Surface Modifications of Wheat Cultivar Bologna upon Treatment with Non-Equilibrium Gaseous Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Material
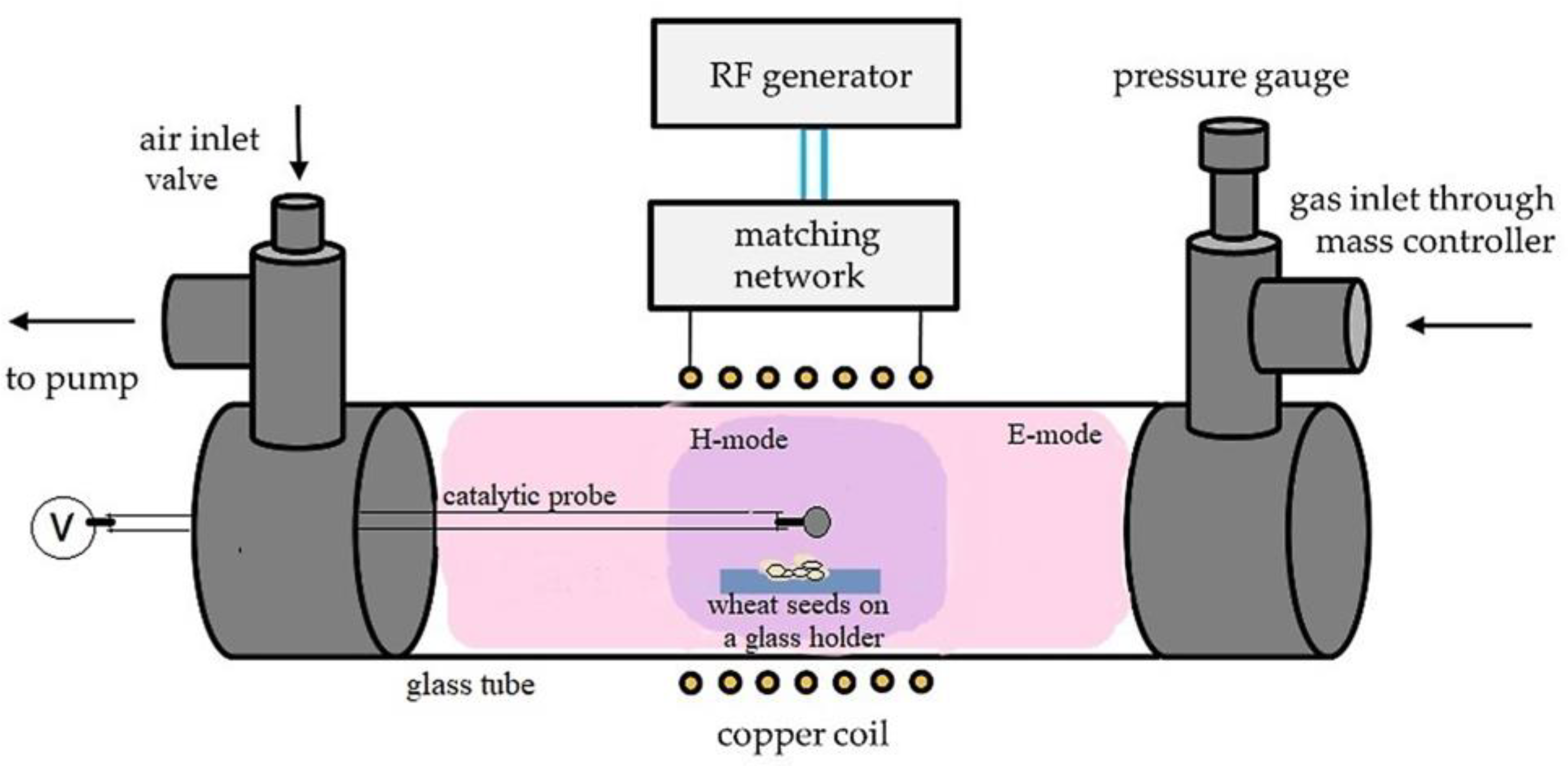
2.2. Plasma Reactor
2.3. Plasma Treatment
2.4. Fluence of Neutral Oxygen Atoms
2.5. X-ray Photoelectron Spectroscopy
2.6. Scanning Electron Microscopy
2.7. Water Contact Angle
2.8. Germination
2.9. Viability of Seeds
2.10. Statistical Analysis
3. Results and Discussion
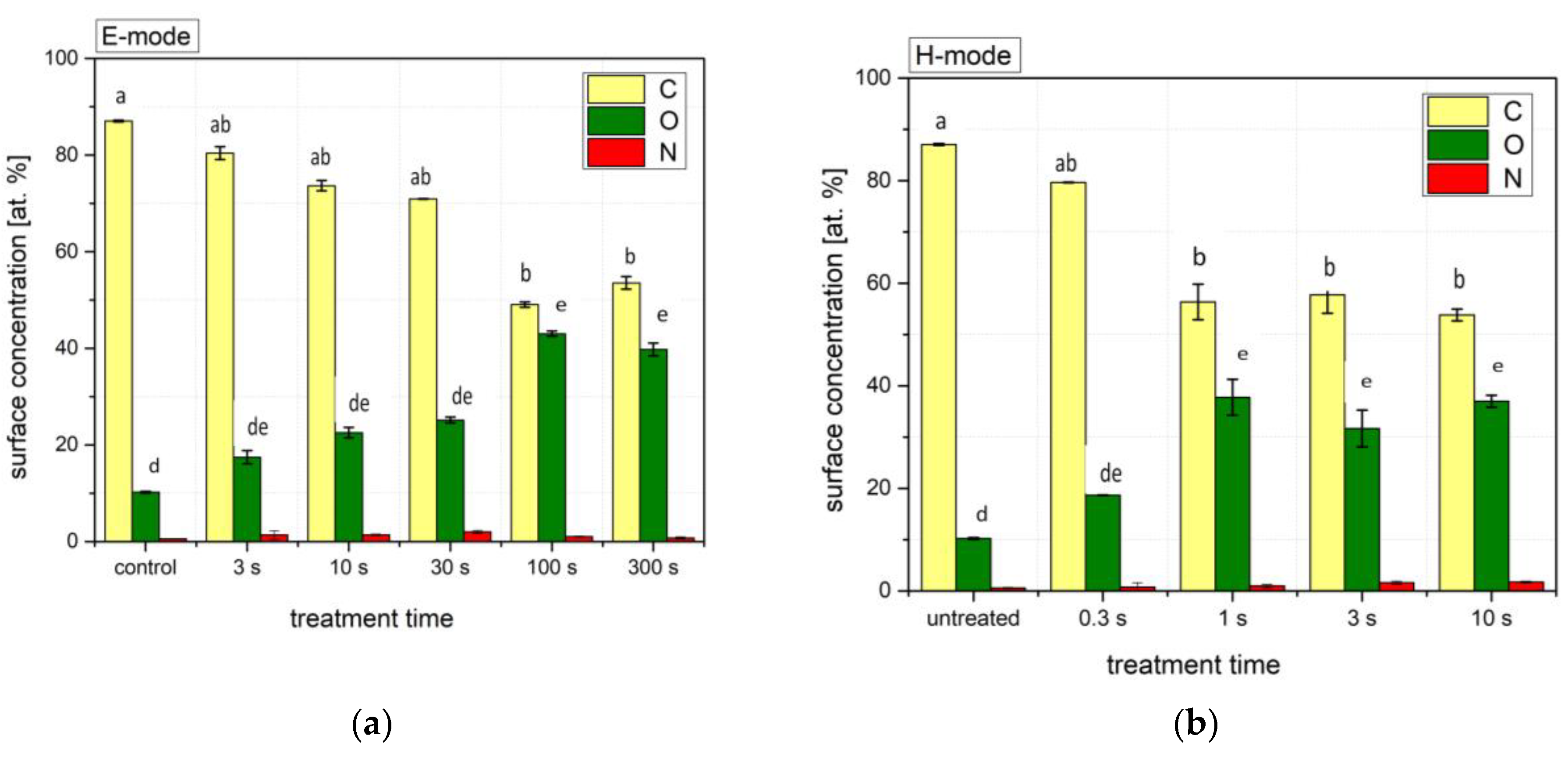
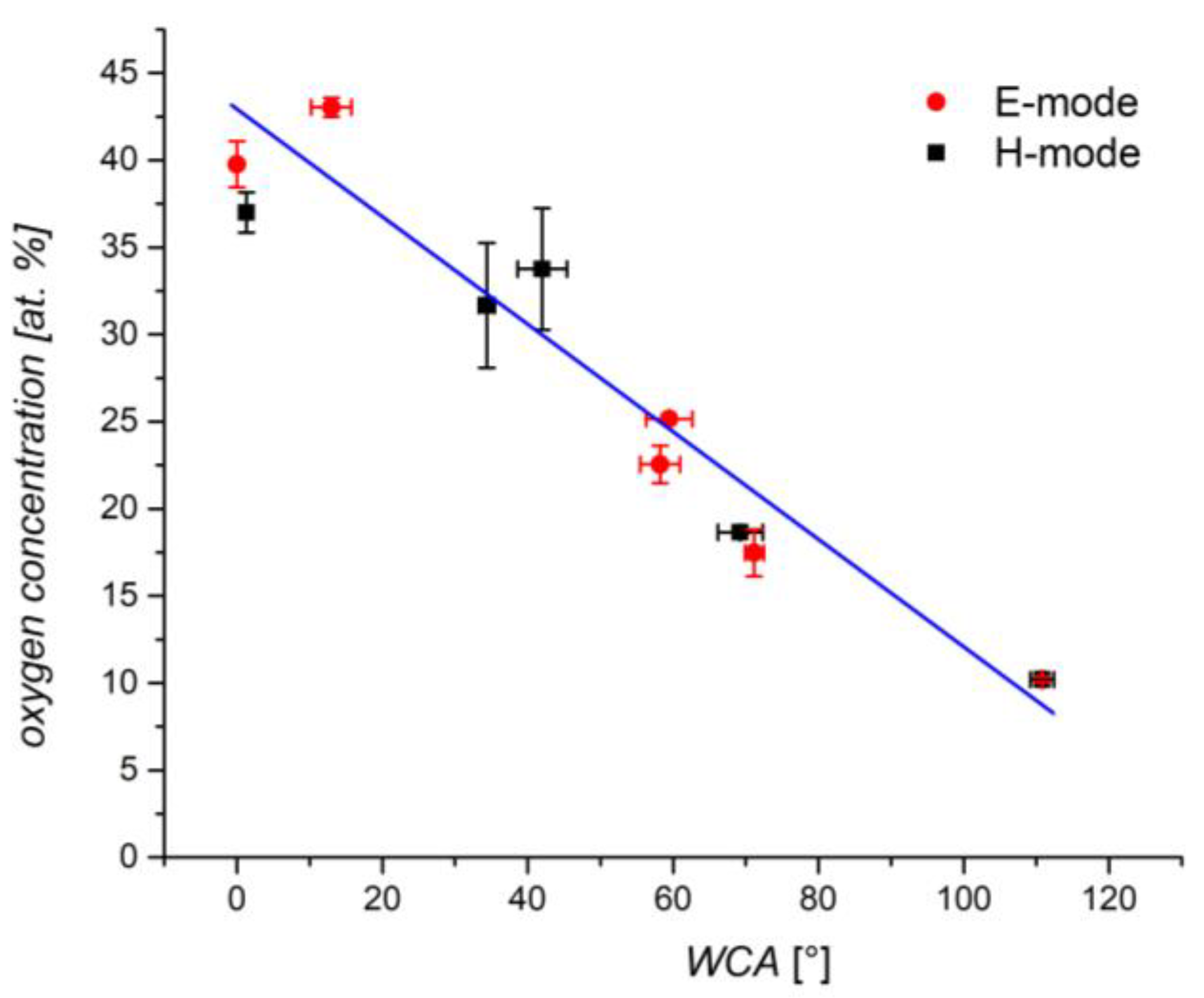
3.1. X-ray Photoelectron Spectroscopy
3.2. Scanning Electron Microscopy
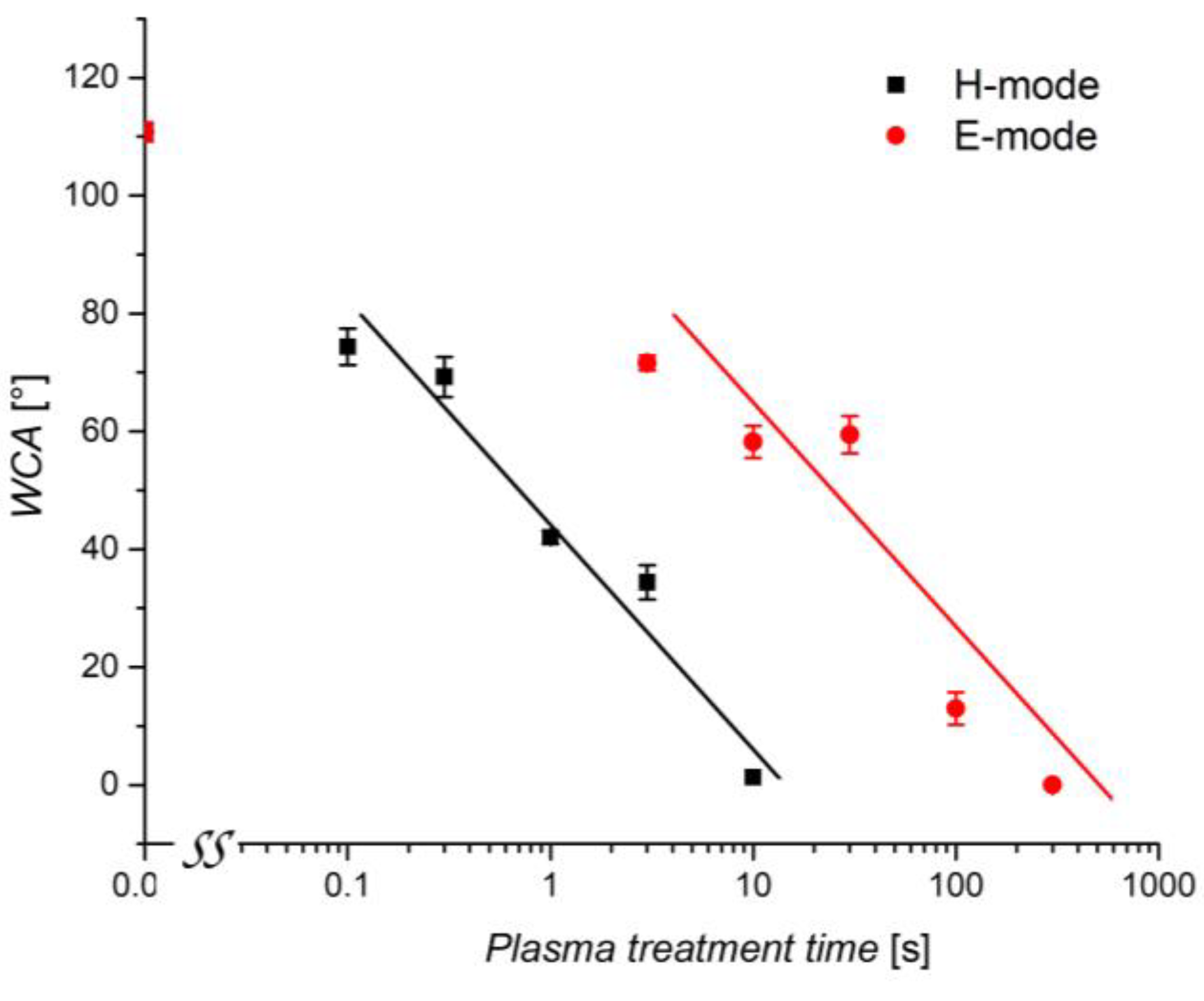
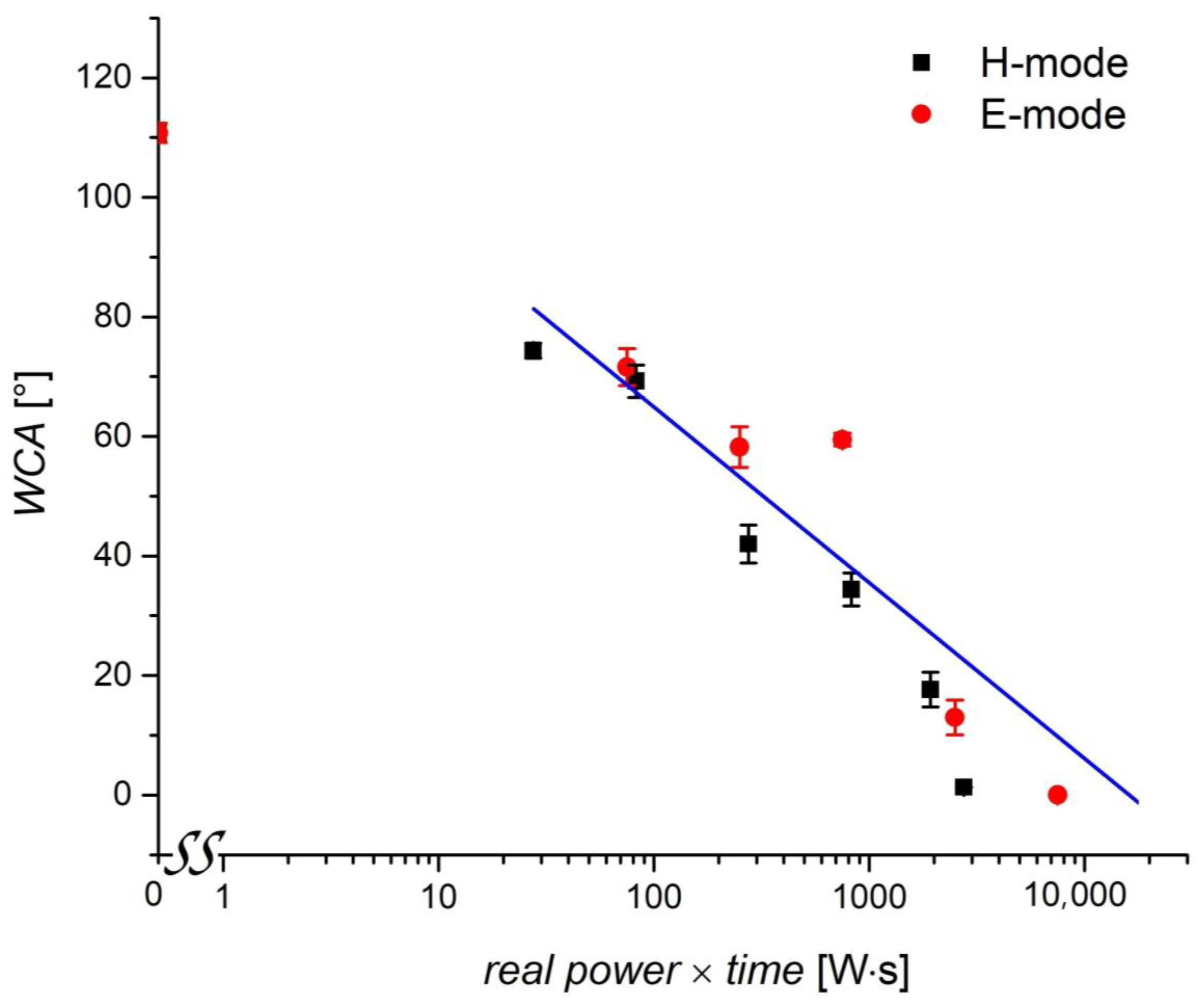
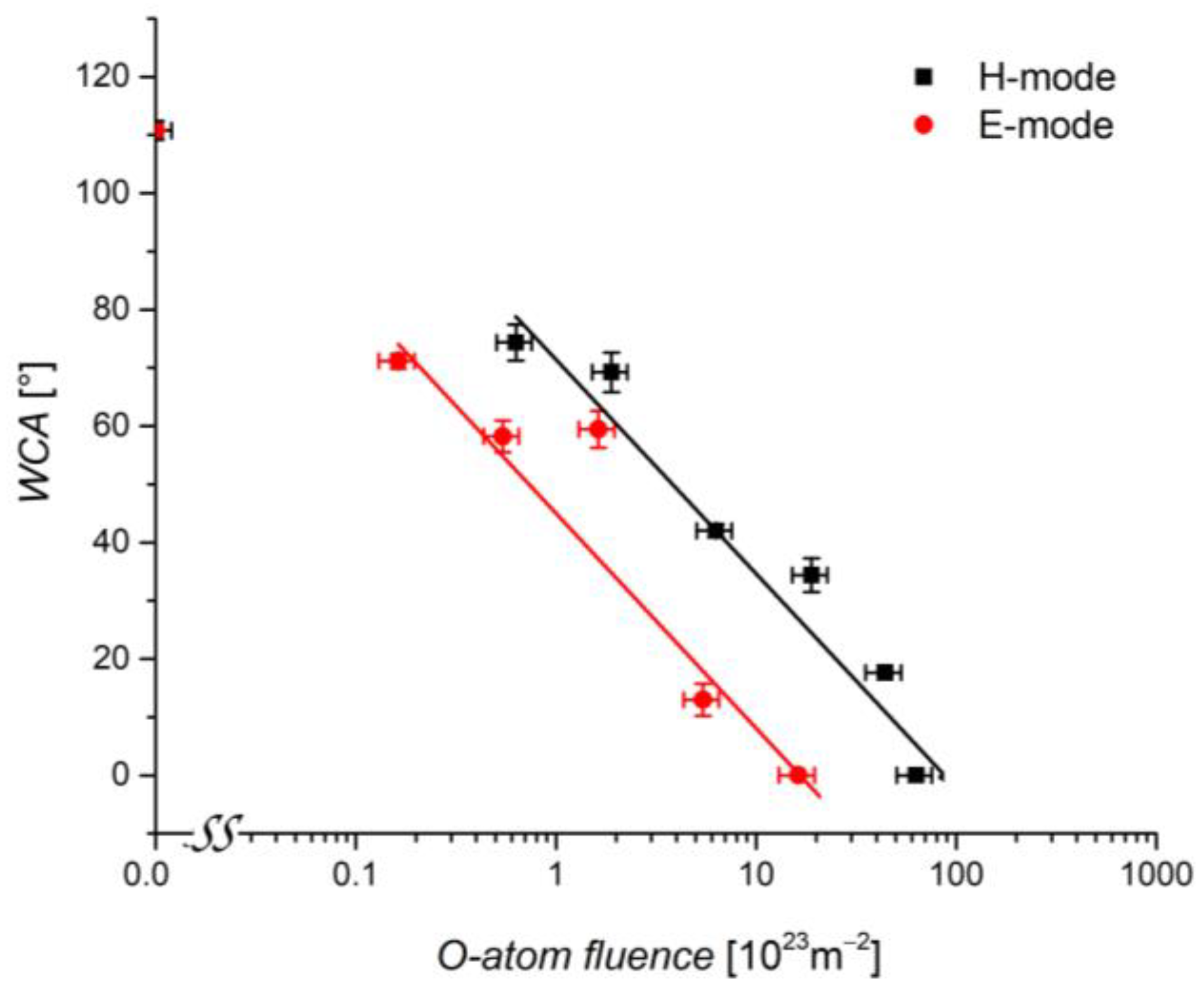
3.3. Water Contact Angle
3.4. Germination
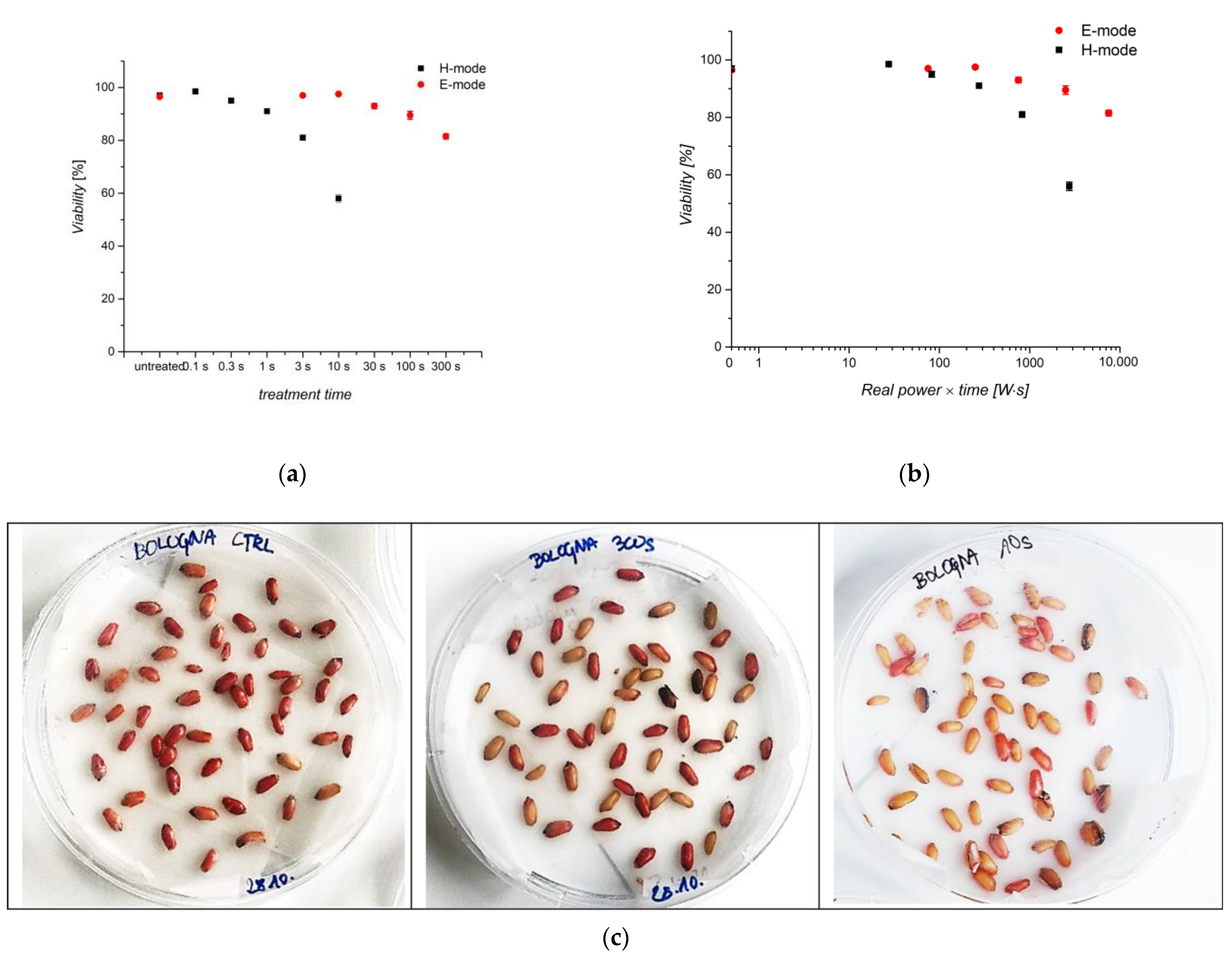
3.5. Viability of Seeds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAT Data. Available online: www.fao.org/faostat/en/#data (accessed on 8 December 2021).
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma Agriculture from Laboratory to Farm: A Review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Loureiro, J.; Amorim, J. Kinetics and Spectroscopy of Low Temperature Plasmas; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Oxford University Press: New York, NY, USA, 2014. [Google Scholar]
- Chateigner-Boutin, A.L.; Bouchet, B.; Alvarado, C.; Bakan, B.; Guillon, F. The Wheat Grain Contains Pectic Domains Exhibiting Specific Spatial and Development-Associated Distribution. PLoS ONE 2014, 9, e89620. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, J.; Gawlak, M.; Szafranek, J.; Szafranek, B.; Synak, E.; Warchalewski, J.R.; Piasecka-Kwiatkowska, D.; Blaszczak, W.; Jelinski, T.; Fornal, J. The effect of wheat grain composition, cuticular lipids and kernel surface microstructure on feeding, egg-laying, and the development of the granary weevil, Sitophilus granarius (L.). J. Stored Prod. Res. 2010, 46, 133–141. [Google Scholar] [CrossRef]
- Filatova, I.; Azharonok, V.; Goncharik, S.; Lushkevich, V.; Zhukovsky, A.; Gadzhieva, G. Effect of RF Plasma Treatment on the Germination and Phytosanitary State of Seeds. J. Appl. Spectrosc. 2014, 81, 250–256. [Google Scholar] [CrossRef]
- Roy, N.C.; Hasan, M.M.; Talukder, M.R.; Hossain, M.D.; Chowdhury, A.N. Prospective Applications of Low Frequency Glow Discharge Plasmas on Enhanced Germination, Growth and Yield of Wheat. Plasma Chem. Plasma Process. 2017, 38, 13–28. [Google Scholar] [CrossRef]
- Sohan, M.S.R.; Hasan, M.; Hossain, M.F.; Sajib, S.A.; Miah, M.M.; Iqbal, M.A.; Karmakar, S.; Alam, M.J.; Khalid-Bin-Ferdaus, K.M.; Kabir, A.H.; et al. Improvement of Seed Germination Rate, Agronomic Traits, Enzymatic Activity and Nutritional Composition of Bread Wheat (Triticum aestivum) Using Low-Frequency Glow Discharge Plasma. Plasma Chem. Plasma Process. 2021, 41, 923–944. [Google Scholar] [CrossRef]
- Meng, Y.; Qu, G.; Wang, T.; Sun, Q.; Liang, D.; Hu, S. Enhancement of Germination and Seedling Growth of Wheat Seed Using Dielectric Barrier Discharge Plasma with Various Gas Sources. Plasma Chem. Plasma Process. 2017, 37, 1105–1119. [Google Scholar] [CrossRef]
- Baldanov, B.B.; Ranzhurov, T.V.; Sordonova, M.N.; Budazhapov, L.V. Changes in the Properties and Surface Structure of Grain Seeds under the Influence of a Glow Discharge at Atmospheric Pressure. Plasma Phys. Rep. 2020, 46, 110–114. [Google Scholar] [CrossRef]
- Starič, P.; Mlakar, S.G.; Junkar, I. Response of Two Different Wheat Varieties to Glow and Afterglow Oxygen Plasma. Plants 2021, 10, 1728. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetič, M. New Developments in Surface Functionalization of Polymers Using Controlled Plasma Treatments. J. Phys. D Appl. Phys. 2017, 50, 293001. [Google Scholar] [CrossRef]
- Brust, H.; Nishime, T.M.C.; Wannicke, N.; Mui, T.S.M.; Horn, S.; Quade, A.; Weltmann, K.D. A medium-scale volume dielectric barrier discharge system for short-term treatment of cereal seeds indicates improved germination performance with long-term effects. J. Appl. Phys. 2021, 129, 044904. [Google Scholar] [CrossRef]
- Molina, R.; Lalueza, A.; López-Santos, C.; Ghobeira, R.; Cools, P.; Morent, R.; de Geyter, N.; González-Elipe, A.R. Physicochemical surface analysis and germination at different irrigation conditions of DBD plasma-treated wheat seeds. Plasma Process. Polym. 2020, 18, e2000086. [Google Scholar] [CrossRef]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold Radiofrequency Plasma Treatment Modifies Wettability and Germination Speed of Plant Seeds. Sci. Rep. 2012, 2, 741. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Y.; Zhang, H.; Qu, G.; Wang, T.; Sun, Q.; Liang, D. Alleviation of adverse effects of drought stress on wheat seed germination using atmospheric dielectric barrier discharge plasma treatment. Sci. Rep. 2017, 7, 16680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotfy, K.; Al-Harbi, N.A.; Abd El-Raheem, H. Cold Atmospheric Pressure Nitrogen Plasma Jet for Enhancement Germination of Wheat Seeds. Plasma Chem. Plasma Process. 2019, 39, 897–912. [Google Scholar] [CrossRef]
- Hoppanová, L.; Medvecká, V.; Dyblíková, J.; Hudecova, D.; Kaliňáková, B.; Kryštofová, S.; Zahoranová, A. Low-temperature plasma applications in chemical fungicide treatment reduction. Acta Chim. Slovaca 2020, 13, 26–33. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Akkermans, S.; Boehm, D.; Cullen, P.J.; Van Impe, J.; Bourke, P. Improving microbiological safety and quality characteristics of wheat and barley by high voltage atmospheric cold plasma closed processing. Food Res. Int. 2018, 106, 509–521. [Google Scholar] [CrossRef] [Green Version]
- Velichko, I.; Gordeev, I.; Shelemin, A.; Nikitin, D.; Brinar, J.; Pleskunov, P.; Choukourov, A.; Pazderů, K.; Pulkrábek, J. Plasma Jet and Dielectric Barrier Discharge Treatment of Wheat Seeds. Plasma Chem. Plasma Process. 2019, 39, 913–928. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Meng, Y.; Qu, G.; Sun, Q.; Liang, D.; Hu, S. Air Atmospheric Dielectric Barrier Discharge Plasma Induced Germination and Growth Enhancement of Wheat Seed. Plasma Chem. Plasma Process. 2017, 37, 1621–1634. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Bourke, P. Investigation of mechanisms involved in germination enhancement of wheat (Triticum aestivum) by cold plasma: Effects on seed surface chemistry and characteristics. Plasma Process. Polym. 2019, 16, e1800148. [Google Scholar] [CrossRef]
- Dobrin, D.; Magureanu, M.; Mandache, N.B.; Ionita, M.-D. The Effect of Non-thermal Plasma Treatment on Wheat Germination and Early Growth. Innov. Food Sci. Emerg. Technol. 2015, 29, 255–260. [Google Scholar] [CrossRef]
- Societa Italiana Sementi. Common Wheats > Bologna. Available online: www.sisonweb.com/en/product-detail.php?idProd=105 (accessed on 8 December 2021).
- Zaplotnik, R.; Vesel, A.; Mozetic, M. Transition from E to H mode in inductively coupled oxygen plasma: Hysteresis and the behaviour of oxygen atom density. EPL (Europhys. Lett.) 2011, 95, 55001. [Google Scholar] [CrossRef] [Green Version]
- Recek, N.; Holc, M.; Vesel, A.; Zaplotnik, R.; Gselman, P.; Mozetič, M.; Primc, G. Germination of Phaseolus vulgaris L. Seeds after a Short Treatment with a Powerful RF Plasma. Int. J. Mol. Sci. 2021, 22, 6672. [Google Scholar] [CrossRef] [PubMed]
- Primc, G.; Lojen, D.; Vesel, A.; Mozetič, M.; Zaplotnik, R. Oxygen atom density in a large reactor powered by four inductively coupled plasma sources. Vacuum 2022, 199, 110964. [Google Scholar] [CrossRef]
- Guerra, V.; Kutasi, K.; Sa, P.A. O2(a1Δg) production in flowing Ar-O2 surface-wave microwave discharges: Possible use for oxygen-iodine laser excitation. Appl. Phys. Lett. 2010, 96, 071503. [Google Scholar] [CrossRef]
- Vesel, A.; Kolar, M.; Recek, N.; Kutasi, K.; Stana-Kleinschek, K.; Mozetic, M. Etching of blood proteins in the early and late flowing afterglow of oxygen plasma. Plasma Process. Polym. 2014, 11, 12–23. [Google Scholar] [CrossRef]
- Holc, M.; Gselman, P.; Primc, G.; Vesel, A.; Mozetič, M.; Recek, N. Wettability and Water Uptake Improvement in Plasma-Treated Alfalfa Seeds. Agriculture 2022, 12, 96. [Google Scholar] [CrossRef]
- Recek, N.; Vesel, A.; Zaplotnik, R.; Paul, D.; Primc, G.; Gselman, P.; Mozetič, M. Hydrophilization of corn seeds by non-equilibrium gaseous plasma. Chem. Biol. Technol. Agric. 2021, 8, 32. [Google Scholar] [CrossRef]
- Zaplotnik, R.; Vesel, A. Effect of VUV radiation on surface modification of polystyrene exposed to atmospheric pressure plasma jet. Polymers 2020, 12, 1136. [Google Scholar] [CrossRef]
- Dhayal, M.; Lee, S.-Y.; Park, S.-U. Using Low-Pressure Plasma for Carthamus tinctorium L. Seed Surface Modification. Vacuum 2006, 80, 499–506. [Google Scholar] [CrossRef]
- Grzegorzewski, F.; Rohn, S.; Kroh, L.W.; Geyer, M.; Schlüter, O. Surface Morphology and Chemical Composition of Lamb’s Lettuce (Valerianella locusta) after Exposure to a Low-pressure Oxygen Plasma. Food Chem. 2010, 122, 1145–1152. [Google Scholar] [CrossRef]
- Chabert, P.; Tsankov, T.V.; Czarnetzki, U. Foundations of capacitive and inductive radio-frequency discharges. Plasma Sources Sci. Technol. 2021, 30, 024001. [Google Scholar] [CrossRef]
- Derzsi, A.; Bruneau, B.; Gibson, A.R.; Johnson, E.; O’Connell, D.; Gans, T.; Booth, J.-P.; Donkó, Z. Power coupling mode transitions induced by tailored voltage waveforms in capacitive oxygen discharges. Plasma Sources Sci. Technol. 2017, 26, 034002. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Graves, D. The effect of VUV radiation from Ar/O2 plasmas on low-k SiOCH films. J. Phys. D Appl. Phys. 2011, 44, 325203. [Google Scholar] [CrossRef]
- Mozetič, M. Surface Modification of Materials Using an Extremely Non-Equilibrium Oxygen Plasma. Mater. Tehnol. 2010, 44, 165–171. [Google Scholar]
- Krištof, J.; Macko, P.; Veis, P. Surface loss probability of atomic oxygen. Vacuum 2012, 86, 614–619. [Google Scholar] [CrossRef]
- Mitsui, Y.; Makabe, T. Review and current status: E⇌h mode transition in low-temperature icp and related electron dynamics. Plasma Sources Sci. Technol. 2021, 30, 023001. [Google Scholar] [CrossRef]
- Memos, G.; Lidorikis, E.; Gogolides, E.; Kokkoris, G. A hybrid modeling framework for the investigation of surface roughening of polymers during oxygen plasma etching. J. Phys. D Appl. Phys. 2021, 54, 175205. [Google Scholar] [CrossRef]
- Tsougeni, K.; Vourdas, N.; Tserepi, A.; Gogolides, E.; Cardinaud, C. Mechanisms of oxygen plasma nanotexturing of organic polymer surfaces: From stable super hydrophilic to super hydrophobic surfaces. Langmuir 2009, 25, 11748–11759. [Google Scholar] [CrossRef]
- Kontziampasis, D.; Constantoudis, V.; Gogolides, E. Plasma directed organization of nanodots on polymers: Effects of polymer type and etching time on morphology and order. Plasma Process. Polym. 2012, 9, 866–872. [Google Scholar] [CrossRef]
- Nishime, T.M.C.; Wannicke, N.; Horn, S.; Weltmann, K.D.; Brust, H. A Coaxial Dielectric Barrier Discharge Reactor for Treatment of Winter Wheat Seeds. Appl. Sci. 2020, 10, 7133. [Google Scholar] [CrossRef]
- Srisonphan, S. Tuning Surface Wettability through Hot Carrier Initiated Impact Ionization in Cold Plasma. ACS Appl. Mater. Interfaces 2018, 10, 11297–11304. [Google Scholar] [CrossRef]
- Holc, M.; Mozetič, M.; Recek, N.; Primc, G.; Vesel, A.; Zaplotnik, R.; Gselman, P. Wettability Increase in Plasma-Treated Agricultural Seeds and Its Relation to Germination Improvement. Agronomy 2021, 11, 1467. [Google Scholar] [CrossRef]
- Filatova, I.; Lyushkevich, V.; Goncharik, S.; Zhukovsky, A.; Krupenko, N.; Kalatskaja, J. The effect of low-pressure plasma treatment of seeds on the plant resistance to pathogens and crop yields. J. Phys. D Appl. Phys. 2020, 53, 244001. [Google Scholar] [CrossRef]
- Kregar, Z.; Bišćan, M.; Milošević, S.; Eleršič, K.; Zaplotnik, R.; Primc, G.; Cvelbar, U. Optical Emission Characterization of Extremely Reactive Oxygen Plasma During Treatment of Graphite Samples. Mater. Tehnol. 2012, 46, 25–30. [Google Scholar]
- De Giorgi, J.; Piskurewicz, U.; Loubery, S.; Utz-Pugin, A.; Bailly, C.; Mène-Saffrané, L.; Lopez-Molina, L. An Endosperm-Associated Cuticle Is Required for Arabidopsis Seed Viability, Dormancy and Early Control of Germination. PLoS Genet. 2015, 11, e1005708. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holc, M.; Vesel, A.; Zaplotnik, R.; Paul, D.; Primc, G.; Mozetič, M.; Gselman, P.; Recek, N. Surface Modifications of Wheat Cultivar Bologna upon Treatment with Non-Equilibrium Gaseous Plasma. Plants 2022, 11, 1552. https://doi.org/10.3390/plants11121552
Holc M, Vesel A, Zaplotnik R, Paul D, Primc G, Mozetič M, Gselman P, Recek N. Surface Modifications of Wheat Cultivar Bologna upon Treatment with Non-Equilibrium Gaseous Plasma. Plants. 2022; 11(12):1552. https://doi.org/10.3390/plants11121552
Chicago/Turabian StyleHolc, Matej, Alenka Vesel, Rok Zaplotnik, Domen Paul, Gregor Primc, Miran Mozetič, Peter Gselman, and Nina Recek. 2022. "Surface Modifications of Wheat Cultivar Bologna upon Treatment with Non-Equilibrium Gaseous Plasma" Plants 11, no. 12: 1552. https://doi.org/10.3390/plants11121552
APA StyleHolc, M., Vesel, A., Zaplotnik, R., Paul, D., Primc, G., Mozetič, M., Gselman, P., & Recek, N. (2022). Surface Modifications of Wheat Cultivar Bologna upon Treatment with Non-Equilibrium Gaseous Plasma. Plants, 11(12), 1552. https://doi.org/10.3390/plants11121552








