Natural Environments; InTech: London, UK, 2011; pp. 139–152. [Google Scholar]
- Blum, A.; Sinmena, B.; Mayer, J.; Golan, G.; Shpiler, L. Stem reserve mobilisation supports wheat-grain filling under heat stress. Aust. J. Plant Physiol. 1994, 21, 771–781. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Halim, A.; Aziz, T. Alleviation of temperature stress by nutrient management in crop plants: A review. J. Soil Sci. Plant Nutr. 2012, 12, 221–244. [Google Scholar] [CrossRef]
- Thiry, D.E.; Sears, R.G.; Shroyer, J.P.; Paulsen, G.M. Planting Date Effect on Tiller Development and Productivity of Wheat. Kansas State University Extension. 2002. Available online: http://www.k-state.edu/historicpublications/Pubs/SRL133.pdf (accessed on 6 June 2022).
- Naeemi, M.; Akbari, G.; Shirani Rad, A.H.; Modares Sanavi, S.A.M.; Sadat-Nuri, S.A.; Jabari, H. Evaluation of drought tolerance in different Canola cultivars based on stress evaluation indices in terminal growth duration. Electron. J. Crop Prod. 2008, 1, 83–98. [Google Scholar]
- Shirani Rad, A.H.S.; Zandi, P. Comparison of winter and spring rapeseed cultivars considering their oil content and fatty acid composition. Am.-Eurasian J. Agric. Environ. Sci. 2012, 12, 775–780. [Google Scholar]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Agarie, S.; Uchida, H.; Agata, W.; Kubota, F.; Kaufamn, B. Effect of silicon on growth, dry matter production and photosynthesis in rice (Oryza sativa L.). Crop Prod. Improve. Technol. 1993, 34, 225–234. [Google Scholar]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A.; et al. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies a review. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2021, 155, 211–234. [Google Scholar] [CrossRef]
- Cuong, T.X.; Ullal, H.; Datta, A.; Hanh, T.C. Effects of silicon-based fertilizer on growth, yield and nutrient uptake of rice in tropical zone of Vietnam. Rice Sci. 2017, 24, 283–290. [Google Scholar] [CrossRef]
- Motzo, R.; Fois, S.; Glunta, F. Protein content and gluten quality of durum wheat (Triticum turgidum subsp. Durum) as affected by sowing date. J. Sci. Food Agric. 2007, 87, 1480–1488. [Google Scholar] [CrossRef]
- Triboï, E.; Marte, P.; Triboï-Blondel, A.M. Environmentally-induced changes in protein composition in developing grains of wheat are related to changes in total protein content. J. Exp. Bot. 2003, 54, 1731–1742. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Protein alterations in tall fescue in response to drought stress and abscisic acid. Crop Sci. 2002, 42, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.A.; Hossain, A.; Teixeira da Silva, J.A.; Zvolinsky, V.P.; Khan, M.M. Yield, protein and starch content of twenty wheat (Triticum aestivum L.) genotypes exposed to high temperature under late sowing conditions. J. Sci. Res. 2012, 4, 477–489. [Google Scholar] [CrossRef]
- Qi, J.C.; Chen, J.X.; Wang, J.M.; Wu, F.B.; Cao, L.P.; Zhang, G.P. Protein and hordein fraction content in barley seeds as affected by sowing date and their relations to malting quality. J. Zhejiang Univ. Sci. 2005, 6B, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Khan, K. Effects of genotype and environment on glutenin polymers and bread making quality. Cereal Chem. 2001, 78, 125–130. [Google Scholar] [CrossRef]
- Futakuchi, K.; Watanabe, H.; Jones, M.P. Relationship of grain protein content to other grain quality traits in interspecific Oryza sativa L. x Oryza glaberrima Steud. Progenies Agric. J. 2008, 3, 50–57. [Google Scholar]
- Distelfeld, A.; Cakmak, I.; Peleg, Z.; Ozturk, L.; Yazici, A.M.; Budak, H.; Saranga, Y.; Fahima, T. Multiple QTL-effects of wheat Gpc-B1 locus on grain protein and micronutrient concentrations. Physiol. Plant 2007, 129, 635–643. [Google Scholar] [CrossRef]
- Soundararajan, P.; Sivanesan, I.; Jana, S.; Jeong, B.R. Influence of silicon supplementa-tion on the growth and tolerance to high temperature in Salvia splendens. Horticult. Environ. Biotechol. 2014, 55, 271–279. [Google Scholar] [CrossRef]
- Randall, P.J.; Moss, H.J. Some effects of temperature regime during grain filling on wheat quality. Aust. J. Agric. Res. 1990, 41, 603–617. [Google Scholar] [CrossRef]
- Panozzo, J.F.; Eagles, H.A. Cultivar and environmental effects on quality characters in wheat. II. Protein. Aust. J. Agric. Res. 2000, 51, 629–636. [Google Scholar] [CrossRef]
- Dupont, F.M.; Hurkman, W.J.; Vensel, W.H.; Tanaka, C.; Kothari, K.M.; Chung, O.K.; Altenbach, S.B. Protein accumulation and composition in wheat grains: Effects of mineral nutrients and high temperature. Eur. J. Agron. 2006, 25, 96–107. [Google Scholar] [CrossRef]
- Hurkman, W.J.; McCue, K.F.; Altenbach, S.B.; Korn, A.; Tanaka, C.K.; Kothari, K.M.; Johnson, E.L.; Bechtel, D.B.; Wilson, J.D.; Anderson, O.D.; et al. Effect of temperature on expression of genes encoding enzymes for starch biosynthesis in developing wheat endosperm. Plant Sci. 2003, 164, 873–881. [Google Scholar] [CrossRef]
- Payne, P.I.; Law, C.N.; Mudd, E.E. Control by homoeologous group 1 chromosomes of the high-molecular-weight subunits of glutenin, a major protein of wheat endosperm. Theor. Appl. Genet. 1980, 58, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Masci, S.; Laino, P.; Janni, M.; Botticella, E.; Carli, M.D.; Benvenuto, E.; Danieli, P.P.; Lilley, K.S.; Lafiandra, D.; D’Ovidio, R. Analysis of Quality-Related Parameters in Mature Kernels of Polygalacturonase Inhibiting Protein (PGIP) Transgenic Bread Wheat Infected with Fusarium graminearum. J. Agric. Food Chem. 2015, 63, 3962–3969. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Anjum, F.M.; Khan, M.R.; Sajjad, M.; Hussain, S.; Muhammad Sajid Arshad, M.S. Electrophoretic Characteristics of Gluten Proteins as Influenced by Crop Year and Variety. Int. J. Food Prop. 2016, 19, 897–910. [Google Scholar] [CrossRef]
- Gianibelli, M.C.; Larroque, O.R.; MacRitchie, F.; Wrigley, C.W. Biochemical, Genetic, and Molecular Characterization of Wheat Glutenin and Its Component Subunits. Cereal Chem. 2001, 78, 635–646. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Fan, R.; Wei, B.; Zhang, X. Structural characterization and evolutionary relationship of high-molecular-weight glutenin subunit genes in Roegneria nakaii and Roegneria alashanica. Int. J. Mol. Sci. 2016, 17, 1115. [Google Scholar] [CrossRef]
- Johansson, E.; Nilsson, H.; Mazhar, H.; Skerritt, J.; MacRitchie, F.; Svensson, G. Seasonal effects on storage proteins and gluten strength in four Swedish wheat cultivars. J. Sci. Food Agric. 2002, 82, 1305–1311. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.K.; Gupta, S.K.; Kaur, N. Effect of sowing time on protein quality and starch pasting characteristics in wheat (Triticum aestivum L.) genotypes grown under irrigated and rain-fed conditions. Food Chem. 2010, 122, 559–565. [Google Scholar] [CrossRef]
- Singh, N.; Virdi, A.S.; Katyal, M.; Kaur, A.; Kaur, D.; Ahlawat, A.K.; Singh, A.M.; Sharma, R.K. Evaluation of heat stress through delayed sowing on physicochemical and functional characteristics of grains, whole meals and flours of India wheat. Food Chem. 2021, 344, 128725. [Google Scholar] [CrossRef]
- Koga, S.H.U.; Aamot, A.K.; Uhlen, T.; Seehusen, E.; Veiseth-Kent, I.S.; Hofgaard, A.; Moldestad, U. Bocker Environmental factors associated with glutenin polymer assembly during grain maturation. J. Cereal Sci. 2020, 91, 102865. [Google Scholar] [CrossRef]
- Siddiqi, R.A.; Singh, T.P.; Rani, M.; Sogi, D.S.; Bhat, M.A. Diversity in grain, flour, amino acid composition, protein profiling, and proportion of total flour proteins of different wheat cultivars of North India. Front. Nutr. 2020, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Anjum, F.M.; Zahoor, T.; Nawaz, H. Biochemical and Technological Characterization of Paksitani Spring Wheats. Pak. J. Agric. Sci. 2009, 46, 271–279. [Google Scholar]
- Plaut, Z.; Butow, B.J.; Blumenthal, A.; Wrigley, C.W. Transport of dry matter into developing wheat kernels and its contribution to grain yield under post-anthesis water deficit and elevated temperature. Field Crops Res. 2004, 86, 185–198. [Google Scholar] [CrossRef]
- Tahir, I.S.A.; Nakata, N.; Yamaguchi, T. Responses of three wheat genotypes to high soil temperature during grain filling. Plant Prod. Sci. 2005, 8, 192–198. [Google Scholar] [CrossRef]
- Yamakawa, H.; Hirose, T.; Kuroda, M.; Yamaguchi, T. Comprehensive expression profiling of rice grain filling-related genes under high temperature using DNA microarray. Plant Physiol. 2007, 144, 258–277. [Google Scholar] [CrossRef]
- Mohapatra, P.; Sarkar, R.; Kuanar, S. Starch synthesizing enzymes and sink strength of grains of contrasting rice cultivars. Plant Sci. 2009, 176, 256–263. [Google Scholar] [CrossRef]
- Tang, T.; Xie, H.; Wang, Y.X.; Lu, B.; Liang, J.S. The effect of sucrose and abscisic acid interaction on sucrose synthase and its relationship to grain filling of rice (Oryza sativa L.). J. Exp. Bot. 2009, 60, 2641–2652. [Google Scholar] [CrossRef]
- Shalaby, T.A.; Abd-Alkarim, E.; El-Aidy, F.; Hamed, E.S.; Sharaf-Eldin, M.; Taha, N.; El-Ramady, H.; Bayoumi, Y.; Dos Reis, A.R. Nano-selenium, silicon and H2O2 boost growth and productivity of cucumber under combined salinity and heat stress. Ecotoxicol. Environ. Saf. 2021, 212, 111962. [Google Scholar] [CrossRef]
- Avila, F.W.; Baliza, D.P.; Eaquin, V.; Araújo, J.L.; Ramos, S.J. Silicon-nitrogen interaction in rice cultivated under nutrient solution. Rev. Ciência Agron. 2010, 41, 184–190. [Google Scholar]
- Desoky, E.S.M.; Mansour, E.; Yasin, M.A.; El Sobky, E.-S.E.; Rady, M.M. Improvement of drought tolerance in five different cultivars of Vicia faba with foliar application of ascorbic acid or silicon. Span. J. Agric. Res. 2020, 18, 16. [Google Scholar] [CrossRef]
- Newberry, M.; Zwart, A.B.; Whan, A.; Mieog, J.C.; Sun, M.; Leyne, E.; Pritchard, J.; Daneri-Castro, S.N.; Ibrahim, K.; Diepeveen, D.; et al. Does late maturity alpha-amylase impact wheat baking quality? Front. Plant Sci. 2018, 9, 1356. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.; Cardone, G.; Nicolodi, A.; Quaglia, L.; Pagani, M.A. Sprouted wheat as an alternative to conventional flour improvers in breadmaking. LWT Food Sci. Technol. 2017, 80, 230–236. [Google Scholar] [CrossRef]
- Nanj, D.R.; Norman, A.G. Pectin estimation of the individual pectic substances in nature. Biochem. J. 1928, 22, 596–604. [Google Scholar]
- Ubbor, S.C.; Akobundu, E.N.T. Quality Characteristics of Cookies from Composite Flours of Watermelon Seed, Cassava and Wheat. Pak. J. Nutr. 2009, 8, 1097–1102. [Google Scholar] [CrossRef]
- Jitngarmkusol, S.; Hongsuwankul, J.; Tananuwong, K. Chemical composition, functional properties and microstructure of defatted macademice flours. Food Chem. 2008, 110, 23–30. [Google Scholar] [CrossRef]
- Garnett, T.P.; Graham, R.D. Distribution and remobilization of iron and copper in wheat. Ann. Bot. 2005, 95, 817–826. [Google Scholar] [CrossRef]
- Dias, A.S.; Lidon, F.C.; Ramalho, J.C. Heat stress in triticum: Kinetics of Ca and Mg accumulation. Braz. J. Plant Physiol. 2009, 21, 123–134. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Drought and heat stress injury to two cool-season turf grasses in relation to antioxidant metabolism and lipid peroxidation. Crop Sci. 2001, 1, 436–442. [Google Scholar] [CrossRef]
- Larkindale, J.; Knight, M.R. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002, 128, 682–693. [Google Scholar] [CrossRef]
- Yasmin, A.; Zeb, A.; Khalil, A.W.; Paracha, G.M.; Khattak, A.B. Effect of processing on anti-nutritional factors of red kidney bean (Phaseolus vulgaris) grains. Food Bioprocess Technol. 2008, 1, 415–419. [Google Scholar] [CrossRef]
- Raboy, V.; Gerbasi, P.F.; Young, K.A.; Stoneberg, S.D.; Pickett, S.G.; Bauman, A.T.; Murthy, P.P.N.; Sheridan, W.F.; Ertl, D.S. Origin and seed phenotype of maize low phytic acid 1-1 and low phytic acid 2-1. Plant Physiol. 2000, 124, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Hannah, L.C. Starch synthesis in the maize seed. In Cellular and Molecular Biology of Plant Seed Development; Larkins, B.A., Vasil, I.K., Eds.; Kluwer: Dordrecht, The Netherlands, 1997; pp. 375–405. [Google Scholar]
- Takahashi, S.; Katagiri, T.; Hirayama, T.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Hyperosmotic stress induces a rapid and transient increase in inositol 1,4,5-trisphosphate independent of abscisic acid in Arabidopsis cell culture. Plant Cell Physiol. 2001, 42, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, S.; Read, N.D.; Trewavas, A.J. Elevation of cytoplasmic calcium by caged calcium or caged inositol triphosphate initiates stomatal closure. Nature 1990, 346, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.J.; Brearley, C.; Elge, S.; Kaplan, B.; Fromm, H.; Mueller-Roeber, B. Arabidopsis inositol polyphosphate 6-/3-kinase is a nuclear protein that complements a yeast mutant lacking a functional ArgR-Mcm1 transcription complex. Plant Cell 2003, 15, 449–463. [Google Scholar] [CrossRef]
- Tuna, A.L.; Kaya, C.; Higgs, D.; Murillo-Amador, B.; Aydemir, S.; Girgin, A.R. Silicon improves salinity tolerance in wheat plants. Environ. Exp. Bot. 2008, 62, 10–16. [Google Scholar] [CrossRef]
- Cabral, C.; Ravnskov, S.; Tringovska, I.; Wollenweber, B. Arbuscular mycorrhizal fungi modify nutrient allocation and composition in wheat (Triticum aestivum L.) subjected to heat-stress. Plant Soil 2016, 408, 385–399. [Google Scholar] [CrossRef]
- Akande, K.E.; Fabiyi, E.F. Effect of processing methods on some antinutritional factors in legume seeds for poultry feeding. Int. J. Poult. Sci. 2010, 9, 996–1001. [Google Scholar] [CrossRef]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Karaman, S.; Tütem, E.; Başkan, K.S.; Apak, R. Comparison of total antioxidant capacity and phenolic composition of some apple juices with combined HPLC–CUPRAC assay. Food Chem. 2010, 120, 1201–1209. [Google Scholar] [CrossRef]
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. J. Agric. Food Chem. 2005, 53, 2297–2306. [Google Scholar] [CrossRef]
- Guo, J.; Xue, C.; Duan, J.A.; Qian, D.; Tang, Y.; You, Y. Anticonvulsant, antidepressant-like activity of Abelmoschus manihot ethanol extract and its potential active components in vivo. Phytomedicine 2011, 18, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Gou, T.; Yang, L.; Hu, W.; Chen, X.; Zhu, Y.; Guo, J.; Gong, H. Silicon improves the growth of cucumber under excess nitrate stress by enhancing nitrogen assimilation and chlorophyll synthesis. Plant Physiol. Biochem. 2020, 152, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Vadiveloo, J.; Phang, O.C. Differences in the nutritive value of two rice straw varieties as influenced by season and location. Anim. Feed Sci. Technol. 1996, 61, 247–258. [Google Scholar] [CrossRef]
- Porteaus, F.; Hill, J.; Ball, A.S.; Pinter, P.J.; Kimball, B.A.; Wall, G.W.; Adamsend, F.J.; Hunsaker, D.J.; LaMorte, R.L.; Leavitt, S.W.; et al. Effect of free air carbon dioxide enrichment (FACE) on the chemical composition and nutritive value of wheat grain and straw. Anim. Feed Sci. Technol. 2009, 149, 322–332. [Google Scholar] [CrossRef]
- Arafa, S.A.; Attia, K.A.; Niedbała, G.; Piekutowska, M.; Alamery, S.; Abdelaal, K.; Alateeq, T.K.; Ali, M.A.M.; Elkelish, A.; Attallah, S.Y. Seed Priming Boost Adaptation in Pea Plants under Drought Stress. Plants 2021, 10, 2201. [Google Scholar] [CrossRef]
- Verma, B.; Hucl, P.; Chibbar, R.N. Phenolic content and anti-oxidant properties of bran in 51 wheat cultivars. Cereal Chem. 2008, 85, 544–549. [Google Scholar] [CrossRef]
- Fischer, R.A.; Murrer, R. Drought resistance in spring wheat cultivars. I. Grain yield response. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Beadle, C.L. Growth analysis. In Photosynthesis and Production in A Changing Environment: A Field and Laboratory Manual; Hall, D.O., Scurlock, J.M.O., Bolhar-Nordenkampf, H.R., Leegood, R.C., Long, S.P., Eds.; Chapman and Hall: London, UK, 1993; pp. 36–46. [Google Scholar]
- AACC. Approved Methods of the American Association of Cereal Chemists, 9th ed.; AACC: St. Paul, MN, USA, 1995; p. 1200. [Google Scholar]
- Sosulski, F.W. The centrifuge method for determining flour absorption in hard red spring wheats. Cereal Chem. 1962, 39, 344–350. [Google Scholar]
- Rutkowski, A.; Kozlowska, H. Preparaty Zywnosciowe Bialka Roslinnego. In Food Preparations from Plant Proteins; Wydamnictwo Naukowo-Techniczne (WNT): Warszawa, Poland, 1981; pp. 318–334. [Google Scholar]
- Beuchat, L.R. Functional and electrophoretic characteristics of succinylated peanut flour protein. J. Agric. Food Chem. 1977, 25, 258–261. [Google Scholar] [CrossRef]
- Blakeney, A.B.; Mutton, L.L. A simple calorimetric method for the determination of sugars in fruits and vegetables. J. Sci. Food Agric. 1980, 31, 889–897. [Google Scholar] [CrossRef]
- Naguib, M.I. Colourimetric estimation of plant polysaccharides. Zucker 1963, 16, 15–18. [Google Scholar]
- Davis, B.D. Occurrence of ct-amylase in the axis of Germinating peas. Plant Physiol. 1977, 60, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Daughaday, W.H.; Lowry, O.H.; Rosebrough, N.J.; Fields, W.S. Determination of cerebrospinal fluid protein with the folin phenol reagent. J. Lab. Clin. Med. 1952, 39, 663–665. [Google Scholar] [PubMed]
- Harborne, J.B. Photochemical Methods: A Guide to Modern Technique of Plant Analysis, 3rd ed.; Champman and Hall: London, UK, 1998; p. 302. [Google Scholar]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histoenzymology; Kalyani Publishers: New Delhi, India, 1980; p. 286. [Google Scholar]
- Oyaizu, M. Studies on products of browning reaction prepared from glucoseamine. Jpn. J. Nutr. 1986, 44, 307–314. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Method of Analysis for Soil, Plants and Waters; Agricultural Science, University of California: Berkeley, CA, USA, 1961; p. 309. [Google Scholar]
- AOAC. Official methods of analysis. In Association of Official Analytical Chemist International, 17th ed.; Pyle, R.A., Ed.; AOAC: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Wheeler, F. A method for phytic acid determination in wheat and wheat fraction. Cereal Chem. 1971, 48, 312–320. [Google Scholar]
- Haug, W.; Lanhzch, H.J. Sensitive method for the rapid determination of phytate in cereals and cereal products. J. Sci. Food Agric. 1983, 34, 1423–1426. [Google Scholar] [CrossRef]
- Day, R.A.; Underwood, A.L. Quantitative Analysis, 5th ed.; Prentice Hall Publication: Hoboken, NJ, USA, 1992; p. 701. [Google Scholar]
- Chinma, C.E.; Igyor, M.A. Micronutrients and antinutritional contents of selected tropical vegetables grown in Southeast, Nigeria. Niger. Food J. 2007, 25, 111–116. [Google Scholar]
- Rittler, G.J.; Seborg, R.; Mitchell, R.I. Factors affecting determination of lignin by 72 percent sulphuric acid method. Ind. Eng. Chem. Ann. 1932, 4, 202. [Google Scholar] [CrossRef]
- Jenkins, S.H. Determination of cellulose in straw. Biochem. J. 1930, 24, 1428. [Google Scholar] [CrossRef]
- Hurkman, W.J.; Tanaka, C.K. Improved methods for separation of wheat endosperm proteins and analysis by two-dimensional gel electrophoresis. J. Cereal Sci. 2004, 40, 295–299. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]



| Treatments | Spike Length (cm) | Spike Weight (g) | Number of Spikelets/Spike | ||
|---|---|---|---|---|---|
| Normal season | Shandaweel 1 | Control | 8.8 ± 0.12 g | 0.66 ± 0.005 i | 28.0 ± 0.33 d |
| 1.5 mM silicon | 10.8 ± 0.14 j | 1.76 ± 0.006 f | 36.0 ± 0.33 g | ||
| 1.66 mM nanosilicon | 10.0 ± 0.03 i | 1.57 ± 0.01 e | 35.0 ± 0.33 g | ||
| Gemmeiza 9 | Control | 7.5 ± 0.05 e | 0.54 ± 0.005 c | 28.0 ± 0.001 d | |
| 1.5 mM silicon | 9.2 ± 0.06 h | 2.07 ± 0.02 h | 38.0 ± 0.33 h | ||
| 1.66 mM nanosilicon | 8.8 ± 0.08 g | 1.82 ± 0.03 g | 30.0 ± 0.33 e | ||
| Late season | Shandaweel 1 | Control | 6.5 ± 0.05 d | 0.43 ± 0.02 b | 26.0 ± 0.33 c |
| 1.5 mM silicon | 7.8 ± 0.03 f | 0.71 ± 0.018 d | 32.0 ± 0.33 f | ||
| 1.66 mM nanosilicon | 6.9 ± 0.15 d | 0.57 ± 0.005 c | 28.0 ± 0.33 d | ||
| Gemmeiza 9 | Control | 4.7 ± 0.08 a | 0.28 ± 0.008 a | 22.0 ± 0.33 a | |
| 1.5 mM silicon | 5.9 ± 0.03 c | 0.45 ± 0.012 b | 28.0 ± 0.33 d | ||
| 1.66 mM nanosilicon | 5.3 ± 0.12 b | 0.38 ± 0.012 a | 24.0 ± 0.66 b | ||
| Treatments | Weight of 1000-Grains | Grain Yield/p | HI (%) | ||
|---|---|---|---|---|---|
| Normal season | Shandaweel 1 | Control | 30.3 ± 0.06 g | 32.1 ± 0.12 e | 94.1 ± 0.12 k |
| 1.5 mM silicon | 37.2 ± 0.08 j | 40.1 ± 0.145 g | 96.3 ± 0.24 l | ||
| 1.66 mM nanosilicon | 35.4 ± 0.03 i | 39.8 ± 0.057 g | 99.7 ± 0.20 e | ||
| Gemmeiza 9 | Control | 29.6 ± 0.08 f | 24.6 ± 0.26 c | 89.1 ± 0.06 c | |
| 1.5 mM silicon | 42.1 ± 0.18 l | 39.0 ± 0.152 h | 98.4 ± 0.14 g | ||
| 1.66 mM nanosilicon | 38.2 ± 0.057 k | 33.9 ± 0.272 f | 92.8 ± 0.94 a | ||
| Late season | Shandaweel 1 | Control | 22.9 ± 0.152 d | 24.4 ± 0.133 b | 88.0 ± 0.26 h |
| 1.5 mM silicon | 32.1 ± 0.08 h | 31.1 ± 0.484 e | 95.3 ± 0.26 i | ||
| 1.66 mM nanosilicon | 26.0 ± 0.03 e | 28.5 ± 0.348 d | 92.8 ± 0.47 b | ||
| Gemmeiza 9 | Control | 14.3 ± 0.115 a | 16.5 ± 0.088 a | 83.7 ± 1.39 f | |
| 1.5 mM silicon | 21.8 ± 0.185 c | 31.3 ± 0.47 f | 97.2 ± 0.43 j | ||
| 1.66 mM nanosilicon | 17.7 ± 0.145 b | 27.8 ± 0.328 c | 95.2 ± 0.21 d | ||
| Treatments | Soluble Sugar | Insoluble Sugar | Starch | α-Amylase | ||
|---|---|---|---|---|---|---|
| Normal season | Shandaweel 1 | Control | 4.98 ± 0.052 c | 23.31 ± 0.057 b | 18.3 ± 0.088 a | 50.4 ± 0.218 k |
| 1.5 mM silicon | 7.64 ± 0.020 g | 31.86 ± 0.061 d | 21.0 ± 0.057 b | 43.6 ± 0.088 h | ||
| 1.66 mM nanosilicon | 6.65 ± 0.023 f | 28.79 ± 0.065 c,d | 20.4 ± 0.116 c | 47.1 ± 0.218 g | ||
| Gemmeiza 9 | Control | 4.82 ± 0.012 c | 25.8 ± 0.057 b,c | 14.4 ± 0.100 a | 48.0 ± 0.240 l | |
| 1.5 mM silicon | 5.30 ± 0.038 d | 30.75 ± 0.125 d | 19.0 ± 0.218 b | 36.9 ± 0.208 j | ||
| 1.66 mM nanosilicon | 5.43 ± 0.028 d | 35.66 ± 0.041 e | 18.3 ± 0.185 d | 35.1 ± 0.088 i | ||
| Late season | Shandaweel 1 | Control | 7.36 ± 0.211 g | 15.65 ± 0.025 a | 10.1 ± 0.266 d | 67.1 ± 0.264 c |
| 1.5 mM silicon | 8.51 ± 0.020 e | 19.44 ± 1.190 a | 16.3 ± 0.1 h | 45.9 ± 0.202 h | ||
| 1.66 mM nanosilicon | 9.05 ± 0.008 e | 18.73 ± 0.312 a | 14.5 ± 0.115 g | 49.0 ± 0.133 e | ||
| Gemmeiza 9 | Control | 5.31 ± 0.006 d | 18.96 ± 0.111 a | 10.6 ± 0.185 e | 69.6 ± 0.066 d | |
| 1.5 mM silicon | 6.73 ± 0.012 a | 28.86 ± 3.290 b,c | 18.4 ± 0.173 f | 37.2 ± 0.218 b | ||
| 1.66 mM nanosilicon | 6.42 ± 0.038 b | 32.73 ± 1.455 d | 17.9 ± 0.360 i | 39.7 ± 0.033 a | ||
| Treatments | Soluble Protein | Insoluble Protein | Total Phenols | Flavonoids | Total Antioxidant Capacity | ||
|---|---|---|---|---|---|---|---|
| Normal season | Shandaweel 1 | Control | 1.45 ± 0.014 g | 2.75 ± 0.017 b | 1.93 ± 0.018 g | 0.49 ± 0.015 f | 0.23 ± 0.036 a |
| 1.5 mM silicon | 2.25 ± 0.043 k | 3.0 ± 0.003 c,d | 1.44 ± 0.017 f | 0.31 ± 0.008 d | 0.38 ± 0.005 c | ||
| 1.66 mM nanosilicon | 2.79 ± 0.012 j | 3.37 ± 0.012 e | 1.32 ± 0.006 e | 0.28 ± 0.006 c | 0.32 ± 0.006 b | ||
| Gemmeiza 9 | Control | 1.33 ± 0.013 a | 2.58 ± 0.012 a | 1.11 ± 0.012 d | 1.03 ± 0.014 g | 0.27 ± 0.001 a | |
| 1.5 mM silicon | 1.71 ± 0.014 d | 2.71 ± 0.017 b | 0.87 ± 0.003 b | 0.44 ± 0.007 e | 0.31 ± 0.016 b | ||
| 1.66 mM nanosilicon | 2.00 ± 0.013 e | 3.0 ± 0.040 d | 0.82 ± 0.016 b | 0.25 ± 0.006 b,c | 0.41 ± 0.001 d | ||
| Late season | Shandaweel 1 | Control | 2.42 ± 0.003 b | 2.97 ± 0.037 c | 0.94 ± 0.012 c | 0.31 ± 0.006 d | 0.52 ± 0.001 f |
| 1.5 mM silicon | 10.0 ± 0.005 f | 3.95 ± 0.035 h | 0.75 ± 0.003 a | 0.21 ± 0.013 b | 0.48 ± 0.008 e | ||
| 1.66 mM nanosilicon | 5.87 ± 0.008 h | 3.67 ± 0.008 g | 0.86 ± 0.006 b | 0.24 ± 0.005 b,c | 0.38 ± 0.002 c,d | ||
| Gemmeiza 9 | Control | 1.66 ± 0.003 c | 3.33 ± 0.005 e | 1.09 ± 0.010 d | 0.48 ± 0.018 e,f | 0.60 ± 0.005 g | |
| 1.5 mM silicon | 2.71 ± 0.026 h | 3.58 ± 0.008 f | 0.76 ± 0.078 b | 0.28 ± 0.012 c | 0.35 ± 0.003 bc | ||
| 1.66 mM nanosilicon | 3.12 ± 0.025 i | 3.95 ± 0.011 h | 0.80 ± 0.032 a,b | 0.12 ± 5.62 a | 0.44 ± 0.001 e | ||
| Treatments | Ca (mg/100 g DW) | Zn (mg/100 g DW) | K (mg/100 g DW) | P (mg/100 g DW) | ||
|---|---|---|---|---|---|---|
| Normal season | Shandaweel 1 | Control | 312 ± 0.333 c | 5.4 ± 0.033 b | 276 ± 0.333 l | 13.2 ± 0.088 a |
| 1.5 mM silicon | 352 ± 0.577 f | 7.32 ± 0.058 c | 216 ± 0.666 k | 36.0 ± 0.240 e | ||
| 1.66 mM nanosilicon | 348 ± 2.00 e | 7.45 ± 0.028 c | 198 ± 0.33 j | 48.0 ± 0.240 g | ||
| Gemmeiza 9 | Control | 264 ± 0.333 a | 4.44 ± 0.127 a | 192 ± 0.66 i | 19.2 ± 0.057 b | |
| 1.5 mM silicon | 372 ± 0.577 g | 5.52 ± 0.058 b | 182 ± 0.33 h | 30.0 ± 0.115 d | ||
| 1.66 mM nanosilicon | 288 ± 0.333 b | 4.68 ± 0.037 a | 174 ± 0.33 g | 24.4 ± 0.202 c | ||
| Late season | Shandaweel 1 | Control | 414 ± 1.00 h | 9.72 ± 0.151 d | 51.6 ± 0.29 f | 44.4 ± 0.272 f |
| 1.5 mM silicon | 489.6 ± 0.290 j | 11.52 ± 0.037 f | 40.8 ± 0.208 d | 148.8 ± 0.152 k | ||
| 1.66 mM nanosilicon | 423.2 ± 0.305 i | 10.8 ± 0.185 e | 37.2 ± 0.115 b | 141.6 ± 0.088 j | ||
| Gemmeiza 9 | Control | 310.8 ± 0.305 c | 7.2 ± 0.057 c | 43.2 ± 0.176 e | 63.6 ± 0.145 i | |
| 1.5 mM silicon | 567.7 ± 0.493 k | 11.28 ± 0.188 f | 34.8 ± 0.233 a | 92.4 ± 0.768 i | ||
| 1.66 mM nanosilicon | 327.2 ± 0.635 d | 9.65 ± 0.060 d | 38.4 ± 0.152 c | 72.0 ± 1.083 h | ||
| Treatments | Inorganic P (mg/100 g DW) | Phytate P (mg/100 g DW) | Phytic Acid (mg/100 g DW) | Oxalic Acid (mg/g DW) | ||
|---|---|---|---|---|---|---|
| Normal season | Shandaweel 1 | Control | 4.8 ± 0.088 a | 47.9 ± 0.264 h | 170.0 ± 0.088 i | 2.20 ± 0.057 a,b |
| 1.5 mM silicon | 11.2 ± 0.033 d | 41.6 ± 0.173 c | 147.6 ± 0.100 c | 2.64 ± 0.003 a,b | ||
| 1.66 mM nanosilicon | 15.6 ± 0.218 f | 41.8 ± 0.284 c | 148.3 ± 0.200 d | 1.76 ± 0.008 a,b | ||
| Gemmeiza 9 | Control | 6.0 ± 0.033 b | 57.5 ± 0.185 i | 204.1 ± 0.145 j | 3.08 ± 0.015 a,b | |
| 1.5 mM silicon | 9.6 ± 0.033 c | 42.6 ± 0.218 d | 151.2 ± 0.057 e | 1.76 ± 0.015 a,b | ||
| 1.66 mM nanosilicon | 14.8 ± 0.185 e | 41.8 ± 0.240 c | 148.3 ± 0.100 d | 1.32 ± 0.025 a | ||
| Late season | Shandaweel 1 | Control | 14.4 ± 0.057 e | 39.1 ± 0.120 b | 138.8 ± 0.088 b | 1.32 ± 0.031 a |
| 1.5 mM silicon | 48.0 ± 0.218 k | 43.8 ± 0.233 e | 155.4 ± 0.033 f | 0.88 ± 0.015 a | ||
| 1.66 mM nanosilicon | 46.8 ± 0.152 j | 44.4 ± 0.115 f | 157.6 ± 0.173 g | 1.02 ± 0.003 a | ||
| Gemmeiza 9 | Control | 31.2 ± 0.200 i | 33.1 ± 0.185 h | 117.5 ± 0.088 a | 1.76 ± 0.003 a,b | |
| 1.5 mM silicon | 30.0 ± 0.218 h | 44.5 ± 0.185 f | 157.9 ± 0.173 g | 1.32 ± 4.026 b | ||
| 1.66 mM nanosilicon | 24.0 ± 0.251 g | 45.5 ± 0.208 g | 161.5 ± 0.088 h | 0.88 ± 0.003 a | ||
| Treatments | Straw Yield (g/Plant) | Soluble Sugar (mg/g DW) | Insoluble Sugar (mg/g DW) | Starch (mg/g DW) | α-Amylase (mg Sugar/Mmin./g DW) | ||
|---|---|---|---|---|---|---|---|
| Normal season | Shandaweel 1 | Control | 34.1 ± 0.014 c | 4.52 ± 0.093 f | 17.6 ± 0.088 | 7.71 ± 0.201 d | 53.0 ± 0.033 h |
| 1.5 mM silicon | 41.6 ± 0.035 e | 6.63 ± 0.095 d | 14.4 ± 0.088 | 0.96 ± 0.012 a | 72.8 ± 0.166 k | ||
| 1.66 mM nanosilicon | 39.9 ± 0.096 i | 5.83 ± 0.159 e | 16.9 ± 0.218 | 0.96 ± 0.025 a | 69.5 ± 0.088 j | ||
| Gemmeiza 9 | Control | 27.7 ± 0.095 f | 4.62 ± 0.121 h | 25.3 ± 0.066 | 7.23 ± 0.029 d | 50.1 ± 0.088 e | |
| 1.5 mM silicon | 39.6 ± 0.061 h | 5.54 ± 0.042 a | 9.6 ± 0.218 | 4.34 ± 0.059 b | 52.1 ± 0.057 g | ||
| 1.66 mM nanosilicon | 36.5 ± 0.159 j | 8.91 ± 0.242 a | 9.0 ± 0.120 | 5.78 ± 0.030 c | 54.8 ± 0.057 i | ||
| Late season | Shandaweel 1 | Control | 27.7 ± 0.076 b | 5.71 ± 0.176 e | 16.9 ± 0.166 | 16.21 ± 0.065 f | 49.8 ± 0.166 d |
| 1.5 mM silicon | 32.6 ± 0.135 d | 4.08 ± 0.031 c | 11.6 ± 0.264 | 24.58 ± 0.126 h | 42.6 ± 0.033 b | ||
| 1.66 mM nanosilicon | 30.7 ± 0.165 g | 4.55 ± 0.125 b | 10.9 ± 0.088 | 23.14 ± 0.117 g | 39.3 ± 0.120 a | ||
| Gemmeiza 9 | Control | 19.7 ± 0.213 a | 7.14 ± 0.078 g | 19.5 ± 0.066 | 13.37 ± 0.095 e | 48.8 ± 0.152 f | |
| 1.5 mM silicon | 32.2 ± 0.161 d | 5.04 ± 0.029 c | 11.8 ± 0.153 | 33.26 ± 0.107 j | 39.6 ± 0.202 a | ||
| 1.66 mM nanosilicon | 34.1 ± 0.014 c | 4.52 ± 0.093 f | 17.6 ± 0.088 | 7.71 ± 0.201 d | 53.0 ± 0.033 h | ||
| Treatments | Soluble Protein (mg/g DW) | Insoluble Protein (mg/g DW) | Total Phenols (mg/g DW) | Flavonoids (mg/g DW) | Total Antioxidant Capacity (µg/g DW) | ||
|---|---|---|---|---|---|---|---|
| Normal season | Shandaweel 1 | Control | 1.67 ± 0.031 c | 3.75 ± 0.008 i | 1.92 ±0.008 a | 0.52 ± 0.033 a | 0.29 ± 0.015 e |
| 1.5 mM silicon | 2.41 ± 0.006 f | 2.21 ± 0.006 d | 3.07 ± 0.017 c | 0.61 ± 0.011 c | 0.16 ± 0.002 b | ||
| 1.66 mM nanosilicon | 2.29 ± 0.008 e | 2.33 ± 0.010 e | 3.17 ± 0.010 d | 0.57 ± 0.006 b | 0.16 ± 0.001 b | ||
| Gemmeiza 9 | Control | 1.58 ± 0.012 a | 3.65 ± 0.005 h | 3.25 ± 0.008 e | 0.59 ± 0.003 b,c | 0.12 ± 0.003 a | |
| 1.5 mM silicon | 2.21 ± 0.003 d | 2.17 ± 0.005 c | 3.72 ± 0.011 h | 0.92 ± 0.017 e | 0.19 ± 0.004 c | ||
| 1.66 mM nanosilicon | 2.63 ± 0.013 g | 2.21 ± 0.003 d | 3.62 ± 0.005 g | 1.17 ± 0.015 h | 0.16 ± 0.008 b | ||
| Late season | Shandaweel 1 | Control | 2.17 ± 0.030 b | 3.34 ± 0.005 g | 2.74 ± 0.003 b | 0.85 ± 0.014 d | 0.56 ± 0.013 f |
| 1.5 mM silicon | 3.00 ± 0.005 h | 2.08 ± 0.016 b | 5.37 ± 0.014 l | 1.05 ± 0.014 f | 0.18 ± 0.001 c | ||
| 1.66 mM nanosilicon | 3.67 ± 0.063 k | 2.17 ± 0.005 c | 3.57 ± 0.003 f | 1.43 ± 0.008 i | 0.19 ± 0.002 c | ||
| Gemmeiza 9 | Control | 2.21 ± 0.003 d | 3.27 ± 0.008 f | 3.84 ± 0.008 i | 1.11 ± 0.008 g | 0.64 ± 0.013 g | |
| 1.5 mM silicon | 3.25 ± 0.006 i | 2.04 ± 0.014 b | 3.98 ± 0.028 j | 1.92 ± 0.027 k | 0.25 ± 0.007 d | ||
| 1.66 mM nanosilicon | 1.67 ± 0.031 c | 3.75 ± 0.008 i | 1.92 ± 0.008 a | 0.52 ± 0.033 a | 0.29 ± 0.015 e | ||
| Treatments | Inorganic P (mg/100 g DW) | Phytate P (mg/100 g DW) | Phytic Acid (mg/100 g DW) | Oxalic Acid (mg/g DW) | ||
|---|---|---|---|---|---|---|
| Normal season | Shandaweel 1 | Control | 37.2 ± 0.115 i | 41.04 ± 0.127 d | 145.6 ± 0.202 c,d | 2.20 ± 0.057 e |
| 1.5 mM silicon | 27.6 ± 0.115 f | 44.3 ± 0.10 a | 157.2 ± 0.633 f,g | 3.52 ± 0.003 g | ||
| 1.66 mM nanosilicon | 33.6 ± 0.066 h | 43.76 ± 0.046 b | 155.3 ± 0.133 f | 2.20 ± 0.033 e | ||
| Gemmeiza 9 | Control | 40.8 ± 0.152 j | 40.1 ± 0.033 c | 142.3 ± 1.874 c | 2.64 ± 0.003 f | |
| 1.5 mM silicon | 37.2 ± 0.115 i | 43.52 ± 0.006 b | 154.4 ± 0.145 f | 2.20 ± 0.057 e | ||
| 1.66 mM nanosilicon | 28.8 ± 0.057 g | 44.94 ± 0.173 b | 159.5 ± 0.735 g | 1.76 ± 0.003 c | ||
| Late season | Shandaweel 1 | Control | 26.4 ± 0.669 e | 35.52 ± 0.103 c | 126.1 ± 1.102 a | 1.32 ± 0.006 a |
| 1.5 mM silicon | 13.2 ± 0.088 d | 42.0 ± 0.033 b | 149.1 ± 0.698 e | 1.76 ± 0.030 c | ||
| 1.66 mM nanosilicon | 7.2 ± 0.115 b | 41.94 ± 0.173 c | 148.8 ± 0.517 d,e | 1.54 ± 0.003 b | ||
| Gemmeiza 9 | Control | 13.2 ± 0.115 d | 36.76 ± 0.046 d | 130.4 ± 0.854 b | 1.32 ± 0.028 a | |
| 1.5 mM silicon | 2.4 ± 0.066 a | 41.4 ± 0.033 c | 146.9 ± 1.047 de | 1.72 ± 0.031 a | ||
| 1.66 mM nanosilicon | 12.0 ± 0.033 c | 41.28 ± 0.052 b | 146.5 ± 0088 c,d,e | 1.98 ± 0.015 d | ||
| Treatments | Ca (mg/100 g DW) | Zn (mg/100 g DW) | P (mg/100 g DW) | K (mg/100 g DW) | ||
|---|---|---|---|---|---|---|
| Normal season | Shandaweel 1 | Control | 176 ± 0.33 c | 24.0 ± 0.033 j | 34.8 ± 0.033 b | 112.8 ± 0.088 g |
| 1.5 mM silicon | 185 ± 0.33 e | 9.72 ± 0.072 f | 40.6 ± 0.033 d | 85.2 ± 0.115 d | ||
| 1.66 mM nanosilicon | 180 ± 0.33 d | 12.0 ± 0.033 h | 51.9 ± 0.033 e | 100.8 ± 3.153 f | ||
| Gemmeiza 9 | Control | 132 ± 0.33 a | 10.92 ± 0.070 g | 30.0 ± 0.033 a | 124.8 ± 0.057 h | |
| 1.5 mM silicon | 132 ± 0.66 a | 9.96 ± 0.111 f | 37.2 ± 0.033 c | 115.2 ± 0.057 g | ||
| 1.66 mM nanosilicon | 144 ± 0.33 b | 9.84 ± 0.140 f | 40.7 ± 0.033 d | 88.8 ± 0.152 e | ||
| Late season | Shandaweel 1 | Control | 228 ± 0.33 h | 12.84 ± 0.140 i | 144 ± 0.33 f | 82.8 ± 0.145 d |
| 1.5 mM silicon | 360 ± 0.33 k | 7.2 ± 0.033 d | 168 ± 0.33 h | 40.8 ± 0.057 c | ||
| 1.66 mM nanosilicon | 292 ± 0.33 j | 5.16 ± 0.157 c | 150 ± 0.33 g | 21.6 ± 0.145 a | ||
| Gemmeiza 9 | Control | 216 ± 0.33 f | 7.68 ± 0.058 e | 150 ± 0.66 g | 84.0 ± 0.088 d | |
| 1.5 mM silicon | 220 ± 0.33 g | 3.48 ± 0.122 b | 180.4 ± 0.115 i | 37.2 ± 0.088 b | ||
| 1.66 mM nanosilicon | 252 ± 0.33 i | 2.61 ± 0.068 a | 186 ± 0.66 j | 40.8 ± 0.088 c | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helal, N.M.; Khattab, H.I.; Emam, M.M.; Niedbała, G.; Wojciechowski, T.; Hammami, I.; Alabdallah, N.M.; Darwish, D.B.E.; El-Mogy, M.M.; Hassan, H.M. Improving Yield Components and Desirable Eating Quality of Two Wheat Genotypes Using Si and NanoSi Particles under Heat Stress. Plants 2022, 11, 1819. https://doi.org/10.3390/plants11141819
Helal NM, Khattab HI, Emam MM, Niedbała G, Wojciechowski T, Hammami I, Alabdallah NM, Darwish DBE, El-Mogy MM, Hassan HM. Improving Yield Components and Desirable Eating Quality of Two Wheat Genotypes Using Si and NanoSi Particles under Heat Stress. Plants. 2022; 11(14):1819. https://doi.org/10.3390/plants11141819
Chicago/Turabian StyleHelal, Nesma M., Hemmat I. Khattab, Manal M. Emam, Gniewko Niedbała, Tomasz Wojciechowski, Inès Hammami, Nadiyah M. Alabdallah, Doaa Bahaa Eldin Darwish, Mohamed M. El-Mogy, and Heba M. Hassan. 2022. "Improving Yield Components and Desirable Eating Quality of Two Wheat Genotypes Using Si and NanoSi Particles under Heat Stress" Plants 11, no. 14: 1819. https://doi.org/10.3390/plants11141819
APA StyleHelal, N. M., Khattab, H. I., Emam, M. M., Niedbała, G., Wojciechowski, T., Hammami, I., Alabdallah, N. M., Darwish, D. B. E., El-Mogy, M. M., & Hassan, H. M. (2022). Improving Yield Components and Desirable Eating Quality of Two Wheat Genotypes Using Si and NanoSi Particles under Heat Stress. Plants, 11(14), 1819. https://doi.org/10.3390/plants11141819









