Low Concentration of Aluminum-Stimulated Pollen Tube Growth of Apples (Malus domestica)
Abstract
:1. Introduction
2. Results
2.1. 20 µM AlCl3 Affected M. domestica Pollen Germination and Pollen Tube Growth
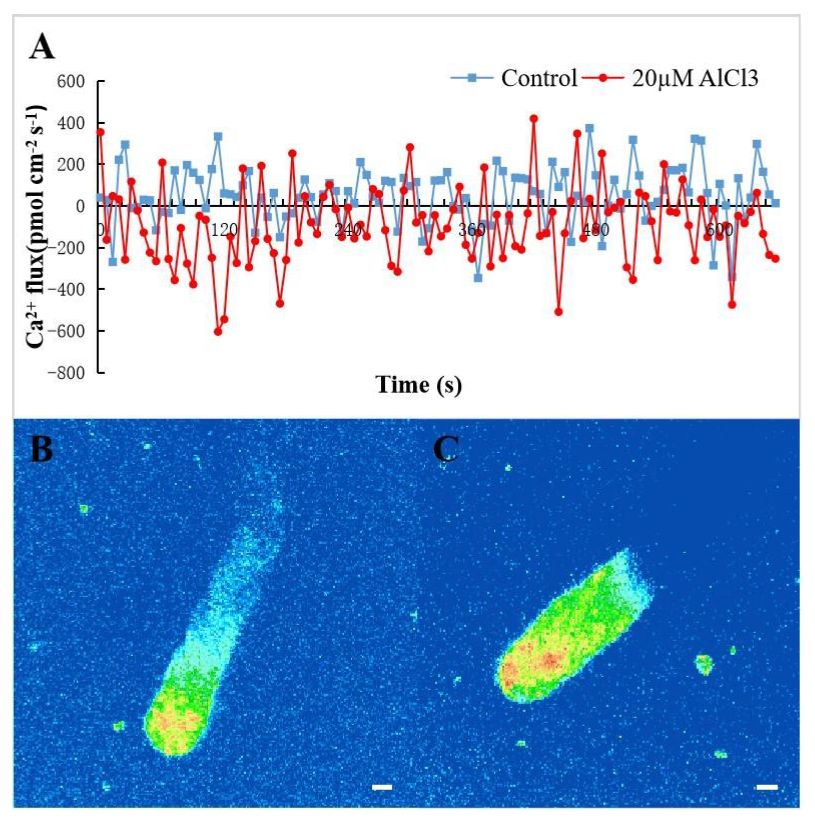
2.2. Effect of 20 µM AlCl3 on Ca2+ Flux and [Ca2+]c in Pollen Tubes of M. domestica
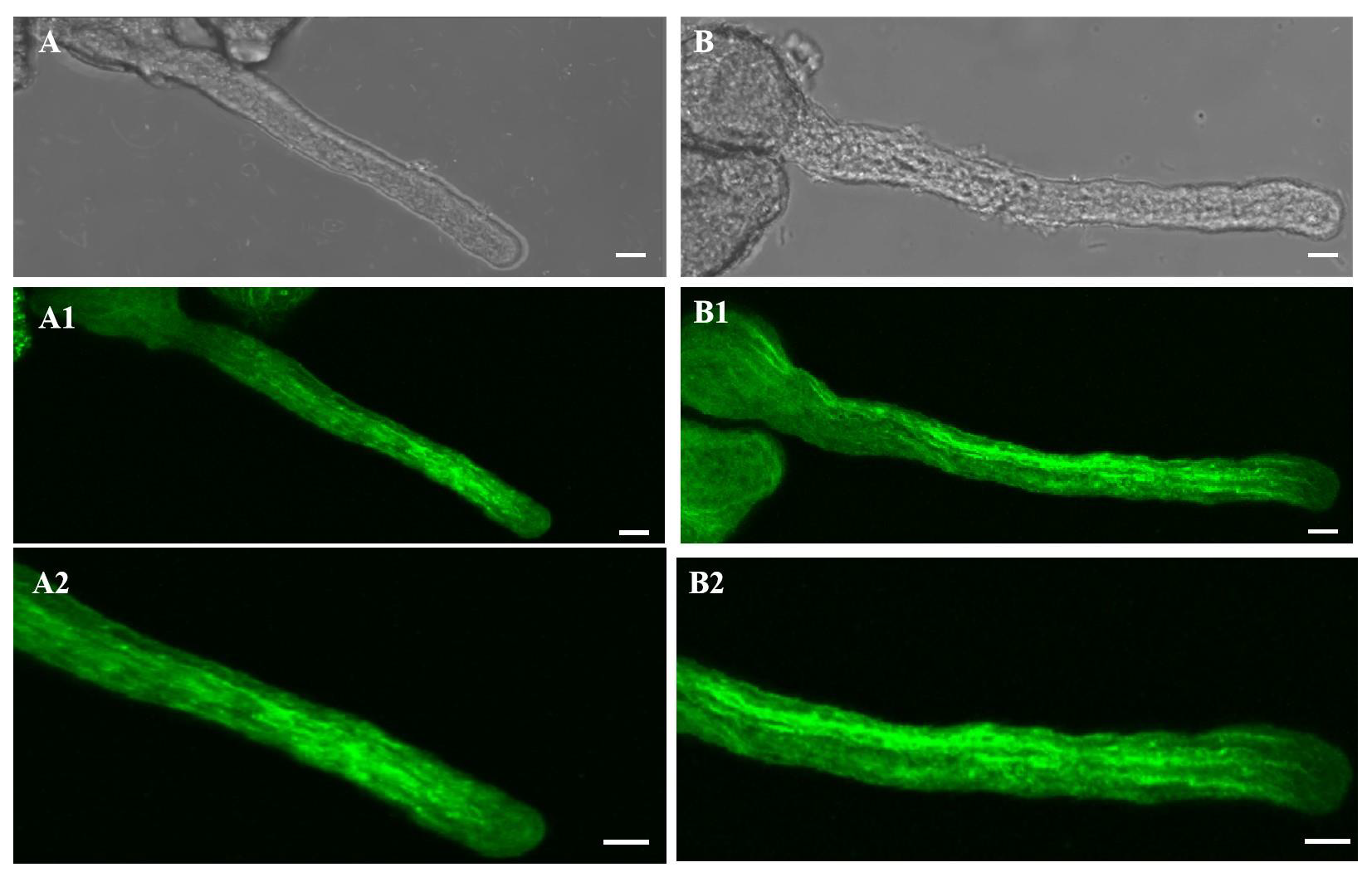
2.3. Effect of 20 µM AlCl3 on Actin Filaments in Pollen Tubes of M. domestica
2.4. Effect of 20 µM AlCl3 on Distribution of Cellulose and Callose in Pollen Tube Cell Wall of M. domestica
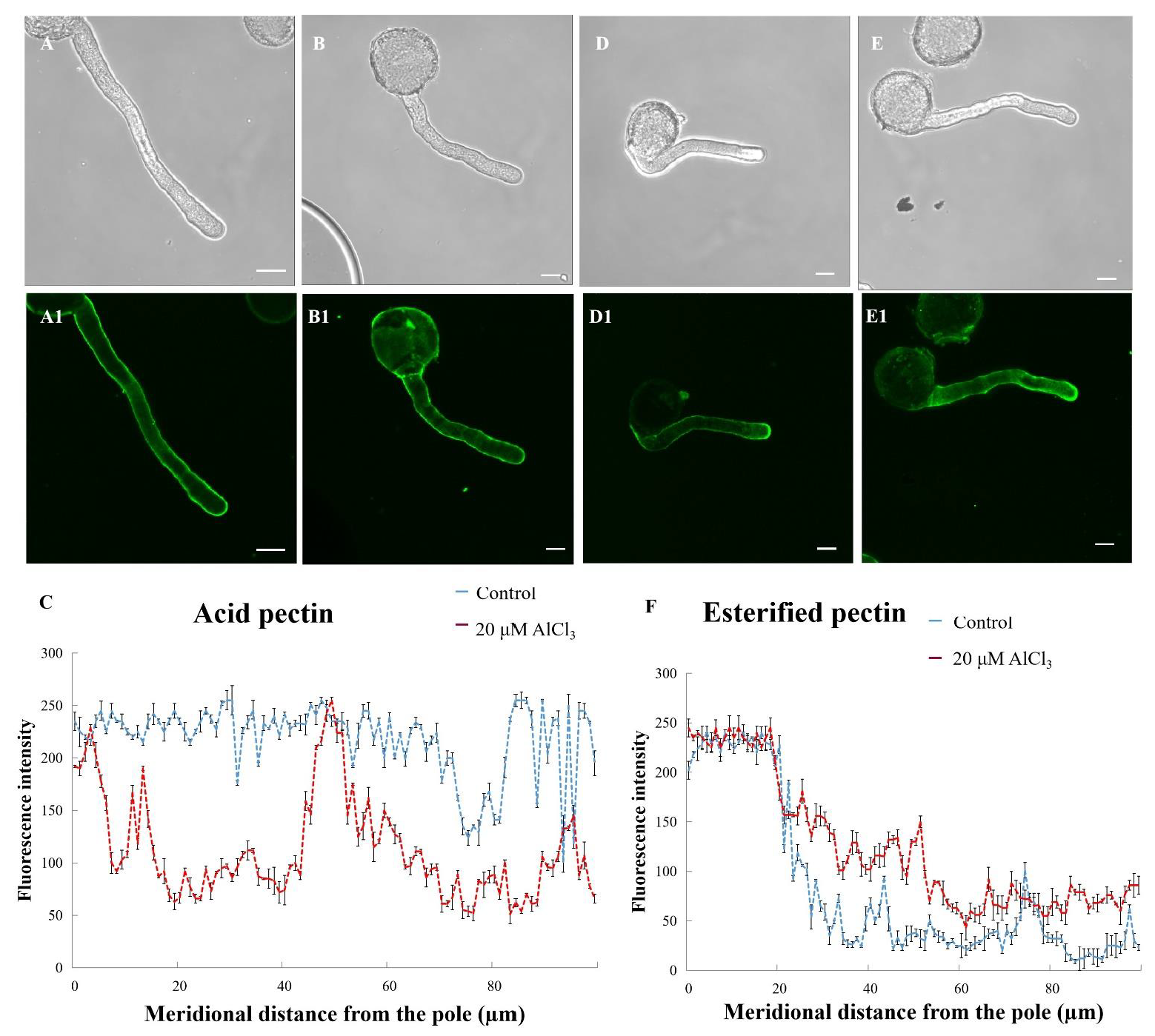
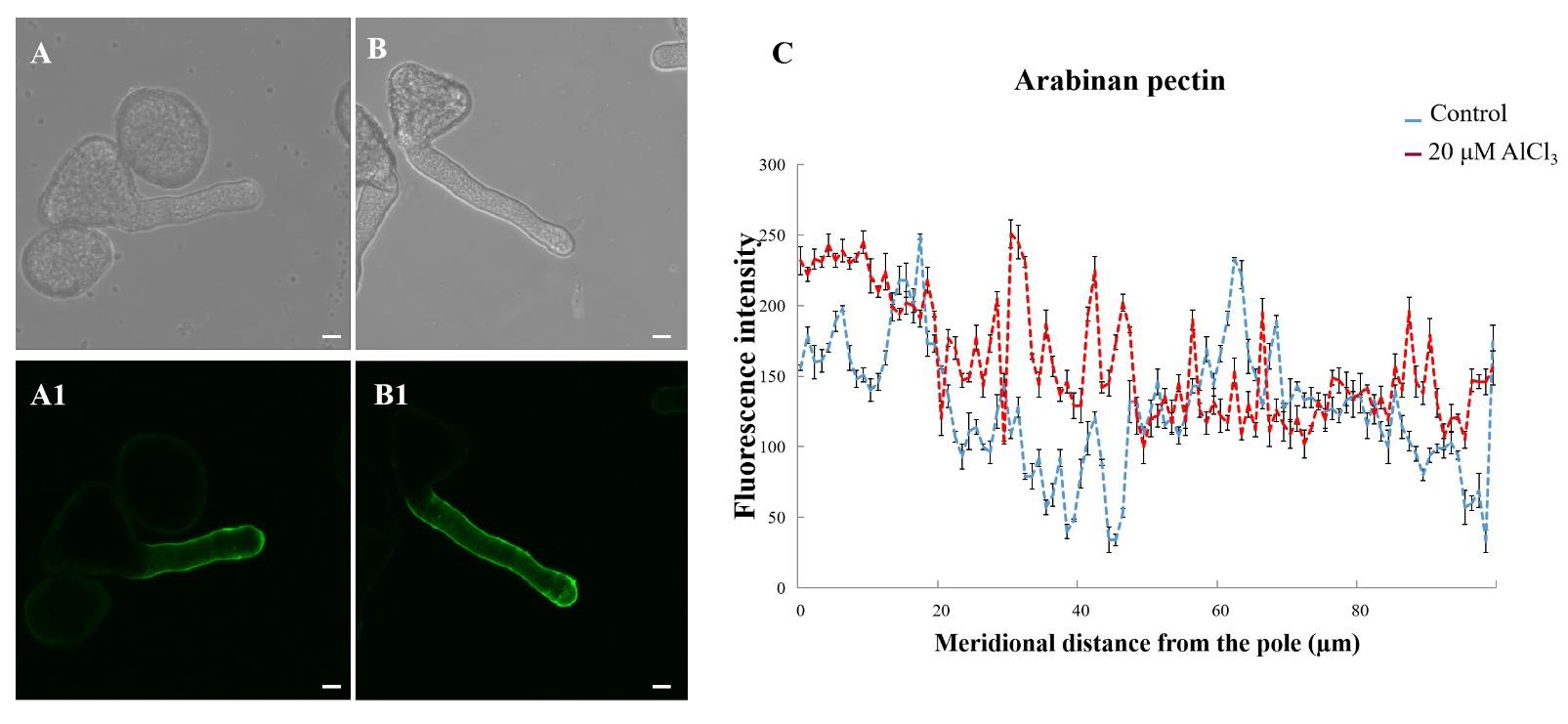
2.5. Effects of 20 µM AlCl3 on Distribution of Pectins in Pollen Tube Cell Wall of M. domestica
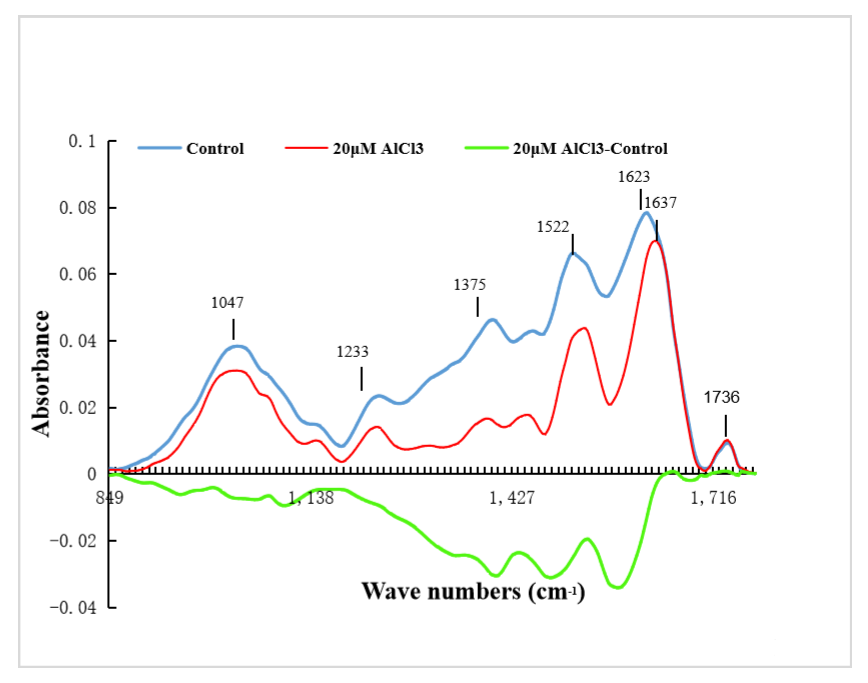
2.6. Effects of 20 µM AlCl3 on Chemical Composition of Pollen Tube Cell Wall of M. domestica
3. Discussion
3.1. Relationship among Al, Ca2+ Flux and [Ca2+]c
3.2. Al Altered the Deposition of Pollen Tube Wall Components
4. Materials and Methods
4.1. Plant Materials and Pollen Culture
4.2. Measurement of Extracellular Ca2+ Flux
4.3. Fluorescence Labeling of Pollen Tube Cytoplasmic [Ca2+]c
4.4. Fluorescence Labeling of Actin Filaments
4.5. Fluorescence Localization and Analysis of Pollen Tube Wall Components
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, K.F.; Xie, P.X.; Zhang, Q.; Xing, Y.; Cao, Q.Q.; Qin, L. Aluminum toxicity-induced pollen tube growth inhibition in apple is mediated by interrupting calcium dynamics and modification of cell wall components. Environ. Exp. Bot. 2020, 171, 1–8. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Sharma, T.R.; Pattanayak, A.; Singh, A.K. Alleviating aluminum toxicity in plants: Implications of reactive oxygen species signaling and crosstalk with other signaling pathways. Plant Physiol. 2021, 173, 1765–1784. [Google Scholar] [CrossRef] [PubMed]
- Osaki, M.T.; Watanabe, T.; Tadano, T. Beneficial effect of aluminum on growth of plants adapted to low pH soils. Soil Sci. Plant Nutr. 1997, 43, 551–563. [Google Scholar] [CrossRef]
- Watanabe, T.; Jansen, S.; Osaki, M. The beneficial effect of aluminium and the role of citrate in Al accumulation in Melastoma malabathricum. New Phytol. 2005, 165, 773–780. [Google Scholar] [CrossRef]
- Du, B.; Nian, H.; Zhang, Z.; Yang, C. Effects of aluminum on superoxide dismutase and peroxidase activities, and lipid peroxidation in the roots and calluses of soybeans differing in aluminum tolerance. Acta Physiol Plant. 2010, 32, 883–890. [Google Scholar] [CrossRef]
- Wang, L.; Fan, X.W.; Pan, J.L.; Huang, Z.B.; Li, Y.Z. Physiological characterization of maize tolerance to low dose of aluminum, highlighted by promoted leaf growth. Planta 2015, 242, 1391–1403. [Google Scholar] [CrossRef] [Green Version]
- Pilon-Smits, E.A.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, C.; Li, Q.; Qiao, X.; Li, X.; Cai, Y.; Wang, P.; Sun, Y.; Zhang, H.; Zhang, S.; et al. Characterization of the pectin methylesterase inhibitor gene family in Rosaceae and role of PbrPMEI23/39/41 in methylesterified pectin distribution in pear pollen tube. Planta 2021, 253, 118. [Google Scholar] [CrossRef]
- Hepler, P.K.; Winship, L.J. The pollen tube clear zone: Clues to the mechanism of polarized growth. J. Integr. Plant Biol. 2015, 57, 79–92. [Google Scholar] [CrossRef]
- Bou, D.F.; Geitmann, A. Actin is involved in pollen tube tropism through redefining the spatial targeting of secretory vesicles. Traffic 2011, 12, 1537–1551. [Google Scholar]
- Zhang, Y.; He, J.; Lee, D.; McCormick, S. Interdependence of endomembrane trafficking and actin dynamics during polarized growth of Arabidopsis pollen tubes. Plant Physiol. 2010, 152, 2200–2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.N.; Cui, X.Y.; Hu, A.N.; Miao, Y.H.; Zhang, L.Y. Characterization and functional analysis of pollen-specific Pw SWEET1 in Picea wilsonii. J. Forestry Res. 2020, 31, 1913–1922. [Google Scholar] [CrossRef] [Green Version]
- Zonia, L.; Munnik, T. Understanding pollen tube growth: The hydrodynamic model versus the cell wall model. Trends Plant Sci. 2011, 16, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Deng, Y.; Kanaoka, M.M.; Okada, K.; Hong, Z. Expression of Arabidopsis callose synthase 5 results in callose accumulation and cell wall permeability alteration. Plant Sci. 2012, 183, 1–8. [Google Scholar] [CrossRef]
- Hepler, P.K.; Winship, L.J. Calcium at the cell wall-cytoplast interface. J. Integr. Plant Biol. 2010, 52, 147–160. [Google Scholar] [CrossRef]
- Sawidis, T.; Reiss, H.D. Effects of heavy metals on pollen tube growth and ultrastructure. Protoplasma 1995, 185, 113–122. [Google Scholar] [CrossRef]
- Sade, H.; Meriga, B.; Surapu, V.; Gadi, J.; Sunita, M.S.; Suravajhala, P.; Kavi Kishor, P.B. Toxicity and tolerance of aluminum in plants: Tailoring plants to suit to acid soils. Biometals 2016, 29, 187–210. [Google Scholar] [CrossRef]
- Zhang, W.H.; Rengel, Z.; Kuo, J.; Yan, G. Aluminium effects on pollen germination and tube growth of Chamelaucium uncinatum. A comparison with other Ca2+ antagonists. Ann. Bot. 1999, 84, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Konishi, S.; Miyamoto, S. Alleviation of aluminum stress and stimulation of tea pollen tube growth by fluorine. Plant Cell Physiol. 1983, 24, 857–862. [Google Scholar] [CrossRef]
- Hajiboland, R.; Barceló, J.; Poschenrieder, C.; Tolrà, R. Amelioration of iron toxicity: A mechanism for aluminum-induced growth stimulation in tea plants. J. Inorg. Biochem. 2013, 128, 183–187. [Google Scholar] [CrossRef]
- Hiromi, Y.; Ikuyo, T.; Fumiko, I.; Miwako, O.; Shigeki, K. Stimulatory effect of aluminum on the growth of tea pollen tubes. Soil Sci. Plant Nutr. 1997, 43, 457–461. [Google Scholar]
- Mollet, J.C.; Leroux, C.; Dardelle, F.; Lehner, A. Cell wall composition, biosynthesis and remodeling during pollen tube growth. Plants 2013, 2, 107–147. [Google Scholar] [CrossRef] [Green Version]
- Shetty, R.; Vidya, C.S.; Prakash, N.B.; Lux, A.; Vaculík, M. Aluminum toxicity in plants and its possible mitigation in acid soils by biochar: A review. Sci. Total Environ. 2021, 765, 142744. [Google Scholar] [CrossRef] [PubMed]
- Le Poder, L.; Mercier, C.; Février, L.; Duong, N.; David, P.; Pluchon, S.; Nussaume, L.; Desnos, T. Uncoupling Aluminum toxicity from Aluminum signals in the STOP1 pathway. Front. Plant Sci. 2022, 13, 785791. [Google Scholar] [CrossRef] [PubMed]
- Bushart, T.J.; Roux, S.J. Conserved features of germination and polarized cell growth: A few insights from a pollen-fern spore comparison. Ann Bot. 2007, 99, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.D.; Callaham, D.A.; Gross, D.J.; Hepler, P.K. Free Ca2+ gradient in growing pollen tubes of lilium. J. Cell Sci. 1992, 101, 7–12. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Messerli, M.A.; Gilroy, S. Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol. 2008, 147, 1690. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.W.; Shaff, J.E.; Grunes, D.L.; Kochian, L.V. Aluminum effects on calcium fluxes at root apex of aluminum-tolerant and aluminum-sensitive wheat cultivars. Plant Physiol. 1992, 98, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Rengel, Z.; Zhang, Z.H. Role of dynamic of intracellular calcium in aluminum toxicity syndrome. New Phytol. 2003, 159, 295–314. [Google Scholar] [CrossRef]
- Very, A.A.; Davies, J.M. Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc. Natl. Acad. Sci. USA 2000, 97, 9801–9806. [Google Scholar] [CrossRef] [Green Version]
- Rengel, Z. Role of calcium in aluminum toxicity. New Phytol. 1992, 121, 499–513. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; Moore, K.L.; Lombi, E.; Gianoncelli, A.; Ferguson, B.J.; Blamey, F.P.C.; Menzies, N.W.; Nicholson, T.M.; McKenna, B.A.; Wang, P.; et al. Identification of the primary lesion of toxic aluminum in plant roots. Plant Physiol. 2015, 167, 1402–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldwin, L.; Domon, J.M.; Flimek, J.F.; Fournet, F.; Sellier, H.; Gillet, F.; Pelloux, J.; Lejeune-Hénaut, I.; Carpita, N.C.; Rayon, C. Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry 2014, 104, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Tian, Q.Y.; Zhao, M.G. Aluminum-induced ethylene production is associated with inhibition of root elongation in Lotus japonicus L. Plant Cell Physiol. 2007, 48, 1229–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.Y.; Kim, J.Y. Callose synthesis in higher plants. Plant Signal Behav. 2009, 4, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Allison, A.V. The ultrastructure of local lesions induced by potato virus x: A sequence of cytological events in the course of infection. Phytopathology 1974, 64, 784. [Google Scholar] [CrossRef]
- Shi, X.; Han, X.; Lu, T.G. Callose synthesis during reproductive development in monocotyledonous and dicotyledonous plants. Plant Signal Behav. 2016, 11, e1062196. [Google Scholar] [CrossRef] [Green Version]
- Parre, E.; Geitmann, A. More than a leak sealant. The mechanical properties of callose in pollen tubes. Plant Physiol. 2005, 137, 274–286. [Google Scholar] [CrossRef] [Green Version]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Nagayama, T.; Nakamura, A.; Yamaji, N.; Satoh, S.; Furukawa, J.; Iwai, H. Changes in the distribution of pectin in root border cells under aluminum stress. Front. Plant Sci. 2019, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Li, Y.Y.; Zhang, Y.J.; Zhang, S.S.; Wu, Y.R.; Wu, P.; Zheng, S.J. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root tip. Plant Physiol. 2008, 146, 602–611. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Nakamura, S.; Kakizoe, S.; Sato, M.; Nakamura, N. Immunocytochemical and chemical analyses of Golgi vesicles isolated from the germinated pollen of Camellia japonica. J. Plant Res. 1998, 111, 421. [Google Scholar] [CrossRef]
- Franklin-Tong, V.E. Signaling and the modulation of pollen tube growth. Plant Cell 1999, 11, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geitmann, A. The rheological properties of the pollen tube cell wall. In Fertilization in Higher Plants; Cresti, M., Cai, G., Moscatelli, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 41, p. 283. [Google Scholar]
- Wu, Q.; Tao, Y.; Zhang, X.L.; Dong, X.Y.; Xia, J.X.; Shen, R.F.; Zhu, X.F. Pectin methylesterases enhance root cell wall phosphorus remobilization in rice. Rice Sci. 2022, 29, 179–190. [Google Scholar]
- Wei, Y.M.; Han, R.R.; Xie, Y.H.; Jiang, C.D.; Yu, Y.X. Recent advances in understanding mechanisms of plant tolerance and response to aluminum toxicity. Sustainability 2021, 13, 1782. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zeng, Z.H.; Yan, J.Y.; Fan, W.; Bian, H.W.; Zhu, M.Y.; Yang, J.L.; Zheng, S.J. Association of specific pectin methylesterases with Al-induced root elongation inhibition in rice. Plant Physiol. 2013, 148, 502–511. [Google Scholar] [CrossRef]
- Fang, K.F.; Zhang, W.W.; Xing, Y.; Zhang, Q.; Yang, L.; Cao, Q.Q.; Qin, L. Boron toxicity causes multiple effects on Malus Domestica pollen tube growth. Front. Plant. Sci. 2016, 7, 208. [Google Scholar] [CrossRef] [Green Version]
- Chebli, Y.; Pujol, L.; Shojaeifard, A.; Brouwer, I.; van Loon, J.J.; Geitmann, A. Cell wall assembly and intracellular trafficking in plant cells are directly affected by changes in the magnitude of gravitational acceleration. PLoS ONE 2013, 8, e58246. [Google Scholar] [CrossRef] [Green Version]
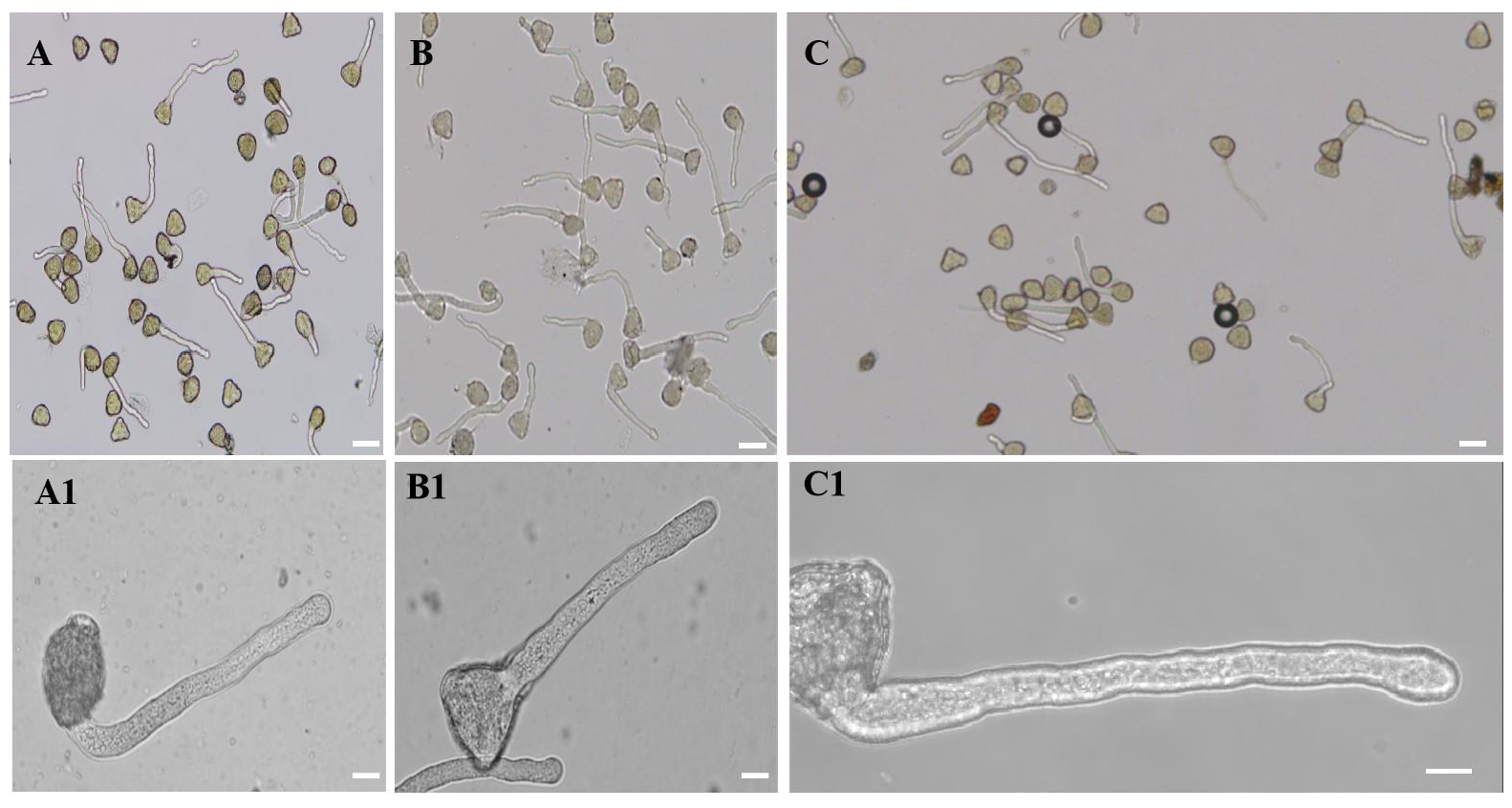

| Concentration of AlCl3/µM | Germination Rate/% | Pollen Tube Length/μm |
|---|---|---|
| 0 | 35.09 ± 2.33 cd | 101.83 ± 8.18 c |
| 10 | 42.07 ± 1.90 bc | 138.46 ± 8.92 b |
| 20 | 57.25 ± 3.29 a | 142.36 ± 4.80 ab |
| 30 | 42.88 ± 1.72 b | 141.88 ± 7.21 ab |
| 50 | 33.89 ± 2.98 d | 144.64 ± 10.47 ab |
| 100 | 31.97 ± 1.37 d | 147.43 ± 7.85 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Xie, P.; Zhang, Q.; Xing, Y.; Cao, Q.; Qin, L.; Fang, K. Low Concentration of Aluminum-Stimulated Pollen Tube Growth of Apples (Malus domestica). Plants 2022, 11, 1705. https://doi.org/10.3390/plants11131705
Zhang C, Xie P, Zhang Q, Xing Y, Cao Q, Qin L, Fang K. Low Concentration of Aluminum-Stimulated Pollen Tube Growth of Apples (Malus domestica). Plants. 2022; 11(13):1705. https://doi.org/10.3390/plants11131705
Chicago/Turabian StyleZhang, Chen, Pengxue Xie, Qing Zhang, Yu Xing, Qingqin Cao, Ling Qin, and Kefeng Fang. 2022. "Low Concentration of Aluminum-Stimulated Pollen Tube Growth of Apples (Malus domestica)" Plants 11, no. 13: 1705. https://doi.org/10.3390/plants11131705
APA StyleZhang, C., Xie, P., Zhang, Q., Xing, Y., Cao, Q., Qin, L., & Fang, K. (2022). Low Concentration of Aluminum-Stimulated Pollen Tube Growth of Apples (Malus domestica). Plants, 11(13), 1705. https://doi.org/10.3390/plants11131705





