Variation in Glucosinolate Accumulation among Different Sprout and Seedling Stages of Broccoli (Brassica oleracea var. italica)
Abstract
:1. Introduction
2. Results
2.1. Identification of GLs
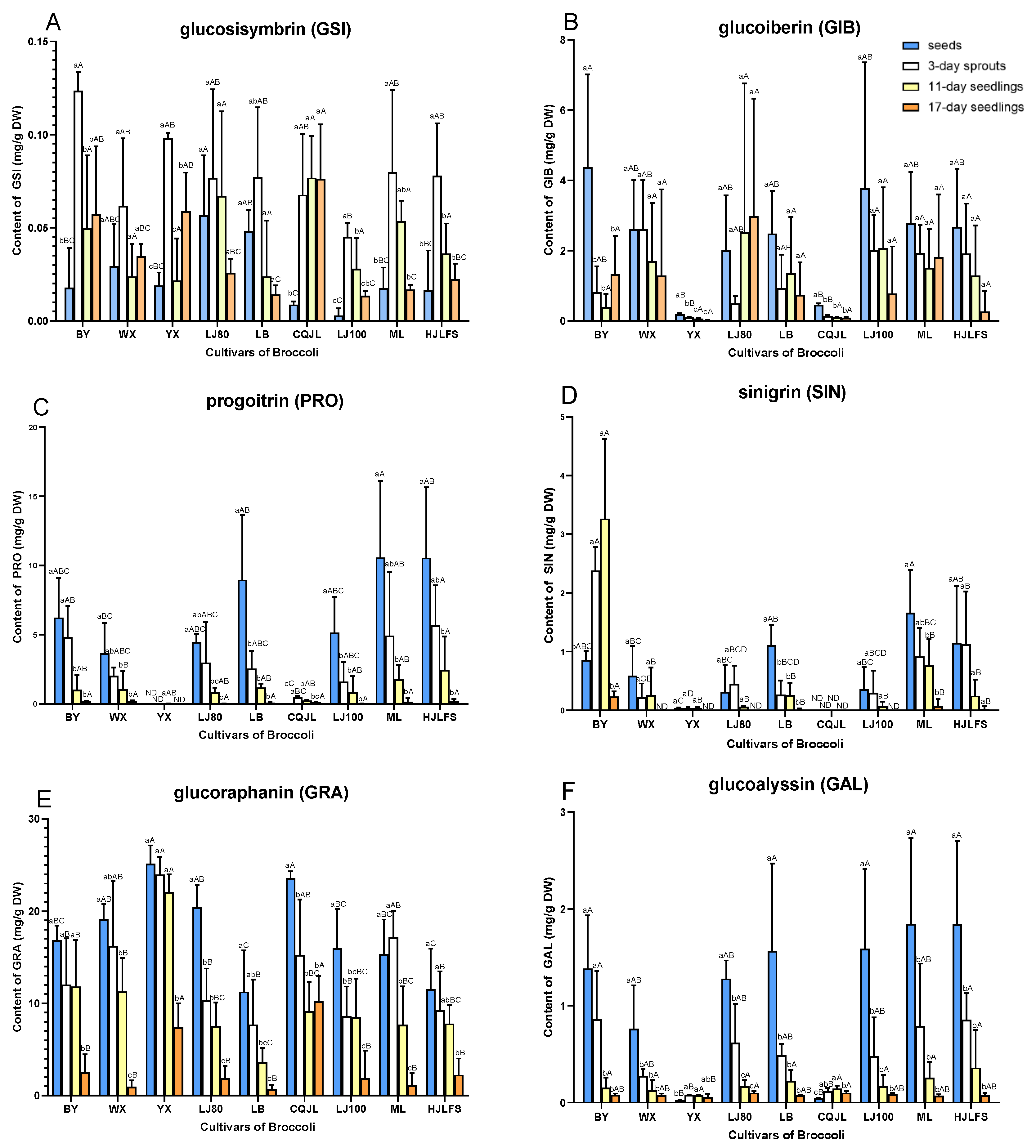
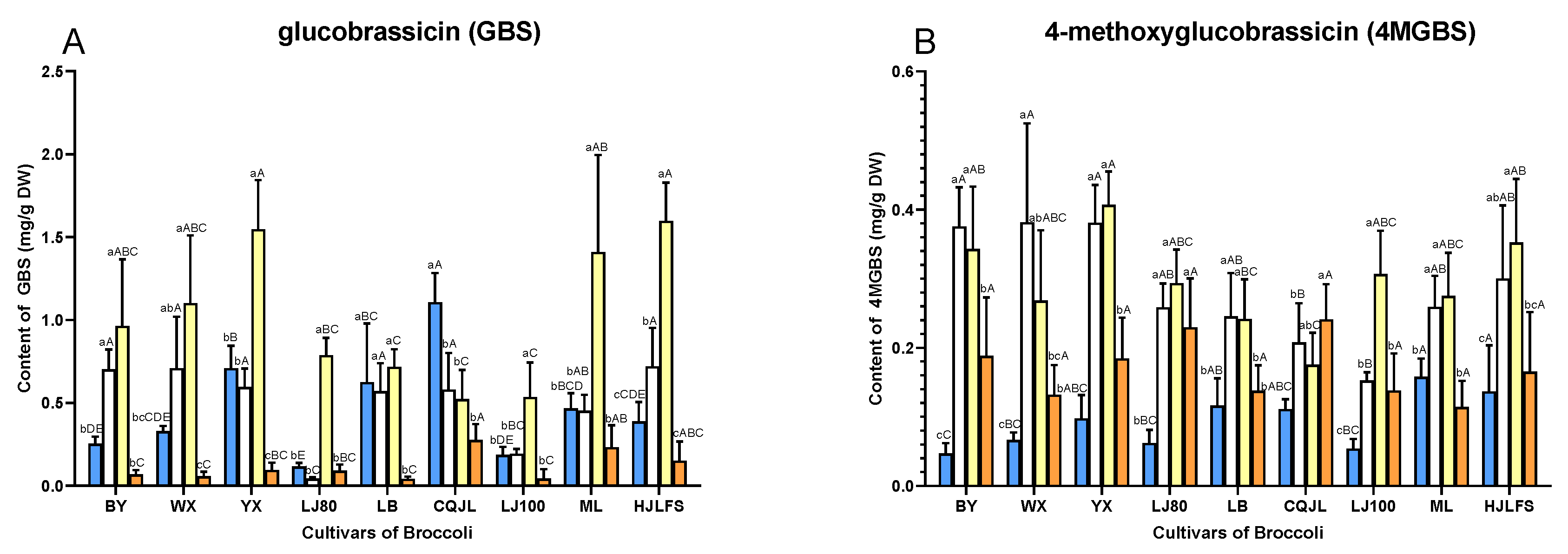
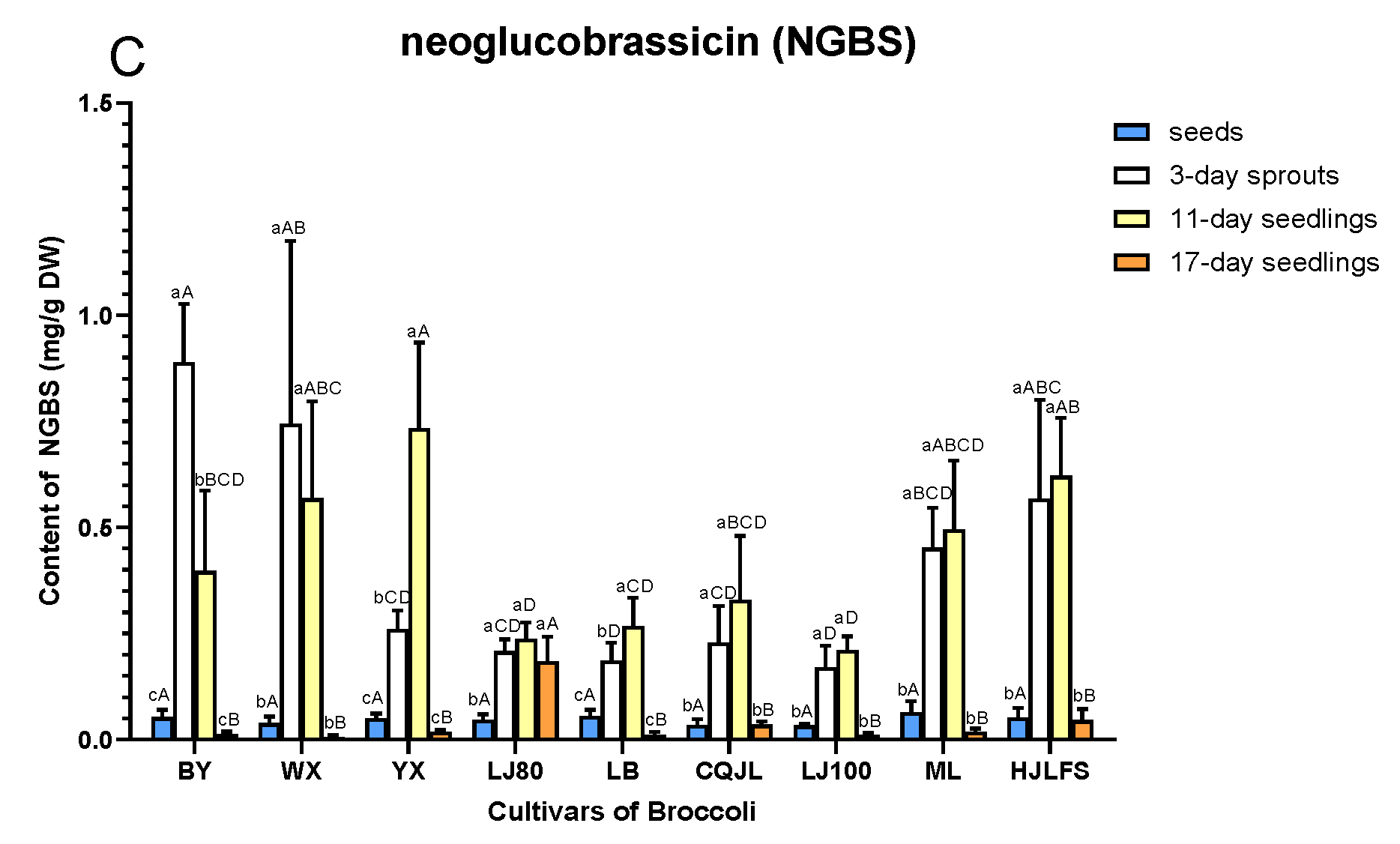
2.2. Quantification of GLs
3. Discussion
4. Material and Methods
4.1. Chemicals
4.2. Plant Material and Culture Condition
4.3. Glucosinolate Extraction and Analysis
4.4. LC-MS Conditions
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Full Name |
| GLs | glucosinolates |
| SF | sulforaphane |
| desulfo-GLs | desulfoglucosinolates |
| GSI | glucosisymbrin |
| GIB | glucoiberin |
| PRO | progoitrin |
| SIN | sinigrin |
| GRA | glucoraphanin |
| GAL | glucoalyssin |
| NAP | gluconapin |
| GIV | glucoibervirin |
| GTP | glucotropaeolin |
| GER | glucoerucin |
| GBS | glucobrassicin |
| 4MGBS | 4-methoxyglucobrassicin |
| NGBS | neoglucobrassicin |
| TDC | tryptophan decarboxylase |
| BY | Biyu |
| WX | Wenxing |
| YX | Youxiu |
| LJ80 | Lvjian80 |
| LB | Lvbao80 |
| CQJL | Chunqiujiali |
| LJ100 | Lvjian100 |
| ML | Meilv |
| HJLFS | Huangjinlvfushi |
| UPLC | ultra-performance liquid chromatography |
| NAS | gluconasturtiin |
| 4HGBS | 4-hydroxy glucobrassicin |
| RPF | relative proportionality factors |
| DW | dry weight. |
| LC-QTOF-MS | liquid chromatography-quadrupole time of flight mass spectrometer |
References
- Johnson, I.T. Cruciferous Vegetables and Risk of Cancers of the Gastrointestinal Tract. Mol. Nutr. Food Res. 2018, 62, e1701000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herr, I.; Büchler, M.W. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat. Rev. 2010, 36, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Tokuhisa, J.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2019, 169, 112100. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Latté, K.P.; Appel, K.-E.; Lampen, A. Health benefits and possible risks of broccoli—An overview. Food Chem. Toxicol. 2011, 49, 3287–3309. [Google Scholar] [CrossRef]
- Juge, N.; Mithen, R.F.; Traka, M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Experientia 2007, 64, 1105–1127. [Google Scholar] [CrossRef]
- Kan, S.F.; Wang, J.; Sun, G.X. Sulforaphane regulates apoptosis- and proliferation-related signaling pathways and synergizes with cisplatin to suppress human ovarian cancer. Int. J. Mol. Med. 2018, 42, 2447–2458. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-R.; Chen, M.; Lee, J.D.; Zhang, J.; Lin, S.-Y.; Fu, T.-M.; Chen, H.; Ishikawa, T.; Chiang, S.-Y.; Katon, J.; et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science 2019, 364, eaau0159. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Korkaya, H.; Liu, S.; Lee, H.-F.; Newman, B.; Yu, Y.; Clouthier, S.G.; Schwartz, S.J.; Wicha, M.S.; et al. Sulforaphane, a Dietary Component of Broccoli/Broccoli Sprouts, Inhibits Breast Cancer Stem Cells. Clin. Cancer Res. 2010, 16, 2580–2590. [Google Scholar] [CrossRef] [Green Version]
- Paul, B.; Li, Y.; Tollefsbol, T.O. The Effects of Combinatorial Genistein and Sulforaphane in Breast Tumor Inhibition: Role in Epigenetic Regulation. Int. J. Mol. Sci. 2018, 19, 1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axelsson, A.S.; Tubbs, E.; Mecham, B.; Chacko, S.; Nenonen, H.A.; Tang, Y.; Fahey, J.W.; Derry, J.M.J.; Wollheim, C.B.; Wierup, N.; et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci. Transl. Med. 2017, 9, eaah4477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, N.I.; Kim, J.K.; Park, W.T.; Cho, J.; Lim, Y.P.; Park, S.U. An efficient protocol for genetic transformation of watercress (Nasturtium officinale) using Agrobacterium rhizogenes. Mol. Biol. Rep. 2010, 38, 4947–4953. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cui, S.; Liu, J.; Tang, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. The recent advances of glucosinolates and their metabolites: Metabolism, physiological functions and potential application strategies. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, L.; Wang, Z.; Zhuang, Y.; Gu, Z. Response surface optimization and identification of isothiocyanates produced from broccoli sprouts. Food Chem. 2013, 141, 1580–1586. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Peñas, E.; Ciska, E.; Piskula, M.K.; Kozlowska, H.; Vidal-Valverde, C.; Frias, J. Time dependence of bioactive compounds and antioxidant capacity during germination of different cultivars of broccoli and radish seeds. Food Chem. 2010, 120, 710–716. [Google Scholar] [CrossRef]
- Clossais-Besnard, N.; Larher, F. Physiological role of glucosinolates in brassica napus. Concentration and distribution pattern of glucosinolates among plant organs during a complete life cycle. J. Sci. Food Agric. 1991, 56, 25–38. [Google Scholar] [CrossRef]
- Hogge, L.R.; Reed, D.W.; Underhill, E.W.; Haughn, G.W. HPLC Separation of Glucosinolates from Leaves and Seeds of Arabidopsis thaliana and Their Identification Using Thermospray Liquid Chramatography/Mass Spectrometry. J. Chromatogr. Sci. 1988, 26, 551–556. [Google Scholar] [CrossRef]
- Lee, J.G.; Bonnema, G.; Zhang, N.; Kwak, J.H.; De Vos, R.C.H.; Beekwilder, J. Evaluation of Glucosinolate Variation in a Collection of Turnip (Brassica rapa) Germplasm by the Analysis of Intact and Desulfo Glucosinolates. J. Agric. Food Chem. 2013, 61, 3984–3993. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Jo, J.S.; Lee, J.G. Comparison of Glucosinolate Profiles in Different Tissues of Nine Brassica Crops. Molecules 2015, 20, 15827–15841. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Rhee, J.; Choi, C.S.; Jo, J.S.; Shin, Y.K.; Lee, J.G. Profiling of Individual Desulfo-Glucosinolate Content in Cabbage Head (Brassica oleracea var. capitata) Germplasm. Molecules 2020, 25, 1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, J.S.; Bhandari, S.R.; Kang, G.H.; Shin, Y.K.; Lee, J.G. Selection of broccoli (Brassica oleracea var. italica) on composition and content of glucosinolates and hydrolysates. Sci. Hortic. 2022, 298, 110984. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, Q.; Zhang, L.; Chen, Z.; Han, Y.; Gu, Z. Physiological and Biochemical Metabolism of Germinating Broccoli Seeds and Sprouts. J. Agric. Food Chem. 2011, 60, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Meng, G.; Li, W.; Fan, D.; Wang, X.; Espinoza-Pinochet, C.A.; Cespedes-Acuña, C.L. Sulforaphane and its antioxidative effects in broccoli seeds and sprouts of different cultivars. Food Chem. 2020, 316, 126216. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Yang, R.; Gu, Z. Cloning of genes related to aliphatic glucosinolate metabolism and the mechanism of sulforaphane accumulation in broccoli sprouts under jasmonic acid treatment. J. Sci. Food Agric. 2016, 96, 4329–4336. [Google Scholar] [CrossRef]
- Gigolashvili, T.; Berger, B.; Flügge, U.I. Specific and coordinated control of indolic and aliphatic glucosinolate biosynthesis by R2R3-MYB transcription factors in Arabidopsis thaliana. Phytochem. Rev. 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Chen, S.; Andreasson, E. Update on glucosinolate metabolism and transport. Plant Physiol. Biochem. 2001, 39, 743–758. [Google Scholar] [CrossRef]
- Chavadej, S.; Brisson, N.; McNeil, J.N.; De Luca, V. Redirection of tryptophan leads to production of low indole glucosinolate canola. Proc. Natl. Acad. Sci. USA 1994, 91, 2166–2170. [Google Scholar] [CrossRef] [Green Version]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Farnham, M.W.; Wilson, P.E.; Stephenson, K.K.; Fahey, J.W. Genetic and environmental effects on glucosinolate content and chemoprotective potency of broccoli. Plant Breed. 2004, 123, 60–65. [Google Scholar] [CrossRef]
- Ishida, M.; Nagata, M.; Ohara, T.; Kakizaki, T.; Hatakeyama, K.; Nishio, T. Small variation of glucosinolate composition in Japanese cultivars of radish (Raphanus sativus L.) requires simple quantitative analysis for breeding of glucosinolate component. Breed. Sci. 2012, 62, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Quiros, C.F. In planta side-chain glucosinolate modification in Arabidopsis by introduction of dioxygenase Brassica homolog BoGSL-ALK. Theor. Appl. Genet. 2002, 106, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, H.; Zhao, Z.; Sheng, X.; Shen, Y.; Gu, H. Natural Variation of Glucosinolates and Their Breakdown Products in Broccoli (Brassica oleracea var. italica) Seeds. J. Agric. Food Chem. 2019, 67, 12528–12537. [Google Scholar] [CrossRef]
- Guo, R.F.; Yuan, G.F.; Wang, Q.M. Effect of NaCl treatments on glucosinolate metabolism in broccoli sprouts. J. Zhejiang Univ. Sci. B 2013, 14, 124–131. [Google Scholar] [CrossRef]
- Wang, J.; Gu, H.; Yu, H.; Zhao, Z.; Sheng, X.; Zhang, X. Genotypic variation of glucosinolates in broccoli (Brassica oleracea var. italica) florets from China. Food Chem. 2012, 133, 735–741. [Google Scholar] [CrossRef]
- Ediage, E.N.; Di Mavungu, J.D.; Scippo, M.; Schneider, Y.; Larondelle, Y.; Callebaut, A.; Robbens, J.; Van Peteghem, C.; De Saeger, S. Screening, identification and quantification of glucosinolates in black radish (Raphanus sativus L. niger) based dietary supplements using liquid chromatography coupled with a photodiode array and liquid chromatography-mass spectrometry. J. Chromatogr. A 2011, 1218, 4395–4405. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, S.; Liu, Y.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H.; Wang, Y.; Xu, D. Characterization of glucosinolates in 80 broccoli genotypes and different organs using UHPLC-Triple-TOF-MS method. Food Chem. 2021, 334, 127519 . [Google Scholar] [CrossRef]
- West, L.G.; Meyer, K.A.; Balch, B.A.; Rossi, F.J.; Schultz, A.M.R.; Haas, G.W. Glucoraphanin and 4-Hydroxyglucobrassicin Contents in Seeds of 59 Cultivars of Broccoli, Raab, Kohlrabi, Radish, Cauliflower, Brussels Sprouts, Kale, and Cabbage. J. Agric. Food Chem. 2004, 52, 916–926. [Google Scholar] [CrossRef]
- Maruyama-Nakashita, A. Metabolic changes sustain the plant life in low-sulfur environments. Curr. Opin. Plant Biol. 2017, 39, 144–151. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for growth and rapid bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]

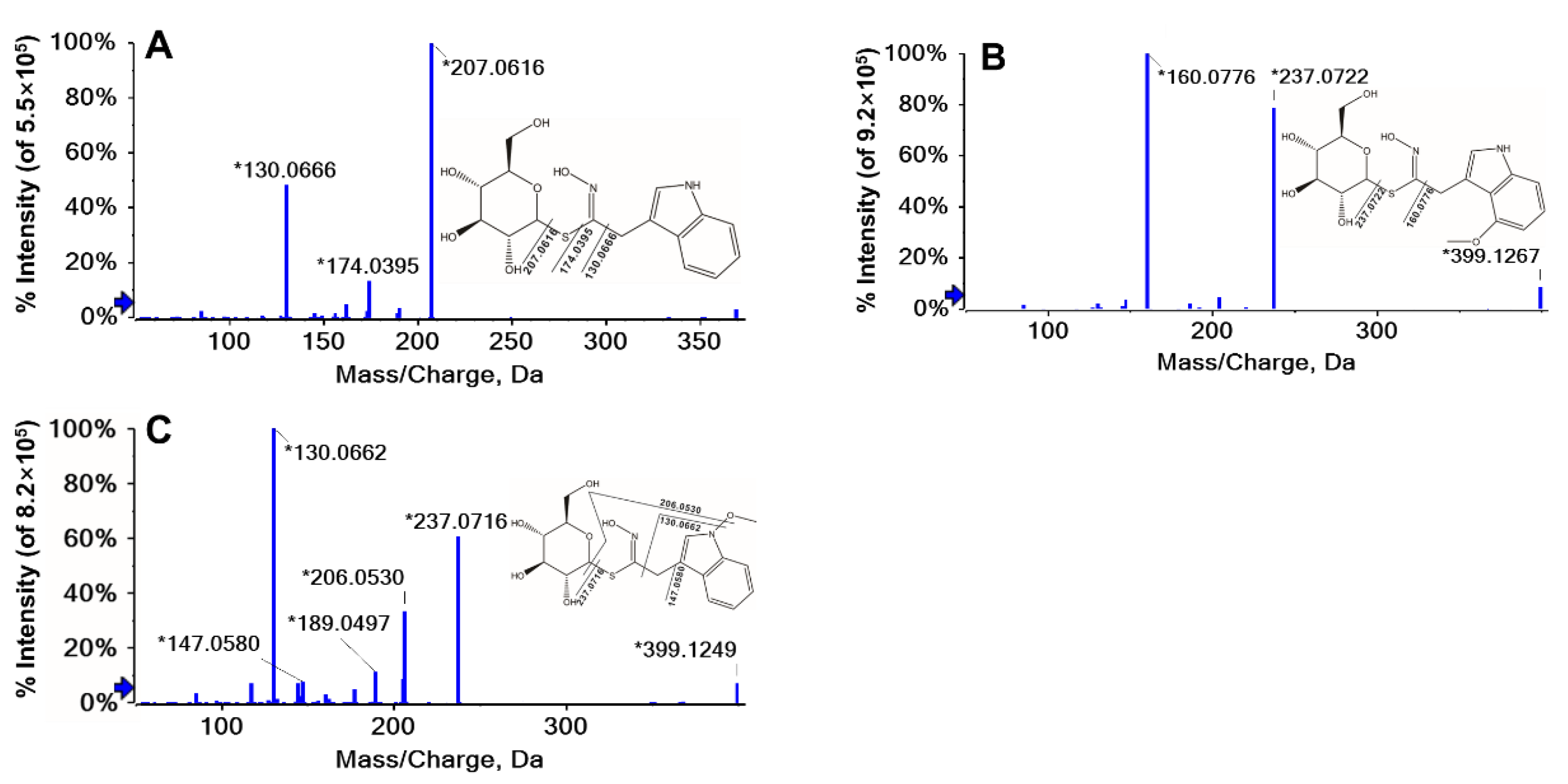

| No. | tR (min) | Molecular Formula | [M]- m/z Expt. | [M]- m/z Calcd. | Error (ppm) | (+) MS/MS m/z Major Diagnostic ions | Relative Response Factor a | Structure | Identification | Abbr. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.00 | C10H19NO7S1 | 298.0955 | 298.0960 | 1.9 | 280.0863, 136.0433, 119.0160 | 1.39 | Aliphatic | Glucosisymbrin | GSI |
| 2 | 1.49 | C11H21NO7S2 | 344.0832 | 344.0847 | 4.3 | 182.0318, 118.0328 | 1.13 | Aliphatic | Glucoiberin | GIB |
| 3 | 1.81 | C11H19NO7S1 | 310.0955 | 310.0965 | 3.2 | 148.0440, 130.0330 | 1.15 | Aliphatic | Progoitrin | PRO |
| 4 | 2.20 | C10H17NO6S1 | 280.0849 | 280.0858 | 3 | 118.0329, 85.0280 | 1.05 | Aliphatic | Sinigrin | SIN |
| 5 | 2.43 | C12H23NO7S2 | 358.0989 | 358.1008 | 5.5 | 196.0476, 132.0488 | 1.13 | Aliphatic | Glucoraphanin | GRA |
| 6 | 3.55 | C13H25NO7S2 | 372.1145 | 372.1163 | 4.8 | 210.0634 | 1.13 | Aliphatic | Glucoalyssin | GAL |
| 7 | 3.87 | C11H19NO6S1 | 294.1006 | 294.1011 | 1.8 | 132.0488, 71.9908 | 1.17 | Aliphatic | Gluconapin | NAP |
| 8 | 4.15 | C11H21NO6S2 | 328.0883 | 328.0899 | 4.8 | 166.0368, 118.0328, 85.0284 | 1.00 | Aliphatic | Glucoibervirin | GIV |
| 9 | 5.90 | C14H19NO6S1 | 330.1006 | 330.1019 | 4 | 168.0491, 134.0609, 91.0547 | 1.00 | Aromatic | Glucotropaeolin b | GTP |
| 10 | 6.16 | C12H23NO6S2 | 342.104 | 342.1061 | 6.3 | 180.0533, 132.0489 | 1.13 | Aliphatic | Glucoerucin | GER |
| 11 | 6.64 | C16H20N2O6S1 | 369.1115 | 369.1136 | 5.7 | 207.0616, 174.0395, 130.0666 | 0.31 | Indole | Glucobrassicin | GBS |
| 12 | 8.41 | C17H22N2O7S1 | 399.1221 | 399.124 | 4.9 | 237.0722, 160.0776 | 0.26 | Indole | 4-Methoxyglucobrassicin | 4MGBS |
| 13 | 9.17 | C17H22N2O7S1 | 399.1221 | 399.124 | 4.9 | 237.0716, 206.0530, 147.0580, 130.0662 | 0.21 | Indole | Neoglucobrassicin | NGBS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Sun, J.; Hu, Z.; Cheng, C.; Lin, S.; Zou, H.; Yan, X. Variation in Glucosinolate Accumulation among Different Sprout and Seedling Stages of Broccoli (Brassica oleracea var. italica). Plants 2022, 11, 1563. https://doi.org/10.3390/plants11121563
Lin H, Sun J, Hu Z, Cheng C, Lin S, Zou H, Yan X. Variation in Glucosinolate Accumulation among Different Sprout and Seedling Stages of Broccoli (Brassica oleracea var. italica). Plants. 2022; 11(12):1563. https://doi.org/10.3390/plants11121563
Chicago/Turabian StyleLin, Haiyan, Jiayi Sun, Zhiwei Hu, Chenxi Cheng, Sue Lin, Huixi Zou, and Xiufeng Yan. 2022. "Variation in Glucosinolate Accumulation among Different Sprout and Seedling Stages of Broccoli (Brassica oleracea var. italica)" Plants 11, no. 12: 1563. https://doi.org/10.3390/plants11121563
APA StyleLin, H., Sun, J., Hu, Z., Cheng, C., Lin, S., Zou, H., & Yan, X. (2022). Variation in Glucosinolate Accumulation among Different Sprout and Seedling Stages of Broccoli (Brassica oleracea var. italica). Plants, 11(12), 1563. https://doi.org/10.3390/plants11121563






