Assessment of Uptake, Accumulation and Degradation of Paracetamol in Spinach (Spinacia oleracea L.) under Controlled Laboratory Conditions
Abstract
:1. Introduction
2. Results
2.1. Paracetamol Uptake and Metabolism in Spinach
2.2. Influence of Paracetamol on Plant Growth Parameters
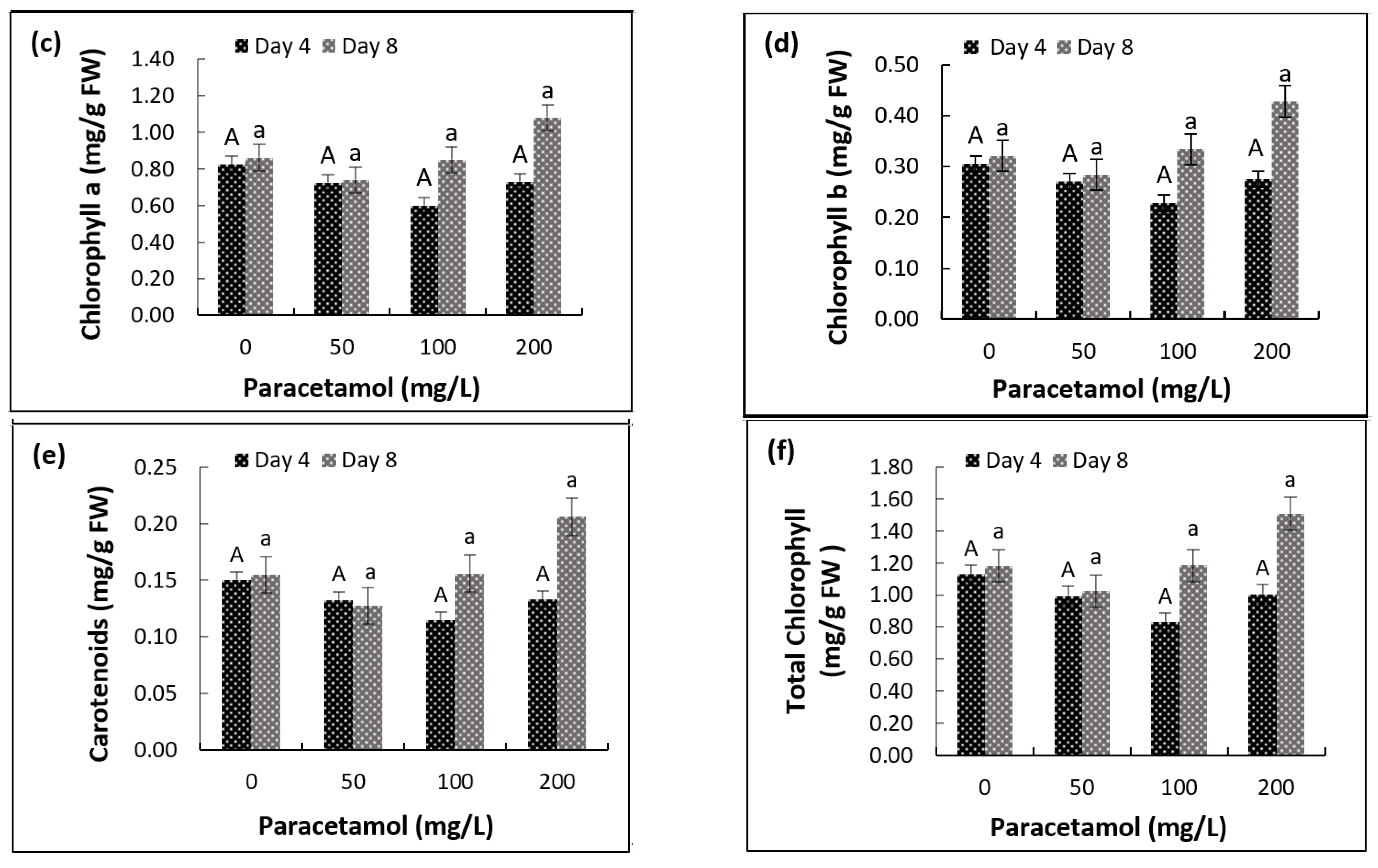
2.3. Impact of Paracetamol on Photosynthetic Pigments and Chlorophyll Fluorescence
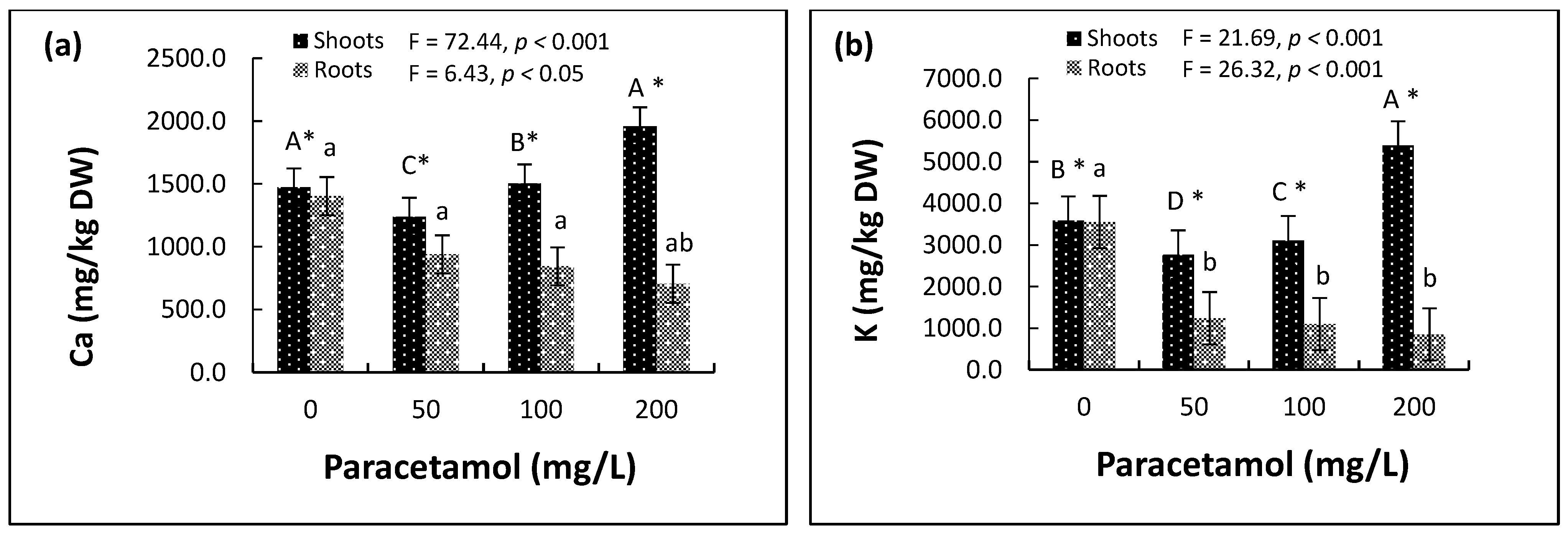
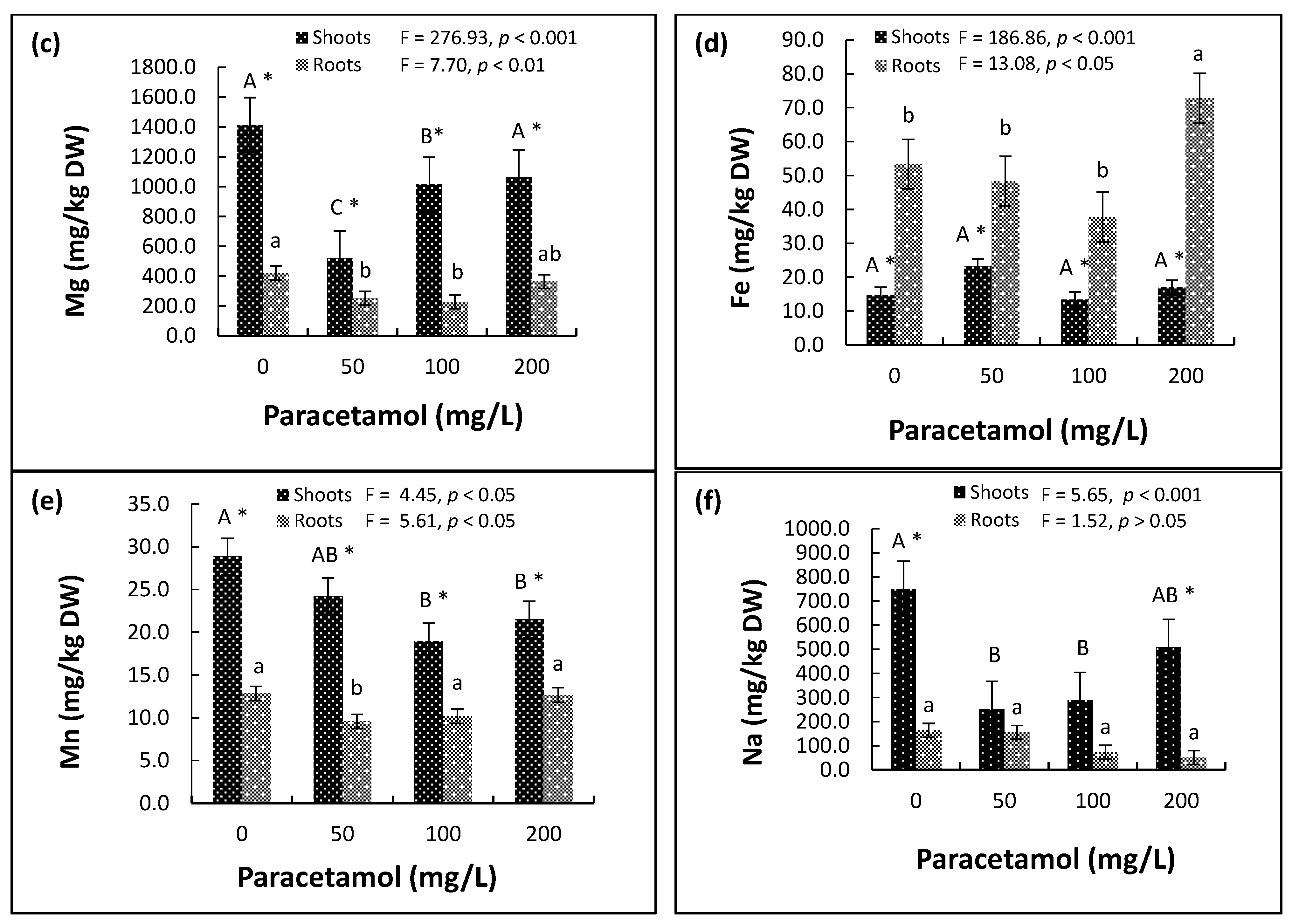
2.4. Plant Nutrients and Elements
2.4.1. Macronutrients
2.4.2. Micronutrients
2.4.3. Sodium
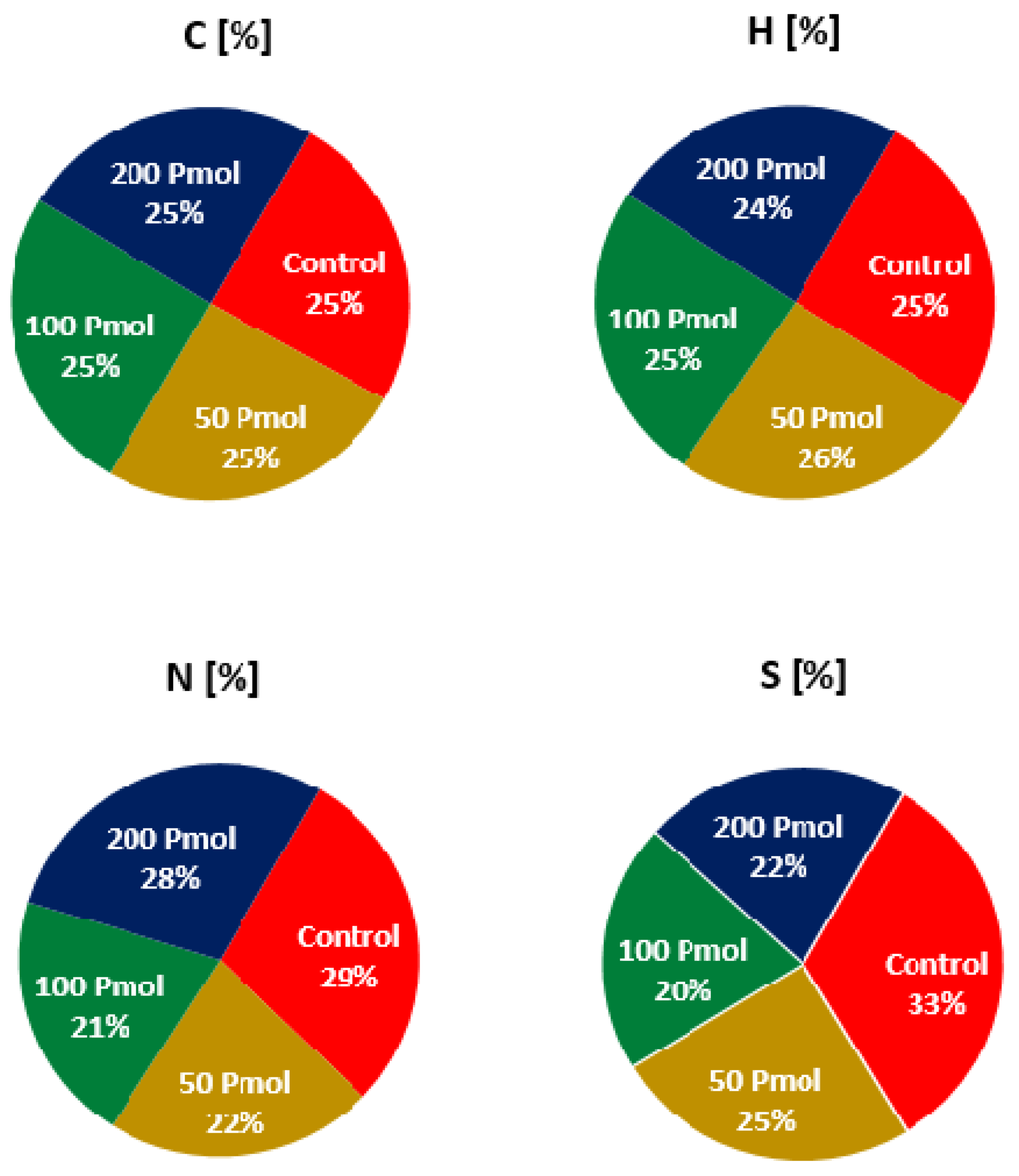
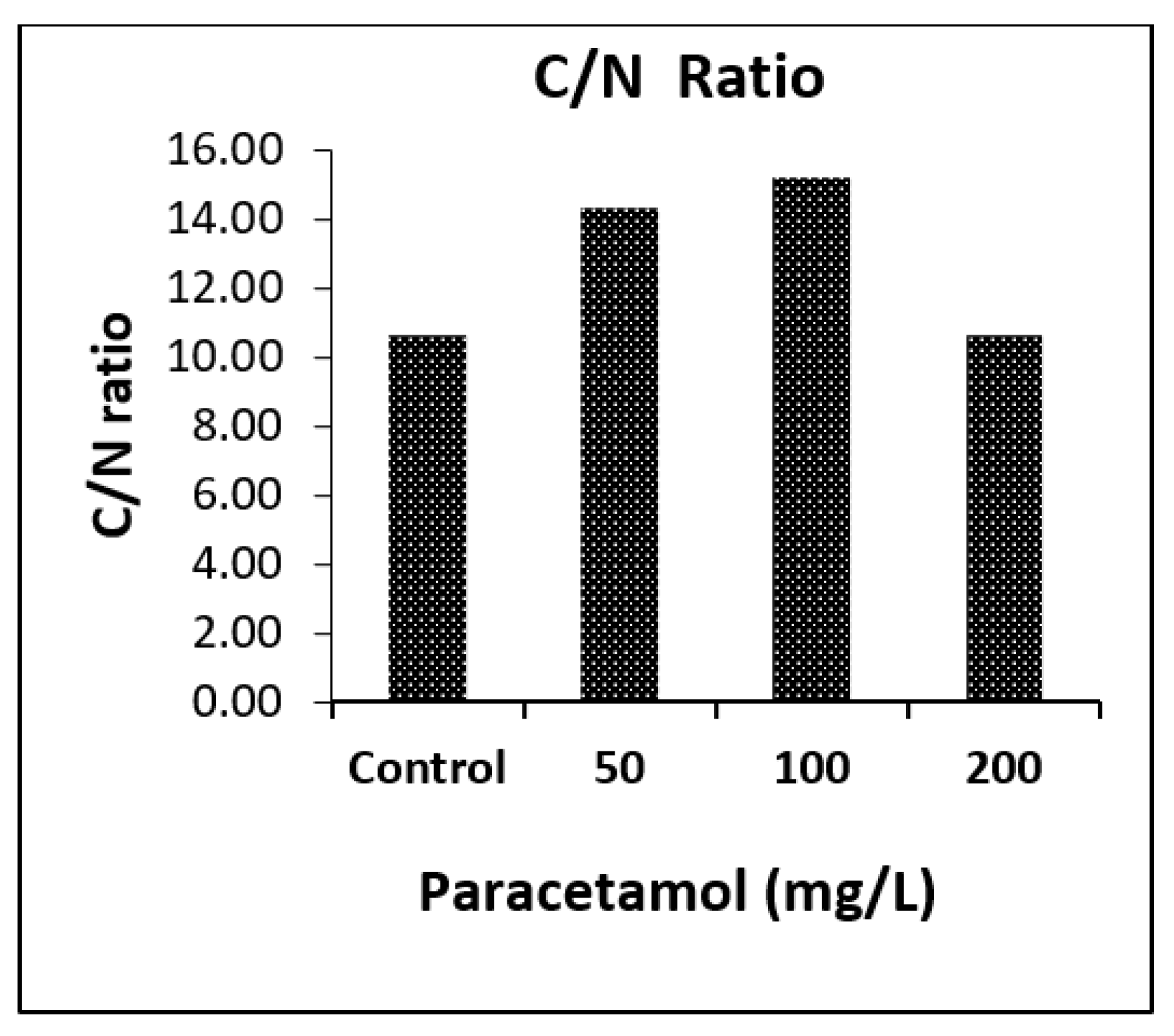
2.4.4. CHNS Analysis
2.5. Microbial Analysis
3. Discussion
4. Material and Methods
4.1. Seed Germination and Seedling Development
4.2. Hydroponic Cultivation and Paracetamol Exposure
4.3. Assessment of Plant Growth Parameters
4.4. The Fate of Paracetamol in Spinach
4.4.1. Liquid Chromatography–Mass Spectrometry (LC–MS) Analysis
4.4.2. Translocation Factor
4.5. Plant Elemental Analysis
4.6. Microbial Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albannay, S.; Kazama, S.; Oguma, K.; Hashimoto, T.; Takizawa, S.; Lo Porto, A. Water Demand Management Based on Water Consumption Data Analysis in the Emirate of Abu Dhabi. Water 2021, 13, 2827. [Google Scholar] [CrossRef]
- Daughton, C.G. Non-Regulated Water Contaminants: Emerging Research. Environ. Impact Assess. Rev. 2004, 24, 711–732. [Google Scholar] [CrossRef]
- Cătălina Vasilachi, I.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and Fate of Emerging Pollutants in Water Environment and Options for Their Removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Bui, X.T.; Vo, T.P.T.; Ngo, H.H.; Guo, W.S.; Nguyen, T.T. Multicriteria Assessment of Advanced Treatment Technologies for Micropollutants Removal at Large-Scale Applications. Sci. Total Environ. 2016, 563–564, 1050–1067. [Google Scholar] [CrossRef] [PubMed]
- Minden, V.; Deloy, A.; Volkert, A.M.; Leonhardt, S.D.; Pufal, G. Antibiotics Impact Plant Traits, Even at Small Concentrations. AoB Plants 2017, 9, plx010. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, P.N.; Basto, M.C.P.; Almeida, C.M.R.; Brix, H. A Review of Plant–Pharmaceutical Interactions: From Uptake and Effects in Crop Plants to Phytoremediation in Constructed Wetlands. Environ. Sci. Pollut. Res. 2014, 21, 11729–11763. [Google Scholar] [CrossRef]
- Coleman, J.O.D.; Blake-Kalff, M.M.A.; Davies, T.G.E. Detoxification of Xenobiotics by Plants: Chemical Modification and Vacuolar Compartmentation. Trends Plant Sci. 1997, 2, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Sauvêtre, A.; Eichhorn, P.; Pérez, S. Metabolism of Pharmaceuticals in Plants and Their Associated Microbiota. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020; Volume 103, pp. 221–264. [Google Scholar] [CrossRef]
- Sandermann, H. Higher Plant Metabolism of Xenobiotics: The ‘Green Liver’ Concept. Pharmacogenetics 1994, 4, 225–241. [Google Scholar] [CrossRef]
- Caragea, G.; Avram, O.; Pauna, A.; Costea, A.; Tudosie, M. Acetaminophen, a Therapeutic or an Extremely Toxic Remedy—A Review. J. Mind Med. Sci. 2022, 9, 102–110. [Google Scholar] [CrossRef]
- Paracetamol Market Research Report [2022–2028] | Industry. Available online: https://www.globenewswire.com/en/news-release/2022/02/15/2384836/0/en/Paracetamol-Market-Research-Report-2022-2028-Industry-Size-Share-Growth-Rate-Business-Strategies-Industry-Revenue-Opportunities-Future-Trends-Leading-Players-Update-Analysis-and-Fo.html (accessed on 14 June 2022).
- Nieto-Juárez, J.I.; Torres-Palma, R.A.; Botero-Coy, A.M.; Hernández, F. Pharmaceuticals and Environmental Risk Assessment in Municipal Wastewater Treatment Plants and Rivers from Peru. Environ. Int. 2021, 155, 106674. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of Drugs in German Sewage Treatment Plants and Rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Eric van Beelen, I.S. Municipal Waste Water Treatment Plant (WWTP) Effluents a Concise Overview of the Occurrence of Organic Substances Association of River Waterworks; RIWA: Nieuwegein, The Netherlands, 2007. [Google Scholar]
- Moreno-González, R.; Rodriguez-Mozaz, S.; Gros, M.; Barceló, D.; León, V.M. Seasonal Distribution of Pharmaceuticals in Marine Water and Sediment from a Mediterranean Coastal Lagoon (SE Spain). Environ. Res. 2015, 138, 326–344. [Google Scholar] [CrossRef]
- Kummerová, M.; Zezulka, Š.; Babula, P.; Tříska, J. Possible Ecological Risk of Two Pharmaceuticals Diclofenac and Paracetamol Demonstrated on a Model Plant Lemna Minor. J. Hazard. Mater. 2016, 302, 351–361. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, Z.; Liu, Y.; Cao, T.; Zhang, H.; Hao, M.; Chen, R.; Dzakpasu, M.; Wang, X.C. Phytoremediation Mechanisms and Plant Eco-Physiological Response to Microorganic Contaminants in Integrated Vertical-Flow Constructed Wetlands. J. Hazard. Mater. 2022, 424, 127611. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Ncube, S.; Chimuka, L. Uptake of Pharmaceuticals by Plants Grown under Hydroponic Conditions and Natural Occurring Plant Species: A Review. Sci. Total Environ. 2018, 636, 477–486. [Google Scholar] [CrossRef]
- Nunes, B. Ecotoxicological Effects of the Drug Paracetamol: A Critical Review of Past Ecotoxicity Assessments and Future Perspectives. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020; Volume 96, pp. 131–145. [Google Scholar] [CrossRef]
- Christou, A.; Papadavid, G.; Dalias, P.; Fotopoulos, V.; Michael, C.; Bayona, J.M.; Piña, B.; Fatta-Kassinos, D. Ranking of Crop Plants According to Their Potential to Uptake and Accumulate Contaminants of Emerging Concern. Environ. Res. 2019, 170, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Sungur, Ş. Pharmaceutical and Personal Care Products in the Environment: Occurrence and Impact on the Functioning of the Ecosystem. In Emerging Contaminants in the Environment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 137–157. [Google Scholar] [CrossRef]
- Wu, X.; Conkle, J.L.; Gan, J. Multi-Residue Determination of Pharmaceutical and Personal Care Products in Vegetables. J. Chromatogr. A 2012, 1254, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Dudley, S.; McGinnis, M.; Trumble, J.; Gan, J. Acetaminophen Detoxification in Cucumber Plants via Induction of Glutathione S-Transferases. Sci. Total Environ. 2019, 649, 431–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tăşchină, M.; Copolovici, D.M.; Bungău, S.; Lupitu, A.I.; Copolovici, L.; Iovan, C. The Influence of Residual Acetaminophen on Phaseolus Vulgaris L. Secondary Metabolites. Farmacia 2017, 65, 709–713. [Google Scholar]
- Bartha, B.; Huber, C.; Harpaintner, R.; Schröder, P. Effects of Acetaminophen in Brassica Juncea L. Czern.: Investigation of Uptake, Translocation, Detoxification, and the Induced Defense Pathways. Environ. Sci. Pollut. Res. 2010, 17, 1553–1562. [Google Scholar] [CrossRef]
- Kotyza, J.; Soudek, P.; Kafka, Z.; Vaněk, T. Phytoremediation of Pharmaceuticals-Preliminary Study. Int. J. Phytoremed. 2010, 12, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Shanableh, A.; Semreen, M.; Semerjian, L.; Abdallah, M.; Mousa, M.; Darwish, N.; Baalbaki, Z. Contaminants of Emerging Concern in Sharjah Wastewater Treatment Plant, Sharjah, UAE. J. Environ. Eng. Sci. 2019, 14, 225–234. [Google Scholar] [CrossRef]
- Semreen, M.H.; Shanableh, A.; Semerjian, L.; Alniss, H.; Mousa, M.; Bai, X.; Acharya, K. Molecules Simultaneous Determination of Pharmaceuticals by Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry: A Case Study from Sharjah Sewage Treatment Plant. Molecules 2019, 24, 633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natarajan, R.; Saikia, K.; Ponnusamy, S.K.; Rathankumar, A.K.; Rajendran, D.S.; Venkataraman, S.; Tannani, D.B.; Arvind, V.; Somanna, T.; Banerjee, K.; et al. Understanding the Factors Affecting Adsorption of Pharmaceuticals on Different Adsorbents—A Critical Literature Update. Chemosphere 2022, 287, 131958. [Google Scholar] [CrossRef]
- Gondi, R.; Kavitha, S.; Yukesh Kannah, R.; Parthiba Karthikeyan, O.; Kumar, G.; Kumar Tyagi, V.; Rajesh Banu, J. Algal-Based System for Removal of Emerging Pollutants from Wastewater: A Review. Bioresour. Technol. 2022, 344, 126245. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.D.; Pereira, P.P.; Costa, D.P.; dos Santos, A.C. Chlorophyll fluorescence as a possible tool for salt-stress tolerance screening in the sunflower. Rev. Ciência Agronômica 2011, 42, 893. [Google Scholar] [CrossRef] [Green Version]
- Nunes, F.V.; De Melo, I.S. Isolation and Characterization of Endophytic Bacteria of Coffee Plants and Their Potential in Caffeine Degradation. WIT Trans. Biomed. Health 2006, 10, 293–297. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cordero, A.; Tuberquia-Sierra, A.; Amell-Jiménez, D. In Vitro Activity of Nitrogen Fixating and Phosphate Solubilizing Bacteria. Agron. Mesoam. 2014, 25, 214–223. [Google Scholar]
- Lopez-Velasco, G.; Welbaum, G.E.; Boyer, R.R.; Mane, S.P.; Ponder, M.A. Changes in Spinach Phylloepiphytic Bacteria Communities Following Minimal Processing and Refrigerated Storage Described Using Pyrosequencing of 16S RRNA Amplicons. J. Appl. Microbiol. 2011, 110, 1203–1214. [Google Scholar] [CrossRef]
- Yang, C.W.; Chen, Y.E.; Chang, B.V. Microbial Communities Associated with Acetaminophen Biodegradation from Mangrove Sediment. Sustainability 2020, 12, 5410. [Google Scholar] [CrossRef]
- Jackson, C.R.; Randolph, K.C.; Osborn, S.L.; Tyler, H.L. Culture Dependent and Independent Analysis of Bacterial Communities Associated with Commercial Salad Leaf Vegetables. BMC Microbiol. 2013, 13, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riggs, P.J.; Chelius, M.K.; Iniguez, A.L.; Kaeppler, S.M.; Triplett, E.W. Enhanced Maize Productivity by Inoculation with Diazotrophic Bacteria. Funct. Plant Biol. 2001, 28, 829. [Google Scholar] [CrossRef]
- White, J.F.; Kingsley, K.I.; Kowalski, K.P.; Irizarry, I.; Micci, A.; Soares, M.A.; Bergen, M.S. Disease Protection and Allelopathic Interactions of Seed-Transmitted Endophytic Pseudomonads of Invasive Reed Grass (Phragmites Australis). Plant Soil 2018, 422, 195–208. [Google Scholar] [CrossRef]
- Saravanakumar, D.; Samiyappan, R. ACC Deaminase from Pseudomonas Fluorescens Mediated Saline Resistance in Groundnut (Arachis Hypogea) Plants. J. Appl. Microbiol. 2007, 102, 1283–1292. [Google Scholar] [CrossRef]
- Sheng, X.F.; Xia, J.J.; Jiang, C.Y.; He, L.Y.; Qian, M. Characterization of Heavy Metal-Resistant Endophytic Bacteria from Rape (Brassica Napus) Roots and Their Potential in Promoting the Growth and Lead Accumulation of Rape. Environ. Pollut. 2008, 156, 1164–1170. [Google Scholar] [CrossRef]
- Tian, T.; Tam, N.F.Y.; Zan, Q.; Cheung, S.G.; Shin, P.K.S.; Wong, Y.S.; Zhang, L.; Chen, Z. Performance and Bacterial Community Structure of a 10-Years Old Constructed Mangrove Wetland. Mar. Pollut. Bull. 2017, 124, 1096–1105. [Google Scholar] [CrossRef]
- Ahmed, R.Z.; Ahmed, N.; Gadd, G.M. Isolation of Two Kocuria Species Capable of Growing on Various Polycyclic Aromatic Hydrocarbons. Afr. J. Biotechnol. 2010, 9, 3611–3617. [Google Scholar]
- Habib, I.; Mohamed, M.Y.I. Foodborne Infections in the Middle East. In Food Safety in the Middle East; Academic Press: Cambridge, MA, USA, 2022; pp. 71–107. [Google Scholar] [CrossRef]
- Scialabba, N.E.-H. Livestock Xenobiotics and Zoonoses. In Managing Health Livestock Production and Consumption; Academic Press: Cambridge, MA, USA, 2022; pp. 45–59. [Google Scholar] [CrossRef]
- Bugg, T.D.H.; Ahmad, M.; Hardiman, E.M.; Singh, R. The Emerging Role for Bacteria in Lignin Degradation and Bio-Product Formation. Curr. Opin. Biotechnol. 2011, 22, 394–400. [Google Scholar] [CrossRef]
- Villarreal Silva, M. Role of Epiphytic Bacteria in the Colonization of Fruits and Leafy Greens by Foodborne Bacterial Pathogens. Ph.D. Thesis, Texas A & M University, College Station, TX, USA, August 2016. [Google Scholar]
- Beyi, A.F.; Hassall, A.; Phillips, G.J.; Plummer, P.J.; Tracking, P.; Kamal, A. Tracking Reservoirs of Antimicrobial Resistance Genes in a Complex Microbial Community Using Metagenomic Hi-C: The Case of Bovine Digital Dermatitis. Antibiotics 2021, 10, 221. [Google Scholar] [CrossRef]
- Pathak, D.; Lone, R.; Nazim, N.; Alaklabi, A.; Khan, S.; Koul, K.K. Plant Growth Promoting Rhizobacterial Diversity in Potato Grown Soil in the Gwalior Region of India. Biotechnol. Rep. 2022, 33, e00713. [Google Scholar] [CrossRef]
- An, J.; Zhou, Q.; Sun, F.; Zhang, L. Ecotoxicological Effects of Paracetamol on Seed Germination and Seedling Development of Wheat (Triticum Aestivum L.). J. Hazard. Mater. 2009, 169, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Dudley, S.; Trumble, J.; Gan, J. Pharmaceutical and Personal Care Products-Induced Stress Symptoms and Detoxification Mechanisms in Cucumber Plants. Environ. Pollut. 2018, 234, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Zezulka, Š.; Kummerová, M.; Babula, P.; Hájková, M.; Oravec, M. Sensitivity of Physiological and Biochemical Endpoints in Early Ontogenetic Stages of Crops under Diclofenac and Paracetamol Treatments. Environ. Sci. Pollut. Res. 2018, 26, 3965–3979. [Google Scholar] [CrossRef] [PubMed]
- Alkimin, G.D.; Daniel, D.; Frankenbach, S.; Serôdio, J.; Soares, A.M.V.M.; Barata, C.; Nunes, B. Evaluation of Pharmaceutical Toxic Effects of Non-Standard Endpoints on the Macrophyte Species Lemna Minor and Lemna Gibba. Sci. Total Environ. 2019, 657, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Leitão, I.; Martins, L.L.; Carvalho, L.; Oliveira, M.C.; Marques, M.M.; Mourato, M.P.; Spagnuolo, V.; Capozzi, F. Acetaminophen Induces an Antioxidative Response in Lettuce Plants. Plants 2021, 10, 1152. [Google Scholar] [CrossRef]
- Al-Muwayhi, M.A.R. Paracetamol Mediated Changes Modifies the Photosynthetic Efficiency of Vigna Radiata. Legum. Res. 2018, 41, 842–845. [Google Scholar] [CrossRef]
- Kudrna, J.; Hnilicka, F.; Kubes, J.; Vachova, P.; Hnilickova, H.; Kuklova, M. Effect of Acetaminophen (Apap) on Physiological Indicators in Lactuca Sativa. Life 2020, 10, 303. [Google Scholar] [CrossRef]
- Soares, C.; Pereira, R.; Martins, M.; Tamagnini, P.; Serôdio, J.; Moutinho-Pereira, J.; Cunha, A.; Fidalgo, F. Glyphosate-Dependent Effects on Photosynthesis of Solanum Lycopersicum L.—An Ecophysiological, Ultrastructural and Molecular Approach. J. Hazard. Mater. 2020, 398, 122871. [Google Scholar] [CrossRef]
- Saidi, I.; Ayouni, M.; Dhieb, A.; Chtourou, Y.; Chaïbi, W.; Djebali, W. Oxidative Damages Induced by Short-Term Exposure to Cadmium in Bean Plants: Protective Role of Salicylic Acid. S. Afr. J. Bot. 2013, 85, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Peng, J.; Lei, Y.; Kanerva, M.; Li, Q.; Song, J.; Guo, J.; Sun, H. Comparison of Oxidative Stress Induced by Clarithromycin in Two Freshwater Microalgae Raphidocelis Subcapitata and Chlorella Vulgaris. Aquat. Toxicol. 2020, 219, 105376. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Ali, M.; Riaz, M.; Javed, S.; Sehar, A.; Abbas, Z.; Rizwan, M.; El-Sheikh, M.A.; et al. Interactive Role of Zinc and Iron Lysine on Spinacia Oleracea L. Growth, Photosynthesis and Antioxidant Capacity Irrigated with Tannery Wastewater. Physiol. Mol. Biol. Plants 2020, 26, 2435–2452. [Google Scholar] [CrossRef] [PubMed]
- Mezanur Rahman, M.; Golam Mostofa, M.; Kumar Das, A.; Rahman Anik, T.; Sultana Keya, S.; Ahsan, S.M.; Arifur Rahman Khan, M.; Ahmed, M.; Abiar Rahman, M.; Motaher Hossain, M.; et al. Ethanol Positively Modulates Photosynthetic Traits, Antioxidant Defense and Osmoprotectant Levels to Enhance Drought Acclimatization in Soybean. Antioxidants 2022, 2022, 516. [Google Scholar] [CrossRef] [PubMed]
- Kvesitadze, G.; Khatisashvili, G.; Sadunishvili, T.; Agmasheneblis, D. Mechanisms to Detoxify Selected Organic Contaminants in Higher Plants and Microbes, and Their Potential Use in Landscape Management; No. 62558; Academy of Sciences Of Georgia (Tbilisi) Durmishidze Institute of Biochemistry and Biotechnology: Tbilisi, Georgia, 2004. [Google Scholar]
- Nazaryuk, V.M.; Klenova, M.I.; Kalimullina, F.R. Ecoagrochemical Approaches to the Problem of Nitrate Pollution in Agroecosystems. Russ. J. Ecol. 2002, 33, 392–397. [Google Scholar] [CrossRef]
- Villette, C.; Maurer, L.; Wanko, A.; Heintz, D. Xenobiotics Metabolization in Salix Alba Leaves Uncovered by Mass Spectrometry Imaging. Metabolomics 2019, 15, 122. [Google Scholar] [CrossRef]
- Rawat, J.; Pandey, N.; Saxena, J. Nitrate Accumulation, Growth and Leaf Quality of Spinach Beet (Beta Vulgaris Linn.) as Affected by NPK Fertilization with Special Reference to Potassium. In Role of Potassium in Abiotic Stress; Springer: Singapore, 2022; Volume 2, pp. 1–14. [Google Scholar] [CrossRef]
- Graciela Maurino, V.; Narayan Misra, A.; Huang, W.; Prasad, S.M.; Singh, R.; Parihar, P. Sulfur and Calcium Simultaneously Regulate Photosynthetic Performance and Nitrogen Metabolism Status in As-Challenged Brassica Juncea L. Seedlings. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Britto, D.T.; Kronzucker, H.J. Futile Cycling at the Plasma Membrane: A Hallmark of Low-Affinity Nutrient Transport. Trends Plant Sci. 2006, 11, 529–534. [Google Scholar] [CrossRef]
- Huber, C.; Bartha, B.; Harpaintner, R.; Schröder, P.; Huber, C.; Bartha, B.; Harpaintner, R.; Schröder, P. Metabolism of Acetaminophen (Paracetamol) in Plants-Two Independent Pathways Result in the Formation of a Glutathione and a Glucose Conjugate. Environ. Sci Pollut. Res. 2009, 16, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Bartha, B. Uptake and Metabolism of Human Pharmaceuticals in Plants: Identification of Metabolites and Specification of the Defense Enzyme Systems under Pharmaceutical Exposure. Ph.D. Thesis, Technische Universität München, Singapore, June 2012. [Google Scholar]
- Martins, L.; Teixeira, J. Gene- and Organ-Specific Impact of Paracetamol on Solanun Nigrum L.’s γ-Glutamylcysteine Synthetase and Glutathione S-Transferase and Consequent Phytoremediation Fitness. Acta Physiol. Plant. 2021, 43, 53. [Google Scholar] [CrossRef]
- Feng, N.X.; Yu, J.; Zhao, H.M.; Cheng, Y.T.; Mo, C.H.; Cai, Q.Y.; Li, Y.W.; Li, H.; Wong, M.H. Efficient Phytoremediation of Organic Contaminants in Soils Using Plant–Endophyte Partnerships. Sci. Total Environ. 2017, 583, 352–368. [Google Scholar] [CrossRef]
- Chandran, V.; Shaji, H.; Mathew, L. Endophytic Microbial Influence on Plant Stress Responses. In Microbial Endophytes: Functional Biology and Applications; Woodhead Publishing: Sawtson, UK, 2020; pp. 161–193. [Google Scholar] [CrossRef]
- Ijaz, A.; Shabir, G.; Khan, Q.M.; Afzal, M. Enhanced Remediation of Sewage Effluent by Endophyte-Assisted Floating Treatment Wetlands. Ecol. Eng. 2015, 84, 58–66. [Google Scholar] [CrossRef]
- Ashraf, S.; Afzal, M.; Naveed, M.; Shahid, M.; Zahir, Z.A. Endophytic Bacteria Enhance Remediation of Tannery Effluent in Constructed Wetlands Vegetated with Leptochloa fusca. Int. J. Phytoremed. 2018, 20, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Żur, J.; Piński, A.; Marchlewicz, A.; Hupert-Kocurek, K.; Wojcieszyńska, D.; Guzik, U. Organic Micropollutants Paracetamol and Ibuprofen—Toxicity, Biodegradation, and Genetic Background of Their Utilization by Bacteria. Environ. Sci. Pollut. Res. Int. 2011, 25, 21498–21524. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Kumar, A.; Sharma, J. Degradation Study of Lindane by Novel Strains Kocuria Sp. DAB-1Y and Staphylococcus Sp. DAB-1W. Bioresour. Bioprocess. 2016, 3, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Hu, J.; Zhu, R.; Zhou, Q.; Chen, J. Degradation of Paracetamol by Pure Bacterial Cultures and Their Microbial Consortium. Appl. Microbiol. Biotechnol. 2013, 97, 3687–3698. [Google Scholar] [CrossRef]
- Weyens, N.; Van Der Lelie, D.; Artois, T.; Smeets, K.; Taghavi, S.; Newman, L.; Carleer, R.; Vangronsveld, J. Bioaugmentation with Engineered Endophytic Bacteria Improves Contaminant Fate in Phytoremediation. Environ. Sci. Technol. 2009, 43, 9413–9418. [Google Scholar] [CrossRef]
- Mosa, K.A.; El-Naggar, M.; Ramamoorthy, K.; Alawadhi, H.; Elnaggar, A.; Wartanian, S.; Ibrahim, E.; Hani, H. Copper Nanoparticles Induced Genotoxicty, Oxidative Stress, and Changes in Superoxide Dismutase (SOD) Gene Expression in Cucumber (Cucumis Sativus) Plants. Front. Plant Sci. 2018, 9, 872. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xin, J.; Ge, W.; Tian, R. Tolerance Mechanism and Phytoremediation Potential of Pistia Stratiotes to Zinc and Cadmium Co-Contamination. Int. J. Phytoremed. 2022, 1–8. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. Secondary Metabolites, Ferulic Acid and p-Hydroxybenzoic Acid Induced Toxic Effects on Photosynthetic Process in Rumex Acetosa L. Biomolecules 2021, 11, 233. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Chuang, Y.H.; Zhang, Y.; Zhang, W.; Boyd, S.A.; Li, H. Comparison of Accelerated Solvent Extraction and Quick, Easy, Cheap, Effective, Rugged and Safe Method for Extraction and Determination of Pharmaceuticals in Vegetables. J. Chromatogr. A 2015, 1404, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kaynar, S.Ç.A.M.; Hİçsönmez, Ü.; Özdemİr, A.; Özdemİr, C. Assessment of Element Concentrations in Soil, Root and Leaf of Spinach Plant (Spinacia Oleracea L.) Grown in Manisa. J. Inst. Sci. Tech. 2018, 8, 131–140. [Google Scholar] [CrossRef]
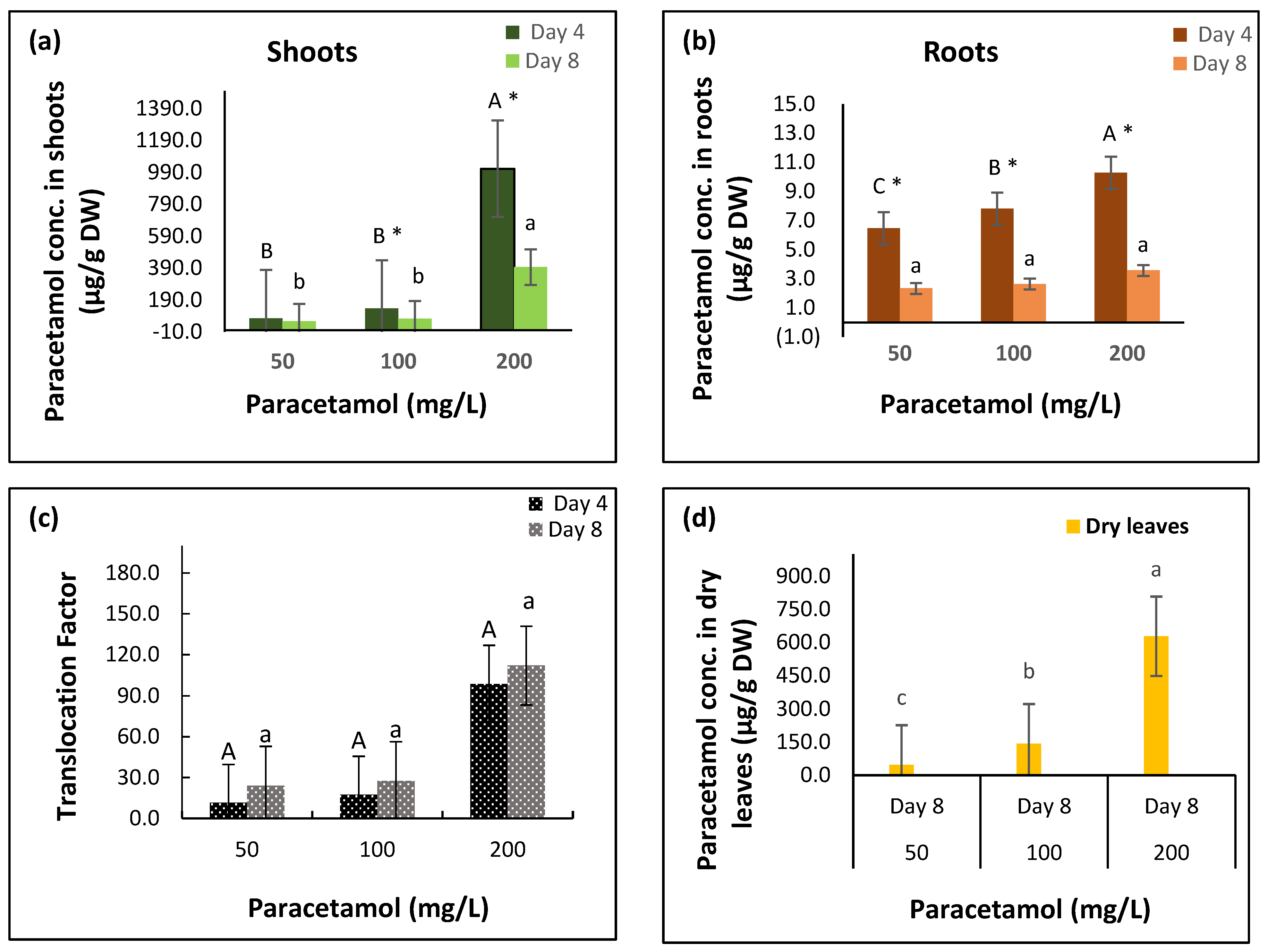
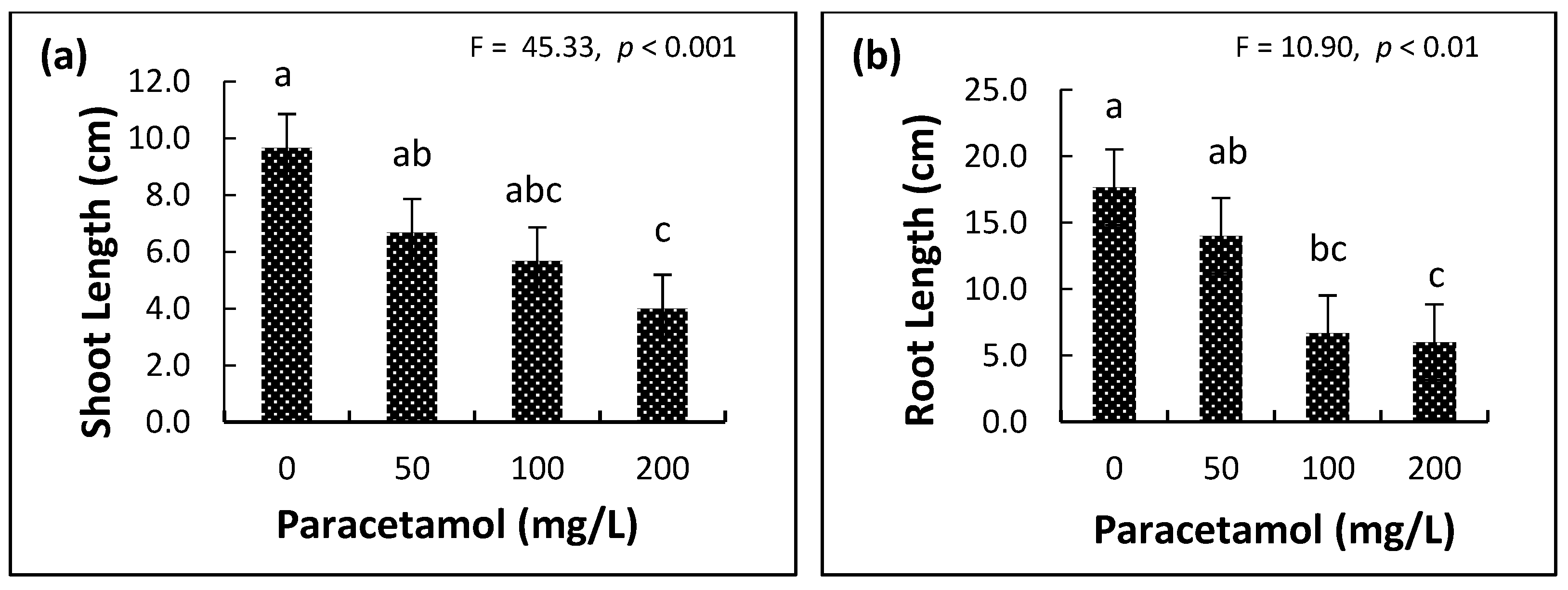
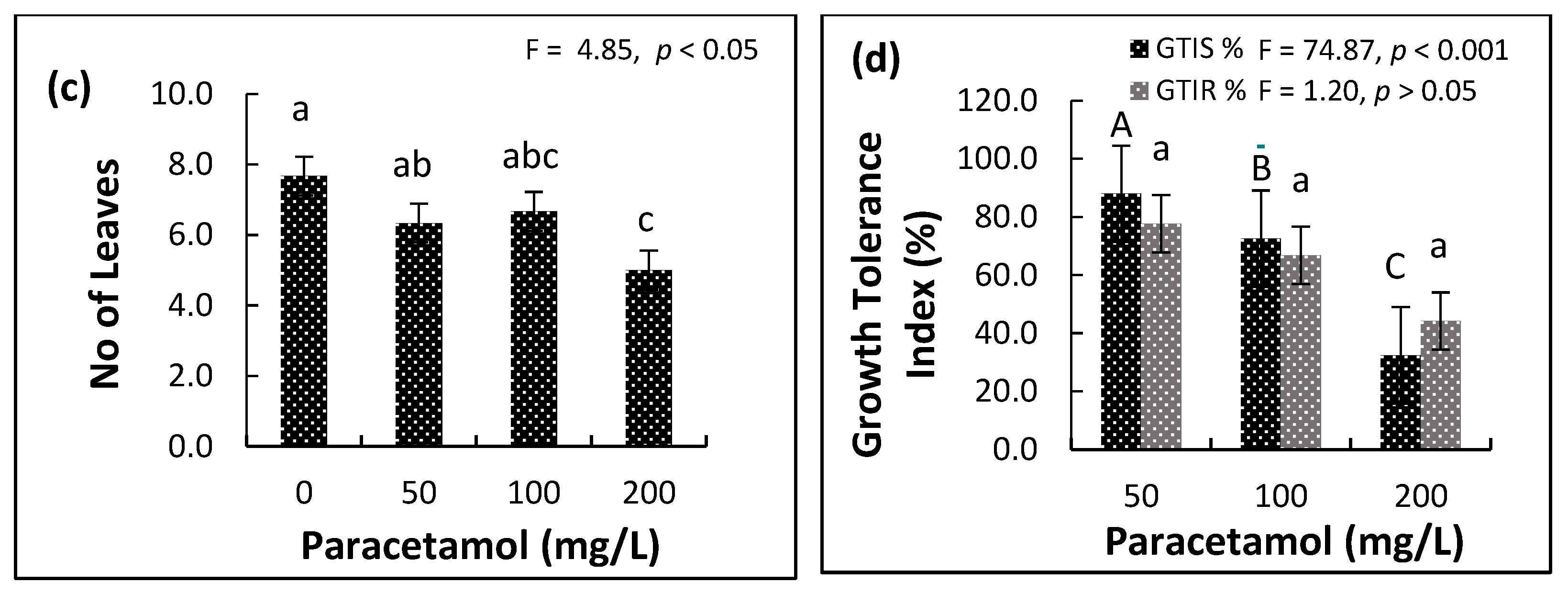
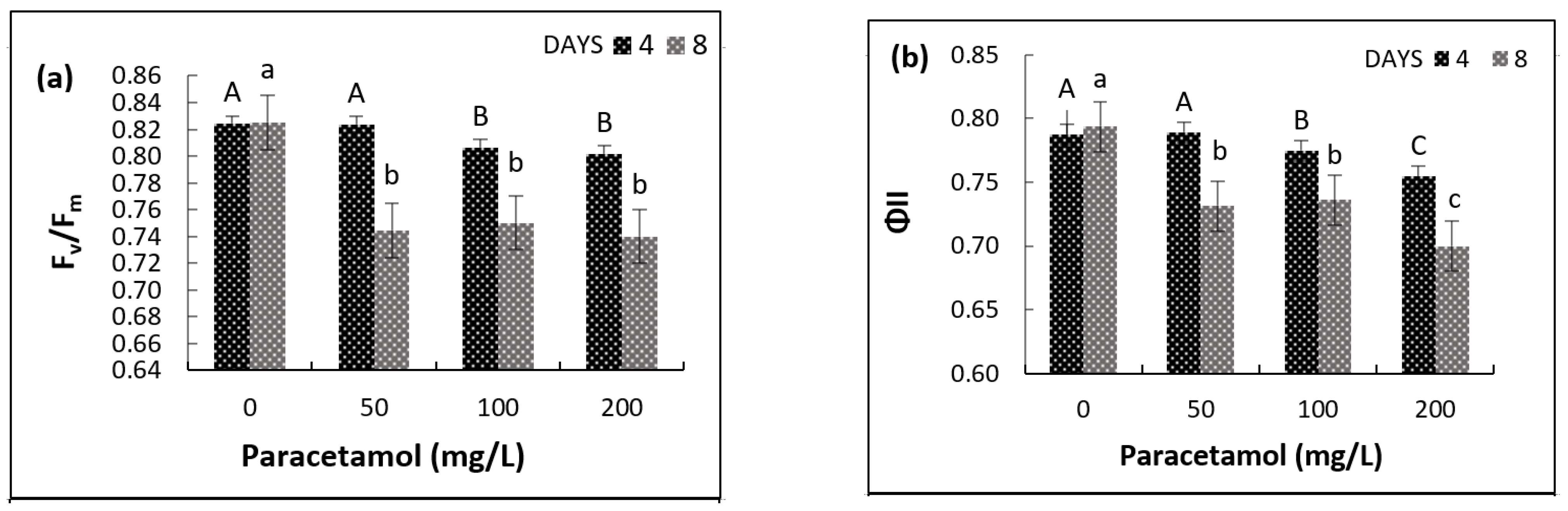
| Variable | Factor | df | F-Ratio | p-Value |
|---|---|---|---|---|
| Shoots | Period (P) | 1 | 488.82 | <0.001 |
| Treatments (T) | 2 | 1538.01 | <0.001 | |
| P × T | 2 | 332.65 | <0.001 | |
| Roots | Period (P) | 1 | 587.76 | <0.001 |
| Treatments (T) | 2 | 45.42 | <0.001 | |
| P × T | 2 | 11.49 | <0.01 | |
| Translocation Factor | Period (P) | 1 | 26.52 | <0.001 |
| Treatments (T) | 2 | 591.35 | <0.001 | |
| P × T | 2 | 0.17 | ns |
| Variable | Factor | df | F-Ratio | p-Value |
|---|---|---|---|---|
| Fv/Fm | Period (P) | 1 | 29.45 | <0.001 |
| Treatment (T) | 3 | 7.07 | <0.01 | |
| P × T | 3 | 3.66 | <0.05 | |
| ΦII | Period (P) | 1 | 21.07 | <0.01 |
| Treatment (T) | 3 | 10.93 | <0.01 | |
| P × T | 3 | 3.43 | <0.05 | |
| Chl a | Period (P) | 1 | 6.19 | <0.05 |
| Treatment (T) | 3 | 1.77 | ns | |
| P × T | 3 | 1.52 | ns | |
| Chl b | Period (P) | 1 | 10.02 | <0.01 |
| Treatment (T) | 3 | 2.32 | ns | |
| P × T | 3 | 2.31 | ns | |
| Carotenoids | Period (P) | 1 | 4.34 | ns |
| Treatment (T) | 3 | 1.70 | ns | |
| P × T | 3 | 1.68 | ns | |
| Total Chlorophyll | Period (P) | 1 | 7.11 | <0.05 |
| Treatment (T) | 3 | 1.89 | ns | |
| P × T | 3 | 1.71 | ns |
| Treatment | Isolate and Colony Description | Organism | Probability (%) | Confidence | Role in Plants |
|---|---|---|---|---|---|
| Control spinach roots | Gram +ve micrococcus, yellowish moist, moderate size | Kocuria kristinae | 98 | Excellent Identification | Antagonist bacteria in spinach [32]. |
| Gram −ve, white, large flat, and dry | Pasteurella pneumotropica | 90 | Good Identification | Endophytic bacterium capable of fixing nitrogen and solubilizing phosphate [33]. | |
| Gram −ve, moderate moist | Comamonas testosteroni | 99 | Excellent Identification | Spinach microbiota [34]. Involved in the degradation of aromatic compounds [35]. | |
| Gram −ve, small moist | Acinetobacter Iwoffii | 99 | Excellent Identification | Spinach microbiota [34,36]. Involved in the degradation of paracetamol and aromatic compounds [35]. | |
| 50 mg/L paracetamol-treated spinach roots | Gram −ve, yellow, large, and dry | Burkhulderia cepacia group | 95 | Very Good Identification | Spinach microbiota [36]. Increased growth parameters in Zea mays [37]. |
| Gram −ve, pale yellow, small, moist | Pseudomonas florescens | 98 | Excellent Identification | Involved in the degradation of paracetamol and aromatic compounds [35], Spinach microbiota [36]. Phosphorus-solubilizing, protease production in Phragimates australis [38]. Salt tolerant in groundnut (Arachishypogaea) [39]. Antifungal, plant growth promotion. Increased growth parameters, increased Pb uptake, root elongation in Brassica napus and Solanum nigrum [40]. | |
| Gram +ve, white, large and moist | Staphylococcus haemolyticus | 95 | Very Good Identification | Spinach microbiota [36]. Increased growth parameters in Triticum aestivum [41]. | |
| Gram −ve rods, pale yellow | Stenotrophomonas maltophilia | 95 | Very Good Identification | Spinach microbiota [36]. Increased growth parameters in Triticum aestivum [41]. | |
| 100 mg/L paracetamol-treated spinach roots | Gram +ve, pale yellow, moderate, moist | Kocuria kristinae | 87 | Acceptable Identification | Antagonist bacteria in spinach [32]. |
| Gram +ve, white, small, round | Kocuria rosea | 98 | Excellent Identification | Capable of growing on naphthalene, phenanthrene and fluoranthene, on all three polycyclic aromatic hydrocarbons (PAHs) [42]. | |
| 200 mg/L paracetamol-treated spinach roots | Gram −ve, coccobacillus, yellow, large and dry colonies | Brucella melitensis- Highly pathogenic | 91 | Good Identification | Foodborne pathogen. Contaminant of leafy vegetables and causes human brucellosis [43,44]. |
| Gram −ve bacilli, yellow, large and moist colonies | Sphingomonas paucimobilis | 92 | Good Identification | Spinach microbiota [35]. Increase in growth parameters in Triticum aestivum [41]. Involved in the degradation of paracetamol and aromatic compounds [35]. Spinach microbiota, involved in PPCP biodegradation, produces lignin-degrading enzymes [36,45]. | |
| Gram +ve, white, moderate size | Staphylococcus auricularis | 98 | Excellent Identification | Spinach microbiota, increases growth parameters in Triticum aestivum [36,41]. |
| Treatment | Isolate and Colony Description | Organism | Probability (%) | Confidence | Role in Plants |
|---|---|---|---|---|---|
| Control spinach shoots | Gram +ve, white moist, moderate size colonies | Staphylococcus hominis ssp hominis | N.A. | Low Discrimination Organism | Epiphytic bacteria from fruits and leafy greens, spinach microbiota [36], potential biocontrol agents, able to reduce the proliferation of E. coli O157:H7 and S. enterica in fruits and vegetables [46]. |
| Gram +ve, white moist, moderate size colonies | Aerococcus viridans | N.A. | Low Discrimination Organism | Epiphytic bacteria on leafy greens, capable of fixing nitrogen and solubilizing phosphate [33]. | |
| Gram −ve, moderate yellow moist colonies | Oligella ureolytica | N.A. | Low Discrimination Organism | Pathogenic bacteria [47]. | |
| Gram −ve, moderate yellow moist colonies | Aeromonas salmonicida | NA | Low Discrimination Organism | Plant growth promoting rhizobacteria, involved in biodegradation of xenobiotic compounds from contaminated water/soil environment [38,48]. | |
| 50 mg/L paracetamol-treated spinach shoots | Gram −ve bacilli, yellow, large and moist colonies | Sphingomonas paucimobilis | 89 | Good Identification | Spinach microbiota, involved in the degradation of paracetamol and aromatic compounds [34,35,36]. |
| Gram +ve, white, moderate size | Staphylococcus hominis ssp hominis | 95 | Very Good Identification | Spinach microbiota [36]. Epiphytic bacteria from fruits and leafy greens are potential biocontrol agents, able to reduce the proliferation of E. coli O157:H7 and S. enterica in fruits and vegetables [46]. | |
| 100 mg/L paracetamol-treated spinach shoots | Gram +ve, pale yellow, moderate, moist | Kocuria rosea | 98 | Excellent Identification | Capable of growing on naphthalene, phenanthrene and fluoranthene, on all three polycyclic aromatic hydrocarbons (PAHs) [42]. |
| Gram −ve, white, moderate size, moist | Escherichia coli | 93 | Very Good Identification | Foodborne pathogen, spinach microbiota [34]. | |
| 200 mg/L paracetamol treated spinach shoots | Gram +ve, white, moderate, moist | Staphylococcus vitulinus | 98 | Excellent Identification | Spinach microbiota [34,36]. |
| Gram +ve, white, moderate, moist | Gamella bergeri | 90 | Good Identification | Epiphytic bacteria from fruits and leafy greens are potential biocontrol agents, able to reduce the proliferation of E. coli 157:H7 and S. enterica in fruits and vegetables [46]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badar, Z.; Shanableh, A.; El-Keblawy, A.; Mosa, K.A.; Semerjian, L.; Mutery, A.A.; Hussain, M.I.; Bhattacharjee, S.; Tsombou, F.M.; Ayyaril, S.S.; et al. Assessment of Uptake, Accumulation and Degradation of Paracetamol in Spinach (Spinacia oleracea L.) under Controlled Laboratory Conditions. Plants 2022, 11, 1626. https://doi.org/10.3390/plants11131626
Badar Z, Shanableh A, El-Keblawy A, Mosa KA, Semerjian L, Mutery AA, Hussain MI, Bhattacharjee S, Tsombou FM, Ayyaril SS, et al. Assessment of Uptake, Accumulation and Degradation of Paracetamol in Spinach (Spinacia oleracea L.) under Controlled Laboratory Conditions. Plants. 2022; 11(13):1626. https://doi.org/10.3390/plants11131626
Chicago/Turabian StyleBadar, Zarreen, Abdallah Shanableh, Ali El-Keblawy, Kareem A. Mosa, Lucy Semerjian, Abdullah Al Mutery, Muhammad Iftikhar Hussain, Sourjya Bhattacharjee, François Mitterand Tsombou, Sefeera Sadik Ayyaril, and et al. 2022. "Assessment of Uptake, Accumulation and Degradation of Paracetamol in Spinach (Spinacia oleracea L.) under Controlled Laboratory Conditions" Plants 11, no. 13: 1626. https://doi.org/10.3390/plants11131626
APA StyleBadar, Z., Shanableh, A., El-Keblawy, A., Mosa, K. A., Semerjian, L., Mutery, A. A., Hussain, M. I., Bhattacharjee, S., Tsombou, F. M., Ayyaril, S. S., Ahmady, I. M., Elnaggar, A., Mousa, M., & Semreen, M. H. (2022). Assessment of Uptake, Accumulation and Degradation of Paracetamol in Spinach (Spinacia oleracea L.) under Controlled Laboratory Conditions. Plants, 11(13), 1626. https://doi.org/10.3390/plants11131626









