Foliar Application of Nano-Silicon Improves the Physiological and Biochemical Characteristics of ‘Kalamata’ Olive Subjected to Deficit Irrigation in a Semi-Arid Climate
Abstract
:1. Introduction
2. Results
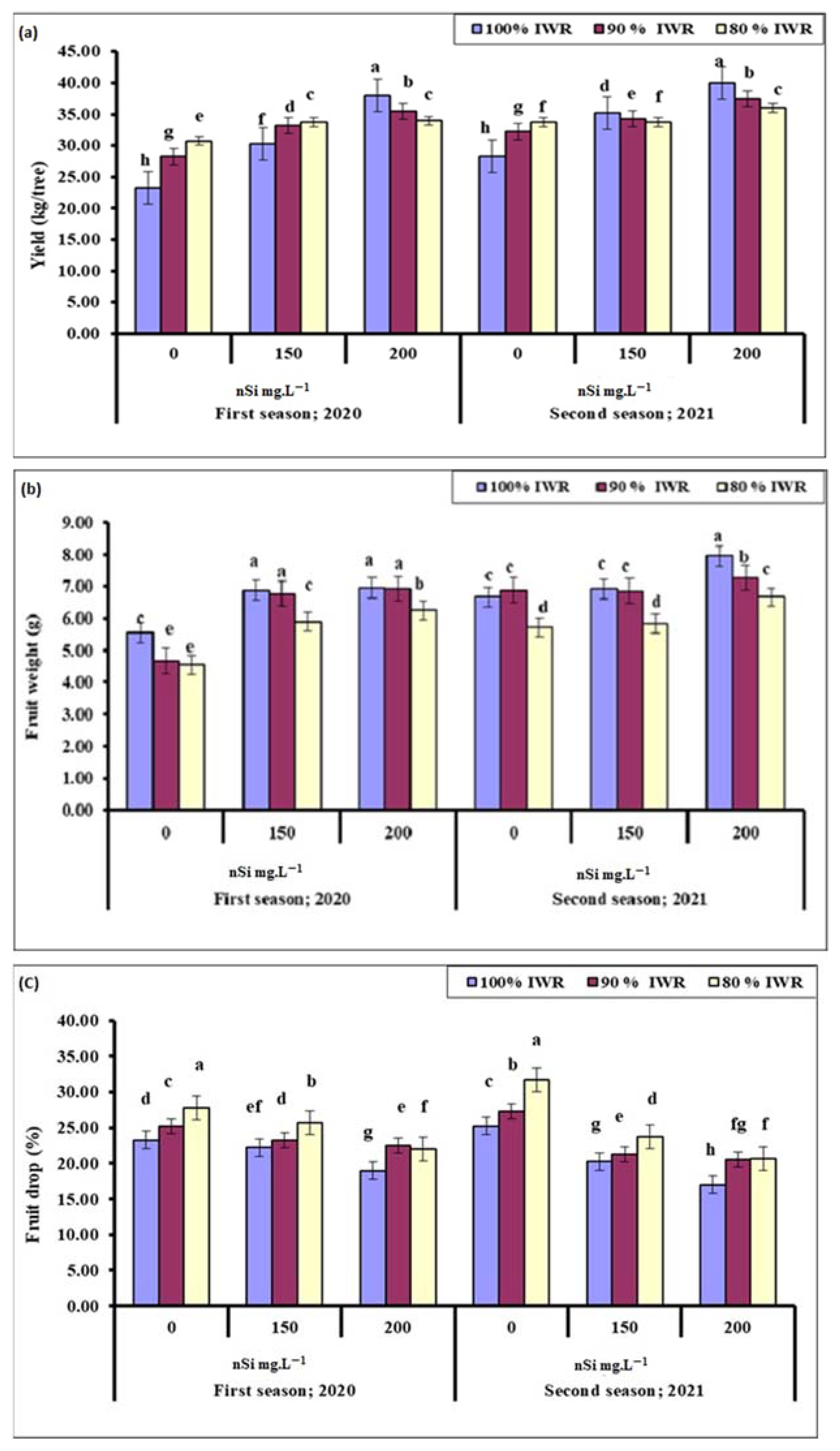
2.1. Yield
2.2. Fruit Weight
2.3. Fruit Drop
2.4. Biochemical Characteristics
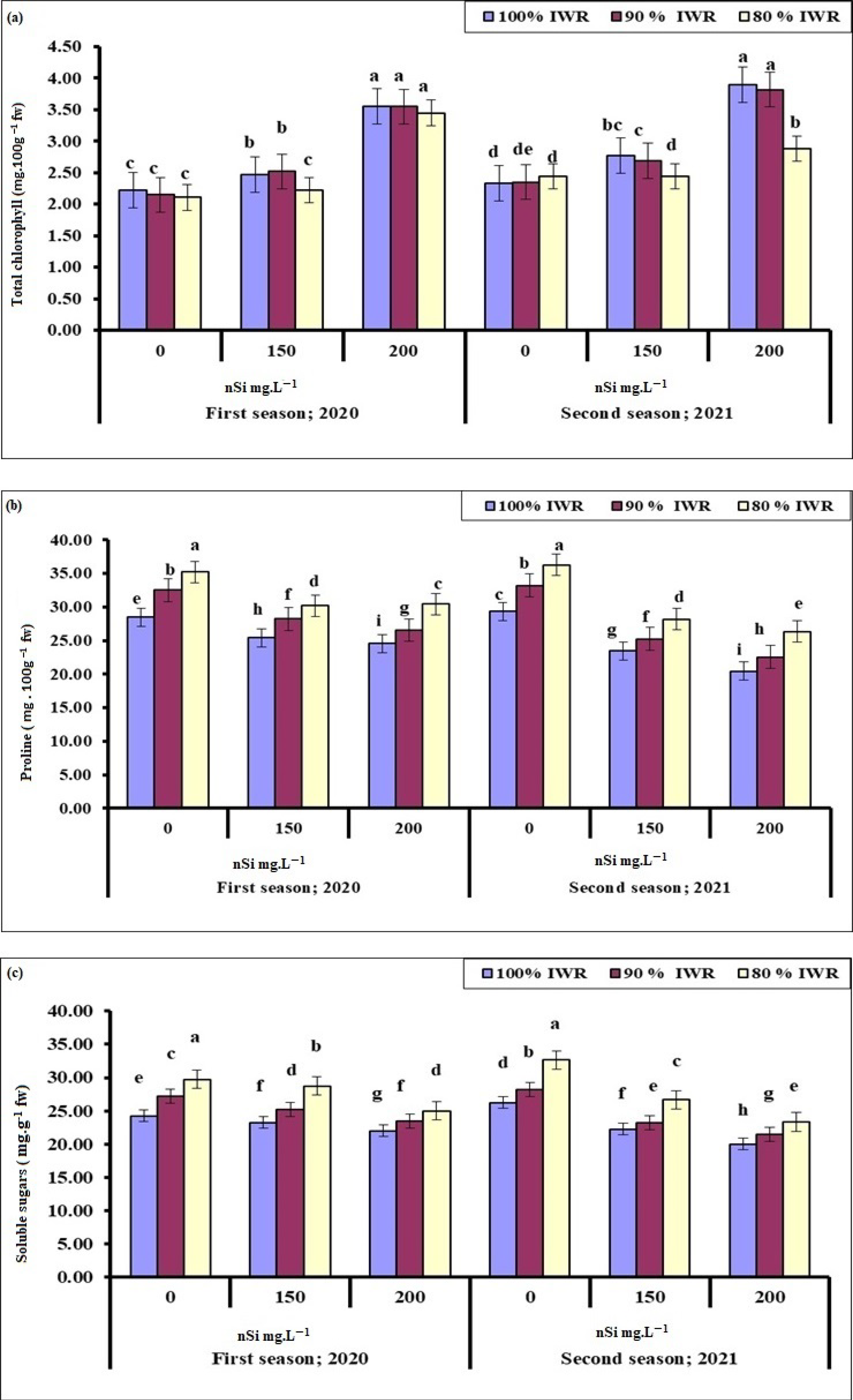
2.4.1. Total Chlorophyll
2.4.2. Proline
2.4.3. Soluble Sugars
2.5. Leaf Water Status and Membrane Damage Indicator
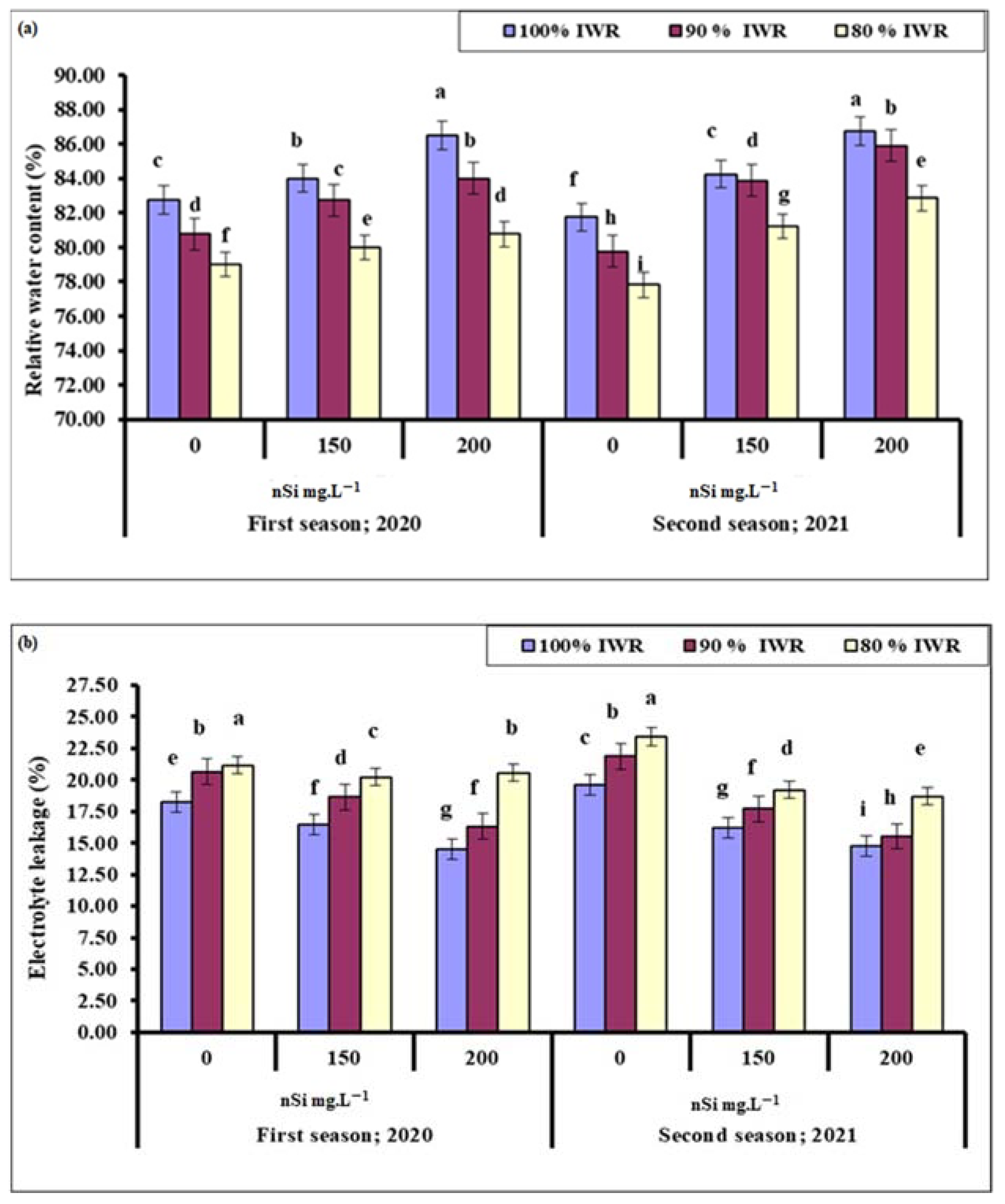
2.5.1. Relative Water Content (RWC)
2.5.2. Electrolyte Leakage
2.6. Oxidative Stress Markers
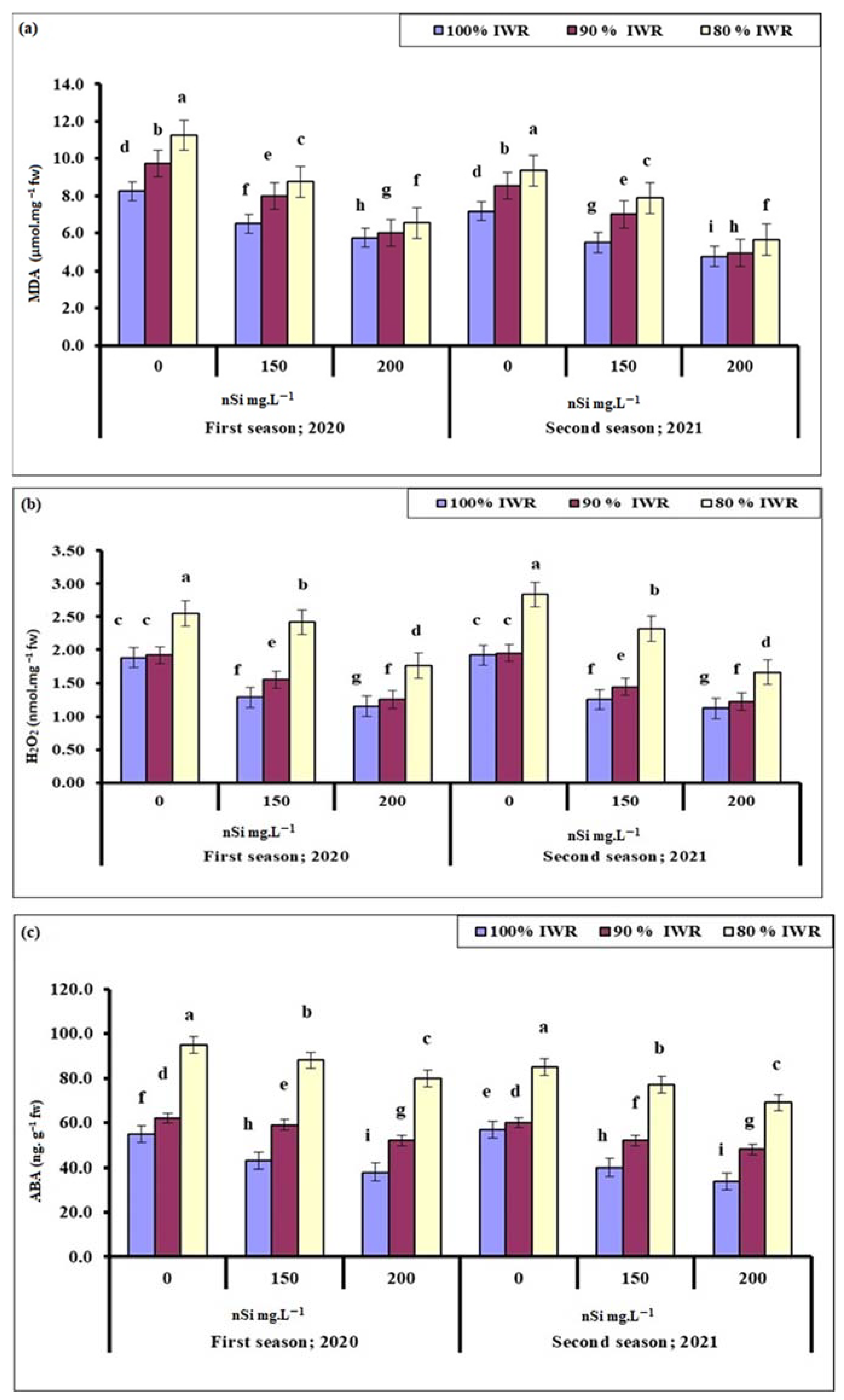
2.6.1. Malondialdehyde (MDA)
2.6.2. Hydrogen Peroxide (H2O2)
2.6.3. Abscisic Acid (ABA)
3. Discussion
4. Materials and Methods
4.1. Experiment
4.2. Yield and Average Fruit Weight
4.3. Fruit Drop
4.4. Leaf Analysis
4.5. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Olive Council (IOC). The Olive Tree; International Olive Council (IOC): Madrid, Spain, 2022; Available online: https://www.internationaloliveoil.org/olive-world/olive-tree/ (accessed on 20 April 2022).
- Food and Agriculture Organization of the United Nations (FAO). FAO Statistics; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2020; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 April 2022).
- Bayoumi, H.; Zaghloul, E.A.; Al-Gebaly, M.R.; Abd el-Ghani, S.S. An economic study of olive crop in North Sinai governorate. Middle East J. Agric. Res. 2014, 3, 994–1001. [Google Scholar]
- Wiesman, Z. Desert Olive Oil Cultivation: Advanced Biotechnologies; Elsevier: New York, NY, USA, 2009; p. 147. [Google Scholar]
- Yacout, D.A.; Soliman, N.F.; Zahran, H.F. Potentials of a Sustainable Olive Industry in Egypt. In Proceedings of the International Conference of Biotechnology and Environment (ICBE 2016), Alexandria, Egypt, 1–3 November 2016; p. 57. [Google Scholar]
- Ogbaga, C.C.; Amir, M.; Bano, H.; Chater, C.C.; Jellason, N.P. Clarity on frequently asked questions about drought measurements in plant physiology. Sci. Afr. 2020, 8, e00405. [Google Scholar] [CrossRef]
- Agricultural Statistics of Egypt. Water Scarcity in Egypt: The Urgent Need for Regional Cooperation among the Nile Basin Countries. Report of the Ministry of Water Resources and Irrigation; Government of Egypt: Cairo, Egypt, 2014; p. 5.
- Ben Ahmed, C.; Ben Rouina, B.; Boukhris, M. Effects of water deficit on olive trees cv. Chemlali under field conditions in arid region in Tunisia. Sci. Hortic. 2007, 113, 267–277. [Google Scholar] [CrossRef]
- Jury, W.A.; Vaux, J.H. The role of science in solving the world’s emerging water problems. Proc. Natl Acad. Sci. USA 2005, 102, 15715–15720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosgrove, W.J.; Loucks, D.P. Water management: Current and future challenges and research directions. Water Resour. Res. 2015, 51, 4823–4839. [Google Scholar] [CrossRef] [Green Version]
- Brito, C.; Dinis, L.-T.; Ferreira, H.; Moutinho-Pereira, J.; Correia, C. The role of nighttime water balance on Olea europaea plants subjected to contrasting water regimes. J. Plant Physiol. 2018, 226, 56–63. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Petridis, A.; Therios, I.; Samouris, G.; Koundouras, S.; Giannakoula, A. Effect of water deficit on leaf phenolic composition, gas exchange, oxidative damage and antioxidant activity of four Greek olive (Olea europaea L.) cultivars. Plant Physiol. Biochem. 2012, 60, 1–11. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Trupiano, D.; Polzella, A.; de Zio, E.; Sassi, M.; Scaloni, A.; Zarrouk, M.; Youssef, N.B.; Scippa, G.S. Unraveling physiological, biochemical and molecular mechanisms involved in olive (Olea europaea L. cv. Chétoui) tolerance to drought and salt stresses. J. Plant Physiol. 2018, 220, 83–95. [Google Scholar] [CrossRef]
- English, M. Deficit irrigation. I. Analytical framework. J. Irrig. Drain. Eng. 1990, 116, 399–412. [Google Scholar] [CrossRef]
- Costa, J.M.; Ortuno, M.F.; Chaves, M.M. Deficit irrigation as a strategy to save water: Physiology and potential application to horticulture. J. Integr. Plant Biol. 2007, 49, 1421–1434. [Google Scholar] [CrossRef]
- Ferenes, E.; Goldhamer, D.A. Deciduous Fruit and Nut Trees. In Irrigation of Agricultural Crops, 1st ed.; Stewart, B.A., Nielsen, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1990; pp. 987–1017. [Google Scholar]
- Panigrahi, P.; Srivastava, A.K. Effective management of irrigation water in citrus orchards under a water scarce hot sub-humid region. Sci. Hortic. 2016, 210, 6–13. [Google Scholar] [CrossRef]
- Zuazo, V.H.D.; Garcia-Tejero, I.F.; Rodriguez, B.C.; Tarifa, D.F.; Ruiz, B.G.; Sacristan, P.C. Deficit irrigation stratigies for subtropical mango farming. A review. Agr. Sustain. Develop. 2021, 41, 13. [Google Scholar] [CrossRef]
- Rapoport, H.F.; Perez-Priego, O.; Orgaz, F.; Martins, P. Water deficiet effects during olive tree inflorescence and flower development. Acta Hortic. 2011, 888, 157–162. [Google Scholar] [CrossRef]
- Connor, D.J.; Fereres, E. The physiology of adaptation and yield expression in olive. Hortic. Rev. 2010, 31, 155–229. [Google Scholar]
- Sofo, A.; Manfreda, S.; Fiorentino, M.; Dichio, B.; Xiloyannis, C. The olive tree: A paradigm for drought tolerance in Mediterranean climates. Hydrol. Earth Syst. Sci. 2008, 12, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, M.A.; Hegazi, A.A.; Hmmam, I.S. Effect of water stress on vegetative characteristics and leaves chemical constituents of some transplants olive cultivars. Am-Eurasian J. Agric. Environ. Sci. 2011, 11, 663–670. [Google Scholar]
- Ahmed, C.B.; Rouina, B.B.; Sensoy, S.; Boukhris, M.; Abdallah, F.B. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Env. Exp. Bot. 2009, 67, 345–352. [Google Scholar] [CrossRef]
- Bolat, I.; Dikilitas, M.; Ikinci, A.; Ercisli, S.; Tonkaz, T. Morphological, physiological, biochemical characteristics and bud success responses of myrobolan 29 c plum rootstock subjected to water stress. Can. J. Plant Sci. 2015, 96, 485–493. [Google Scholar] [CrossRef]
- Saiki, S.T.; Ishida, A.; Yoshimura, K.; Yazaki, K. Physiological mechanisms of drought-induced tree die-off in relation to carbon, hydraulic and respiratory stress in a drought-tolerant woody plants. Sci. Rep. 2017, 7, 2995. [Google Scholar] [CrossRef] [Green Version]
- Tahir, F.M.; Ibrahim, M.; Hamid, K. Effect of drought stress on vegetative and reproductive growth behavior of mango (Mangifera indica L.). Asian J. Plant Sci. 2003, 2, 116–118. [Google Scholar] [CrossRef] [Green Version]
- Tadina, N.; Germ, M.; Kreft, I.; Breznik, B.; Gaberščik, A. Effects of water deficit and selenium on common buckwheat (Fagopyrum esculentum Moench.) plants. Photosynthetica 2007, 45, 472–476. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010, 48, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.H.; Abou Leila, B.; Gaballah, M.E.L.; Wakeel, H. Effect of antioxidants on Citrus leaf anatomical structure grown under saline irrigation water. Plant Arch. 2019, 19, 840–845. [Google Scholar]
- Ahmadipour, S.; Arji, I.; Ebadi, A.; Abdossi, V. Physiological and biochemical responses of some olive cultivars (Olea europaea L.) to water stress. Cell. Molec. Biol. 2018, 64, 20–29. [Google Scholar] [CrossRef]
- Boughalleb, F.; Hajlaoui, H. Physiological and anatomical changes induced by drought in two olive cultivars (cv Zalmati and Chemlali). Acta Physiol Plant 2011, 33, 53–65. [Google Scholar] [CrossRef]
- Fernandez, V.; Sotiropoulos, T.; Brown, P.H. Foliar Fertilization: Scientific Principles and Field Practices, 1st ed.; International Fertilizer Industry Association (IFA): Paris, France, 2013; p. 140. [Google Scholar]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc oxide and silicon nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- Regni, L.; Del Buono, D.; Micheli, M.; Facchin, S.L.; Tolisano, C.; Proietti, P. Effects of Biogenic ZnO Nanoparticles on Growth, Physiological, Biochemical Traits and Antioxidants on Olive Tree In Vitro. Horticulturae 2022, 8, 161. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Meybodi, N.D.H.; da Silva, J.A.T. Foliar application of selenium and nano-selenium affects pomegranate (Punica granatum cv. Malase Saveh) fruit yield and quality. South Afr. J. Bot. 2019, 124, 350–358. [Google Scholar] [CrossRef]
- Scott, N.; Chen, H. Nanoscale Science and Engineering for Agriculture and Food Systems. A Report Submitted to Cooperative State Research, Education and Extension Service; National Planning Workshop, The United States Department of Agriculture (USDA): Washington, DC, USA, 2003; p. 62. Available online: http://www.nseafs.cornel.edu/web.roadmap.pdf (accessed on 25 April 2022).
- Mosanna, R.; Khalilvand, B.E. Morpho-physiological response of maize (Zea mays L.) to zinc nano-chelate foliar and soil application at different growth stages. J. New Biol. Rep. 2015, 4, 46–50. [Google Scholar]
- Boutchuen, A.; Zimmerman, D.; Aich, N.; Masud, A.M.; Arabshahi, A.; Palchoudhury, S. Increased Plant Growth with Hematite Nanoparticle Fertilizer Drop and Determining Nanoparticle Uptake in Plants Using Multimodal Approach. J. Nanomater. 2019, 2019, 6890572. [Google Scholar] [CrossRef] [Green Version]
- Helaly, M.N.; El-Hoseiny, H.; El-Sheery, N.I.; Rastogi, A.; Kalaji, H.M. Regulation and physiological role of silicon in alleviating drought stress of mango. Plant Physiol. Biochem. 2017, 118, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, M.; Rizwan, M.S.; Mushtaq, M.A.; Ashraf, M.; Shahzad, S.M.; Yousaf, B.; Tu, S. Silicon occurrence, uptake, transport and mechanisms of heavy metals, minerals and salinity enhanced tolerance in plants with future prospects: A review. J. Environ. Manag. 2016, 183, 521–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.F.; Takahasi, E. Soil, Fertilizer, and Plant Silicon Research in Japan, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2002; p. 294. [Google Scholar]
- Ma, J.F.; Miyake, Y.; Takahashi, E. Silicon as a beneficial element for crop plants. In Silicon in Agriculture. Studies in Plant Science, 8; Datnoff, L.E., Snyder, G.H., Korndorfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 17–39. [Google Scholar]
- Laane, H.-M. The Effects of Foliar Sprays with Different Silicon Compounds. Plants 2018, 7, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivanesan, I.; Park, S.W. The role of silicon in plant tissue culture. Front. Plant Sci. 2014, 5, 571. [Google Scholar] [CrossRef] [Green Version]
- Artyszak, A. Effect of Silicon Fertilization on Crop Yield Quantity and Quality—A Literature Review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Balakhnina, T.; Borkowska, A. Effects of silicon on plant resistance to environmental stresses: Review. Int. Agrophys. 2013, 27, 225–232. [Google Scholar] [CrossRef]
- Ashkavand, P.; Zarafshar, M.; Tabari, M.; Mirzaie, J.; Nikpour, A.; Bordbar, S.K.; Struve, D.; Striker, G.G. Application of SiO2 nanoparticles as pretreatment alleviates the impact of drought on the physiological performance of Prunus mahaleb L. (Rosaceae). Bol. Soc. Argent. Bot. 2018, 53, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Behboudi, F.; Tahmasebi Sarvestani, Z.; Kassaee, M.Z.; Modares Sanavi, S.; Sorooshzadeh, A. Improving growth and yield of wheat under drought stress via application of SiO2 nanoparticles. J. Agric. Sci. Technol. 2018, 20, 1479–1492. [Google Scholar]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Byrt, C.S. Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy 2019, 9, 246. [Google Scholar] [CrossRef] [Green Version]
- Hellala, F.; Amerb, A.K.; El-Sayeda, S.; El-Azab, K. Mitigation The negative effect of water stress on barley by nano silica application. Plant Arch. 2020, 20, 3224–3231. [Google Scholar]
- Desoky, E.-S.M.; Mansour, E.; El-Sobky, E.-S.E.; Abdul-Hamid, M.I.; Taha, T.F.; Elakkad, H.A.; Arnaout, S.M.; Eid, R.S.; El-Tarabily, K.A.; Yasin, M.A. Physio-biochemical and agronomic responses of faba beans to exogenously applied nano-silicon under drought stress conditions. Front. Plant. Sci. 2021, 12, 637783. [Google Scholar] [CrossRef] [PubMed]
- Bayati, P.; Karimmojeni, H.; Razmjoo, J.; Pucci, M.; Abate, G.; Baldwin, T.C.; Mastinu, A. Physiological, Biochemical, and Agronomic Trait Responses of Nigella sativa Genotypes to Water Stress. Horticulturae 2022, 8, 193. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Sunoj, V.; Wen, Y.; Zhu, J.; Muralidharan, G.; Cao, K. Foliar application of nanoparticles mitigates the chilling effect on photosynthesis and photoprotection in sugarcane. Plant Physiol. Biochem. 2020, 149, 50–60. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop Protec. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Matussin, S.; Harunsani, M.H.; Tan, A.L.; Khan, M.M. Plant-extract-mediated SnO2 nanoparticles: Synthesis and applications. ACS Sustain. Chem. Eng. 2020, 8, 3040–3054. [Google Scholar] [CrossRef]
- El-Dengawy, E.; EL-Abbasy, U.; El-Gobba, M.H. Influence of nano-silicon treatment on growth behavior of ‘Sukkary’and ‘Gahrawy’mango root-stocks under salinity stress. J. Plant Prod. 2021, 12, 49–61. [Google Scholar]
- Al-Wasfy, M.M. Response of Sakkoti date palms to foliar application of royal jelly, silicon and vitamins B. J. Amer. Sci. 2013, 9, 315–321. [Google Scholar]
- Zhang, W.; Xie, Z.; Wang, L.; Li, M.; Lang, D.; Zhang, X. Silicon alleviates salt and drought stress of Glycyrrhiza uralensis seedling by altering antioxidant metabolism and osmotic adjustment. J. Plant. Res. 2017, 130, 611–624. [Google Scholar] [CrossRef]
- Ismail, L.M.; Soliman, M.I.; Abd El-Aziz, M.H.; Abdel-Aziz, H.M. Impact of Silica Ions and Nano Silica on Growth and Productivity of Pea Plants under Salinity Stress. Plants 2022, 11, 494. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Dutt, M.; Shalan, A.M.; El-Kady, M.E.; El-Boray, M.S.; Shabana, Y.M.; Grosser, J.W. Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress. S. Afr. J. Bot. 2020, 132, 155–163. [Google Scholar] [CrossRef]
- Molahoseini, H.; Feizian, M.; Mehdi Pour, E.; Davazdah Emami, S. Investigating the Effect of Coated Nanosilicon Oxide with Humic Acid on Yield, Ion Composition and Salinity Tolerance of Black Cumin (Nigella sativa L.). Iran. J. Soil Water Res. 2020, 51, 2711–2723. [Google Scholar]
- Muhammad, H.M.D.; Abbas, A.; Ahmad, R. Fascinating Role of Silicon Nanoparticles to Mitigate Adverse Effects of Salinity in Fruit Trees: A Mechanistic Approach. Silicon 2022, 9, 1–8. [Google Scholar] [CrossRef]
- Andreotti, C.; Rouphael, Y.; Colla, G.; Basile, B. Rate and Timing of Application of Biostimulant Substances to Enhance Fruit Tree Tolerance toward Environmental Stresses and Fruit Quality. Agronomy 2022, 12, 603. [Google Scholar] [CrossRef]
- Attia, E.A.; Elhawat, N. Combined foliar and soil application of silica nanoparticles enhances the growth, flowering period and flower characteristics of marigold (Tagetes erecta L.). Sci. Hortic. 2021, 282, 110015. [Google Scholar] [CrossRef]
- González-García, Y.; Cárdenas-Álvarez, C.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Cabrera-de-la-Fuente, M.; Sandoval-Rangel, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Effect of three nanoparticles (Se, Si and Cu) on the bioactive compounds of bell pepper fruits under saline stress. Plants 2021, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- González-Moscoso, M.; Martínez-Villegas, N.V.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Rivera-Cruz, M.d.C.; González-Morales, S.; Juárez-Maldonado, A. Impact of silicon nanoparticles on the antioxidant compounds of tomato fruits stressed by arsenic. Foods 2019, 8, 612. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, L.M.; Shalan, A.M.; El-Boray, M.S.; Vincent, C.I.; El-Kady, M.E.; Grosser, J.W.; Dutt, M. Application of silicon nanoparticles enhances oxidative stress tolerance in salt stressed ‘Valencia’ sweet orange plants. Sci. Hortic. 2022, 295, 110856. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef]
- Fatemi, H.; Esmaiel Pour, B.; Rizwan, M. Foliar application of silicon nanoparticles affected the growth, vitamin C, flavonoid, and antioxidant enzyme activities of coriander (Coriandrum sativum L.) plants grown in lead (Pb)-spiked soil. Environ. Sci. Pollut. Res. 2021, 28, 1417–1425. [Google Scholar] [CrossRef]
- Hassan, I.F.; Gaballah, M.S.; Ogbaga, C.C.; Murad, S.A.; Brysiewicz, A.; Bakr, B.M.; Mira, A.; Alam-Eldein, S.M. Does melatonin improve the yield attributes of field-droughted banana under Egyptian semi-arid conditions? J. Water Land Dev. 2022, 52, 221–231. [Google Scholar]
- Helaly, M.N.; El-Hoseiny, H.M.; Elsheery, N.I.; Kalaji, H.M.; Santos-Villalobos, S.d.l.; Wróbel, J.; Hassan, I.F.; Gaballah, M.S.; Abdelrhman, L.A.; Mira, A.M. 5-Aminolevulinic acid and 24-epibrassinolide improve the drought stress resilience and productivity of banana plants. Plants 2022, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.B.; Tounsi, S.; Gharsallah, H.; Hammami, A.; Frikha-Gargouri, O. Lipopeptides from Bacillus amyloliquefaciens strain 32a as promising biocontrol compounds against the plant pathogen Agrobacterium tumefaciens. Environ. Sci. Pollut. Res. 2018, 25, 36518–36529. [Google Scholar] [CrossRef] [PubMed]
- Ashkavand, P.; Tabari, M.; Zarafshar, M.; Tomásková, I.; Struve, D. Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. Leśne Pr. Badaw. 2015, 76, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C.M. Drought stress effects and olive tree acclimation under a changing climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef] [Green Version]
- Maghsoudi, K.; Emam, Y.; Pessarakli, M. Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. J. Plant Nutr. 2016, 39, 1001–1015. [Google Scholar] [CrossRef]
- Patumi, M.; d’Andria, R.; Marsilio, V.; Fontanazza, G.; Morelli, G.; Lanza, B. Olive and olive oil quality after intensive monocone olive growing (Olea europaea L., cv. Kalamata) in different irrigation regimes. Food Chem. 2002, 77, 27–34. [Google Scholar] [CrossRef]
- Greven, M.; Neal, S.; Green, S.; Dichio, B.; Clothier, B. The effects of drought on the water use, fruit development and oil yield from young olive trees. Agric. Water Manag. 2009, 96, 1525–1531. [Google Scholar] [CrossRef]
- Machado, M.; Felizardo, C.; Fernandes-Silva, A.A.; Nunes, F.M.; Barros, A. Polyphenolic compounds, antioxidant activity and l-phenylalanine ammonia-lyase activity during ripening of olive cv. “Cobrançosa” under different irrigation regimes. Food Res. Int. 2013, 51, 412–421. [Google Scholar] [CrossRef]
- Caruso, G.; Gucci, R.; Urbani, S.; Esposto, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Servili, M. Effect of different irrigation volumes during fruit development on quality of virgin olive oil of cv. Frantoio. Agric. Water Manag. 2014, 134, 94–103. [Google Scholar] [CrossRef]
- Hassan, I.F.; Gaballah, M.S.; El-Hoseiny, H.M.; El-Sharnouby, M.E.; Alam-Eldein, S.M. Deficit Irrigation to Enhance Fruit Quality of the ‘African Rose’Plum under the Egyptian Semi-Arid Conditions. Agronomy 2021, 11, 1405. [Google Scholar] [CrossRef]
- Karimi, S.; Rahemi, M.; Rostami, A.A.; Sedaghat, S. Drought effects on growth, water content and osmoprotectants in four olive cultivars with different drought tolerance. Int. J. Fruit Sci. 2018, 18, 254–267. [Google Scholar] [CrossRef]
- Sharp, R.E.; LeNoble, M.E.; Else, M.A.; Thome, E.T.; Gherardi, F. Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: Evidence for an interaction with ethylene. J. Exp. Bot. 2000, 51, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.C.; Li, Q.F.; Gao, Y.B.; Xin, T.R. Effects of silicon application on drought resistance of cucumber plants. Soil Sci. Plant Nutr. 2004, 50, 623–632. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, M.B.; Methenni, K.; Nouairi, I.; Zarrouk, M.; Youssef, N.B. Drought priming improves subsequent more severe drought in a drought-sensitive cultivar of olive cv. Chétoui. Sci. Hortic. 2017, 221, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Ogbaga, C.C.; Stepien, P.; Johnson, G.N. Sorghum (Sorghum bicolor) varieties adopt strongly contrasting strategies in response to drought. Phys. Plant. 2014, 152, 389–401. [Google Scholar] [CrossRef]
- Noor, R.; Yasmin, H.; Ilyas, N.; Nosheen, A.; Hassan, M.N.; Mumtaz, S.; Khan, N.; Ahmad, A.; Ahmad, P. Comparative analysis of iron oxide nanoparticles synthesized from ginger (Zingiber officinale) and cumin seeds (Cuminum cyminum) to induce resistance in wheat against drought stress. Chemosphere 2022, 292, 133201. [Google Scholar] [CrossRef]
- Sreelakshmi, B.; Induja, S.; Adarsh, P.; Rahul, H.; Arya, S.; Aswana, S.; Haripriya, R.; Aswathy, B.; Manoj, P.; Vishnudasan, D. Drought stress amelioration in plants using green synthesised iron oxide nanoparticles. Mater. Today Proc. 2021, 41, 723–727. [Google Scholar] [CrossRef]
- Shallan, M.A.; Hassan, H.M.; Namich, A.A.; Ibrahim, A.A. Biochemical and physiological effects of TiO2 and SiO2 nanoparticles on cotton plant under drought stress. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1540–1551. [Google Scholar]
- Ma, C.; Liu, H.; Guo, H.; Musante, C.; Coskun, S.H.; Nelson, B.C.; White, J.C.; Xing, B.; Dhankher, O.P. Defense mechanisms and nutrient displacement in Arabifopsis thaliana upon exposure to CeO2 and In2O3 nanoparticles. Environ. Sci. Nano 2016, 3, 1369–1379. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Mohammadi, H.; Kariman, K. Nanosilicon-based recovery of barley (Hordeum vulgare) plants subjected to drought stress. Environ. Sci. Nano 2020, 7, 443–461. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Parveen, A.; Liu, W.; Hussain, S.; Asghar, J.; Perveen, S.; Xiong, Y. Silicon priming regulates morpho-physiological growth and oxidative metabolism in maize under drought stress. Plants 2019, 8, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradshahi, A.; Salehi, E.A.B.; Khold, B.B. Some Physiological Responses of Canola (Brassica napus L.) to Water Deficit Stress Under Laboratory Conditions. Iran. J. Sci. Technol. 2004, 28, 43–50. [Google Scholar]
- Ghafoor, R.; Akram, N.A.; Rashid, M.; Ashraf, M.; Iqbal, M.; Lixin, Z. Exogenously applied proline induced changes in key anatomical features and physio-biochemical attributes in water stressed oat (Avena sativa L.) plants. Physiol. Mol. Biol. Plants 2019, 25, 1121–1135. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Nitrogen containing compounds and adaptation of plants to salinity stress. Biol. Plant. 2000, 43, 491–500. [Google Scholar] [CrossRef]
- Meister, A. Biochemistry of the Amino Acids, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2012; ISBN 0323161472. [Google Scholar]
- El Sayed, O.M.; El Gammal, O.H.M.; Salama, A.S.M. Effect of proline and tryptophan amino acids on yield and fruit quality of Manfalouty pomegranate variety. Sci. Hortic. 2014, 169, 1–5. [Google Scholar] [CrossRef]
- Caronia, A.; Gugliuzza, G.; Inglese, P. Influence of L-proline on citrus sinensis (L.) [’new hall’ and ’tarocco Sciré’] fruit quality. Acta Hortic. 2010, 884, 423–426. [Google Scholar] [CrossRef]
- Kahlaoui, B.; Hachicha, M.; Misle, E.; Fidalgo, F.; Teixeira, J. Physiological and biochemical responses to the exogenous application of proline of tomato plants irrigated with saline water. J. Saudi Soc. Agric. Sci. 2018, 17, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Karimi, S.; Abbaspour, H.; Sinak, J.; Makarian, H. Evaluation of drought stress and foliar chitosan on biochemical characterices of castor bean (Ricinus communis L.). Res. J. Biol. Sci. 2012, 7, 117–122. [Google Scholar] [CrossRef]
- Akhtar, N.; Ilyas, N.; Hayat, R.; Yasmin, H.; Noureldeen, A.; Ahmad, P. Synergistic effects of plant growth promoting rhizobacteria and silicon dioxide nano-particles for amelioration of drought stress in wheat. Plant Physiol. Biochem. 2021, 166, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.W.M.; Abdeldaym, E.A.; Abdelaziz, S.M.; El-Sawy, M.B.; Mottaleb, S.A. Synergetic effects of zinc, boron, silicon, and zeolite nanoparticles on confer tolerance in potato plants subjected to salinity. Agronomy 2019, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Apel, K.; Hirt, H. Reactive oxygen species, metabolism, oxidative stress and signal transduction. Ann. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, A. Osmotic adjustment is a prime drought stressadaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Sen, A. Oxidative Stress Studies in Plant Tissue Culture. In Antioxidant Enzyme; World’s Largest Science, Technology and Medicine Open Access Book; Chapter 3; El-Missiry, M.A., Ed.; INTECH: Rijeka, Croatia, 2012; pp. 59–88. Available online: https://doi.org/10.5772/2895 (accessed on 17 February 2021).
- Gadallah, M.A.A. Effect of proline and glycinebetaine on Vicia faba responses to salt stress. Biol. Plant. 1999, 42, 249–257. [Google Scholar] [CrossRef]
- Diaz-Mula, H.M.; Zapata, P.J.; Guillen, F.; Castilo, S.; Martinez-Romero, D.; Valero, D.; Serrano, M. Changes in phytochemical and nutritive parameters and bioactive compounds during development and on-tree ripening of eight plum cultivars: A comparative study. J. Sci. Food Agric. 2008, 88, 2499–2507. [Google Scholar] [CrossRef]
- Norman, S.M.; Maier, V.P.; Pon, D.L. Abscisic acid accumulation and carotenoid and chlorophyll content in relation to water stress and leaf age of different types of citrus. J. Agric. Food Chem. 1990, 38, 1326–1334. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E. Abscisic acid induced changes in production of primary and secondary metabolites, photosynthetic cabacity, antioxidant capability, antioxidant enzymes and lipoxygenase inhibitory activity of Orthosiphon stamineus Benth. Molecules 2013, 18, 7957–7976. [Google Scholar] [CrossRef]
- Shu, S.; Gao, P.; Li, L.; Yuan, Y.; Sun, J.; Guo, S. Abscisic acid-induced H2O2 accumulation enhances antioxidant capacity in pumpkin-grafted cucumber leaves under Ca(NO3)2 stress. Front. Plant Sci. 2016, 7, 1489. [Google Scholar] [CrossRef] [PubMed]
- Ming-Yi, J.; Jian-Hua, Z. Abscisic acid and antioxidant defense in plant cells. Acta Bot. Sin. 2004, 1, 1–9. [Google Scholar]
- Hegazi, E.S.; El-Motaium, R.A.; Yehia, T.A.; Hashem, M.E. Effect of foliar boron application on boron, chlorophyll, phenol, sugars and hormones concentration of olive (Olea europea L.) buds, leaves, and fruits. J. Plant Nutr. 2018, 41, 749–765. [Google Scholar] [CrossRef]
- Johnson, R.S.; Handley, D.F.; Day, K.R. Postharvest water stress of an early maturing plum. J. Hort. Sci. 1994, 69, 1035–1041. [Google Scholar] [CrossRef]
- Aung, L.H.; Houch, L.G.; Norman, S.M. The abscisic acid content of citrus with special references to lemon. J. Exp. Bot. 1991, 42, 1083–1088. [Google Scholar] [CrossRef]
- Pinillos, V.; Ibáñez, S.; Cunha, J.M.; Hueso, J.J.; Cuevas, J. Postveraison deficit irrigation effects on fruit quality and yield of “Flame Seedless” table grape cultivated under greenhouse and net. Plants 2020, 9, 1437. [Google Scholar] [CrossRef]
- Rasmussen, G.K. Gibberellin and cell-wall hydrolysis as related to the low response of ‘Valencia’ oranges to abscission chemicals. HortScience 1981, 16, 497–498. [Google Scholar]
- Burns, J.K.; Lewandowski, D.J. Genetics and Expression of Pectinmethylesterase, Endo-B-Glucanase and Polygalacturonase Genes in Valencia Oranges; Proc. 1st Int.; Citrus Biotechnology Symposium: Eilat, Israel, 2000; pp. 65–80. [Google Scholar]
- Kazokas, W.C.; Burns, J.K. Cellulase activity and gene expression in citrus fruit abscission zones during and after ethylene treatment. J. Amer. Soc. Hort. Sci. 1998, 123, 781–786. [Google Scholar] [CrossRef] [Green Version]
- Wilde, S.A.; Corey, R.B.; Lyer, J.G.; Voight, G.K. Soil and Plant Analysis for Tree Culture, 3rd ed.; Oxford and IBH. Puplishing Co.: New Delhi, India, 1985; pp. 93–106. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. FAO Irrigation and drainage paper No. 56. Rome Food Agric. Organ. United Nations 1998, 56, e156. [Google Scholar]
- Saini, R. Laboratory Manual of Analytical Techniques in Horticulture; Agrobios: Jodhpur, India, 2001. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Sergiev, I.; Alexieva, V.; Karanov, E. Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt. Rend. Acad. Bulg. Sci. 1997, 51, 121–124. [Google Scholar]
- Koshioka, M.; Harada, J.M.; Noma, T.; Sassa, T.; Ogiama, K.; Taylor, S.; Rood, S.B.; Legge, R.L.; Pharis, R.P.K. Reversed-phase C18 high performance liquid chromatography of acidic and conjugated gibberellins. J. Chromatog. 1983, 256, 101–115. [Google Scholar] [CrossRef]
- Clarke, G.M. Introduction to the Design and Analysis of Experiments; Arnold: New York, NY, USA, 1997. [Google Scholar]
- CoHort Software. Statistics Software. CoStat; Version 4.20; CoHort Software: Berkeley, CA, USA, 1990. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1990; p. 593. [Google Scholar]
- Duncan, D.B. Multiple ranges and multiple F. test. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
| Soil (0–40 cm) | Water | |
|---|---|---|
| pH | 8.22 | 7.01 |
| Sand (%) | 92.0 | – |
| Silt (%) | 5.0 | – |
| Clay (%) | 3.0 | – |
| EC (dS/m) | 1.82 | 1.56 |
| CaCO3 (%) | 3.4 | – |
| Ca2+ (meq·100 g−1) | 8.6 | 9.4 |
| Mg2+ (meq·100 g−1) | 3.2 | 4.3 |
| Na+ (meq·100 g−1) | 6.9 | 9.80 |
| K+ (meq·100 g−1) | 1.5 | 0.22 |
| Cl− (meq·100 g−1) | 8.2 | 6.46 |
| SO42– (meq·100 g−1) | 6.4 | 14.3 |
| CO3– (meq·100 g−1) | 0.0 | – |
| HCO3– (meq·100 g−1) | 5.6 | 3.0 |
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sept | Oct | Nov | Dec | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. mean Max (°C) | 20.7 | 25.5 | 25.7 | 27.1 | 32.9 | 33.8 | 34.8 | 34.9 | 32.8 | 28 | 23.2 | 20.7 |
| Temp. mean Min (°C) | 9.1 | 8.98 | 11.1 | 13.7 | 16.7 | 19.5 | 20.2 | 22.7 | 20.2 | 17 | 10 | 9.3 |
| Temp. average (°C) | 14.9 | 17.24 | 19.4 | 20.4 | 24.3 | 26.65 | 27.45 | 28.5 | 26.4 | 23.4 | 20.05 | 15 |
| Relative humidity (%) | 65.1 | 62.5 | 62.56 | 58 | 58.1 | 59.2 | 58.8 | 59.9 | 63.1 | 62 | 65.1 | 65.2 |
| Evaporation (mm·day−1) | 6.2 | 7.7 | 9.8 | 12.5 | 13.8 | 15 | 14.3 | 12.7 | 10.5 | 8.6 | 6.1 | 5.1 |
| ETo (mm·day−1) | 2.80 | 3.30 | 4.0 | 4.80 | 5.30 | 5.80 | 6.10 | 5.40 | 4.40 | 3.10 | 3.02 | 2.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, I.F.; Ajaj, R.; Gaballah, M.S.; Ogbaga, C.C.; Kalaji, H.M.; Hatterman-Valenti, H.M.; Alam-Eldein, S.M. Foliar Application of Nano-Silicon Improves the Physiological and Biochemical Characteristics of ‘Kalamata’ Olive Subjected to Deficit Irrigation in a Semi-Arid Climate. Plants 2022, 11, 1561. https://doi.org/10.3390/plants11121561
Hassan IF, Ajaj R, Gaballah MS, Ogbaga CC, Kalaji HM, Hatterman-Valenti HM, Alam-Eldein SM. Foliar Application of Nano-Silicon Improves the Physiological and Biochemical Characteristics of ‘Kalamata’ Olive Subjected to Deficit Irrigation in a Semi-Arid Climate. Plants. 2022; 11(12):1561. https://doi.org/10.3390/plants11121561
Chicago/Turabian StyleHassan, Islam F., Rahaf Ajaj, Maybelle S. Gaballah, Chukwuma C. Ogbaga, Hazem M. Kalaji, Harlene M. Hatterman-Valenti, and Shamel M. Alam-Eldein. 2022. "Foliar Application of Nano-Silicon Improves the Physiological and Biochemical Characteristics of ‘Kalamata’ Olive Subjected to Deficit Irrigation in a Semi-Arid Climate" Plants 11, no. 12: 1561. https://doi.org/10.3390/plants11121561
APA StyleHassan, I. F., Ajaj, R., Gaballah, M. S., Ogbaga, C. C., Kalaji, H. M., Hatterman-Valenti, H. M., & Alam-Eldein, S. M. (2022). Foliar Application of Nano-Silicon Improves the Physiological and Biochemical Characteristics of ‘Kalamata’ Olive Subjected to Deficit Irrigation in a Semi-Arid Climate. Plants, 11(12), 1561. https://doi.org/10.3390/plants11121561








