Use of traC Gene to Type the Incidence and Distribution of pXFAS_5235 Plasmid-Bearing Strains of Xylella fastidiosa subsp. fastidiosa ST1 in Spain
Abstract
:1. Introduction
2. Results
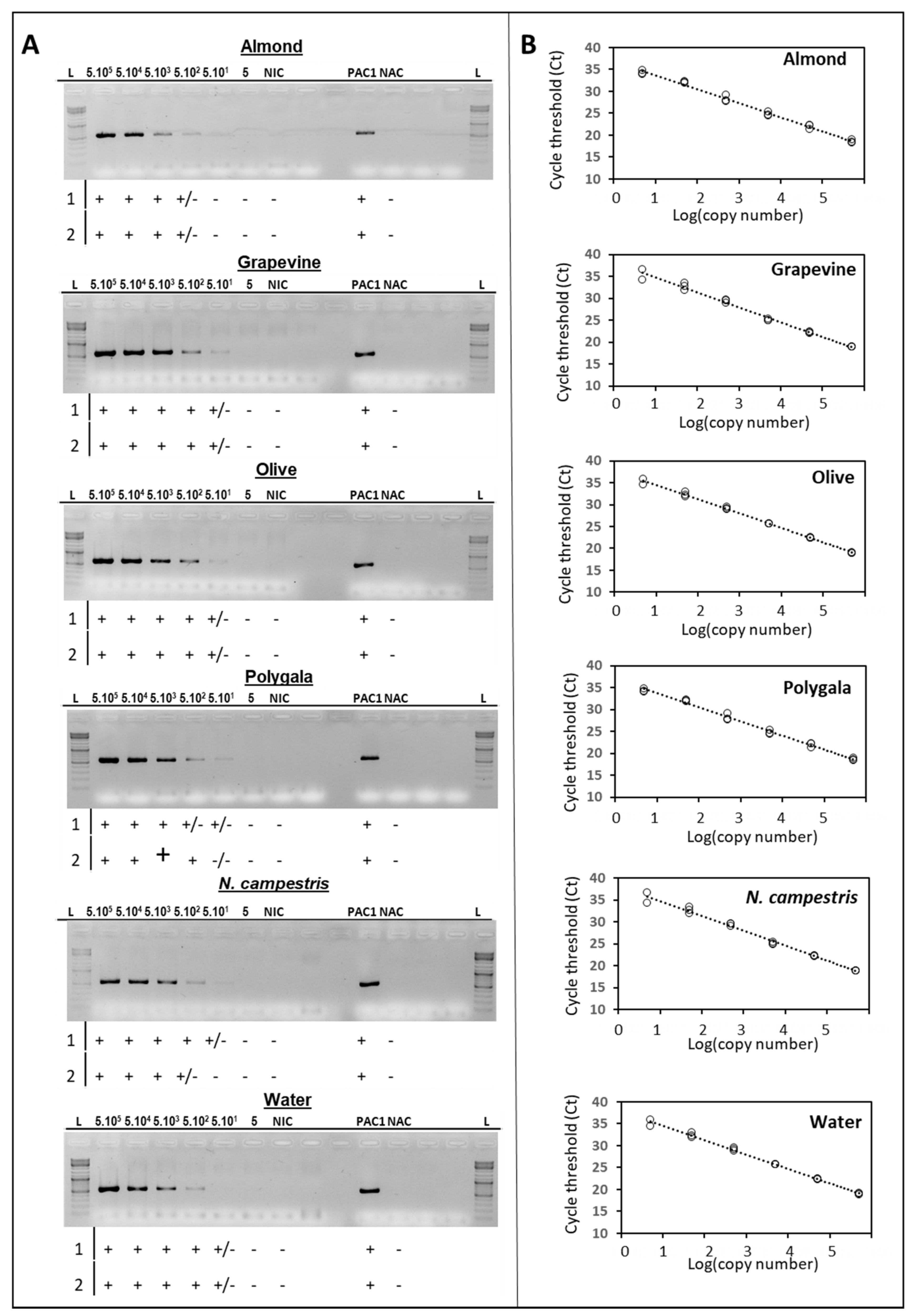
2.1. Sensitivity of the traC PCR-Based Plasmid Typing Protocol
2.2. PCR-Based Plasmid Typing of Xylella fastidiosa Strains and Naturally-Infected Plant Samples
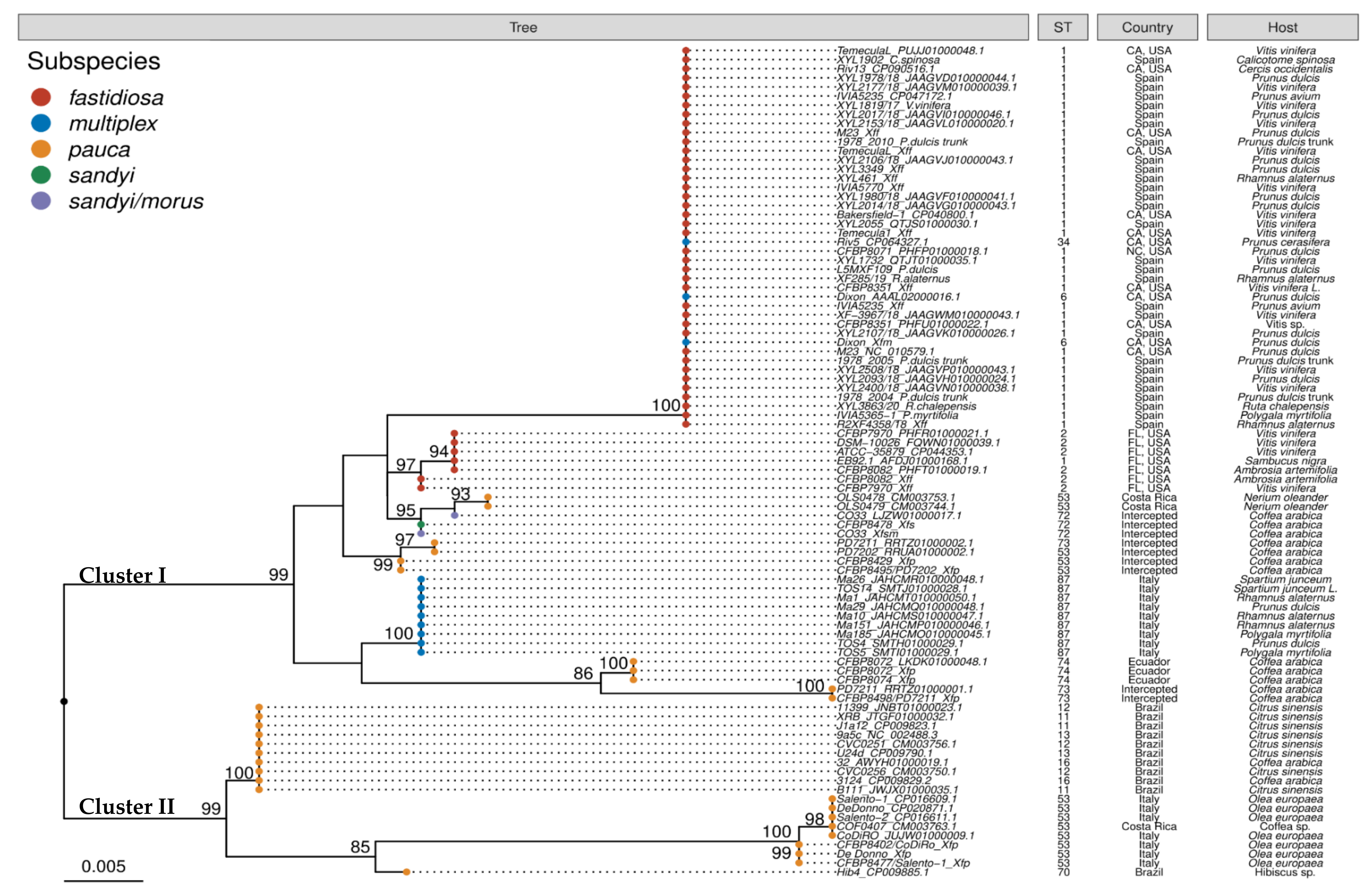
2.3. Phylogenetic Analysis of traC Gene
3. Discussion
4. Materials and Methods
4.1. Bacterial and Plant Sample Sources and DNA Extraction Procedures
4.2. PCR-Based Plasmid Typing
4.3. Limit of Detection of the PCR-Based Plasmid Typing
4.4. Sequencing and Phylogenetic Analysis of traC
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority (EFSA); Delbianco, A.; Gibin, D.; Pasinato, L.; Morelli, M. Scientific Report on the Update of the Xylella spp. Host Plant Database–Systematic Literature Search up to 30 June 2021. EFSA J. 2022, 20, 67. [Google Scholar] [CrossRef]
- Nunney, L.; Yuan, X.; Bromley, R.; Hartung, J.; Montero-Astúa, M.; Moreira, L.; Ortiz, B.; Stouthamer, R. Population genomic analysis of a bacterial plant pathogen: Novel insight into the Origin of Pierce’s Disease of Grapevine in the U.S. PLoS ONE 2010, 5, e15488. [Google Scholar] [CrossRef] [PubMed]
- Nunney, L.; Vickerman, D.B.; Bromley, R.E.; Russell, S.A.; Hartman, J.R.; Morano, L.D.; Stouthamer, R. Recent Evolutionary radiation and host plant specialization in the Xylella fastidiosa subspecies native to the United States. Appl. Environ. Microbiol. 2013, 79, 2189–2200. [Google Scholar] [CrossRef]
- Schaad, N.W.; Postnikova, E.; Lacy, G.; Fatmi, M.; Chang, C.-J. Xylella fastidiosa subspecies: X. fastidiosa subsp. fastidiosasubsp. nov., X. fastidiosa subsp. multiplex subsp. nov., and X. fastidiosa subsp. pauca subsp. nov. Syst. Appl. Microbiol. 2004, 27, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Nunney, L.; Schuenzel, E.L.; Scally, M.; Bromley, R.E.; Stouthamer, R. Large-Scale Intersubspecific Recombination in the Plant-Pathogenic Bacterium Xylella fastidiosa Is Associated with the Host Shift to Mulberry. Appl. Environ. Microbiol. 2014, 80, 3025–3033. [Google Scholar] [CrossRef] [PubMed]
- Schuenzel, E.L.; Scally, M.; Stouthamer, R.; Nunney, L. A Multigene Phylogenetic Study of Clonal Diversity and Divergence in North American Strains of the Plant Pathogen Xylella fastidiosa. Appl Env. Microbiol 2005, 71, 3832–3839. [Google Scholar] [CrossRef] [PubMed]
- Denancé, N.; Briand, M.; Gaborieau, R.; Gaillard, S.; Jacques, M.-A. Identification of Genetic Relationships and Subspecies Signatures in Xylella fastidiosa. BMC Genom. 2019, 20, 239. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Morano, L.; Bromley, R.; Spring-Pearson, S.; Stouthamer, R.; Nunney, L. Multilocus Sequence Typing of Xylella fastidiosa Causing Pierce’s Disease and Oleander Leaf Scorch in the United States. Phytopathology 2010, 100, 601–611. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-Access Bacterial Population Genomics: BIGSdb Software, the PubMLST.Org Website and Their Applications. Wellcome Open Res 2018, 3, 124. [Google Scholar] [CrossRef]
- Saponari, M.; Boscia, D.; Altamura, G.; Loconsole, G.; Zicca, S.; D’Attoma, G.; Morelli, M.; Palmisano, F.; Saponari, A.; Tavano, D.; et al. Isolation and Pathogenicity of Xylella fastidiosa Associated to the Olive Quick Decline Syndrome in Southern Italy. Sci. Rep. 2017, 7, 17723. [Google Scholar] [CrossRef]
- Mang, S.M.; Frisulo, S.; Elshafie, H.S.; Camele, I. Diversity evaluation of Xylella fastidiosa from infected olive trees in Apulia (Southern Italy). Plant Pathol. J. 2016, 32, 102–111. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on Protective Measures against Pests of Plants, Amending Regulations (EU) No 228/2013, (EU) No 652/2014 and (EU) No 1143/2014 of the European Parliament and of the Council Directives 69/464/EEC, 74/647/EEC, 93/85/EEC, 98/57/EC, 2000/29/EC, 2006/91/EC and 2007/33/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32016R2031 (accessed on 12 May 2022).
- European Commission. Commission Implementing Regulation (EU) 2020/1201 of 14 August 2020 of the European Parliament and of the Council as Regards Measures to Prevent the Introduction into and the Spread within the Union of Xylella fastidiosa (Wells et al.). Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX:32020R1201 (accessed on 12 May 2022).
- Denancé, N.; Legendre, B.; Briand, M.; Olivier, V.; de Boisseson, C.; Poliakoff, F.; Jacques, M.-A. Several Subspecies and Sequence Types Are Associated with the Emergence of Xylella fastidiosa in Natural Settings in France. Plant Pathol. 2017, 66, 1054–1064. [Google Scholar] [CrossRef]
- Landa, B.B. Emergence of Xylella fastidiosa in Spain: Current Situation. In Proceedings of the European Conference on Xylella fastidiosa: Finding Answers to a Global Problem, Palma de Mallorca, Spain, 13–15 November 2017. [Google Scholar] [CrossRef]
- Landa, B.B.; Castillo, A.I.; Giampetruzzi, A.; Kahn, A.; Román-Écija, M.; Velasco-Amo, M.P.; Navas-Cortés, J.A.; Marco-Noales, E.; Barbé, S.; Moralejo, E.; et al. Emergence of a Plant Pathogen in Europe Associated with Multiple Intercontinental Introductions. Appl. Environ. Microbiol. 2019, 86, e01521-19. [Google Scholar] [CrossRef]
- Latest Developments of Xylella fastidiosa in the EU Territory. Available online: https://ec.europa.eu/food/plants/plant-health-and-biosecurity/legislation/control-measures/xylella-fastidiosa/latest-developments-xylella-fastidiosa-eu-territory_en (accessed on 29 March 2022).
- Moralejo, E.; Borràs, D.; Gomila, M.; Montesinos, M.; Adrover, F.; Juan, A.; Nieto, A.; Olmo, D.; Seguí, G.; Landa, B.B. Insights into the epidemiology of Pierce’s disease in vineyards of Mallorca, Spain. Plant Pathol. 2019, 68, 1458–1471. [Google Scholar] [CrossRef]
- Olmo, D.; Nieto, A.; Borràs, D.; Montesinos, M.; Adrover, F.; Pascual, A.; Gost, P.A.; Quetglas, B.; Urbano, A.; García, J.d.D.; et al. Landscape Epidemiology of Xylella fastidiosa in the Balearic Islands. Agronomy 2021, 11, 473. [Google Scholar] [CrossRef]
- Moralejo, E.; Gomila, M.; Montesinos, M.; Borràs, D.; Pascual, A.; Nieto, A.; Adrover, F.; Gost, P.A.; Seguí, G.; Busquets, A.; et al. Phylogenetic Inference Enables Reconstruction of a Long-Overlooked Outbreak of Almond Leaf Scorch Disease (Xylella fastidiosa) in Europe. Commun. Biol. 2020, 3, 560. [Google Scholar] [CrossRef]
- Landa, B.B.; Velasco-Amo, M.P.; Marco-Noales, E.; Olmo, D.; López, M.M.; Navarro, I.; Monterde, A.; Barbé, S.; Montes-Borrego, M.; Román-Écija, M.; et al. Draft Genome Sequence of Xylella fastidiosa Subsp. fastidiosa Strain IVIA5235, Isolated from Prunus avium in Mallorca Island, Spain. Microbiol. Resour. Announc. 2018, 7, e01222-18. [Google Scholar] [CrossRef]
- Arias-Giraldo, L.F.; Giampetruzzi, A.; Metsis, M.; Marco-Noales, E.; Imperial, J.; Velasco-Amo, M.P.; Román-Écija, M.; Landa, B.B. Complete Circularized Genome Data of Two Spanish Strains of Xylella fastidiosa (IVIA5235 and IVIA5901) Using Hybrid Assembly Approaches. Phytopathology 2020, 110, 969–972. [Google Scholar] [CrossRef]
- Rogers, E.E.; Stenger, D.C. A Conjugative 38 KB Plasmid Is Present in Multiple Subspecies of Xylella fastidiosa. PLoS ONE 2012, 7, e52131. [Google Scholar] [CrossRef]
- Pierry, P.M.; Uceda-Campos, G.; Feitosa-Junior, O.R.; Martins-Junior, J.; de Santana, W.O.; Coletta-Filho, H.D.; Zaini, P.A.; da-Silva, A.M. Genetic Diversity of Xylella fastidiosa Plasmids Assessed by Comparative Genomics. Trop. Plant Pathol. 2020, 45, 342–360. [Google Scholar] [CrossRef]
- Otten, L.; Canaday, J.; Gérard, J.-C.; Fournier, P.; Crouzet, P.; Paulus, F. Evolution of Agrobacteria and Thie Ti Plasmids-A Review. Mol. Plant-Microbe Interact. 1992, 5, 279–287. [Google Scholar] [CrossRef]
- Sundin, G.W. Genomic Insights into the Contribution of Phytopathogenic Bacterial Plasmids to the Evolutionary History of Their Hosts. Annu. Rev. Phytopathol. 2007, 45, 129–151. [Google Scholar] [CrossRef]
- Palmer, K.L.; Kos, V.N.; Gilmore, M.S. Horizontal Gene Transfer and the Genomics of Enterococcal Antibiotic Resistance. Curr. Opin. Microbiol. 2010, 13, 632–639. [Google Scholar] [CrossRef]
- Cesbron, S.; Dupas, E.; Beaurepère, Q.; Briand, M.; Montes-Borrego, M.; Velasco-Amo, M.d.P.; Landa, B.B.; Jacques, M.-A. Development of A Nested-MultiLocus Sequence Typing Approach for A Highly Sensitive and Specific Identification of Xylella fastidiosa Subspecies Directly from Plant Samples. Agronomy 2020, 10, 1099. [Google Scholar] [CrossRef]
- Harper, S.J.; Ward, L.I.; Clover, G.R.G. Development of LAMP and Real-Time PCR Methods for the Rapid Detection of Xylella fastidiosa for Quarantine and Field Applications. Phytopathology 2010, 100, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Giampetruzzi, A.; Velasco-Amo, M.P.; Marco-Noales, E.; Montes-Borrego, M.; Román-Écija, M.; Navarro, I.; Monterde, A.; Barbé, S.; Almeida, R.P.P.; Saldarelli, P.; et al. Draft Genome Resources of Two Strains (“ESVL” and “IVIA5901”) of Xylella fastidiosa Associated with Almond Leaf Scorch Disease in Alicante, Spain. Phytopathology 2019, 109, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.V.; da Silva, A.M.; Gomes, S.L. Genetic Organization of Plasmid PXF51 from the Plant Pathogen Xylella fastidiosa. Plasmid 2001, 45, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Coletta-Filho, H.D.; Francisco, C.S.; Lopes, J.R.S.; Muller, C.; Almeida, R.P.P. Homologous Recombination and Xylella fastidiosa Host–Pathogen Associations in South America. Phytopathology 2017, 107, 305–312. [Google Scholar] [CrossRef]
- Kahn, A.K.; Almeida, R.P.P. Phylogenetics of Historical Host Switches in a Bacterial Plant Pathogen. Appl. Environ. Microbiol. 2022, 88, e0235621. [Google Scholar] [CrossRef]
- Firrao, G.; Scortichini, M.; Pagliari, L. Orthology-Based Estimate of the Contribution of Horizontal Gene Transfer from Distantly Related Bacteria to the Intraspecific Diversity and Differentiation of Xylella fastidiosa. Pathogens 2021, 10, 46. [Google Scholar] [CrossRef]
- Burbank, L.P.; Van Horn, C.R. Conjugative Plasmid Transfer in Xylella fastidiosa Is Dependent on Tra and Trb Operon Functions. J. Bacteriol. 2017, 199, e00388-17. [Google Scholar] [CrossRef]
- Wells, J.M.; Raju, B.C.; Nyland, G.; Lowe, S.K. Medium for Isolation and Growth of Bacteria Associated with Plum Leaf Scald and Phony Peach Diseases. Appl. Environ. Microbiol. 1981, 42, 357–363. [Google Scholar] [CrossRef]
- Davis, M.J.; Purcell, A.H.; Thomson, S.V. Isolation Media for the Pierce’s Disease Bacterium. Phytopathology 1980, 70, 425–429. [Google Scholar] [CrossRef]
- EPPO. PM 7/24 (4) Xylella fastidiosa. EPPO Bull. 2019, 49, 175–227. [Google Scholar] [CrossRef]
- Burbank, L.P.; Ortega, B.C. Novel Amplification Targets for Rapid Detection and Differentiation of Xylella fastidiosa Subspecies fastidiosa and Multiplex in Plant and Insect Tissues. J. Microbiol. Methods 2018, 155, 8–18. [Google Scholar] [CrossRef]
- Stenger, D.C.; Lee, M.W.; Rogers, E.E.; Chen, J. Plasmids of Xylella fastidiosa Mulberry-Infecting Strains Share Extensive Sequence Identity and Gene Complement with PVEIS01 from the Earthworm Symbiont Verminephrobacter Eiseniae. Physiol. Mol. Plant Pathol. 2010, 74, 238–245. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New Capabilities and Interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. TrimAl: A Tool for Automated Alignment Trimming in Large-Scale Phylogenetic Analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A New and Scalable Tool for the Selection of DNA and Protein Evolutionary Models. Mol. Biol. Evol. 2020, 37, 291–294. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Yu, G. Using Ggtree to Visualize Data on Tree-Like Structures. Curr. Protoc. Bioinform. 2020, 69, e96. [Google Scholar] [CrossRef] [PubMed]
| Subspecies a | ST a | Strain | Origin | Host | traC Gene b |
|---|---|---|---|---|---|
| fastidiosa | 1 | IVIA5235 | Balearic Island, Spain | Prunus avium | + |
| IVIA5770 | Balearic Island, Spain | Vitis vinifera | + | ||
| R2XF4358/18 | Balearic Island, Spain | Rhamnus alaternus | + | ||
| XYL461 | Balearic Island, Spain | Rhamnus alaternus | + | ||
| XYL3349 | Balearic Island, Spain | Prunus dulcis | + | ||
| CFBP8351 | California, USA | Vitis vinifera | + | ||
| Temecula1 | California, USA | Vitis vinifera | + | ||
| TemeculaL | California, USA | Vitis vinifera | + | ||
| M23 | California, USA | Prunus dulcis | + | ||
| fastidiosa | 2 | CFBP7970 | Florida, USA | Vitis vinifera | + |
| CFBP8082 | Florida, USA | Ambrosia artemifolia | + | ||
| WM1-1 | Georgia, USA | Vitis vinifera | − | ||
| CFBP7969 | North Carolina, USA | Vitis rotundifolia | − | ||
| CFBP8083 | North Carolina, USA | Vitis vinifera | − | ||
| fastidiosa | 75 | CFBP8073 | Mexico | Coffea canephora | − |
| morus | 29 | CFBP8084 | Georgia, USA | Morus alba | − |
| multiplex | 6 | Dixon | California, USA | Prunus dulcis | + |
| ESVL | Valencian Community, Spain | Prunus dulcis | − | ||
| IVIA6902 | Valencian Community, Spain | Prunus dulcis | − | ||
| IAS-AXF212H7 | Valencian Community, Spain | Prunus dulcis | − | ||
| IAS-AXF235T1 | Valencian Community, Spain | Prunus dulcis | − | ||
| IAS-AXF235T10 | Valencian Community, Spain | Prunus dulcis | − | ||
| IAS-AXF64H11 | Valencian Community, Spain | Prunus dulcis | − | ||
| IAS-AXF64T12 | Valencian Community, Spain | Prunus dulcis | − | ||
| IAS-AXF64T13 | Valencian Community, Spain | Prunus dulcis | − | ||
| IAS-AXF64T14 | Valencian Community, Spain | Prunus dulcis | − | ||
| IVIA5901 | Valencian Community, Spain | Prunus dulcis | − | ||
| IVIA6586-2 | Valencian Community, Spain | Helicrysum italicum | − | ||
| IVIA6629 | Valencian Community, Spain | Rhamnus alaternus | − | ||
| CFBP8417 | Corsica, France | Spartium junceum | − | ||
| CFBP8418 | Corsica, France | Spartium junceum | − | ||
| multiplex | 7 | CFBP8416 | Corsica, France | Polygala myrtifolia | − |
| M12 | California, USA | Prunus dulcis | − | ||
| LM10 | California, USA | Olea europaea | − | ||
| RH1 | California, USA | Olea europaea | − | ||
| multiplex | 10 | CFBP8070 | Georgia, USA | Prunus sp. | − |
| multiplex | 27 | CFBP8075 | California, USA | Prunus sp. | − |
| multiplex | 41 | CFBP8173 | Georgia, USA | Prunus saliciana | − |
| CFBP8068 | Washington DC, USA | Ulmus sp. | − | ||
| multiplex | 42 | AlmaEm3 | Georgia, USA | Vaccinium sp. | − |
| multiplex | 43 | BB08-1 | Florida, USA | Vaccinium corymbosum | − |
| multiplex | 51 | CFBP8078 | Florida, USA | Vinca sp. | − |
| multiplex | 81 | XYL1981/17 | Balearic Islands, Spain | Ficus carica | − |
| XYL1966/18 | Balearic Islands, Spain | Olea europaea | − | ||
| XYL468 | Balearic Islands, Spain | Olea europaea | − | ||
| XYL466/19 | Balearic Islands, Spain | Olea europaea | − | ||
| XF3348 | Balearic Islands, Spain | Prunus dulcis | − | ||
| XYL1752/17 | Balearic Islands, Spain | Prunus dulcis | − | ||
| Santa29b | Balearic Islands, Spain | Santolina chamaecyparissus | − | ||
| Fillmore | California, USA | Olea europaea | − | ||
| pauca | 53 | DeDonno | Apulia, Italy | Olea europaea | + |
| CFBP8477/Salento-1 | Apulia, Italy | Olea europaea | + | ||
| CFBP8402/CoDiRo | Apulia, Italy | Olea europaea | + | ||
| CFBP8495/PD7202 | Intercepted, Costa Rica c | Coffea arabica | + | ||
| CFBP8429 | Intercepted, unknown c | Coffea arabica | + | ||
| pauca | 73 | CFBP8498/PD7211 | Intercepted, Costa Rica c | Coffea arabica | + |
| pauca | 74 | CFBP8072 | Ecuador | Coffea arabica | + |
| CFBP8074 | Ecuador | Coffea arabica | + | ||
| pauca | 80 | XYL1961 | Balearic Island, Spain | Olea europaea | − |
| IAS-XYL1513-1 | Balearic Island, Spain | Prunus dulcis | − | ||
| IAS-XYL1518 | Balearic Island, Spain | Prunus dulcis | − | ||
| sandyi | 5 | Ann-1 | California, USA | Nerium oleander | − |
| sandyi | 72 | CFBP8478 | Intercepted, Costa Rica c | Coffea arabica | + |
| sandyi | 76 | CFBP8356 | Intercepted, Costa Rica c | Coffea arabica | − |
| sandyi/morus | 72 | CO33 | Intercepted, Costa Rica c | Coffea arabica | + |
| Subspecies a | ST a | Origin | Host b | Ct c | Number of Samples d | traC Gene e |
|---|---|---|---|---|---|---|
| fastidiosa | 1 | Mallorca | Calicotome spinosa | 23, 27 | 2 | 2 |
| fastidiosa | 1 | Mallorca | Genista lucida | 20 | 1 | 1 |
| fastidiosa | 1 | Mallorca | Juglans regia | 29 | 1 | 1 |
| fastidiosa | 1 | Mallorca | Polygala myrtifolia | 23–28 | 4 | 4 |
| fastidiosa | 1 | Mallorca | Prunus dulcis | 21–31 | 23 | 19 |
| fastidiosa | 1 | Mallorca | Prunus dulcis TR < 2004 | 25 | 2 | 2 |
| fastidiosa | 1 | Mallorca | Prunus dulcis TR 2005–2009 | 22 | 2 | 2 |
| fastidiosa | 1 | Mallorca | Prunus dulcis TR 2010–2017 | 22 | 5 | 5 |
| fastidiosa | 1 | Mallorca | Prunus dulcis TR 1996–2015 | 25–26 | 14 | 0 |
| fastidiosa | 1 | Mallorca | Rhamnus alaternus | 20–27 | 8 | 8 |
| fastidiosa | 1 | Mallorca | Ruta chalepensis | 17–27 | 3 | 3 |
| fastidiosa | 1 | Mallorca | Teucrium capitatum | 33 | 1 | 0 |
| fastidiosa | 1 | Mallorca | Vitis vinifera | 21–25 | 9 | 9 |
| multiplex | 6 | Alicante | Prunus dulcis | 18–29 | 22 | 0 |
| multiplex | 7 | Mallorca | Polygala myrtifolia | 28 | 1 | 0 |
| multiplex | 7 | Mallorca | Prunus dulcis | 24, np | 2 | 0 |
| multiplex | 81 | Mallorca | Acacia saligna | 29 | 1 | 0 |
| multiplex | 81 | Mallorca | Calicotome spinosa | 25 | 1 | 0 |
| multiplex | 81 | Mallorca | Cistus albidus | 33, 34 | 2 | 0 |
| multiplex | 81 | Mallorca | Ficus carica | 26–32 | 4 | 0 |
| multiplex | 81 | Mallorca | Fraxinus angustifolia | 24–30 | 9 | 0 |
| multiplex | 81 | Mallorca | Genista valdes-bermejoi | 24 | 1 | 0 |
| multiplex | 81 | Mallorca | Helichrysum stoechas | 28 | 1 | 0 |
| multiplex | 81 | Mallorca | Lavandula dentata | 25, 28 | 2 | 0 |
| multiplex | 81 | Mallorca | Olea europaea | 20–33 | 22 | 0 |
| multiplex | 81 | Mallorca | Phagnalon saxatile | 31 | 1 | 0 |
| multiplex | 81 | Mallorca | Phillyrea angustifolia | 32 | 1 | 0 |
| multiplex | 81 | Mallorca | Polygala myrtifolia | 22 | 1 | 0 |
| multiplex | 81 | Mallorca | Prunus dulcis | 25, 26 | 2 | 0 |
| multiplex | 81 | Mallorca | Rhamnus alaternus | 27 | 2 | 0 |
| multiplex | 81 | Mallorca | Rosmarinus officinalis | 33 | 1 | 0 |
| multiplex | 81 | Mallorca | Salvia officinalis | 23 | 1 | 0 |
| multiplex | 81 | Mallorca | Santolina chamaecyparissus | 20, 22 | 2 | 0 |
| multiplex | 81 | Mallorca | Spartium junceum | 21, 24 | 2 | 0 |
| multiplex | 81 | Menorca | Cistus albidus | 25, 26 | 2 | 0 |
| multiplex | 81 | Menorca | Clematis cirrhosa | 30 | 1 | 0 |
| multiplex | 81 | Menorca | Ficus carica | 28 | 1 | 0 |
| multiplex | 81 | Menorca | Helichrysum stoechas | 21, 27 | 2 | 0 |
| multiplex | 81 | Menorca | Olea europaea | 24–30 | 11 | 0 |
| multiplex | 81 | Menorca | Phlomis italica | 25, 27 | 2 | 0 |
| multiplex | 81 | Menorca | Rhamnus alaternus | 24, 35 | 2 | 0 |
| multiplex | 81 | Menorca | Rosmarinus officinalis | 26, 29 | 2 | 0 |
| multiplex | 81 | Menorca | Santolina chamaecyparissus | 24–31 | 5 | 0 |
| multiplex | 81 | Menorca | Santolina magonica | 29 | 1 | 0 |
| multiplex | 81 | Menorca | Vitex agnus-castus | 31 | 1 | 0 |
| pauca | 80 | Ibiza | Acacia saligna | 23, 24 | 2 | 0 |
| pauca | 80 | Ibiza | Cistus albidus | 25–30 | 3 | 0 |
| pauca | 80 | Ibiza | Genista hirsuta | 22 | 2 | 0 |
| pauca | 80 | Ibiza | Lavandula angustifolia | 23 | 1 | 0 |
| pauca | 80 | Ibiza | Lavandula dentata | 29 | 1 | 0 |
| pauca | 80 | Ibiza | Olea europaea | 24–32 | 15 | 0 |
| pauca | 80 | Ibiza | Polygala myrtifolia | 28, 31 | 2 | 0 |
| pauca | 80 | Ibiza | Prunus dulcis | 29–33 | 9 | 0 |
| pauca | 80 | Ibiza | Rosmarinus officinales | 29, np | 3 | 0 |
| pauca | 80 | Ibiza | Ulex parviflorus | 21–25 | 2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco-Amo, M.P.; Arias-Giraldo, L.F.; Olivares-García, C.; Denancé, N.; Jacques, M.-A.; Landa, B.B. Use of traC Gene to Type the Incidence and Distribution of pXFAS_5235 Plasmid-Bearing Strains of Xylella fastidiosa subsp. fastidiosa ST1 in Spain. Plants 2022, 11, 1562. https://doi.org/10.3390/plants11121562
Velasco-Amo MP, Arias-Giraldo LF, Olivares-García C, Denancé N, Jacques M-A, Landa BB. Use of traC Gene to Type the Incidence and Distribution of pXFAS_5235 Plasmid-Bearing Strains of Xylella fastidiosa subsp. fastidiosa ST1 in Spain. Plants. 2022; 11(12):1562. https://doi.org/10.3390/plants11121562
Chicago/Turabian StyleVelasco-Amo, María Pilar, Luis F. Arias-Giraldo, Concepción Olivares-García, Nicolás Denancé, Marie-Agnès Jacques, and Blanca B. Landa. 2022. "Use of traC Gene to Type the Incidence and Distribution of pXFAS_5235 Plasmid-Bearing Strains of Xylella fastidiosa subsp. fastidiosa ST1 in Spain" Plants 11, no. 12: 1562. https://doi.org/10.3390/plants11121562
APA StyleVelasco-Amo, M. P., Arias-Giraldo, L. F., Olivares-García, C., Denancé, N., Jacques, M.-A., & Landa, B. B. (2022). Use of traC Gene to Type the Incidence and Distribution of pXFAS_5235 Plasmid-Bearing Strains of Xylella fastidiosa subsp. fastidiosa ST1 in Spain. Plants, 11(12), 1562. https://doi.org/10.3390/plants11121562






