Effect of Regulated Deficit Irrigation on Agronomic Parameters of Three Plum Cultivars (Prunus salicina L.) under Semi-Arid Climate Conditions
Abstract
:1. Introduction
2. Results
2.1. Environmental Conditions
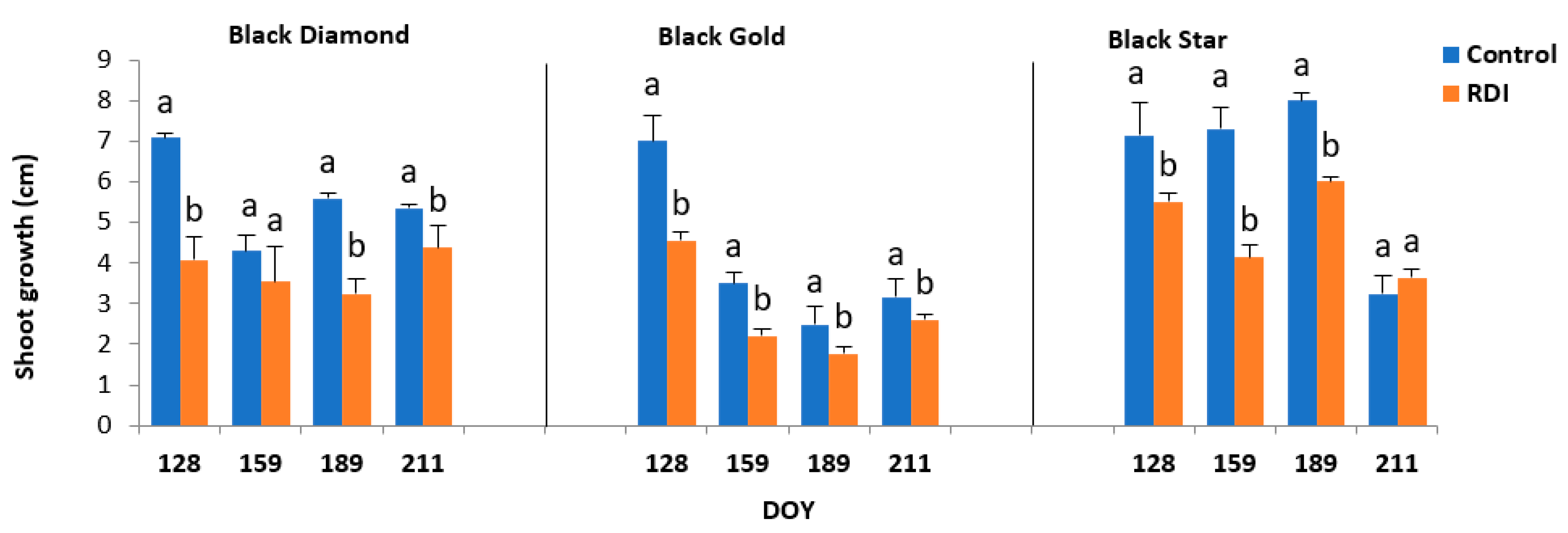
2.2. Tree Growth Pattern
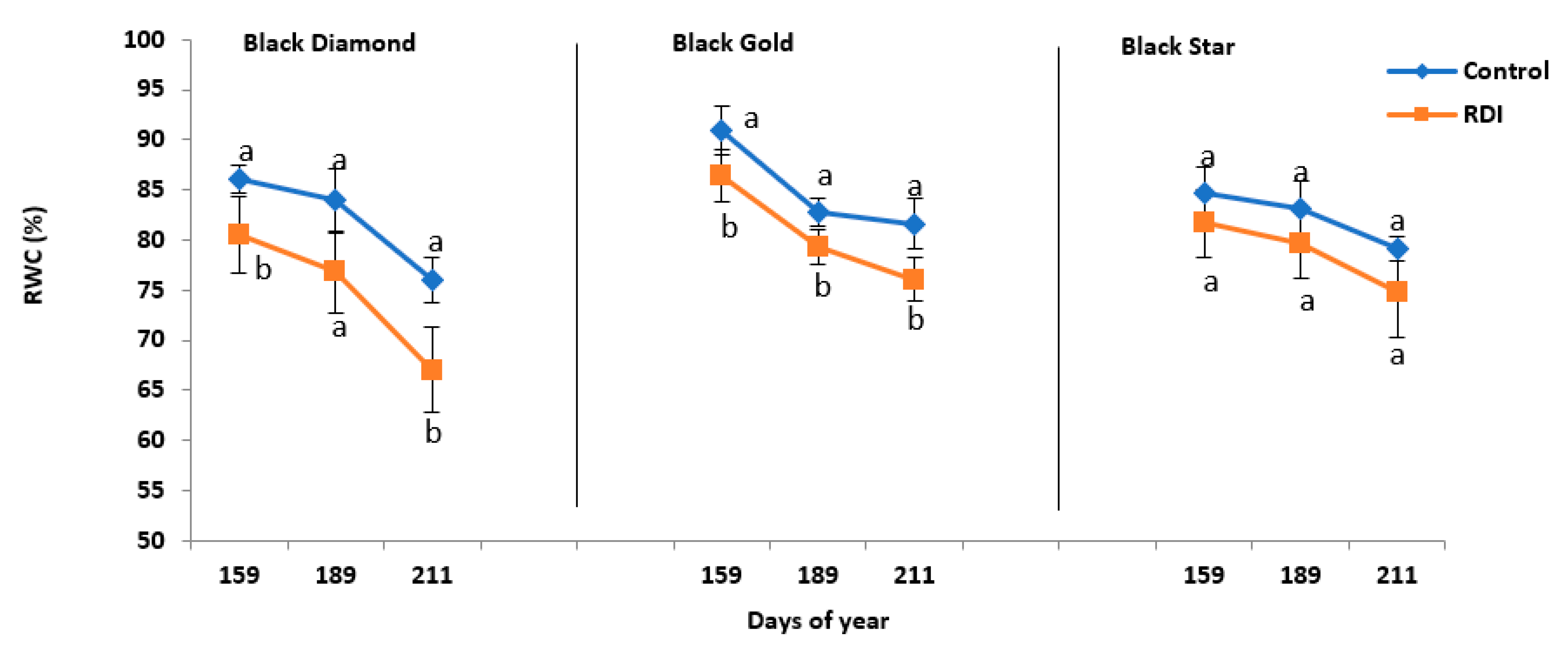
2.3. Water Status
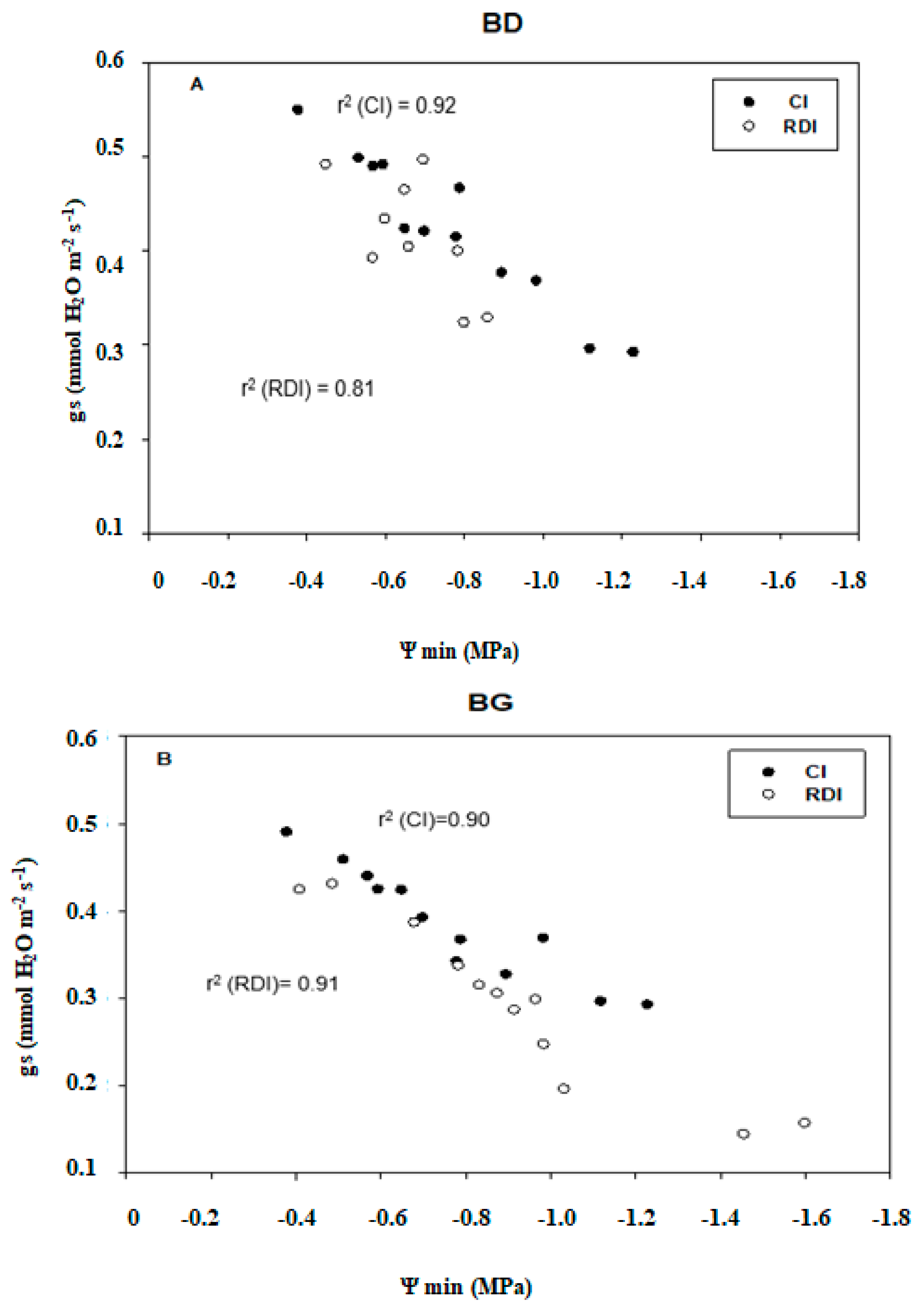
2.4. Gas Exchange
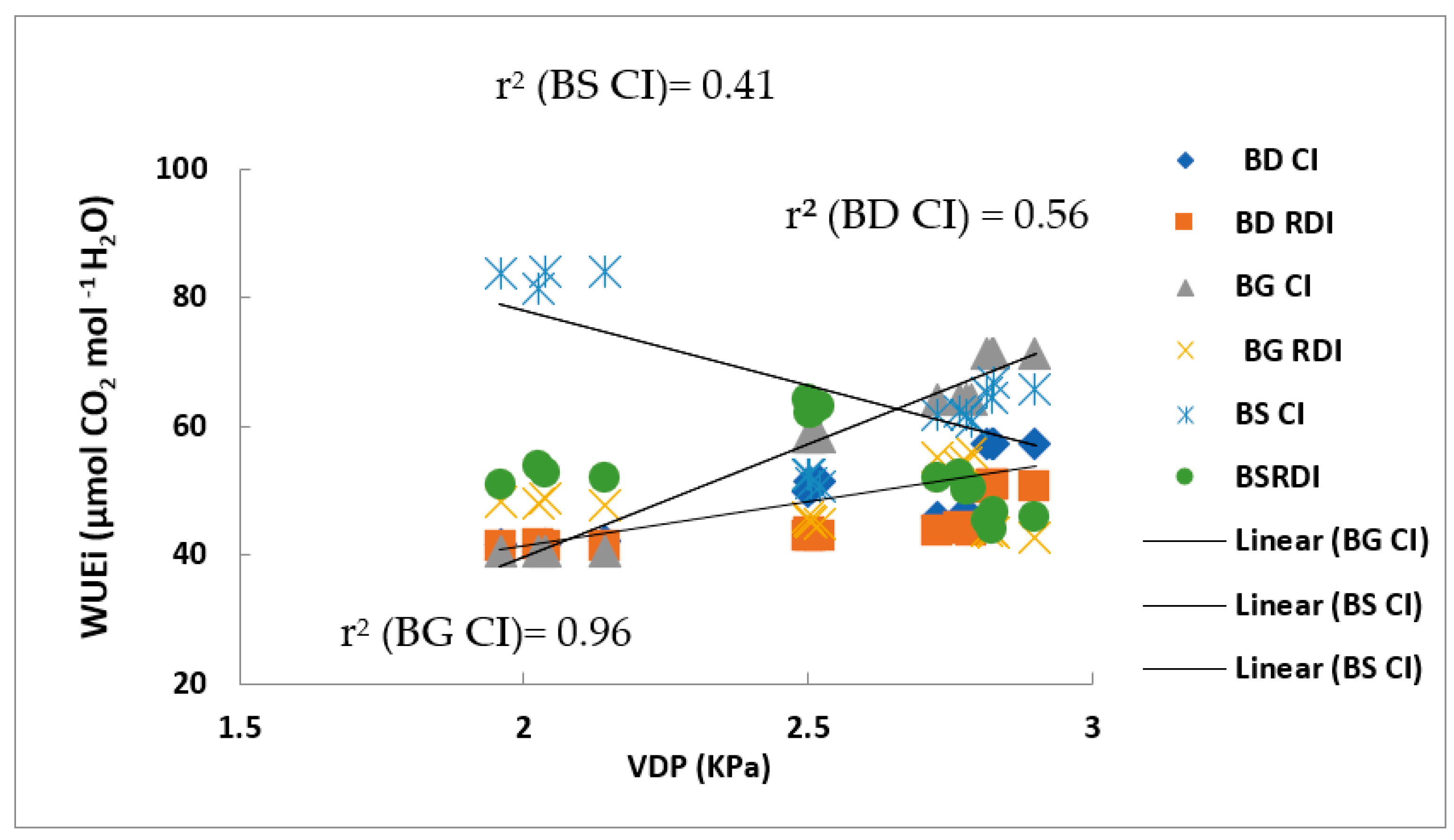
2.5. Correlations
2.6. Plum Yield and Fruit Quality Evaluation
3. Discussion
4. Materials and Methods
4.1. Plant Material, Experimental Conditions and Irrigation Treatments
4.2. Soil Analysis
4.3. Measurements of Shoot Growth
4.4. Tree Water Status
4.5. Osmotic Potential (Ψos)
4.6. Gas-Exchange MEASUREMENTS
4.7. The Yield Determination
4.8. Pomological Parameters
4.8.1. Sample Processing
4.8.2. Fruit Quality Parameters
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AN | Net CO2 assimilation rate |
| BD | Black Diamond |
| BG | Black Gold |
| BS | Black Star |
| CI | Control irrigation |
| DI | Deficit irrigation |
| DOY | Day of year |
| DW | Dry weight |
| ETo | Reference evapotranspiration |
| ETc | Crop evapotranspiration |
| FY | Fruit yield |
| FW | fresh weight |
| gs | Stomatal conductance |
| Kc | Crop coefficient |
| RDI | Regulated deficit irrigation |
| ROS | Reactive oxygen species |
| RWC | Relative water content |
| SSC | Soluble solid content |
| VDP | Vapor pressure deficit |
| Ψmin | Leaf water potential |
| Ψos | Leaf osmotic potential |
| WUEi | Intrinsic water use efficiency |
References
- Cattivelli, L.; Rizza, F.; Badeck, F.-W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Mare, C.; Tondelli, A.; Stanca, A.M. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crop. Res. 2008, 115, 1–14. [Google Scholar] [CrossRef]
- Houghton, J.T.; Ding, Y.; Griggs, D.J.; Noguer, M.; Linden, P.J.; Dai, X.; Maskell, K.; Johnson, C.A. Climate Change; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Ripoll, J.; Urban, L.; Staudt, M.; Lopez-Lauri, F.; Bidel, L.P.R.; Bertin, N. Water shortage and quality of fleshy fruits—Making the most of the unavoidable. J. Exp. Bot. 2014, 65, 4097–4117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, J.S. Increasing agricultural water use efficiency to meet future food production. Agric. Ecosyst Environ. 2000, 82, 105–119. [Google Scholar] [CrossRef]
- Chalmers, D.J.; Mitchell, P.D.; Van, H.L. Control of peach tree growth and productivity by regulated water supply, tree density and summer pruning. J. Am. Soc. Hortic. Sci. 1981, 106, 307–312. [Google Scholar]
- Behboudian, M.H.; Mills, T.M. Deficit irrigation in deciduous orchards. Hortic. Rev. 1997, 21, 105–131. [Google Scholar]
- Stikic, R.; Popovic, S.; Srdic, M.; Savic, D.; Jovanovic, Z.; Prokic, L.; Zdravkovic, J. Partial root drying (PRD): A new technique for growing plants that saves water and improves the quality of fruit. Bulg. J. Plant Physiol. 2003, 9, 164–171. [Google Scholar]
- Meddi, M.; Eslamian, S. Uncertainties in Rainfall and Water Resources in Maghreb Countries under Climate Change. In African Handbook of Climate Change Adaptation; Oguge, N., Ayal, D., Adeleke, L., da Silva, I., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Henao, A. Agroecology and the design of climate change-resilient farming systems. Agron. Sustain. Dev. 2015, 35, 869–890. [Google Scholar] [CrossRef] [Green Version]
- Vadell, J.; Medrano, H. Effect of drought on subterranean clover. 2, Genetic variability of photosynthesis, transpiration and stomatal conductance. Photosynthetica 1992, 27, 433–440. [Google Scholar]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Kramer, P.J.; Boyer, J.S. Water Relations of Plants and Soils; Academic Press: San Diego, CA, USA, 1995; p. 495. [Google Scholar]
- Ruiz-Sanchez, M.C.; Domingo, R.; Castel, J.R. Deficit irrigation in fruit trees and vines in Spain. Span. J. Agric. Res. 2010, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Alscher, R.G.; Cumming, J.R. Stress Responses in Plants: Adaptation and Acclimation Mechanisms. 1990. Available online: https://www.journals.uchicago.edu/doi/abs/10.1086/417281 (accessed on 30 September 2021).
- Domingo, R.; Ruiz-Sanchez, M.C.; Sfinchez-Blanco, M.; Torrecillas, A. Water relations, growth and yield of Fino lemon trees under regulated deficit irrigation. Irrig. Sci. 1996, 16, 115–123. [Google Scholar] [CrossRef]
- Torrecillas, A.; Alarcon, J.J.; Domingo, R.; Planes, J.; Sànchez-Blanco, M.J. Strategies for drought resistance in leaves of two almond cultivars. Plant Sci. 1996, 118, 135–143. [Google Scholar] [CrossRef]
- Dong, G.F.; Cheng, Z.Y.; Zhang, Z.H.; Wang, X.J.; Liu, X.R.; Zhang, R. Effects of RDI on water use efficiency and quality of alfalfa. Trans. CSAE 2006, 5, 201–203. [Google Scholar]
- Shi, W.J.; Hu, X.T.; Kang, S.Z. The status and prospect of RDI technique in water stress conditions. Agric. Res. 1998, 16, 84–88. [Google Scholar]
- Liu, M.C.; Kojim, A.T.; Tanaka, M.; Chen, H. Effect of soil moisture on plant growth and fruit properties of strawberry. Acta Hortic. 2001, 28, 307–311. [Google Scholar]
- Guizani, M.; Dabbou, S.; Maatallah, S.; Montevecchi, G. Physiological responses and fruit quality of four peach cultivars under sustained and cyclic deficit irrigation in center-west of Tunisia. Agric. Water Manag. 2019, 217, 81–97. [Google Scholar] [CrossRef]
- Guizani, M.; Dabbou, S.; Maatallah, S.; Montevecchi, G.; Antonelli, A.; Serrano, M.; Hajlaoui, H.; Rezig, M.; Kilani-Jaziric, S. Evaluation of Two Water Deficit Models on Phenolic Profiles and Antioxidant Activities of Different Peach Fruits Parts. Chem. Biodivers. 2022, 19, e202100851. [Google Scholar] [CrossRef]
- Grassman, J.; Susanne, H.; Erich, F.E. Plant’s defense and its benefits for animals and medicine: Role of phenolics and terpenoids in avoiding oxygen stress. Plant Physiol. Biochem. 2002, 40, 471–478. [Google Scholar] [CrossRef]
- Du, S.; Kang, S.; Li, F.; Du, T. Water use efficiency is improved by alternate partial root-zone irrigation of apple in arid northwest China. Agric. Water Manag. 2017, 179, 184–192. [Google Scholar] [CrossRef]
- Li, S.H. The Response of sensitive periods of fruit tree growth, yield and quality to water stress and water saving irrigation. Plant Physiol. 1993, 29, 10–16. [Google Scholar]
- Marsal, J.; Lopez, G.; Mata, M.; Arbones, A. Girona Recommendations for water conservation in peachorchards in mediterraneanclimate zone using combined regulated deficit irrigation. Acta Hortic. 2004, 664, 391–397. [Google Scholar]
- Intrigliolo, D.S.; Castel, J.R. Performance of various water stress indicators for prediction of fruit size response to deficit irrigation in plum. Agric. Water Manag. 2006, 83, 173–180. [Google Scholar] [CrossRef]
- Rahmati, M.; Davarynejad, G.H.; Génard, M.; Bannayan, M.; Azizi, M.; Vercambre, G. Peach water relations, gas exchange, growth and shoot mortality under water deficit in semi-arid weather conditions. PLoS ONE 2015, 10, e0120246. [Google Scholar] [CrossRef] [Green Version]
- Damour, G. Bases Théoriques et Approches Expérimentales de la Modélisation des Effets de la Contrainte Hydrique sur les Echanges Gazeux Foliaires du Manguier et du Litchi. Ph.D. Thesis, Universite de la Reunion Faculté des Sciences et Technologies Ecole Doctorale interdisciplinaire EDI n°0445 Avec la participation de CIRAD-PERSYST UR Production Fruitière Intégrée Bassin Plat, Saint Pierre, France, 2010; p. 273. [Google Scholar]
- Ennajeh, M.; Vadel, A.M.; Khemira, H.; Mimoun, M.B.; Hellali, R. Defense mechanisms against water deficit in two olive (Olea europaea L.) cultivars ‘Meski’ and ‘Chemlali’. J. Hortic. Sci. Biotechnol. 2006, 81, 99–104. [Google Scholar] [CrossRef]
- Fathi, H.; Imani, A.; Amiri, M.E.; Hajilou, J.; Nikbakht, J. Response of almond genotypes/cultivars grafted on GN15 ‘Garnem’ rootstock in Deficit-Irrigation Stress conditions. J. Nuts 2017, 8, 123–135. [Google Scholar]
- Lawlor, D.W.; Cornis, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficit in Higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [Green Version]
- Besset, J.M.; Génard, T.; Girard, V.; Serra, C. Effect of water stress applied during the final stage of rapid growth on peach trees (cv. Big-Top). Sci. Hortic. 2001, 91, 289–303. [Google Scholar] [CrossRef]
- Girona, J.; Marsal, J.; Mata, M.; Arbonés, A.; Mata, A. The combined effect of fruit load and water stress in different peach fruit growth stages (Prunus persica L. Batsch). Acta Hortic. 2002, 584, 149–152. [Google Scholar] [CrossRef]
- Attia, F. Effet du stress hydrique sur le comportement ecophysiologique et la maturité phénolique de la vigne Vitis vinifera L. In Etude de Cinq Cépages Autochtones de Midi-Pyrenees; National Polytechnic Institute of Toulouse: Toulouse, France, 2007. [Google Scholar]
- Schaffer, B.; Urban, L.; Lu, P.; Whiley, A.W. The Mango. Botany, Production and Uses; CABI: Cambridge, UK, 2009; pp. 170–209. [Google Scholar]
- Goldhamer, D.A.; Viveros, M. Effects of pre-harvest irrigation cut off durations and post-harvest water deprivation on almond tree performance. Irrig. Sci. 2000, 19, 125–131. [Google Scholar] [CrossRef]
- Xiloyannis, C.; Uriu, K.; Martin, G.C. Seasonal and diurnal variations in abscisic acid, water potential, and diffusive resistance in Leaves from irrigated and non-irrigated peach trees. J. Am. Soc. Hortic. Sci. 1980, 105, 412–415. [Google Scholar]
- Lovisolo, C.; Perrone, I.; Hartung, W.; Schubert, A. An abscisic acid-related reduced transpiration promotes gradual embolism repair when grapevines are rehydrated after drought. New Phytol. 2008, 180, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Silva, A.A.; Ferreira, T.C.; Correia, C.M.; Malheiro, A.C.; Villalobos, F.J. Influence of different irrigation regimes on crop yield and water use efficiency of olive. Plant Soil. 2010, 333, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Mac Farlane, C.; White, D.A.; Adams, M.A. The apparent feed-forward response to vapour pressure deficit of stomata in droughted, field-grown Eucalyptus globules Labill. Plant Cell Environ. 2004, 27, 1268–1280. [Google Scholar] [CrossRef]
- Esparza, G.T.M.; Dejong, S.A. Weinbaum and I. Klein. Effects of irrigation deprivation during the harvest period on yield determinants in mature almond trees. Tree Physiol. 2001, 21, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Moutinho-Pereira, J.M.; Correia, C.M.; Gonçalves, B.M.; Bacelar, E.A.; Torres-Pereira, J.M. Leaf gas exchange and water relations of grapevines grown in three different conditions. Photosynthetica 2004, 42, 81–86. [Google Scholar] [CrossRef]
- Cheng, F.H.; Li, S.H.; Meng, Z.Q. Study on the effect of RDI on the vegetative growth, cropping and fruit quality of Yali pear variety. J. Fruit Sci. 2003, 20, 22–26. [Google Scholar]
- Mirás-Avalos, J.M.; Pérez-Sarmiento, F.; Alcobendas, R.; Alarcón, J.J.; Mounzer, O.; Nicolás, E. Using midday stem water potential for scheduling deficit irrigation in mid–late maturing peach trees under Mediterranean conditions. Irrig. Sci. 2016, 34, 161–173. [Google Scholar] [CrossRef]
- María José, M.; Fernando, B.C.; Antonio, V.; Oscar, G.B.; María, H.P. Evaluation of different deficit irrigation strategies in the late-maturing Japanese plum cultivar ‘Angeleno’. Agric. Water Manag. 2020, 234, 106–111. [Google Scholar]
- Chen, J.; Vercambr, G.; Kang, S.; Bertin, N.; Gautier, H.; Génard, M. Fruit water content as an indication of sugar metabolism improves simulation of carbohydrate accumulation in tomato fruit. J. Exp. Bot. 2020, 71, 5010–5026. [Google Scholar] [CrossRef]
- Mpelasoka, B.S.; Behboudian, M.H.; Dixon, J.; Neal, S.M. Improvement of fruit quality and storage potential of ‘Braeburn’ apple through deficit irrigation. J. Hortic. Sci. Biotechnol. 2000, 75, 615–621. [Google Scholar] [CrossRef]
- Lopes, C.M.; Santos, T.P.; Monteiroa, A.; Rodrigues, M.L.; Costa, J.M.; Chaves, M.M. Combining cover cropping with deficit irrigation in a Mediterranean low vigor vineyard. Sci. Hortic. 2011, 129, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Pérez-pastor, A.; Ruiz-sánchez, M.C.; Martínez, J.A.; Nortes, P.; Artés, F.; Domingo, R. Effect of deficit irrigation on apricot fruit quality at harvest and during storage. J. Sci. Food Agric. 2007, 87, 2409–2415. [Google Scholar] [CrossRef]
- Velez, J.E.; Intrigliolo, D.S.; Castel, J.R. Scheduling deficit irrigation of citrus trees with maximum daily trunk shrinkage. Agric. Water Manag. 2007, 90, 197–204. [Google Scholar] [CrossRef]
- Pérez-Pérez, J.G.; Romero, P.; Navarro, J.M.; Botía, P. Response of sweet orange cv ‘Lanelate’ to deficit irrigation in tworootstocks. I: Water relations, leaf gas exchange and vegetative growth. Irrig. Sci. 2008, 26, 415–425. [Google Scholar] [CrossRef]
- Yakushiji, H.; Morinaga, K.; Nonami, H. Sugar accumulation and partitioning in Satsuma mandarin tree tissues and fruit in response to drought stress. J. Am. Soc. Hortic. Sci. 1998, 123, 719–726. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://modis.gsfc.nasa.gov/about/ (accessed on 30 September 2013).
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Henningsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Nobel, P.S. Physicochemical and Environmental Plant Physiology; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Von, C.S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Plants 1981, 153, 376–387. [Google Scholar]



| Depth (cm) | K2O (g kg−1) | CaO+ (g kg−1) | Na2O+ (g kg−1) | K2O/MgO | Fe+ (mg kg−1) | Mn+ (mg kg−1) | Cu+ (mg kg−1) | B (mg kg−1) | Zn+ (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| 0–25 | 0.58 | 8.97 | 0.35 | 0.9 | 6 | 3.1 | 4.4 | 2.9 | 1 |
| 25–50 | 0.28 | 7.95 | 0.14 | 0.4 | 4 | 2 | 2.1 | 1.1 | 0.3 |
| Cultivars | Treatments | Day of Years | Ψ min (MPa) | Ψ os (MPa) |
|---|---|---|---|---|
| BS | Control | 128 | −1.9 ± 0.08 a | −0.63 ± 0.02 a |
| 159 | −2.2 ± 0.05 a | −0.81 ± 0.04 a | ||
| 189 | −3.1 ± 0.05 a | −0.91 ± 0.03 a | ||
| 211 | −2.2 ± 0.01 a | −1.40 ± 0.04 a | ||
| RDI | 128 | −1.9 ± 0.03 a | −0.83 ± 0.02 b | |
| 159 | −2.6 ± 0.07 b | −1.07 ± 0.01 b | ||
| 189 | −3.6 ± 0.02 b | −1.16 ± 0.08 b | ||
| 211 | −3.1 ± 0.01 b | −2.38 ± 0.07 b | ||
| BD | Control | 128 | −2.3 ± 0.04 a | −0.75 ± 0.09 a |
| 159 | −1.6 ± 0.03 a | −0.87 ± 0.06 a | ||
| 189 | −2.1 ± 0.07 a | −1.20 ± 0.01 a | ||
| 211 | −1.7 ± 0.09 a | −1.51 ± 0.08 a | ||
| RDI | 128 | −2.3 ± 0.02 a | −0.94 ± 0.07 b | |
| 159 | −1.86 ± 0.07 b | −1.20 ± 0.05 b | ||
| 189 | −2.5 ± 0.06 b | −1.56 ± 0.02 b | ||
| 211 | −2.6 ± 0.05 b | −2.22 ± 0.03 b | ||
| BG | Control | 128 | −1.8 ± 0.02 a | −1.61 ± 0.02 a |
| 159 | −1.8 ± 0.04 a | −1.58 ± 0.05 a | ||
| 189 | −2.5 ± 0.05 a | −1.56 ± 0.01 a | ||
| 211 | −2.1 ± 0.01 a | −1.65 ± 0.01 a | ||
| RDI | 128 | −1.8 ± 0.02 a | −2.21 ± 0.09 b | |
| 159 | −2.3 ± 0.04 b | −2.18 ± 0.08 b | ||
| 189 | −3.3 ± 0.07 b | −2.32 ± 0.01 b | ||
| 211 | −2.6 ± 0.04 b | −2.58 ± 0.05 b |
| Varieties | Treatment | Fruit Weight (g) | Size (mm) | Yield (kg Tree−1) | Firmness (kg 0.5 cm−2) | Titratable Acidity (% Malic Acid) | SSC (°Brix) |
|---|---|---|---|---|---|---|---|
| BD | CI | 96.33 ± 4.90 a | 59.24 ± 1.45 a | 62.5 ± 1.5 a | 5.22 ± 0.28 a | 3.96 ± 0.08 a | 16.66 ± 0.12 b |
| RDI | 78.4 ± 2.14 b | 53.04 ± 0.69 b | 54 ± 2 b | 5.58 ± 0.68 a | 3.75 ± 0.03 b | 17.8 ± 0.15 a | |
| BG | CI | 89.61 ± 2.82 a | 55.06 ± 0.67 a | 55 ± 1.8 a | 6.46 ± 0.11 b | 3.7 ± 0.09 a | 14.66 ± 0.06 a |
| RDI | 77.87 ± 3.02 b | 52.30 ± 0.86 b | 45 ± 1.8 b | 7.57 ± 0.19 a | 3.54 ± 0.06 b | 14.93 ± 0.10 a | |
| BS | CI | 104.57 ± 2.79 a | 57.41 ± 0.71 a | 67.5 ± 0.9 a | 2.11 ± 0.23 b | 3.65 ± 0.03 a | 15.13 ± 0.16 a |
| RDI | 90.64 ± 3.99 b | 55.39 ± 0.56 a | 49.5 ± 1 b | 2.79 ± 0.03 a | 3.49 ± 0.01 b | 15.73 ± 0.16 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajlaoui, H.; Maatallah, S.; Guizani, M.; Boughattas, N.E.H.; Guesmi, A.; Ennajeh, M.; Dabbou, S.; Lopez-Lauri, F. Effect of Regulated Deficit Irrigation on Agronomic Parameters of Three Plum Cultivars (Prunus salicina L.) under Semi-Arid Climate Conditions. Plants 2022, 11, 1545. https://doi.org/10.3390/plants11121545
Hajlaoui H, Maatallah S, Guizani M, Boughattas NEH, Guesmi A, Ennajeh M, Dabbou S, Lopez-Lauri F. Effect of Regulated Deficit Irrigation on Agronomic Parameters of Three Plum Cultivars (Prunus salicina L.) under Semi-Arid Climate Conditions. Plants. 2022; 11(12):1545. https://doi.org/10.3390/plants11121545
Chicago/Turabian StyleHajlaoui, Hichem, Samira Maatallah, Monia Guizani, Nour El Houda Boughattas, Anis Guesmi, Mustapha Ennajeh, Samia Dabbou, and Félicie Lopez-Lauri. 2022. "Effect of Regulated Deficit Irrigation on Agronomic Parameters of Three Plum Cultivars (Prunus salicina L.) under Semi-Arid Climate Conditions" Plants 11, no. 12: 1545. https://doi.org/10.3390/plants11121545
APA StyleHajlaoui, H., Maatallah, S., Guizani, M., Boughattas, N. E. H., Guesmi, A., Ennajeh, M., Dabbou, S., & Lopez-Lauri, F. (2022). Effect of Regulated Deficit Irrigation on Agronomic Parameters of Three Plum Cultivars (Prunus salicina L.) under Semi-Arid Climate Conditions. Plants, 11(12), 1545. https://doi.org/10.3390/plants11121545






