Effect of Light Intensity on Morphology, Photosynthesis and Carbon Metabolism of Alfalfa (Medicago sativa) Seedlings
Abstract
:1. Introduction
2. Results
2.1. Morphological Characteristics
2.2. Leaf Pigment and Nitrogen Content
2.3. Photosynthetic and Chlorophyll Fluorescence Characteristics
2.4. Leaf Non-Structural Carbohydrate Contents
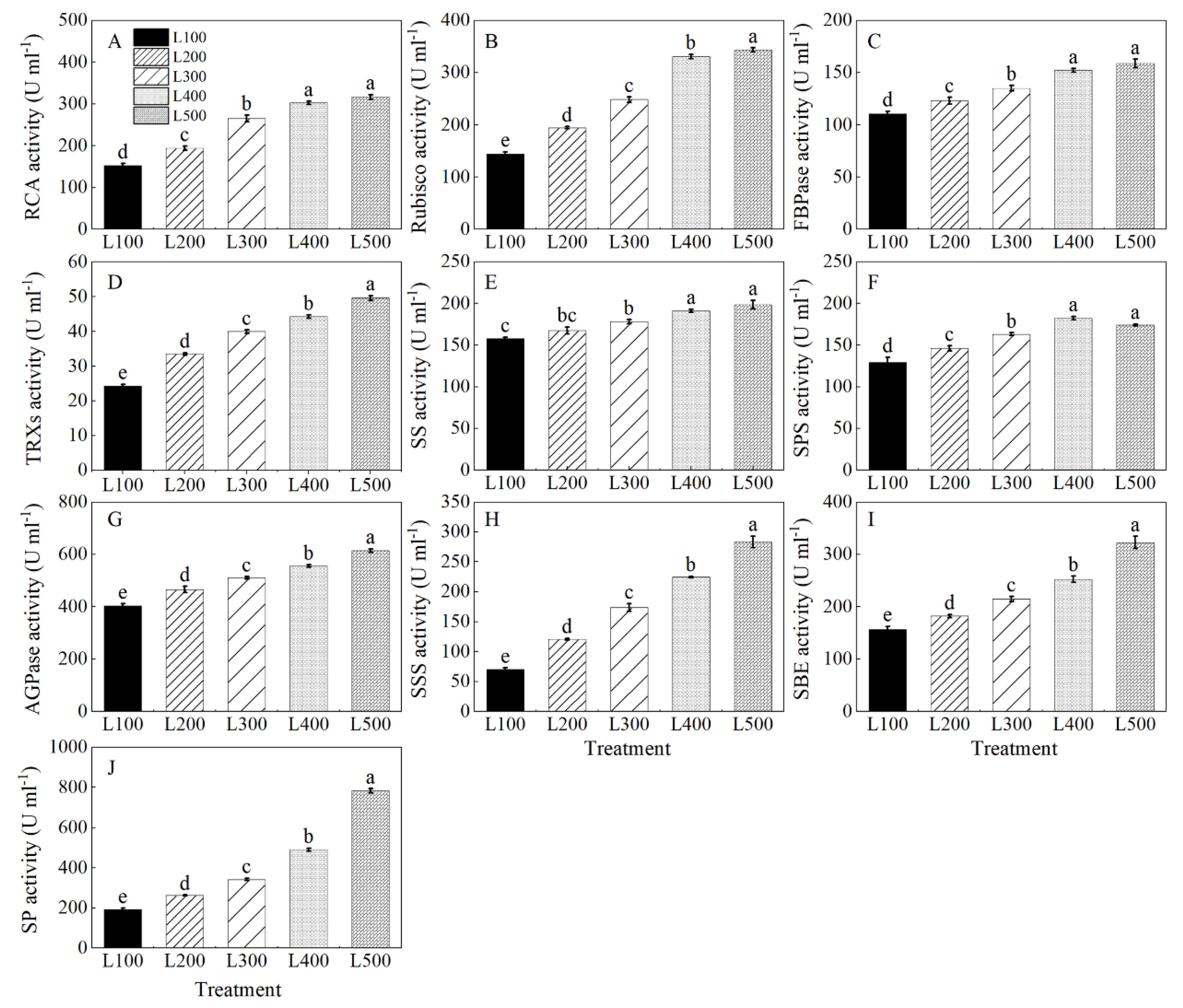
2.5. Gene Expression and Enzymatic Activity
3. Discussion
3.1. Light Intensity Affects Morphological Characteristics
3.2. Effect of Light Intensity on Chlorophyll Content and Chlorophyll Fluorescence Characteristics
3.3. Effect of Light Intensity on Photosynthetic Characteristics and Carbohydrate Accumulation
3.4. Effect of Light Intensity on Enzymatic Activity
4. Conclusions
5. Materials and Methods
5.1. Plant Material and Growth Conditions
5.2. Plant Morphology Parameters
5.3. Photosynthetic Pigment Content and Leaf Nitrogen Content
5.4. Photosynthesis Parameters
5.5. Chlorophyll Fluorescence Measurements
5.6. Soluble Sugar, Sucrose and Starch Content
5.7. Enzyme Activity
5.8. Real-Time Quantitative PCR Verification
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, F.; Feng, L.; Liu, Q.; Wu, X.; Fan, Y.; Raza, M.A.; Cheng, Y.; Chen, J.; Wang, X.; Yong, T.; et al. Effect of interactions between light intensity and red-to- far-red ratio on the photosynthesis of soybean leaves under shade condition. Environ. Exp. Bot. 2018, 150, 79–87. [Google Scholar] [CrossRef]
- Wu, Y.S.; Gong, W.Z.; Yang, W.Y. Shade Inhibits leaf size by controlling cell proliferation and enlargement in soybean. Sci. Rep. 2017, 7, 9259. [Google Scholar] [CrossRef]
- Ciolfi, A.; Sessa, G.; Sassi, M.; Possenti, M.; Salvucci, S.; Carabelli, M.; Morelli, G.; Ruberti, I. Dynamics of the shade-avoidance response in Arabidopsis. Plant Physiol. 2013, 163, 331–353. [Google Scholar] [CrossRef] [Green Version]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Hitz, T.; Hartung, J.; Graeff-Honninger, S.; Munz, S. Morphological response of soybean (Glycine max (L.) Merr.) cultivars to light intensity and red to far-red ratio. Agronomy 2019, 9, 428. [Google Scholar] [CrossRef] [Green Version]
- Mauser, H.; King, W.A.; Gready, J.E.; Andrews, T.J. CO2 fixation by Rubisco: Computational dissection of the key steps of carboxylation, hydration, and C-C bond cleavage. J. Am. Chem. Soc. 2001, 123, 10821–10829. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Pang, T.; Iqbal, N.; Shafiq, I.; Skalicky, M.; Brestic, M.; Safdar, M.E.; Mumtaz, M.; Ahmad, A.; Asghar, M.A.; et al. Acclimation strategy and plasticity of different soybean genotypes in intercropping. Funct. Plant Biol. 2020, 47, 592–610. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.A.; Feng, L.Y.; Iqbal, N.; Ahmed, M.; Chen, Y.K.; Bin Khalid, M.H.; Din, A.M.U.; Khan, A.; Ijaz, W.; Hussain, A.; et al. Growth and development of soybean under changing light environments in relay intercropping system. PeerJ 2019, 7, 7262. [Google Scholar] [CrossRef]
- Chen, T.T.; Zhang, H.J.; Zeng, R.E.; Wang, X.Y.; Huang, L.P.; Wang, L.D.; Wang, X.W.; Zhang, L. Shade effects on peanut yield associate with physiological and expressional regulation on photosynthesis and sucrose metabolism. Int. J. Mol. Sci. 2020, 21, 5284. [Google Scholar] [CrossRef]
- Patel, S.; Bartel, C.A.; Lenssen, A.W.; Moore, K.J.; Berti, M.T. Stem density, productivity, and weed community dynamics in corn-alfalfa intercropping. Agronomy 2021, 11, 1696. [Google Scholar] [CrossRef]
- Rascher, U.; Liebig, M.; Lüttge, U. Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ. 2010, 23, 1397–1405. [Google Scholar] [CrossRef]
- Hu, L.L.; Liao, W.B.; Dawuda, M.M.; Yu, J.H.; Lv, J. Appropriate NH4+: NO3− ratio improves low light tolerance of mini Chinese cabbage seedlings. BMC Plant Biol. 2017, 17, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, X.L.; Mao, S.L.; Wang, L.H.; Zhang, B.X.; Zhang, Z.X. Effect of low light on the characteristics of photosynthesis and chlorophyll a fluorescence during leaf development of sweet pepper. J. Integr. Agric. 2012, 11, 1633–1643. [Google Scholar] [CrossRef]
- Sheth, B.P.; Thaker, V.S. In silico analyses of Rubisco enzymes from different classes of algae. Int. Res. J. Biol. Sci. 2014, 3, 11–17. [Google Scholar]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef]
- Redondo-Gomez, S.; Mateos-Naranjo, E.; Davy, A.J.; Fernandez-Munoz, F.; Castellanos, E.M.; Luque, T.; Figueroa, M.E. Growth and photosynthetic responses to salinity of the salt-marsh shrub Atriplex portulacoides. Ann. Bot. 2007, 100, 555–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.L.; Sui, X.L.; Huang, H.Y.; Wang, S.X.; Wei, Y.X.; Zhang, Z.X. Low light stress down-regulated Rubisco gene expression and photosynthetic capacity during cucumber (Cucumis sativus L.) leaf development. J. Integr. Agric. 2014, 13, 997–1007. [Google Scholar] [CrossRef] [Green Version]
- Pan, T.; Wang, Y.; Wang, L.; Ding, J.; Cao, Y.; Qin, G.; Yan, L.; Xi, L.; Zhang, J.; Zou, Z. Increased CO2 and light intensity regulate growth and leaf gas exchange in tomato. Physiol. Plant. 2020, 168, 694–708. [Google Scholar] [CrossRef]
- Feng, L.Y.; Raza, M.A.; Li, Z.C.; Chen, Y.K.; Khalid, M.H.B.; Du, J.B.; Liu, W.G.; Wu, X.L.; Song, C.; Yu, L.; et al. The influence of light Intensity and leaf movement on photosynthesis characteristics and carbon balance of soybean. Front. Plant Sci. 2019, 9, 1952. [Google Scholar] [CrossRef]
- Liu, M.G.; Mu, L.; Lu, Y.X.; Yang, H.M. Forage accumulation and radiation use of alfalfa under deficit irrigation. Crop Sci. 2021, 61, 2190–2202. [Google Scholar] [CrossRef]
- Vasileva, V.; Kostov, O. Effect of mineral and organic fertilization on alfalfa forage and soil fertility. Emir. J. Food Agric. 2015, 27, 678–686. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.X.; Zhao, H.M.; Liu, G.B.; Li, Y.; Li, S.J.; Zhang, Y.J.; Liu, N.; Ma, L. Alfalfa and silage maize intercropping provides comparable productivity and profitability with lower environmental impacts than wheat-maize system in the North China plain. Agric. Syst. 2022, 195, 103305. [Google Scholar] [CrossRef]
- Xu, R.X.; Zhao, H.M.; Liu, G.B.; You, Y.L.; Ma, L.; Liu, N.; Zhang, Y.J. Effects of nitrogen and maize plant density on forage yield and nitrogen uptake in an alfalfa-silage maize relay intercropping system in the North China Plain. Field Crops Res. 2021, 263, 108068. [Google Scholar] [CrossRef]
- Lorenzo, C.D.; Iserte, J.A.; Lamas, M.S.; Antonietti, M.S.; Gagliardi, P.G.; Hernando, C.E.; Dezar, C.A.A.; Vazquez, M.; Casal, J.J.; Yanovsky, M.J.; et al. Shade delays flowering in Medicago sativa. Plant J. 2019, 99, 7–22. [Google Scholar] [CrossRef] [Green Version]
- Pan, T.H.; Ding, J.J.; Qin, G.G.; Wang, Y.L.; Xi, L.J.; Yang, J.W.; Li, J.M.; Zhang, J.; Zou, Z.R. Interaction of supplementary light and CO2 enrichment improves growth, photosynthesis, yield, and quality of tomato in autumn through spring greenhouse production. HortScience 2019, 54, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Larbi, A.; Vazquez, S.; El-Jendoubi, H.; Msallem, M.; Abadia, J.; Abadia, A.; Morales, F. Canopy light heterogeneity drives leaf anatomical, eco-physiological, and photosynthetic changes in olive trees grown in a high-density plantation. Photosynth. Res. 2015, 123, 141–155. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Khalid, M.; Jan, F.; Rahman, S.U.; Jiang, X.; Yu, X. Effects of light-regulation and intensity on the growth, physiological and biochemical properties of Aralia elata (Miq.) seedlings. S. Afr. J. Bot. 2019, 121, 456–462. [Google Scholar] [CrossRef]
- Wu, Y.S.; Gong, W.Z.; Yang, F.; Wang, X.C.; Yong, T.W.; Yang, W.Y. Responses to shade and subsequent recovery of soybean in maize-soybean relay strip intercropping. Plant Prod. Sci. 2016, 19, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Song, K.S.; Jeon, K.S.; Choi, K.S.; Chang, H.K.; Kim, J.J. Characteristics of photosynthesis and leaf growth of Peucedanum japonicum by leaf mold and shading level in forest farming. Korean J. Med. Crop Sci. 2015, 23, 43–48. [Google Scholar] [CrossRef]
- Dong, J.; Li, X.; Duan, Z.Q. Biomass allocation and organs growth of cucumber (Cucumis sativus L.) under elevated CO2 and different N supply. Korean J. Med. Crop Sci. 2016, 62, 277–288. [Google Scholar]
- da Cruz, P.J.R.; Santos, M.V.; da Silva, L.D.; Ferreira, E.A.; Magalhaes, M.A.; Martuscello, J.A.; de Fonseca, D.M. Morphogenetic, physiological, and productive of forage peanut responses to shading. Pesquisa Agropecuária Brasileira 2020, 55, e01746. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Bawa, G.; Feng, L.Y.; Chen, G.P.; Chen, H.; Hu, Y.; Pu, T.; Cheng, Y.J.; Shi, J.Y.; Xiao, T.; Zhou, W.G.; et al. Gibberellins and auxin regulate soybean hypocotyl elongation under low light and high-temperature interaction. Physiol. Plant. 2020, 170, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Gao, M.F.; He, R.; Zhang, Y.T.; Song, S.W.; Su, W.; Liu, H.C. Far-red light suppresses glucosinolate profiles of Chinese kale through inhibiting genes related to glucosinolate biosynthesis. Environ. Exp. Bot. 2021, 188, 104507. [Google Scholar] [CrossRef]
- Wang, Y.B.; Huang, R.D.; Zhou, Y.F. Effects of shading stress during the reproductive stages on photosynthetic physiology and yield characteristics of peanut (Arachis hypogaea Linn.). J. Integr. Agric. 2021, 20, 1250–1265. [Google Scholar] [CrossRef]
- Ren, B.Z.; Cui, H.Y.; Camberato, J.J.; Dong, S.T.; Liu, P.; Zhao, B.; Zhang, J.W. Effects of shading on the photosynthetic characteristics and mesophyll cell ultrastructure of summer maize. Sci. Nat. 2016, 103, 67. [Google Scholar] [CrossRef]
- Wang, X.; Chen, G.D.; Du, S.J.; Wu, H.X.; Fu, R.; Yu, X.B. Light Intensity Influence on Growth and Photosynthetic Characteristics of Horsfieldia hainanensis. Front. Ecol. Evol. 2021, 9, 636804. [Google Scholar] [CrossRef]
- Yi, Z.H.; Cui, J.J.; Fu, Y.M.; Liu, H. Effect of different light intensity on physiology, antioxidant capacity and photosynthetic characteristics on wheat seedlings under high CO2 concentration in a closed artificial ecosystem. Photosynth. Res. 2020, 144, 23–34. [Google Scholar] [CrossRef]
- Neda, L.; Michael, K.; Jörg, F. Interaction effect between elevated CO2 and fertilization on biomass, gas exchange and C/N ratio of European beech (Fagus sylvatica L.). Plants 2016, 5, 38. [Google Scholar]
- Zhong, X.M.; Shi, Z.S.; Li, F.H.; Huang, H.J. Photosynthesis and chlorophyll fluorescence of infertile and fertile stalks of paired near-isogenic lines in maize (Zea mays L.) under shade conditions. Photosynthetica 2014, 52, 597–603. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Z.; Zhao, B.; Liu, P.; Zhang, J.W. Physiological and comparative proteomic analysis provides new insights into the effects of shade stress in maize (Zea mays L.). BMC Plant Biol. 2020, 20, 60. [Google Scholar] [CrossRef] [PubMed]
- Loconsole, D.; Cocetta, G.; Santoro, P.; Ferrante, A. Optimization of LED lighting and quality evaluation of romaine lettuce grown in an innovative indoor cultivation system. Sustainability 2019, 11, 841. [Google Scholar] [CrossRef] [Green Version]
- Jafarikouhini, N.; Pradhan, D.; Sinclair, T.R. Basis of limited-transpiration rate under elevated vapor pressure deficit and high temperature among sweet corn cultivars. Environ. Exp. Bot. 2020, 179, 104205. [Google Scholar] [CrossRef]
- Michalska, J.; Zauber, H.; Buchanan, B.B.; Cejudo, F.J.; Geigenberger, P. NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc. Natl. Acad. Sci. USA 2009, 106, 9908–9913. [Google Scholar] [CrossRef] [Green Version]
- Dayer, S.; Murcia, G.; Prieto, J.A.; Duran, M.; Martinez, L.; Piccoli, P.; Pena, J.P. Non-structural carbohydrates and sugar export in grapevine leaves exposed to different light regimes. Physiol. Plant. 2021, 171, 728–738. [Google Scholar] [CrossRef]
- Fernandez, O.; Ishihara, H.; George, G.M.; Mengin, V.; Flis, A.; Sumner, D.; Arrivault, S.; Feil, R.; Lunn, J.E.; Zeeman, S.C. Foliar starch turnover occurs in long days and in falling light at the end of the day. Plant Physiol. 2017, 174, 00601. [Google Scholar] [CrossRef] [Green Version]
- Breuer, G.; Martens, D.E.; Draaisma, R.B.; Wijffels, R.H.; Lamers, P.P. Photosynthetic efficiency and carbon partitioning in nitrogen-starved Scenedesmus obliquus. Algal Res. 2015, 9, 254–262. [Google Scholar] [CrossRef]
- Schmitz, J.; Heinrichs, L.; Scossa, F.; Fernie, A.R.; Oelze, M.L.; Dietz, K.J.; Rothbart, M.; Grimm, B.; Flugge, U.I.; Hausler, R.E. The essential role of sugar metabolism in the acclimation response of Arabidopsis thaliana to high light intensities. J. Exp. Bot. 2014, 65, 1619–1636. [Google Scholar] [CrossRef]
- Jian, Y.; Wu, G.L.; Zhou, D.H.; Hu, Z.Q.; Quan, Z.X.; Zhou, B.Y. Effects of shading on carbohydrates of Syzygium samarangense. Not. Bot. Horti Agrobot. 2019, 47, 1252–1257. [Google Scholar] [CrossRef] [Green Version]
- Mengin, V.; Pyl, E.T.; Moraes, T.A.; Sulpice, R.; Krohn, N.; Encke, B.; Stitt, M. Photosynthate partitioning to starch in Arabidopsis thaliana is insensitive to light intensity but sensitive to photoperiod due to a restriction on growth in the light in short photoperiods. Plant Cell Environ. 2017, 40, 2608–2627. [Google Scholar] [CrossRef]
- Kono, T.; Mehrotra, S.; Endo, C.; Kizu, N.; Matusda, M.; Kimura, H.; Mizohata, E.; Inoue, T.; Hasunuma, T.; Yokota, A.; et al. A RuBisCO-mediated carbon metabolic pathway in methanogenic archaea. Nat. Commun. 2017, 8, 14007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyagi, A.K.; Gaur, T. Light regulation of nuclear photosynthetic genes in higher plants. Crit. Rev. Plant Sci. 2003, 22, 417–452. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Cun, Z.; Chen, J.W. Photosynthetic performance and photosynthesis-related gene expression coordinated in a shade-tolerant species Panax notoginseng under nitrogen regimes. BMC Plant Biol. 2020, 20, 273. [Google Scholar] [CrossRef] [PubMed]
- Carmo-Silva, A.E.; Salvucci, M.E. The regulatory properties of rubisco activase differ among species and affect photosynthetic induction during light transitions. Plant Physiol. 2013, 161, 1645–1655. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Kallis, R.P.; Ewy, R.G.; Portis, A.R. Light modulation of Rubisco in Arabidopsis requires a capacity for redox regulation of the larger Rubisco activase isoform. Proc. Natl. Acad. Sci. USA 2002, 99, 3330–3334. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, E.; Galvis, V.C.; Armbruster, U. Efficient photosynthesis in dynamic light environments: A chloroplast’s perspective. Biochem. J. 2019, 476, 2725–2741. [Google Scholar] [CrossRef] [Green Version]
- Eliyahu, E.; Rog, I.; Inbal, D.; Danon, A. ACHT4-driven oxidation of APS1 attenuates starch synthesis under low light intensity in Arabidopsis plants. Proc. Natl. Acad. Sci. USA 2015, 112, 12876–12881. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.H.; Zhang, Y.; Xiao, Y.; Liu, F.F.; Wang, M.H.; Zhu, X.G.; Liu, P.; Sun, Q.; Wang, W.Q.; Peng, M.; et al. Transcriptome response of cassava leaves under natural shade. Sci. Rep. 2016, 6, 31673. [Google Scholar] [CrossRef] [Green Version]
- Bahaji, A.; Sanchez-Lopez, A.M.; De Diego, N.; Munoz, F.J.; Baroja-Fernandez, E.; Li, J.; Ricarte-Bermejo, A.; Baslam, M.; Aranjuelo, I.; Almagro, G.; et al. Plastidic phosphoglucose isomerase is an important determinant of starch accumulation in mesophyll cells, growth, photosynthetic capacity, and biosynthesis of plastidic cytokinins in Arabidopsis. PLoS ONE 2015, 10, e0119641. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.Y.; Chen, J.J.; Sun, X.M.; Li, J.X.; Chen, N.L. Inhibition of sucrose and galactosyl-sucrose oligosaccharide metabolism in leaves and fruits of melon (Cucumis melo L.) under low light stress. Sci. Hortic. 2019, 244, 343–351. [Google Scholar] [CrossRef]
- Wang, J.; Shi, K.; Lu, W.P.; Lu, D.L. Effects of post-silking shading stress on enzymatic activities and phytohormone contents during grain development in spring maize. J. Plant Growth Regul. 2021, 40, 1060–1073. [Google Scholar] [CrossRef]
- Pashkovskiy, P.; Kreslavski, V.D.; Ivanov, Y.; Ivanova, A.; Kartashov, A.; Shmarev, A.; Strokina, V.; Kuznetsov, V.V.; Allakhverdiev, S.I. Influence of light of different spectral compositions on the growth, photosynthesis, and expression of light-dependent genes of scots pine seedlings. Cells 2021, 10, 3284. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Baskin, C.C.; Baskin, J.M.; Nan, Z.B. Plastic film mulching improves seed germination, seedling development and potential for perenniality of Vicia unijuga under subalpine climate conditions. Crop Pasture Sci. 2020, 71, 592–609. [Google Scholar] [CrossRef]
- Shi, H.R.; Wang, B.; Yang, P.J.; Li, Y.B.; Miao, F. Differences in sugar accumulation and mobilization between sequential and non-sequential senescence wheat cultivars under natural and drought conditions. PLoS ONE 2016, 11, e0166155. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xin, G.F.; Liu, C.; Shi, Q.H.; Yang, F.J.; Wei, M. Effects of red and blue light on leaf anatomy, CO2 assimilation and the photosynthetic electron transport capacity of sweet pepper (Capsicum annuum L.) seedlings. BMC Plant Biol. 2020, 20, 318. [Google Scholar] [CrossRef]
- Kenneth, J.L.; Thomas, D.S. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2002, 25, 402–408. [Google Scholar]
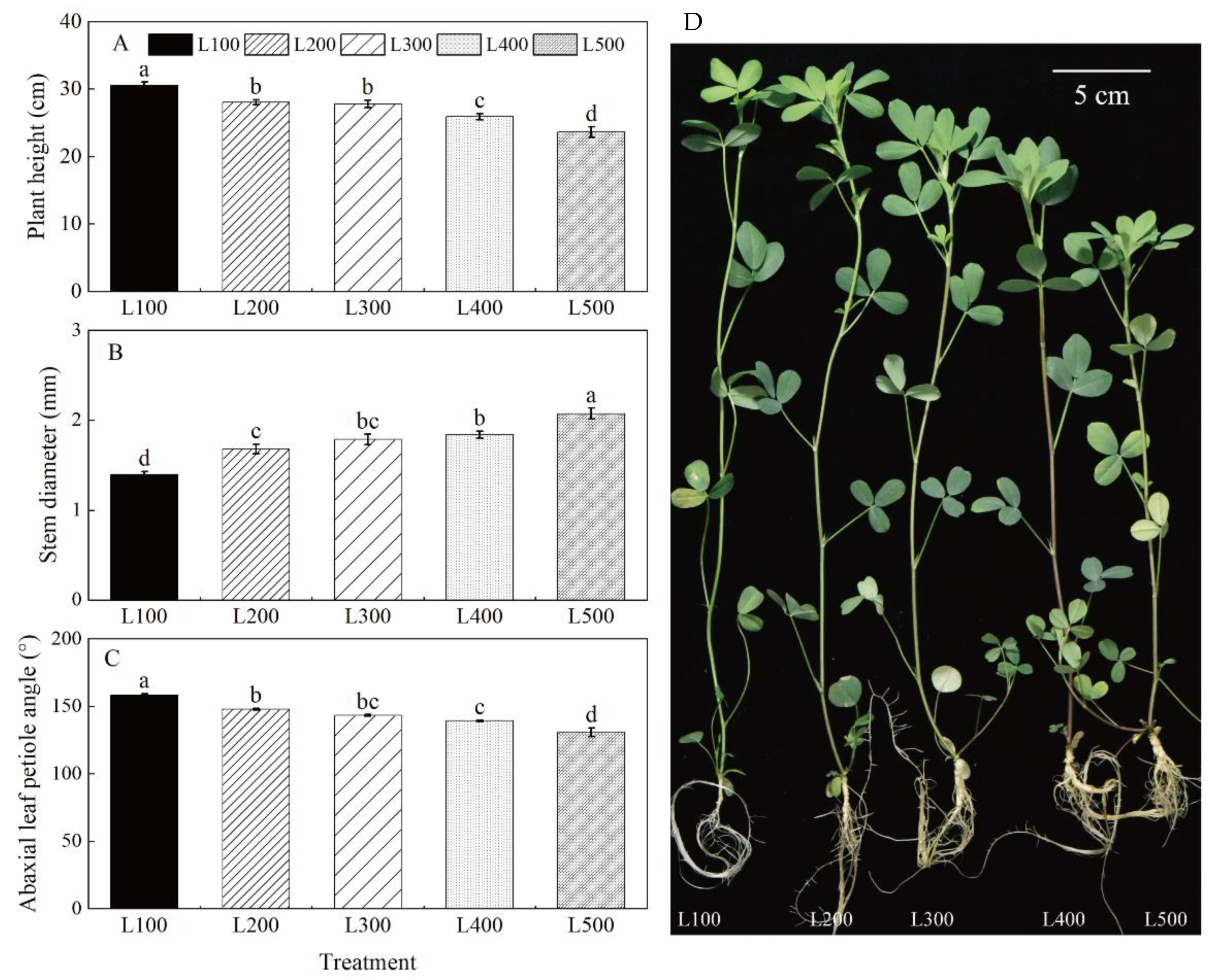
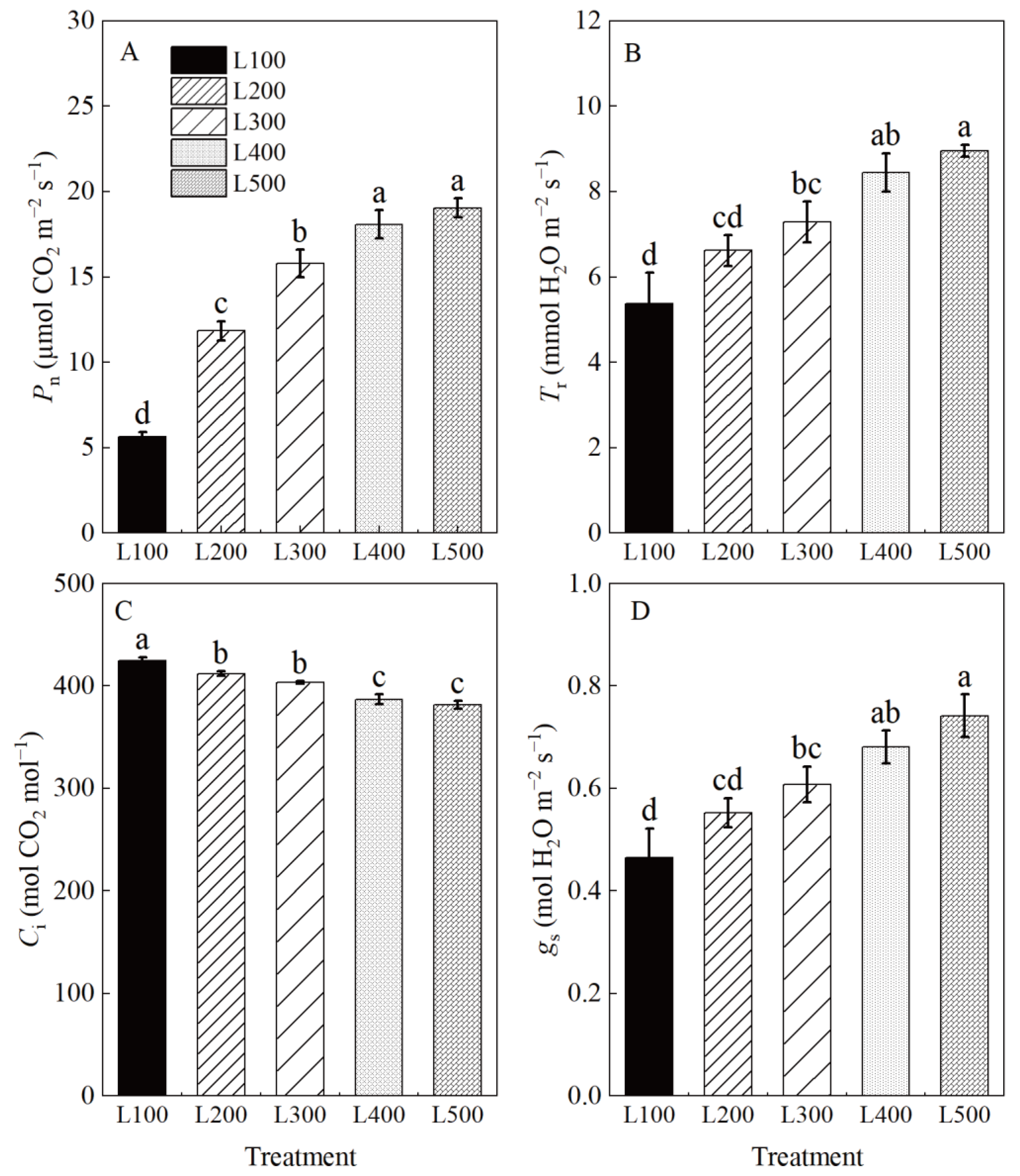

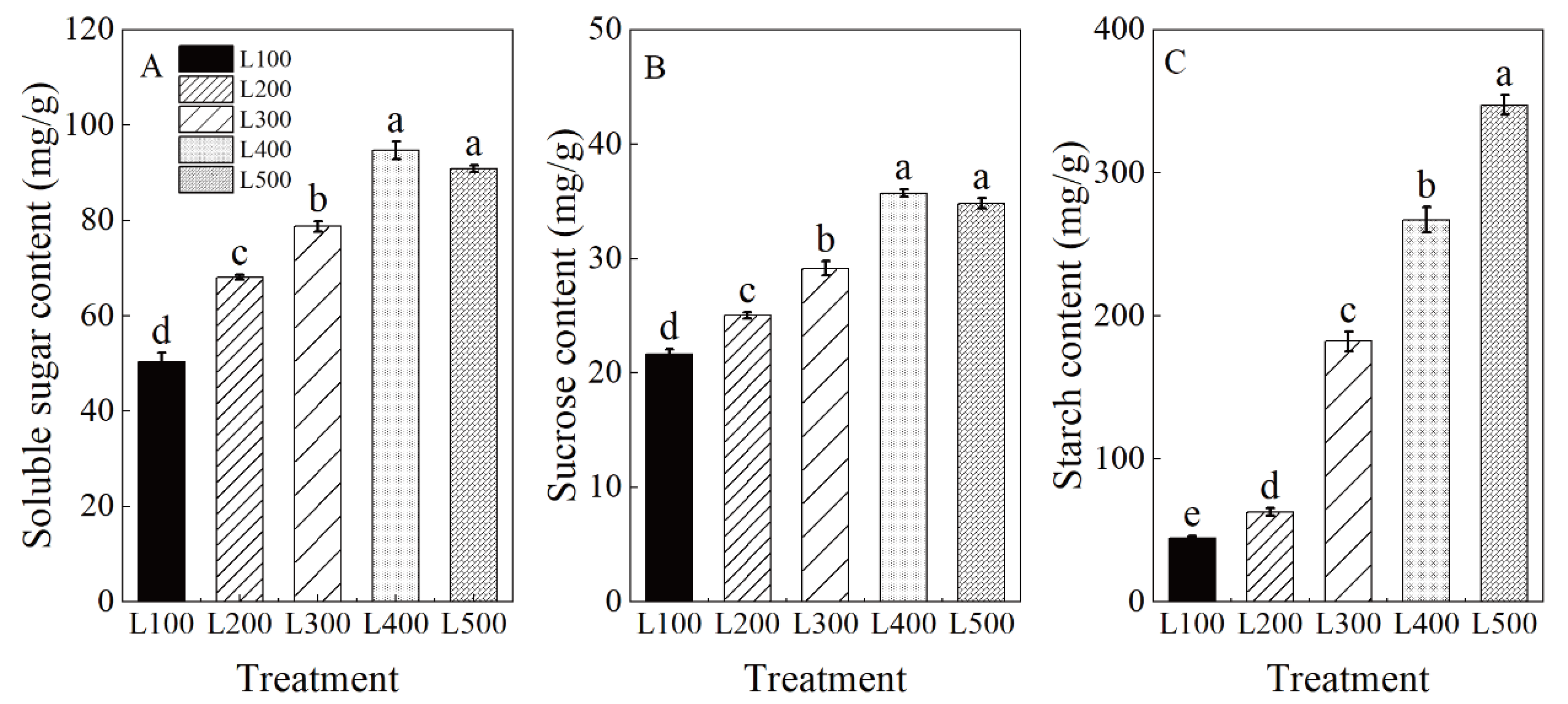
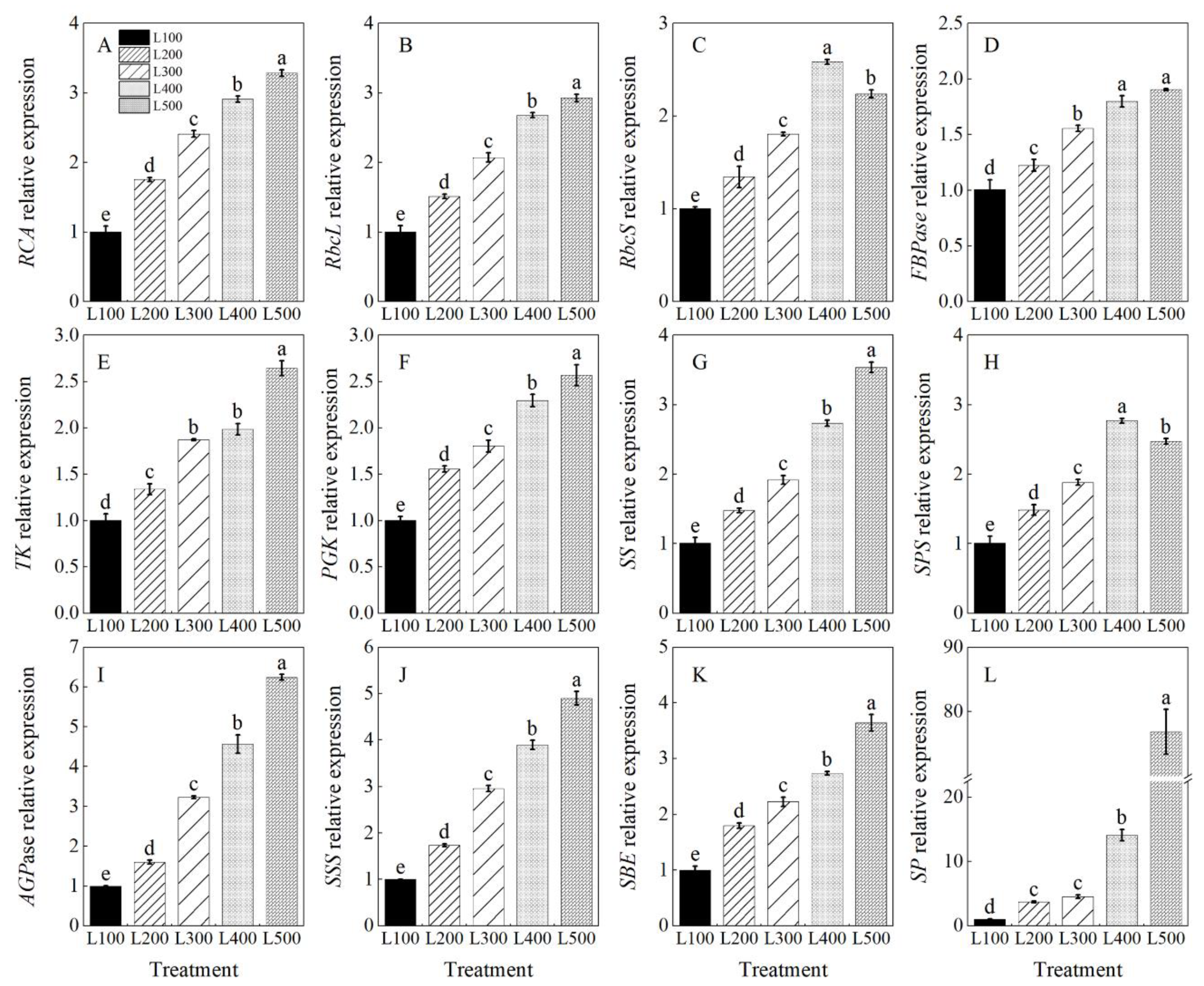
| Treatment a | SLA | SDM | RDM | RSR |
|---|---|---|---|---|
| L100 | 0.474a (0.023) | 205.4d (11.8) | 22.0e (1.3) | 0.108c (0.007) |
| L200 | 0.371b (0.021) | 227.6d (17.7) | 30.6d (2.2) | 0.137b (0.013) |
| L300 | 0.269c (0.015) | 320.4c (21.5) | 54.2c (2.6) | 0.170a (0.004) |
| L400 | 0.125d (0.004) | 379.4b (10.6) | 70.0b (3.2) | 0.185a (0.007) |
| L500 | 0.117d (0.002) | 461.4a (15.8) | 82.2a (1.8) | 0.179a (0.007) |
| Treatment a | RL | RSA | RV | RD |
|---|---|---|---|---|
| L100 | 206.4e (7.2) | 18.6e (0.7) | 0.229e (0.011) | 0.243c (0.013) |
| L200 | 397.1d (6.2) | 44.4d (1.0) | 0.787d (0.009) | 0.325b (0.006) |
| L300 | 426.8c (8.7) | 58.0c (2.2) | 1.298c (0.081) | 0.418a (0.012) |
| L400 | 474.6b (11.2) | 67.0b (2.8) | 1.650b (0.083) | 0.430a (0.009) |
| L500 | 583.1a (5.7) | 84.4a (2.7) | 2.349a (0.179) | 0.449a (0.010) |
| Treatment a | Chl a | Chl b | Car | Chl a + b | Chl a/b | LNC |
|---|---|---|---|---|---|---|
| L100 | 36.5a (1.7) | 13.1a (0.3) | 6.91a (0.24) | 49.6a (1.8) | 2.80b (0.14) | 38.0a (1.1) |
| L200 | 34.1ab (2.0) | 11.5ab (0.9) | 6.19b (0.12) | 45.6ab (2.9) | 2.98ab (0.07) | 28.0b (0.5) |
| L300 | 31.0bc (1.7) | 10.3bc (0.8) | 6.15b (0.21) | 41.9bc (2.4) | 3.10ab (0.12) | 18.7c (0.2) |
| L400 | 29.7bc (1.1) | 9.4c (0.4) | 6.11b (0.13) | 39.1c (1.4) | 3.18a (0.12) | 17.4c (0.6) |
| L500 | 28.6c (1.2) | 8.7c (0.5) | 5.49c (0.29) | 37.3c (1.6) | 3.29a (0.12) | 18.8c (0.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Guo, H.; Baskin, C.C.; Xiong, W.; Yang, C.; Li, Z.; Song, H.; Wang, T.; Yin, J.; Wu, X.; et al. Effect of Light Intensity on Morphology, Photosynthesis and Carbon Metabolism of Alfalfa (Medicago sativa) Seedlings. Plants 2022, 11, 1688. https://doi.org/10.3390/plants11131688
Tang W, Guo H, Baskin CC, Xiong W, Yang C, Li Z, Song H, Wang T, Yin J, Wu X, et al. Effect of Light Intensity on Morphology, Photosynthesis and Carbon Metabolism of Alfalfa (Medicago sativa) Seedlings. Plants. 2022; 11(13):1688. https://doi.org/10.3390/plants11131688
Chicago/Turabian StyleTang, Wei, Haipeng Guo, Carol C. Baskin, Wangdan Xiong, Chao Yang, Zhenyi Li, Hui Song, Tingru Wang, Jianing Yin, Xueli Wu, and et al. 2022. "Effect of Light Intensity on Morphology, Photosynthesis and Carbon Metabolism of Alfalfa (Medicago sativa) Seedlings" Plants 11, no. 13: 1688. https://doi.org/10.3390/plants11131688
APA StyleTang, W., Guo, H., Baskin, C. C., Xiong, W., Yang, C., Li, Z., Song, H., Wang, T., Yin, J., Wu, X., Miao, F., Zhong, S., Tao, Q., Zhao, Y., & Sun, J. (2022). Effect of Light Intensity on Morphology, Photosynthesis and Carbon Metabolism of Alfalfa (Medicago sativa) Seedlings. Plants, 11(13), 1688. https://doi.org/10.3390/plants11131688







