Acaricidal Activity of Bufadienolides Isolated from Drimia pancration against Tetranychus urticae, and Structural Elucidation of Arenobufagin-3-O-α-L-rhamnopyranoside
Abstract
:1. Introduction
2. Results and Discussion
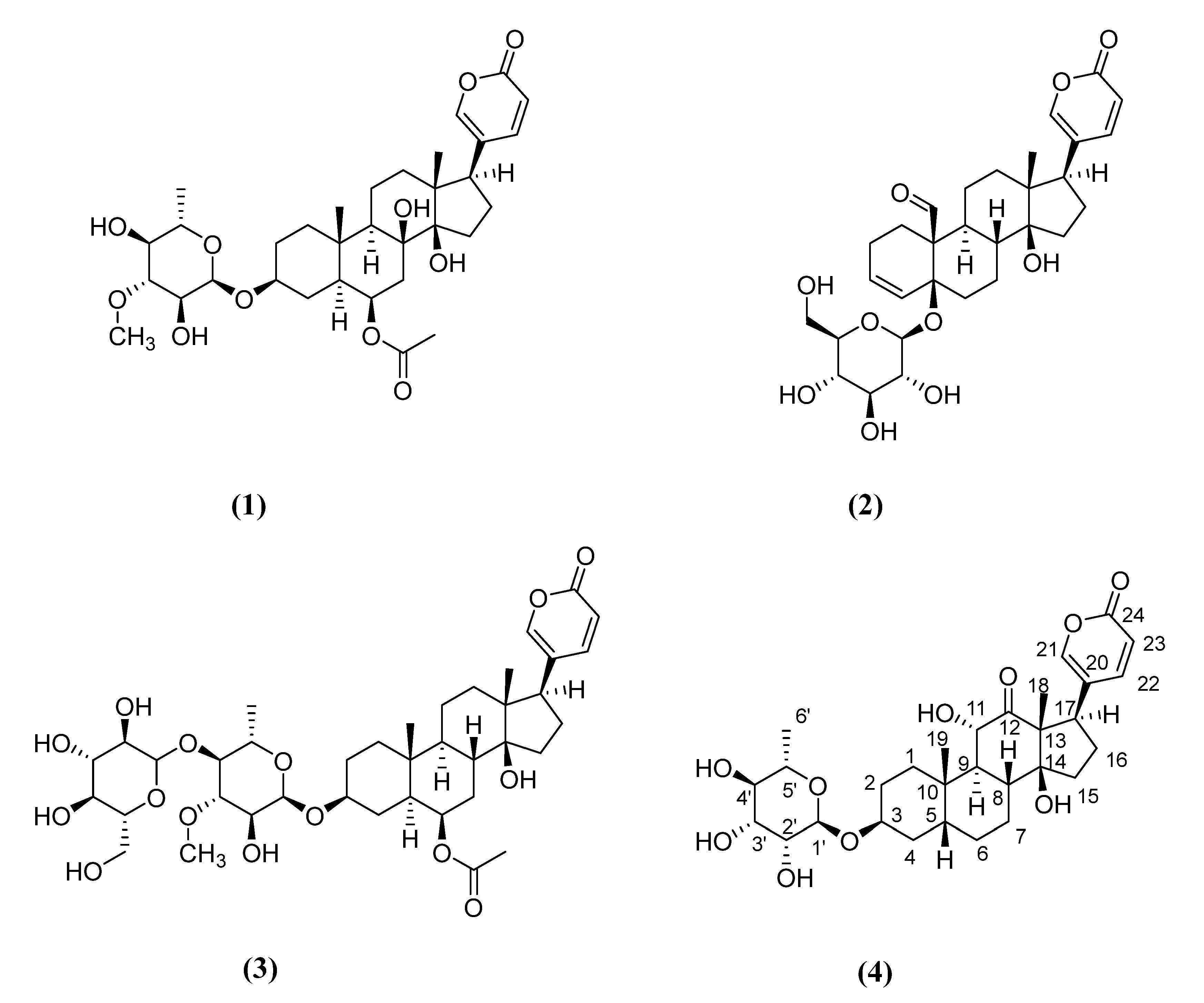
2.1. Chemical Compounds
2.2. Acaricidal Effect
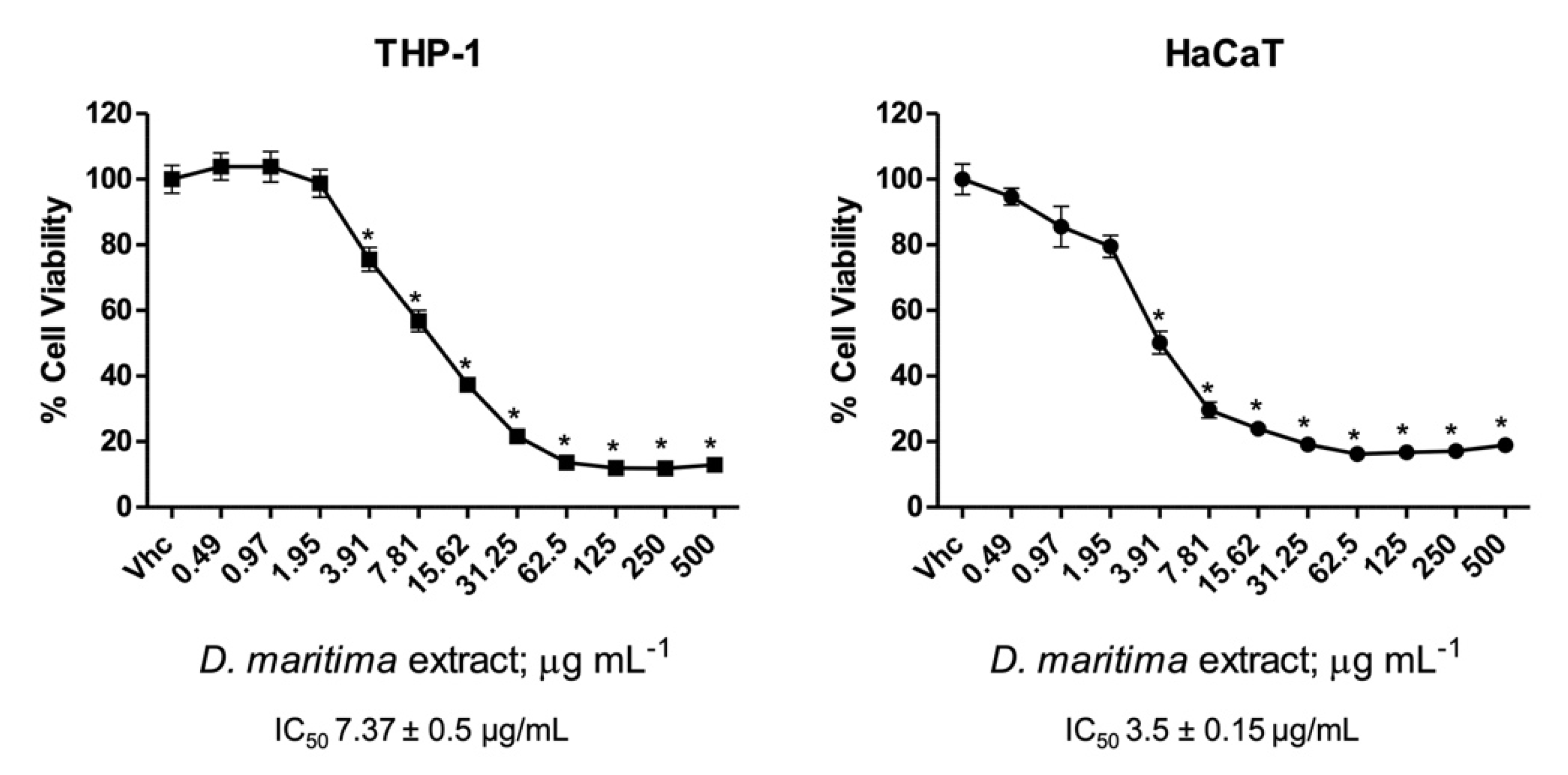
2.3. Cytotoxicity Assay
3. Materials and Methods
3.1. Plant Material
3.2. Extraction, Isolation, and General Experimental Procedures
Arenobufagin-3-O-α-L-rhamnopyranoside (4)
3.3. Mites
3.4. Acaricidal Activity
3.5. Cytotoxicity on Vertebrate Cells
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Xu, X.; Ullah, F.; Ding, Q.; Gao, X.; Desneux, N.; Song, D. Comparison of full-length transcriptomes of different imidacloprid-resistant strains of Rhopalosiphum padi (L.). Entomol. Gen. 2021, 41, 289–304. [Google Scholar] [CrossRef]
- Leska, A.; Nowak, A.; Nowak, I.; Gòrczynska, A. Effects of insecticides and microbiological contaminants on Apis mellifera health. Molecules 2021, 26, 5080. [Google Scholar] [CrossRef]
- Ricupero, M.; Desneux, N.; Zappalà, L.; Biondi, A. Target and non-target impact of systemic insecticides on a polyphagous aphid pest and its parasitoid. Chemosphere 2020, 247, 125728. [Google Scholar] [CrossRef] [PubMed]
- Passos, L.C.; Soares, M.A.; Collares, L.J.; Malagoli, I.; Desneux, N.; Carvalho, G.A. Lethal, sublethal and transgenerational effects of insecticides on Macrolophus basicornis, predator of Tuta absoluta. Entomol. Gen. 2018, 38, 127–143. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Dermauw, W.; Tirry, L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochem. Mol. Biol. 2010, 40, 563–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adesanya, A.W.; Lavine, M.D.; Moural, T.W.; Lavine, L.C.; Zhu, F.; Walsh, D.B. Mechanisms and management of acaricide resistance for Tetranychus urticae in agroecosystems. J. Pest Sci. 2021, 94, 639–663. [Google Scholar] [CrossRef]
- Whalon, M.E.; Mota-Sanchez, R.M.; Hollingworth, R.M.; Duynslager, L. Artrhopods Resistant to Pesticides Database (ARPD). Available online: http://www.pesticideresistance.org (accessed on 2 May 2022).
- Van Leeuwen, T.; Tirry, L.; Yamamoto, A.; Nauen, R.; Dermauw, W. The economic importance of acaricides in the control of phytophagous mites and an update on recent acaricide mode of action research. Pestic. Biochem. Physiol. 2015, 121, 12–21. [Google Scholar] [CrossRef]
- Migeon, A.; Nouguier, E.; Dorkeld, F. Spider Mites Web: A comprehensive database for the Tetranychidae. In Trends in Acarology; Springer: Dordrecht, The Netherlands, 2010; pp. 557–560. [Google Scholar]
- Cazaux, M.; Navarro, M.; Bruinsma, K.A.; Zhurov, V.; Negrave, T.; Van Leeuwen, T.; Grbic, M. Application of two-spotted spider mite Tetranychus urticae for plant-pest interaction studies. J. Vis. Exp. 2014, 89, e51738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavela, R.; Morshedloo, M.R.; Mumivand, H.; Khorsand, G.J.; Karami, A.; Maggi, F.; Benelli, G. Phenolic monoterpene-rich essential oils from Apiaceae and Lamiaceae species: Insecticidal activity and safety evaluation on non-target earthworms. Entomol. Gen. 2020, 40, 421–435. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Canale, A.; Nicoletti, M.; Petrelli, R.; Cappellacci, L.; Galassi, R.; Maggi, F. Isofuranodiene and germacrone from Smyrnium olusatrum essential oil as acaricides and oviposition inhibitors against Tetranychus urticae: Impact of chemical stabilization of isofuranodiene by interaction with silver triflate. J. Pest Sci. 2017, 90, 693–699. [Google Scholar] [CrossRef]
- Pavela, R.; Murugan, K.; Canale, A.; Benelli, G. Saponaria officinalis-synthesized silver nanocrystals as effective biopesticides and oviposition inhibitors against Tetranychus urticae Koch. Ind. Crop. Prod. 2017, 97, 338–344. [Google Scholar] [CrossRef]
- Pavela, R.; Dall’Acqua, S.; Sut, S.; Baldan, V.; Kamte, S.L.N.; Nya, P.C.B.; Benelli, G. Oviposition inhibitory activity of the Mexican sunflower Tithonia diversifolia (Asteraceae) polar extracts against the two-spotted spider mite Tetranychus urticae (Tetranychidae). Physiol. Mol. Plant Pathol. 2018, 101, 85–92. [Google Scholar] [CrossRef]
- Sut, S.; Pavela, R.; Kolarčik, V.; Cappellacci, L.; Petrelli, R.; Maggi, F.; Benelli, G. Identification of Onosma visianii roots extract and purified shikonin derivatives as potential acaricidal agents against Tetranychus urticae. Molecules 2017, 22, 1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavela, R.; Benelli, G. Essential oils as eco-friendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Euro Med Plant Base. Available online: https://ww2.bgbm.org/EuroPlusMed/query.asp (accessed on 30 April 2022).
- Mitsuhashi, H.; Tanaka, O.; Nozoe, S.; Nagai, M. Chemistry of Organic Natural Products, 4th ed.; Nankoudou Press: Tokyo, Japan, 1994; pp. 168–174. [Google Scholar]
- O’connor, M.G.; Buck, R.E.; Fellers, C.R. Red squill investigations properties, toxicity, and palatability of red squill and powder baits to rats. Ind. Eng. Chem. 1935, 27, 1377–1380. [Google Scholar] [CrossRef]
- Saadane, F.Z.; Habbachi, W.; Habbachi, S.; Boublata, N.E.I.; Slimani, A.; Tahraoui, A. Toxic effects of Drimia maritima (Asparagaceae) ethanolic extracts on the mortality, development, sexual behaviour and oviposition behaviour of Drosophila melanogaster (Diptera: Drosophilidae). J. Anim. Behav. Biometeorol. 2020, 9, 2102. [Google Scholar] [CrossRef]
- Kopp, B.; Krenn, L.; Draxler, M.; Hoyer, A.; Terkola, R.; Vallaster, P.; Robien, W. Bufadienolides from Urginea maritima from Egypt. Phytochemistry 1996, 42, 513–522. [Google Scholar] [CrossRef]
- Krenn, L.; Jelovina, M.; Kopp, B. New bufadienolides from Urginea maritima sensu strictu. Fitoterapia 2000, 71, 126–129. [Google Scholar] [CrossRef]
- Fernandez, M.; Vega, F.A.; Arrupe, T.; Renedo, J. Flavonoids of squill, Urginea maritima. Phytochemistry 1972, 11, 1534. [Google Scholar] [CrossRef]
- Iizuka, M.; Warashina, T.; Noro, T. Bufadienolides and a new lignan from the bulbs of Urginea maritima. Chem. Pharm. Bull 2001, 49, 282–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulholland, D.A.; Schwikkardab, S.L.; Crouch, N.R. The chemistry and biological activity of the Hyacinthaceae. Nat. Prod. Rep. 2013, 30, 1165–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, B.; Unterluggauer, M.; Robien, W.; Kubelka, W. Bufadienolides from Urginea pancration. Planta Med. 1990, 56, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Krenn, L.; Bamberger, M.; Kopp, B. A new bufadienolide from Urginea pancration. Plant Med. 1992, 58, 284–285. [Google Scholar] [CrossRef]
- Badalamenti, N.; Rosselli, S.; Zito, P.; Bruno, M. Phytochemical profile and insecticidal activity of Drimia pancration (Asparagaceae) against adults of Stegobium paniceum (Anobiidae). Nat. Prod. Res. 2021, 35, 4468–4478. [Google Scholar] [CrossRef]
- Rosselli, S.; Tundis, R.; Bruno, M.; Leporini, M.; Falco, T.; Candela, R.G.; Badalamenti, N.; Loizzo, M.R. Ceiba speciosa (A. St.-Hil.) seeds oil: Fatty acids profiling by GC-MS and NMR and bioactivity. Molecules 2020, 25, 1037. [Google Scholar] [CrossRef] [Green Version]
- Sut, S.; Maggi, F.; Bruno, S.; Badalamenti, N.; Quassinti, L.; Bramucci, M.; Beghelli, D.; Lupidi, G.; Dall’Acqua, S. Hairy garlic (Allium subhirsutum) from Sicily (Italy): LC-DAD-MSn analysis of secondary metabolites and in vitro biological properties. Molecules 2020, 25, 2837. [Google Scholar] [CrossRef]
- Badalamenti, N.; Russi, S.; Bruno, M.; Maresca, V.; Vaglica, A.; Ilardi, V.; Zanfardino, A.; Di Napoli, M.; Varcamonti, M.; Cianciullo, P.; et al. Dihydrophenanthrenes from a Sicilian accession of Himantoglossum robertianum (Loisel.) P. Delforge showed antioxidant, antimicrobial, and antiproliferative activities. Plants 2021, 10, 2776. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Schicchi, R.; Geraci, A.; Leporini, M.; Gervasi, L.; Tundis, R.; Loizzo, M.R. Chemical compositions and antioxidant activities of essential oils, and their combinations, obtained from flavedo by-product of seven cultivars of Sicilian Citrus aurantium L. Molecules 2022, 27, 1580. [Google Scholar]
- Badalamenti, N.; Ilardi, V.; Bruno, M.; Pavela, R.; Boukouvala, M.C.; Kavallieratos, N.G.; Maggi, F.; Canale, A.; Benelli, G. Chemical composition and broad-spectrum insecticidal activity of the flower essential oil from an ancient Sicilian food plant, Ridolfia segetum. Agriculture 2021, 11, 304. [Google Scholar] [CrossRef]
- D’Agostino, G.; Giambra, B.; Palla, F.; Bruno, M.; Badalamenti, N. The application of the essential oils of Thymus vulgaris L. and Crithmum maritimum L. as biocidal on two Tholu bommalu indian leather puppets. Plants 2021, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Basile, S.; Badalamenti, N.; Riccobono, O.; Guarino, S.; Ilardi, V.; Bruno, M.; Peri, E. Chemical composition and evaluation of insecticidal activity of Calendula incana subsp. maritima and Laserpitium siler subsp. siculum essential oils against stored products pests. Molecules 2022, 27, 588. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Villalobos, M.J. Anti-insect activity of bufadienolides from Urginea maritima. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002; pp. 564–566. [Google Scholar]
- Dagne, E.; Mammo, W.; Alemu, M.; Casser, I. Two bufadienolides from Drimia altissima (Urginea altissima). Bull. Chem. Soc. Ethiop. 1994, 8, 85–89. [Google Scholar]
- Ferreira, C.B.S.; Andrade, F.H.N.; Rodrigues, A.R.S.; Siqueira, H.A.A.; Gondim, M.G.C. Resistance in field populations of Tetranychus urticae to acaricides and characterization of the inheritance of abamectin resistance. Crop Prot. 2015, 67, 77–83. [Google Scholar] [CrossRef]
- Rincón, R.A.; Rodríguez, D.; Coy-Barrera, E. Botanicals against Tetranychus urticae Koch under laboratory conditions: A survey of alternatives for controlling pest mites. Plants 2019, 8, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, F.; Maurer, C.; Friedberg, R.C. International Organization for Standardization (ISO) 15189. Ann. Lab. Med. 2017, 37, 365–370. [Google Scholar] [CrossRef]
- Pavela, R. Acaricidal properties of extracts and major furanochromenes from the seeds of Ammi visnaga Linn. against Tetranychus urticae Koch. Ind. Crop. Prod. 2015, 67, 108–113. [Google Scholar] [CrossRef]
- Marris, J.W. The Toxicity of Hexythiazox to Twospotted Spider Mite (Tetranychus urticae Koch) Adults and Eggs. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 1988. [Google Scholar]
- Havasi, M.; Sangak Sani Bozhgani, N.; Golmohmmadi, G.; Kheradmand, K. Impact of hexythiazox on life table parameters of the Amblyseius swirskii (Acari: Phytoseiidae) and its prey Tetranychus urticae. J. Crop. Prot. 2021, 10, 295–308. [Google Scholar]
- Pavela, R.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Cappellacci, L.; Petrelli, R.; Spinozzi, E.; Aguzzi, C.; Zeppa, L.; Ubaldi, M.; et al. Encapsulation of Carlina acaulis essential oil and carlina oxide to develop long-lasting mosquito larvicides: Microemulsions versus nanoemulsions. J. Pest Sci. 2021, 94, 899–915. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve; Cambridge University Press: Cambridge, UK, 1952. [Google Scholar]
- Nabissi, M.; Morelli, M.B.; Offidani, M.; Amantini, C.; Gentili, S.; Soriani, A.; Cardinali, C.; Leoni, P.; Santoni, G. Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration. Oncotarget 2016, 7, 77543–77557. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Tested Acaricide | Mortality ** | Lethal Dose (for 96 h) | ||||
|---|---|---|---|---|---|---|
| At 24 h (Dose 100 µg/cm2) | At 96 h (Dose 100 µg/cm2) | LD50 (µg/cm2) | LD90 (µg/cm2) | χ2 | p-Value | |
| 1 | 100.0 ± 0.0e | 100.0 ± 0.0d | 0.28 (0.22–0.34) | 1.08 (0.84–1.60) | 1.485 | 0.685 |
| 2 | 47.4 ± 11.7c | 83.3 ± 4.7c | 1.41 (0.62–2.48) | 198.58 (86.01–786.52) | 1.836 | 0.968 |
| 3 | 16.4 ± 5.6b | 46.7 ± 12.5b | ˃100 | |||
| 4 | 10.6 ± 5.4ab | 70.0 ± 8.2c | 29.61 (13.20–48.35) | 1862.15 (648.5–2651.24) | 0.407 | 0.981 |
| D. pancration extract | 5.5 ± 2.5a | 78.9 ± 6.3c | 8.5 (5.9–10.6) | 118.8 (103.3–129.5) | 0.852 | 0.384 |
| Positive control Hexythiazox | 82.5 ± 12.5d | 100.0 ± 0.0d | 18.7 (12.7–21.5) | 132.5 (111.7–142.8) | 1.234 | 0.251 |
| Negative control | 0.0 ± 0.0a | 6.7 ± 4.7a | ||||
| ANOVA F6,28, p-value * | 381.72; 0.000 | 273.57; 0.000 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badalamenti, N.; Bruno, M.; Pavela, R.; Maggi, F.; Marinelli, O.; Zeppa, L.; Benelli, G.; Canale, A. Acaricidal Activity of Bufadienolides Isolated from Drimia pancration against Tetranychus urticae, and Structural Elucidation of Arenobufagin-3-O-α-L-rhamnopyranoside. Plants 2022, 11, 1629. https://doi.org/10.3390/plants11131629
Badalamenti N, Bruno M, Pavela R, Maggi F, Marinelli O, Zeppa L, Benelli G, Canale A. Acaricidal Activity of Bufadienolides Isolated from Drimia pancration against Tetranychus urticae, and Structural Elucidation of Arenobufagin-3-O-α-L-rhamnopyranoside. Plants. 2022; 11(13):1629. https://doi.org/10.3390/plants11131629
Chicago/Turabian StyleBadalamenti, Natale, Maurizio Bruno, Roman Pavela, Filippo Maggi, Oliviero Marinelli, Laura Zeppa, Giovanni Benelli, and Angelo Canale. 2022. "Acaricidal Activity of Bufadienolides Isolated from Drimia pancration against Tetranychus urticae, and Structural Elucidation of Arenobufagin-3-O-α-L-rhamnopyranoside" Plants 11, no. 13: 1629. https://doi.org/10.3390/plants11131629
APA StyleBadalamenti, N., Bruno, M., Pavela, R., Maggi, F., Marinelli, O., Zeppa, L., Benelli, G., & Canale, A. (2022). Acaricidal Activity of Bufadienolides Isolated from Drimia pancration against Tetranychus urticae, and Structural Elucidation of Arenobufagin-3-O-α-L-rhamnopyranoside. Plants, 11(13), 1629. https://doi.org/10.3390/plants11131629












