Natural Sources and Pharmacological Properties of Pinosylvin
Abstract
:1. Introduction
2. Sources of Pinosylvin
3. Technology of Extraction and Purification
3.1. Pinosylvin Production in Callus Cultures and Cell Suspension Cultures
3.2. Microbial Biosynthesis of Pinosylvin
4. Technology of Identification and Characterization
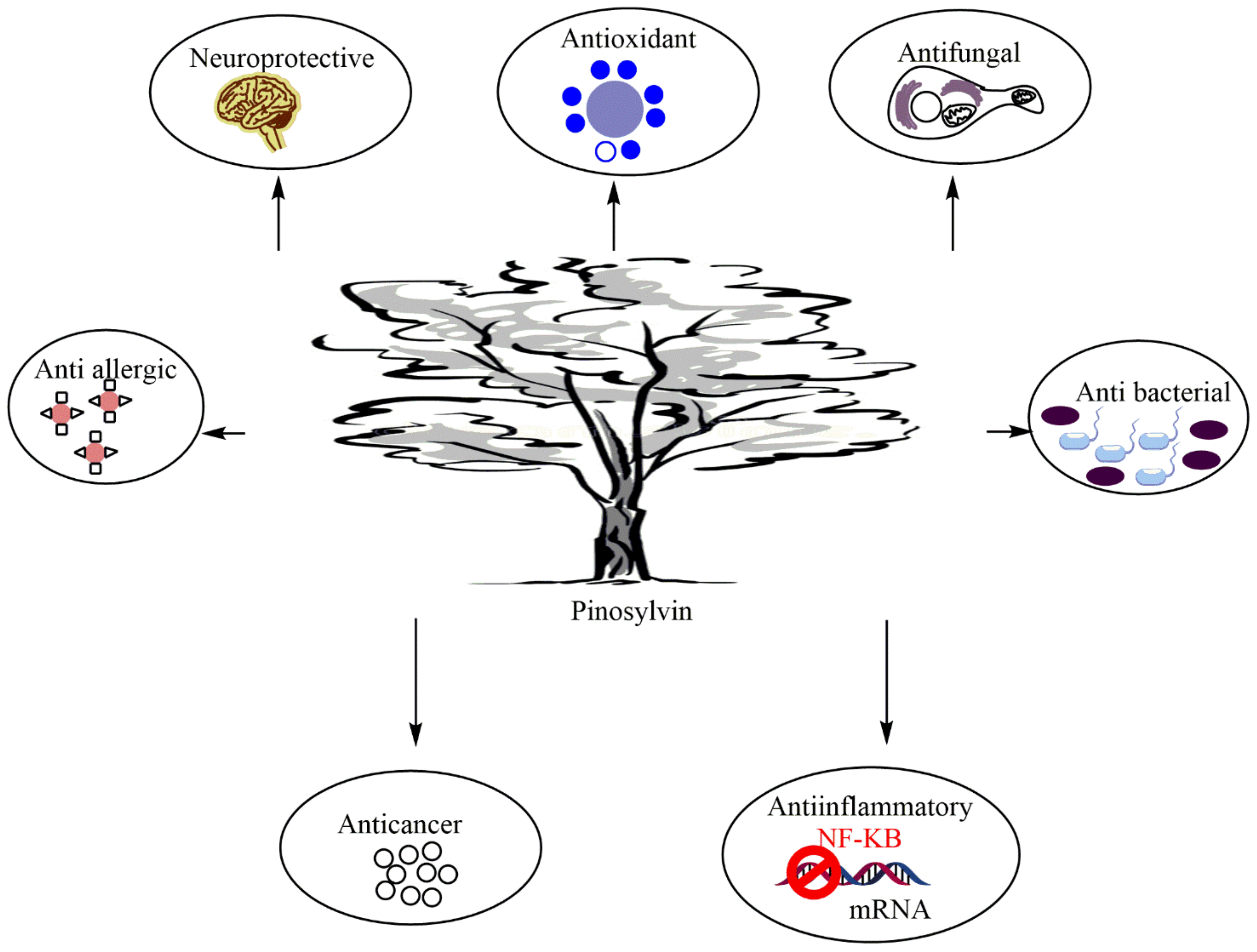
5. Biological and Pharmacological Properties
5.1. Antimicrobial Activity
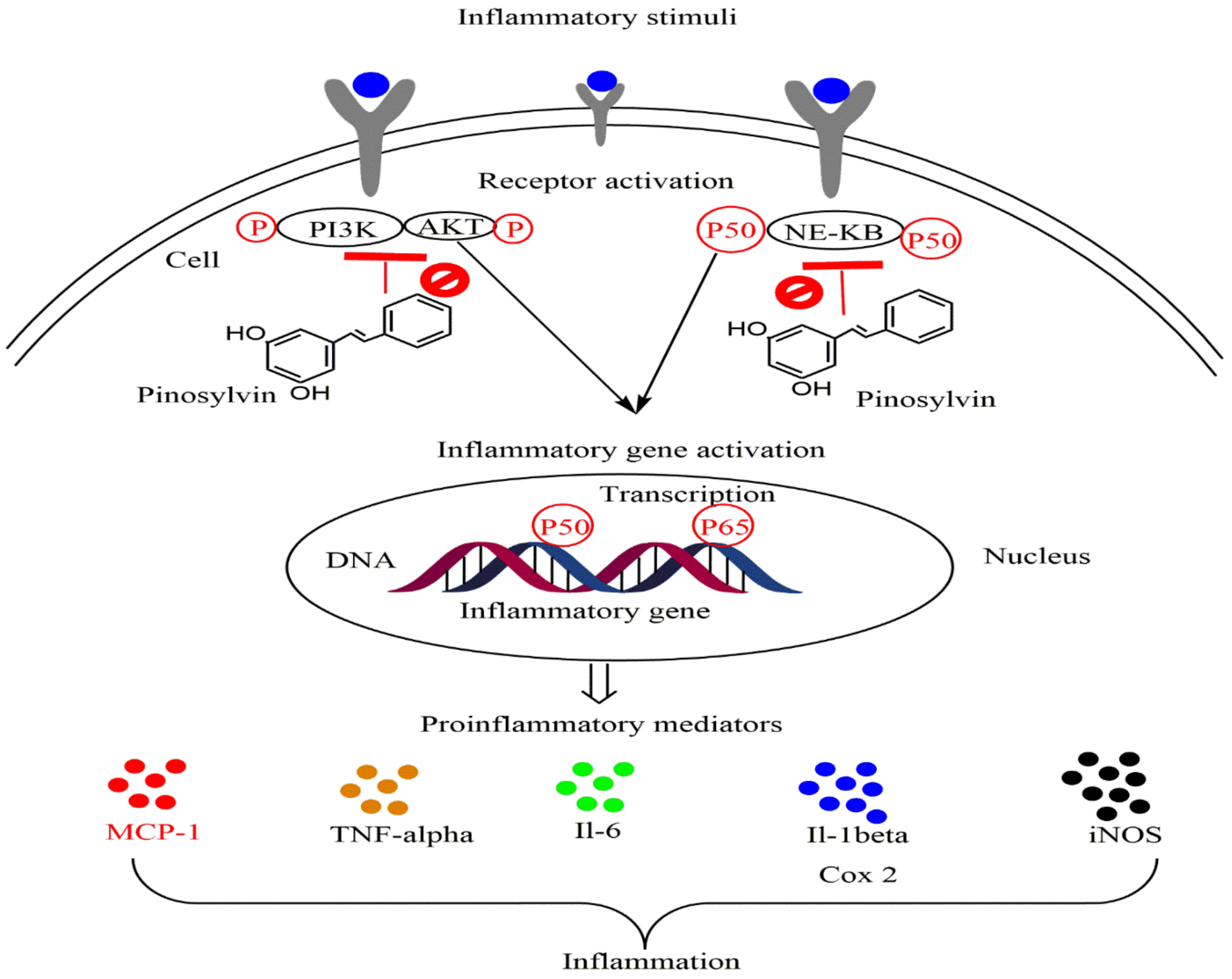
5.2. Anti-Inflammatory Activity
5.3. Antioxidant Activity
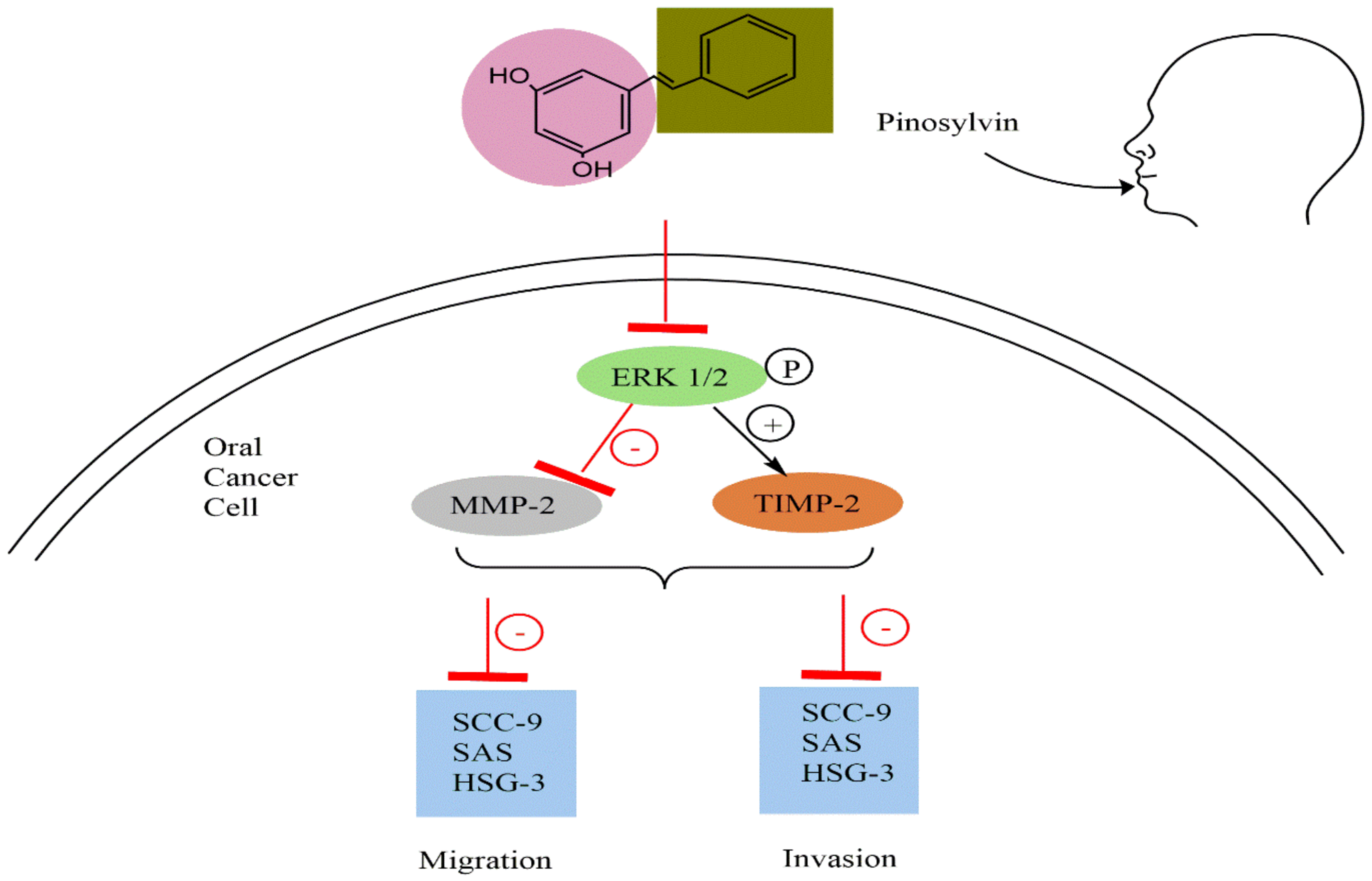
5.4. Anticancer Activity
5.5. Neuroprotective Activity
5.6. Anti-Allergic Activity
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| MAPK | Mitogen-activated protein kinase |
| JNK | Jun amino-terminal kinase |
| NF-κB | Nuclear factor kappa B |
| Nrf2 | Nuclear erythroid 2-related factor 2 |
| ARE | Antioxidant response element |
| HCT | Colorectal cancer cell |
| FAK | Focal adhesion kinase |
| ERK | Extracellular signal-regulated kinase |
| GSK3 | Glycogen synthase kinase-3 |
| PI3K/AKT | Phosphatidylinositol 3-kinase/Protein kinase B |
| MMP | Matrix metalloproteinase |
| CX | Cyclohexane |
| EtOAc | Ethyl acetate |
| DRIFT | Diffuse reflectance fourier transform infrared spectroscopy |
| NIR | Near-infrared spectroscopy |
| FID | Flame ionization detection |
| GC | Gas chromatography |
| GC-MS | Gas chromatography-mass spectrometry |
| MIC | Minimal inhibitory concentration |
| EtBr | Ethidium bromide |
| Eps | Efflux pumps |
| MRSA | Methicillin-resistant Staphylococcus aureus; |
| ATTC | American type culture collection; |
| PGE2 | Prostaglandin E2 |
| NO | Nitric oxide |
| COX | Cyclooxygenase |
| iNOS | Inducible nitric oxide synthase |
| IL6 | Interleukin 6 |
| MCP1 | Monocyte chemotactic protein 1 |
| LPS | Lipopolysaccharide |
| IRF-3 | Interferon regulatory factor 3 |
| IFN-E | Interferon-E |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| HO-1 | Heme oxygenase-1 |
| MTX | Methotrexate |
| TBARS | Plasmatic thiobarbituric acid-reactive substances |
| LOX | Lipoxygenase |
| TRPA1 | Ankyrin subtype 1 protein |
| AA | Adjuvant arthritis |
| GGT | Glutamyltransferase |
| CRP | C-Reactive protein |
| PKC | Protein kinase C |
| GSTP1 | Glutathione S-transferase pi 1 |
| qRT-PCR | Quantitative reverse transcription polymerase chain reaction |
| HPV | Hind paw volume |
| CL | Chemiluminescence |
| MPO | Myeloperoxidase |
| LH | Luteinizing hormone |
| FSH | Follicle-stimulating hormone |
| ELISA | Enzyme-linked immunosorbent assay |
| NFE2L2 | Nuclear factor erythroid 2 like 2 |
| TIMP-2 | Tissue inhibitor of metalloproteinase-2 |
| NPC | Nasopharyngeal carcinoma |
| CRPC | Castration-resistant prostate cancer |
| PSME | Pinosylvin methyl ether |
| HQ | Hydroquinone |
| CDK2 | Cyclin-dependent kinase 2 |
| pRb | Retinoblastoma protein |
| AMPK | AMP-activated protein kinase |
| eNOS | Endothelial nitric oxide synthase |
| RBL | Rat basophilic leukemia |
| PCA | Passive cutaneous anaphylaxis |
| LC3-II | Microtubule associated protein 1 light chain 3-II |
References
- Jeong, E.; Lee, H.-R.; Pyee, J.; Park, H. Pinosylvin Induces Cell Survival, Migration and Anti-Adhesiveness of Endothelial Cells via Nitric Oxide Production: Pinosylvin is a vasoregulating compound. Phytother. Res. 2013, 27, 610–617. [Google Scholar] [CrossRef]
- Riviere, C.; Pawlus, A.D.; Merillon, J.-M. Natural Stilbenoids: Distribution in the Plant Kingdom and Chemotaxonomic Interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef] [PubMed]
- Castelli, G.; Bruno, F.; Vitale, F.; Roberti, M.; Colomba, C.; Giacomini, E.; Guidotti, L.; Cascio, A.; Tolomeo, M. In Vitro Antileishmanial Activity of Trans-Stilbene and Terphenyl Compounds. Exp. Parasitol. 2016, 166, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinwumi, B.C.; Bordun, K.-A.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.-J.; Park, H.J.; Chung, H.-J.; Shin, Y.; Min, H.-Y.; Hong, J.-Y.; Kang, Y.-J.; Ahn, Y.-H.; Pyee, J.-H.; Kook Lee, S. Antimetastatic Activity of Pinosylvin, a Natural Stilbenoid, Is Associated with the Suppression of Matrix Metalloproteinases. J. Nutr. Biochem. 2012, 23, 946–952. [Google Scholar] [CrossRef]
- Plumed-Ferrer, C.; Väkeväinen, K.; Komulainen, H.; Rautiainen, M.; Smeds, A.; Raitanen, J.-E.; Eklund, P.; Willför, S.; Alakomi, H.-L.; Saarela, M. The Antimicrobial Effects of Wood-Associated Polyphenols on Food Pathogens and Spoilage Organisms. Int. J. Food Microbiol. 2013, 164, 99–107. [Google Scholar] [CrossRef]
- Silva, F.; Domingues, F.C.; Nerín, C. Control Microbial Growth on Fresh Chicken Meat Using Pinosylvin Inclusion Complexes Based Packaging Absorbent Pads. LWT 2018, 89, 148–154. [Google Scholar] [CrossRef]
- Sousa, V.; Luís, Â.; Oleastro, M.; Domingues, F.; Ferreira, S. Polyphenols as Resistance Modulators in Arcobacter Butzleri. Folia Microbiol. 2019, 64, 547–554. [Google Scholar] [CrossRef]
- Celimene, C.C.; Micales, J.A.; Ferge, L.; Young, R.A. Efficacy of Pinosylvins against White-Rot and Brown-Rot Fungi. Holzforschung 1999, 53, 491–497. [Google Scholar] [CrossRef]
- Lee, D.G.; Lee, S.J.; Rodriguez, J.P.; Kim, I.H.; Chang, T.; Lee, S. Antifungal Activity of Pinosylvin from Pinus Densiflora on Turfgrass Fungal Diseases. J. Appl. Biol. Chem. 2017, 60, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Eräsalo, H.; Hämäläinen, M.; Leppänen, T.; Mäki-Opas, I.; Laavola, M.; Haavikko, R.; Yli-Kauhaluoma, J.; Moilanen, E. Natural Stilbenoids Have Anti-Inflammatory Properties in Vivo and down-Regulate the Production of Inflammatory Mediators NO, IL6, and MCP1 Possibly in a PI3K/Akt-Dependent Manner. J. Nat. Prod. 2018, 81, 1131–1142. [Google Scholar] [CrossRef]
- Kivimäki, K.; Leppänen, T.; Hämäläinen, M.; Vuolteenaho, K.; Moilanen, E. Pinosylvin Shifts Macrophage Polarization to Support Resolution of Inflammation. Molecules 2021, 26, 2772. [Google Scholar] [CrossRef]
- Laavola, M.; Nieminen, R.; Leppänen, T.; Eckerman, C.; Holmbom, B.; Moilanen, E. Pinosylvin and Monomethylpinosylvin, Constituents of an Extract from the Knot of Pinus Sylvestris, Reduce Inflammatory Gene Expression and Inflammatory Responses in Vivo. J. Agric. Food Chem. 2015, 63, 3445–3453. [Google Scholar] [CrossRef]
- Modi, S.; Yaluri, N.; Kokkola, T. Strigolactone GR24 and Pinosylvin Attenuate Adipogenesis and Inflammation of White Adipocytes. Biochem. Biophys. Res. Commun. 2018, 499, 164–169. [Google Scholar] [CrossRef]
- Moilanen, L.J.; Hämäläinen, M.; Lehtimäki, L.; Nieminen, R.M.; Muraki, K.; Moilanen, E. Pinosylvin Inhibits TRPA 1-Induced Calcium Influx In Vitro and TRPA 1-Mediated Acute Paw Inflammation In Vivo. Basic Clin. Pharmacol. Toxicol. 2016, 118, 238–242. [Google Scholar] [CrossRef]
- Bauerova, K.; Acquaviva, A.; Ponist, S.; Gardi, C.; Vecchio, D.; Drafi, F.; Arezzini, B.; Bezakova, L.; Kuncirova, V.; Mihalova, D.; et al. Markers of Inflammation and Oxidative Stress Studied in Adjuvant-Induced Arthritis in the Rat on Systemic and Local Level Affected by Pinosylvin and Methotrexate and Their Combination. Autoimmunity 2015, 48, 46–56. [Google Scholar] [CrossRef]
- Drafi, F.; Bauerova, K.; Kuncirova, V.; Ponist, S.; Mihalova, D.; Fedorova, T.; Harmatha, J.; Nosal, R. Pharmacological Influence on Processes of Adjuvant Arthritis: Effect of the Combination of an Antioxidant Active Substance with Methotrexate. Interdiscip. Toxicol. 2012, 5, 84–91. [Google Scholar] [CrossRef]
- Rodríguez-Bonilla, P.; Gandía-Herrero, F.; Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Comparative Study of the Antioxidant Capacity of Four Stilbenes Using ORAC, ABTS+, and FRAP Techniques. Food Anal. Methods 2017, 10, 2994–3000. [Google Scholar] [CrossRef]
- Wang, C.; Sang, M.; Gong, S.; Yang, J.; Cheng, C.Y.; Sun, F. Two Resveratrol Analogs, Pinosylvin and 4,4′-Dihydroxystilbene, Improve Oligoasthenospermia in a Mouse Model by Attenuating Oxidative Stress via the Nrf2-ARE Pathway. Bioorg. Chem. 2020, 104, 104295. [Google Scholar] [CrossRef]
- Chen, M.-K.; Liu, Y.-T.; Lin, J.-T.; Lin, C.-C.; Chuang, Y.-C.; Lo, Y.-S.; Hsi, Y.-T.; Hsieh, M.-J. Pinosylvin Reduced Migration and Invasion of Oral Cancer Carcinoma by Regulating Matrix Metalloproteinase-2 Expression and Extracellular Signal-Regulated Kinase Pathway. Biomed. Pharmacother. 2019, 117, 109160. [Google Scholar] [CrossRef]
- Park, E.-J.; Chung, H.-J.; Park, H.J.; Kim, G.D.; Ahn, Y.-H.; Lee, S.K. Suppression of Src/ERK and GSK-3/β-Catenin Signaling by Pinosylvin Inhibits the Growth of Human Colorectal Cancer Cells. Food Chem. Toxicol. 2013, 55, 424–433. [Google Scholar] [CrossRef]
- Park, J.; Pyee, J.; Park, H. Pinosylvin at a High Concentration Induces AMPK-Mediated Autophagy for Preventing Necrosis in Bovine Aortic Endothelial Cells. Can. J. Physiol. Pharmacol. 2014, 92, 993–999. [Google Scholar] [CrossRef]
- Ketola, K.; Viitala, M.; Kohonen, P.; Fey, V.; Culig, Z.; Kallioniemi, O.; Iljin, K. High-Throughput Cell-Based Compound Screen Identifies Pinosylvin Methyl Ether and Tanshinone IIA as Inhibitors of Castration-Resistant Prostate Cancer. J. Mol. Biochem. 2016, 5, 12–22. [Google Scholar]
- Chambers, V.H. British Bees and Wind-Borne Pollen. Nature 1945, 155, 145. [Google Scholar] [CrossRef]
- Ioannidis, K.; Melliou, E.; Alizoti, P.; Magiatis, P. Identification of Black Pine (Pinus nigra Arn.) Heartwood as a Rich Source of Bioactive Stilbenes by QNMR. J. Sci. Food Agric. 2017, 97, 1708–1716. [Google Scholar] [CrossRef]
- Rowe, J.W.; Bower, C.L.; Wagner, E.R. Extractives of Jack Pine Bark: Occurrence of Cis- and Trans-Pinosylvin Dimethyl Ether and Ferulic Acid Esters. Phytochemistry 1969, 8, 235–241. [Google Scholar] [CrossRef]
- Seppänen, S.K.; Syrjälä, L.; Von Weissenberg, K.; Teeri, T.H.; Paajanen, L.; Pappinen, A. Antifungal Activity of Stilbenes in in Vitro Bioassays and in Transgenic Populus Expressing a Gene Encoding Pinosylvin Synthase. Plant Cell Rep. 2004, 22, 584–593. [Google Scholar] [CrossRef]
- Pietarinen, S.P.; Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Holmbom, B.R. Knotwood and Bark Extracts: Strong Antioxidants from Waste Materials. J. Wood Sci. 2006, 52, 436–444. [Google Scholar] [CrossRef]
- Hovelstad, H.; Leirset, I.; Oyaas, K.; Fiksdahl, A. Screening Analyses of Pinosylvin Stilbenes, Resin Acids and Lignans in Norwegian Conifers. Molecules 2006, 11, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Vek, V.; Poljanšek, I.; Humar, M.; Willför, S.; Oven, P. In Vitro Inhibition of Extractives from Knotwood of Scots Pine (Pinus sylvestris) and Black Pine (Pinus nigra) on Growth of Schizophyllum commune, Trametes versicolor, Gloeophyllum trabeum and Fibroporia Vaillantii. Wood Sci. Technol. 2020, 54, 1645–1662. [Google Scholar] [CrossRef]
- Lindberg, L.E.; Willför, S.M.; Holmbom, B.R. Antibacterial Effects of Knotwood Extractives on Paper Mill Bacteria. J. Ind. Microbiol. Biotechnol. 2004, 31, 137–147. [Google Scholar] [CrossRef]
- Verkasalo, E.; Möttönen, V.; Roitto, M.; Vepsäläinen, J.; Kumar, A.; Ilvesniemi, H.; Siwale, W.; Julkunen-Tiitto, R.; Raatikainen, O.; Sikanen, L. Extractives of Stemwood and Sawmill Residues of Scots Pine (Pinus sylvestris L.) for Biorefining in Four Climatic Regions in Finland-Phenolic and Resin Acid Compounds. Forests 2021, 12, 192. [Google Scholar] [CrossRef]
- Bergström, B. Chemical and Structural Changes during Heartwood Formation in Pinus sylvestris. Forestry 2003, 76, 45–53. [Google Scholar] [CrossRef]
- François, S.; Jean, L.; Serge, L.; Vakhtang, M.; André, P. Inhibition of Cholinesterase and Amyloid-&bgr; Aggregation by Resveratrol Oligomers from Vitis amurensis. Phytother. Res. 2008, 22, 544–549. [Google Scholar] [CrossRef]
- Kodan, A.; Kuroda, H.; Sakai, F. A Stilbene Synthase from Japanese Red Pine (Pinus densiflora): Implications for Phytoalexin Accumulation and down-Regulation of Flavonoid Biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 3335–3339. [Google Scholar] [CrossRef] [Green Version]
- Dumas, M.T.; Hubbes, M.; Strunz, G.M. Identification of Some Compounds Associated with Resistance of Pinus densiflora to Fomes annosus. Eur. J. For. Pathol. 1983, 13, 151–160. [Google Scholar] [CrossRef]
- Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Reunanen, M.H.T.; Eklund, P.C.; Sjöholm, R.E.; Eckerman, C.S.E.; Pohjamo, S.P.; Holmbom, B.R. Antioxidant Activity of Knotwood Extractives and Phenolic Compounds of Selected Tree Species. J. Agric. Food Chem. 2003, 51, 7600–7606. [Google Scholar] [CrossRef]
- Raiber, S.; Schröder, G.; Schröder, J. Molecular and Enzymatic Characterization of Two Stilbene Synthases from Eastern White Pine (Pinus strobus) A Single Arg/His Difference Determines the Activity and the PH Dependence of the Enzymes. FEBS Lett. 1995, 361, 299–302. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.S.; Han, J.Y.; Choi, Y.E. Enhanced Accumulation of Pinosylvin Stilbenes and Related Gene Expression in Pinus Strobus after Infection of Pine Wood Nematode. Tree Physiol. 2021, 41, 1972–1987. [Google Scholar] [CrossRef]
- Koo, H.B.; Hwang, H.S.; Han, J.Y.; Cheong, E.J.; Kwon, Y.S.; Choi, Y.E. Enhanced Production of Pinosylvin Stilbene with Aging of Pinus strobus Callus and Nematicidal Activity of Callus Extracts against Pinewood Nematodes. Sci. Rep. 2022, 12, 770. [Google Scholar] [CrossRef]
- Hemingway, R.W.; Mcgraw, G.W.; Barras, S.J. Polyphenols in Ceratocystis Minor Infected Pinus taeda: Fungal Metabolites, Phloem and Xylem Phenols. J. Agric. Food Chem. 1977, 25, 717–722. [Google Scholar] [CrossRef]
- Gabaston, J.; Leborgne, C.; Waffo-Téguo, P.; Pedrot, E.; Richard, T.; Mérillon, J.M.; Valls Fonayet, J. Separation and Isolation of Major Polyphenols from Maritime Pine (Pinus pinaster) Knots by Two-Step Centrifugal Partition Chromatography Monitored by LC-MS and NMR Spectroscopy. J. Sep. Sci. 2020, 43, 1080–1088. [Google Scholar] [CrossRef]
- Gabaston, J.; Richard, T.; Cluzet, S.; Palos Pinto, A.; Dufour, M.C.; Corio-Costet, M.F.; Mérillon, J.M. Pinus pinaster Knot: A Source of Polyphenols against Plasmopara viticola. J. Agric. Food Chem. 2017, 65, 8884–8891. [Google Scholar] [CrossRef]
- Conde, E.; Fang, W.; Hemming, J.; Willför, S.; Domínguez, H.; Parajó, J.C. Recovery of Bioactive Compounds from Pinus pinaster Wood by Consecutive Extraction Stages. Wood Sci. Technol. 2014, 48, 311–323. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, M.; Randy, A.; Nho, C.W. Inhibitory Effect of the Branches of Hovenia dulcis Thunb. and Its Constituent Pinosylvin on the Activities of IgE-Mediated Mast Cells and Passive Cutaneous Anaphylaxis in Mice. Food Funct. 2015, 6, 1361–1370. [Google Scholar] [CrossRef]
- Wollenweber, E.; Stevens, J.F.; Dörr, M.; Rozefelds, A.C. Taxonomic Significance of Flavonoid Variation in Temperate Species of Nothofagus. Phytochemistry 2003, 62, 1125–1131. [Google Scholar] [CrossRef]
- Gyeltshen, T.; Jordan, G.J.; Smith, J.A.; Bissember, A.C. Natural Products Isolation Studies of the Paleoendemic Plant Species Nothofagus gunnii and Nothofagus cunninghamii. Fitoterapia 2022, 156, 105088. [Google Scholar] [CrossRef]
- Kostecki, K.; Engelmeier, D.; Pacher, T.; Hofer, O.; Vajrodaya, S.; Greger, H. Dihydrophenanthrenes and Other Antifungal Stilbenoids from Stemona cf. Pierrei. Phytochemistry 2004, 65, 99–106. [Google Scholar] [CrossRef]
- Schöppner, A.; Kindl, H. Purification and Properties of a Stilbene Synthase from Induced Cell Suspension Cultures of Peanut. J. Biol. Chem. 1984, 259, 6806–6811. [Google Scholar] [CrossRef]
- Poljanšek, I.; Oven, P.; Vek, V.; Raitanen, J.E.; Hemming, J.; Willför, S. Isolation of Pure Pinosylvins from Industrial Knotwood Residue with Non-Chlorinated Solvents. Holzforschung 2019, 73, 475–484. [Google Scholar] [CrossRef]
- Jorgensen, E.; Balsillie, D. Formation of Heartwood Phenols in Callus Tissue Cultures of Red Pine (Pinus resinosa). Can. J. Bot. 1969, 47, 1015–1016. [Google Scholar] [CrossRef]
- Lange, B.M.; Trost, M.; Heller, W.; Langebartels, C.; Sandermann, H. Elicitor-Induced Formation of Free and Cell-Wall-Bound Stilbenes in Cell-Suspension Cultures of Scots Pine (Pinus sylvestris L.). Planta 1994, 194, 143–148. [Google Scholar] [CrossRef]
- Makrides, S.C. Strategies for Achieving High-Level Expression of Genes in Escherichia coli. Microbiol. Rev. 1996, 60, 512–538. [Google Scholar] [CrossRef]
- Wu, J.; Liu, P.; Fan, Y.; Bao, H.; Du, G.; Zhou, J.; Chen, J. Multivariate Modular Metabolic Engineering of Escherichia coli to Produce Resveratrol from L-Tyrosine. J. Biotechnol. 2013, 167, 404–411. [Google Scholar] [CrossRef]
- Watts, K.T.; Lee, P.C.; Schmidt-Dannert, C. Biosynthesis of Plant-Specific Stilbene Polyketides in Metabolically Engineered Escherichia coli. BMC Biotechnol. 2006, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Van Summeren-Wesenhagen, P.V.; Marienhagen, J. Metabolic Engineering of Escherichia coli for the Synthesis of the Plant Polyphenol Pinosylvin. Appl. Environ. Microbiol. 2015, 81, 840–849. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.Y.; Xu, Y.; Chu, X.; Tan, M.; Ye, B.C. Protein Acylation Affects the Artificial Biosynthetic Pathway for Pinosylvin Production in Engineered E. coli. ACS Chem. Biol. 2018, 13, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-L.; Guo, L.-Q.; Lin, J.-F.; He, Z.-Q.; Cai, F.-J.; Chen, J.-F. A Novel Process for Obtaining Pinosylvin Using Combinatorial Bioengineering in Escherichia coli. World J. Microbiol. Biotechnol. 2016, 32, 102. [Google Scholar] [CrossRef] [PubMed]
- Salas-Navarrete, C.; Hernández-Chávez, G.; Flores, N.; Martínez, L.M.; Martinez, A.; Bolívar, F.; Barona-Gomez, F.; Gosset, G. Increasing Pinosylvin Production in Escherichia coli by Reducing the Expression Level of the Gene FabI-Encoded Enoyl-Acyl Carrier Protein Reductase. Electron. J. Biotechnol. 2018, 33, 11–16. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Funa, N.; Horinouchi, S. Precursor-Directed Biosynthesis of Stilbene Methyl Ethers in Escherichia coli. Biotechnol. J. 2007, 2, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, S.; Xiao, A.; Rasmussen, M.; Skidmore, C.; Zhan, J. Metabolic Engineering of Escherichia coli for the Biosynthesis of Various Phenylpropanoid Derivatives. Metab. Eng. 2015, 29, 153–159. [Google Scholar] [CrossRef]
- Holmgren, A.; Bergström, B.; Gref, R.; Ericsson, A. Detection of Pinosylvins in Solid Wood of Scots Pine Using Fourier Transform Raman and Infrared Spectroscopy. J. Wood Chem. Technol. 1999, 19, 139–150. [Google Scholar] [CrossRef]
- Roupe, K.; Halls, S.; Davies, N.M. Determination and Assay Validation of Pinosylvin in Rat Serum: Application to Drug Metabolism and Pharmacokinetics. J. Pharm. Biomed. Anal. 2005, 38, 148–154. [Google Scholar] [CrossRef]
- Ekeberg, D.; Flæte, P.O.; Eikenes, M.; Fongen, M.; Naess-Andresen, C.F. Qualitative and Quantitative Determination of Extractives in Heartwood of Scots Pine (Pinus sylvestris L.) by Gas Chromatography. J. Chromatogr. A 2006, 1109, 267–272. [Google Scholar] [CrossRef]
- Preusz, M.; Tříska, J.; Vrchotová, N.; Vilímek, J.; Enei, F.; Preusz, K. Chemical Profile of Organic Residues from Ancient Amphoras Found in Pyrgi and Castrum Novum, Tyrrhenian Sea (Italy). J. Archaeol. Sci. Rep. 2019, 24, 565–573. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, H.J.; Min, H.Y.; Park, E.J.; Lee, K.M.; Ahn, Y.H.; Cho, Y.J.; Pyee, J.H. Antibacterial and Antifungal Activity of Pinosylvin, a Constituent of Pine. Fitoterapia 2005, 76, 258–260. [Google Scholar] [CrossRef]
- Xu, H.; Deng, R.; Li, E.T.S.; Shen, J.; Wang, M. Pinosylvin Provides Neuroprotection against Cerebral Ischemia and Reperfusion Injury through Enhancing PINK1/Parkin Mediated Mitophagy and Nrf2 Pathway. J. Funct. Foods 2020, 71, 104019. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Dey, A.; Koirala, N.; Shaheen, S.; El Omari, N.; Salehi, B.; Goloshvili, T.; Silva, N.C.C.; Bouyahya, A.; Vitalini, S. Cinnamomum Species: Bridging Phytochemistry Knowledge, Pharmacological Properties and Toxicological Safety for Health Benefits. Front. Pharmacol. 2021, 12, 600139. [Google Scholar] [CrossRef]
- Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.-E.; Balahbib, A.; El Omari, N.; Taha, D.; Belmehdi, O.; Ghokhan, Z.; El Menyiy, N. Traditional Use, Phytochemistry, Toxicology, and Pharmacology of Origanum majorana L. J. Ethnopharmacol. 2021, 265, 113318. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.-E.; Balahbib, A.; El-Shazly, M.; Chamkhi, I. Ethnomedicinal Use, Phytochemistry, Pharmacology, and Toxicology of Ajuga iva (L.) Schreb. J. Ethnopharmacol. 2020, 258, 112875. [Google Scholar] [CrossRef]
- Marmouzi, I.; Bouyahya, A.; Ezzat, S.M.; El Jemli, M.; Kharbach, M. The Food Plant Silybum marianum (L.) Gaertn.: Phytochemistry, Ethnopharmacology and Clinical Evidence. J. Ethnopharmacol. 2021, 265, 113303. [Google Scholar] [CrossRef]
- De Bruijn, W.J.; Araya-Cloutier, C.; Bijlsma, J.; de Swart, A.; Sanders, M.G.; de Waard, P.; Gruppen, H.; Vincken, J.-P. Antibacterial Prenylated Stilbenoids from Peanut (Arachis hypogaea). Phytochem. Lett. 2018, 28, 13–18. [Google Scholar] [CrossRef]
- Silva, F.; Nerín, C.; Domingues, F.C. Stilbene Phytoallexins Inclusion Complexes: A Natural-Based Strategy to Control Foodborne Pathogen Campylobacter. Food Control 2015, 54, 66–73. [Google Scholar] [CrossRef]
- Bouyahya, A.; Omari, N.E.; El Hachlafi, N.; Jemly, M.E.; Hakkour, M.; Balahbib, A.; El Menyiy, N.; Bakrim, S.; Naceiri Mrabti, H.; Khouchlaa, A. Chemical Compounds of Berry-Derived Polyphenols and Their Effects on Gut Microbiota, Inflammation, and Cancer. Molecules 2022, 27, 3286. [Google Scholar] [CrossRef]
- Bouyahya, A.; Guaouguaou, F.-E.; El Omari, N.; El Menyiy, N.; Balahbib, A.; El-Shazly, M.; Bakri, Y. Anti-Inflammatory and Analgesic Properties of Moroccan Medicinal Plants: Phytochemistry, in Vitro and in Vivo Investigations, Mechanism Insights, Clinical Evidences and Perspectives. J. Pharm. Anal. 2021, 12, 35–37. [Google Scholar] [CrossRef]
- Park, J.-H.; Choi, G.J.; Jang, K.S.; Lim, H.K.; Kim, H.T.; Cho, K.Y.; Kim, J.-C. Antifungal Activity against Plant Pathogenic Fungi of Chaetoviridins Isolated from Chaetomium globosum. FEMS Microbiol. Lett. 2005, 252, 309–313. [Google Scholar] [CrossRef] [Green Version]
- Koskela, A.; Reinisalo, M.; Hyttinen, J.M.; Kaarniranta, K.; Karjalainen, R.O. Pinosylvin-Mediated Protection against Oxidative Stress in Human Retinal Pigment Epithelial Cells. Mol. Vis. 2014, 20, 760. [Google Scholar]
- Park, E.-J.; Min, H.-Y.; Ahn, Y.-H.; Bae, C.-M.; Pyee, J.-H.; Lee, S.K. Synthesis and Inhibitory Effects of Pinosylvin Derivatives on Prostaglandin E2 Production in Lipopolysaccharide-Induced Mouse Macrophage Cells. Bioorg. Med. Chem. Lett. 2004, 14, 5895–5898. [Google Scholar] [CrossRef]
- Park, E.-J.; Min, H.-Y.; Chung, H.-J.; Ahn, Y.-H.; Pyee, J.-H.; Lee, S.K. Pinosylvin Suppresses LPS-Stimulated Inducible Nitric Oxide Synthase Expression via the MyD88-Independent, but TRIF-Dependent Downregulation of IRF-3 Signaling Pathway in Mouse Macrophage Cells. Cell. Physiol. Biochem. 2011, 27, 353–362. [Google Scholar] [CrossRef]
- Mačičková, T.; Drábiková, K.; Nosal, R.; Bauerová, K.; Mihálová, D.; Harmatha, J.; Pečivová, J. In Vivo Effect of Pinosylvin and Pterostilbene in the Animal Model of Adjuvant Arthritis. Neuroendocrinol. Lett. 2010, 31, 91. [Google Scholar]
- Jančinová, V.; Perečko, T.; Nosáľ, R.; Harmatha, J.; Šmidrkal, J.; Drábiková, K. The Natural Stilbenoid Pinosylvin and Activated Neutrophils: Effects on Oxidative Burst, Protein Kinase C, Apoptosis and Efficiency in Adjuvant Arthritis. Acta Pharmacol. Sin. 2012, 33, 1285–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, O.; Seo, Y.; Park, H. Pinosylvin Exacerbates LPS-Induced Apoptosis via ALOX 15 Upregulation in Leukocytes. BMB Rep. 2018, 51, 302. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.; Holzer, W.; Doerfler, H.; Weckwerth, W.; Viernstein, H.; Okonogi, S.; Mueller, M. Cajanus Cajan–a Source of PPARγ Activators Leading to Anti-Inflammatory and Cytotoxic Effects. Food Funct. 2016, 7, 3798–3806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.-J.; Ahn, Y.-H.; Pyee, J.-H.; Park, H.J.; Chung, H.-J.; Min, H.-Y.; Hong, J.-Y.; Kang, Y.-J.; Bae, I.-K.; Lee, S.K. Suppressive Effects of Pinosylvin, a Natural Stilbenoid, on Cyclooxygenase-2 and Inducible Nitric Oxide Synthase and the Growth Inhibition of Cancer Cells. Cancer Res. 2005, 65, 176. [Google Scholar]
- Tamminen, T.; Koskela, A.; Toropainen, E.; Gurubaran, I.S.; Winiarczyk, M.; Liukkonen, M.; Paterno, J.J.; Lackman, P.; Sadeghi, A.; Viiri, J.; et al. Pinosylvin Extract Retinari™ Sustains Electrophysiological Function, Prevents Thinning of Retina, and Enhances Cellular Response to Oxidative Stress in NFE2L2 Knockout Mice. Oxid. Med. Cell. Longev. 2021, 2021, 8028427. [Google Scholar] [CrossRef]
- Song, J.; Seo, Y.; Park, H. Pinosylvin Enhances Leukemia Cell Death via Down-Regulation of AMPKα Expression: Anti-Cancer Activity of Pinosylvin. Phytother. Res. 2018, 32, 2097–2104. [Google Scholar] [CrossRef]
- Jina, S.; Jinsun, P.; Eunsil, J.; A-Young, S.; Jaeho, P.; Heonyong, P. Apoptotic Effect of Pinosylvin at a High Concentration Regulated by C-Jun N-Terminal Kinase in Bovine Aortic Endothelial Cells. J. Life Sci. 2015, 25, 416–424. [Google Scholar] [CrossRef] [Green Version]
- Skinnider, L.; Stoessl, A. The Effect of the Phytoalexins, Lubimin, (−)-Maackiain, Pinosylvin, and the Related Compounds Dehydroloroglossol and Hordatine M on Human Lymphoblastoid Cell Lines. Experientia 1986, 42, 568–570. [Google Scholar] [CrossRef]
- Simard, F.; Legault, J.; Lavoie, S.; Mshvildadze, V.; Pichette, A. Isolation and Identification of Cytotoxic Compounds from the Wood of Pinus resinosa. Phytother. Res. 2008, 22, 919–922. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Hsieh, M.-C.; Lin, C.-C.; Lo, Y.-S.; Ho, H.-Y.; Hsieh, M.-J.; Lin, J.-T. Pinosylvin Inhibits Migration and Invasion of Nasopharyngeal Carcinoma Cancer Cells via Regulation of Epithelial-mesenchymal Transition and Inhibition of MMP-2. Oncol. Rep. 2021, 46, 143. [Google Scholar] [CrossRef]
| Species | Extract/Essential Oil | References |
|---|---|---|
| Pinus sylvestris | Extract | [13,24,25,26,27,28,29,30,31,32,33] |
| Pinus resinosa | Extract | [9,28,31,34] |
| Pinus banksiana | Extract | [26,28,31] |
| Pinus nigra Arn. | Extract | [25,30] |
| Pinus densiflora | Extract | [35,36] |
| Pinus sibirica | Extract | [37] |
| Pinus contorta | Extract | [37] |
| Pinus strobus | Extract | [38,39,40] |
| Pinus taeda | Extract | [41] |
| Pinus cembra | Extract | [37] |
| Pinus pinaster | Extract | [42,43,44] |
| Hovenia dulcis Thunb. | Extract | [45] |
| Picea glauca | Extract | [9] |
| Nothofagus (Southern beeches) | Extract | [46,47] |
| Stemona cf. pierrei | Extract | [48] |
| Arachis hypogaea | Extract | [49] |
| Experimental Approaches | Key Results | References |
|---|---|---|
| Western blot analysis and reverse transcription-polymerase chain reaction (RT-PCR) | Inhibited COX-2, iNOS protein and gene expression | [76] |
| Murine adipocytes model, cytotoxicity assays, lipid staining, western blotting, and ELISA assays | Attenuated adipogenesis and inflammation through downregulation of the expression of PPARγ, C/EBP and TNF-a-stimulated IL-6 secretion | [14] |
| Cell viability and RNA interference analysis | Protected (10 µM) cell survival from oxidative damage by promoting HO-1 induction | [77] |
| LPS-induced mouse macrophage RAW 264.7 cells | Suppressed COX-2-mediated PGE2 production (IC50 = 10.6 µM) | [78] |
| LPS-stimulated Macrophage cells Western blot analysis RT-PCR analysis | Inhibited LPS-induced iNOS protein and mRNA expression in dose-dependent manner (IC50 = 39.9 μM) Decreased the expression levels of interferon regulatory factor 3 (IRF-3) and interferon-E (IFN-E) | [79] |
| AITC-induced acute paw inflammation in mice model Fluo-3-AM assay and patch clamping | Reduced paw inflammation formation by inhibiting and attenuating IL-6 production at the site of inflammation | [15] |
| Adjuvant-induced arthritis in rats | Pinosylvin + MTX reduced oxidative stress by upregulating HO-1 expression in lungs and reducing plasma activity of thiobarbituricacid reactive substances (TBARS) and lipoxygenase (LOX) in the lungs | [16] |
| Primary cultures of human OA chondrocytes | Increased aggrecan expression Inhibited IL-6 production by attenuating NF-κB activity | [13] |
| AA in rats Chemiluminescence (CL) of the joint and myeloperoxidase (MPO) activity | Decreased HPV Reduced CL of the joint and MPO activity of the joint homogenate | [80] |
| Carrageenan-induced paw edema in male C57BL/6 mice | Reduced inflammatory response by downregulating the production of inflammatory cytokines IL6, MCP1, and NO | [11] |
| AA was induced in Lewis rats Fresh human blood neutrophils as model | Reduced the formation of oxidants, both extra- and intra-cellular Suppressed PKC activation induced by phorbol myristate acetate Reduced neutrophil countDecreased the amount of ROS (in vivo) | [81] |
| LPS-triggered apoptosis in the leukocyte | Enhanced apoptosis of LPS-preconditioned leukocytes via decreasing ALOX 15 expression mediated by ERK and JNK pathways | [82] |
| Humane monocytic THP-1 cell lines Western blotting analysis | Suppressed proinflammatory enzymes TNF-α and IL-8 by the inhibition of NF-κB activation | [66] |
| Murine and U937 Human macrophages model qRT-PCR and ELISA | Changed macrophage polarization from the proinflammatory M1 phenotype to the M2 phenotype Promoted resolution of inflammation and repair Enhanced PPAR-γ expression in IL-4 treated macrophages | [12] |
| LPS-induced mouse macrophage RAW 264.7 cells | Decreased inflammation on LPS-stimulated macrophages Inhibited PPARγ activity in vitro | [83] |
| Antigen-stimulated mast cell-like cell line rat basophilic leukemia (RBL)-2H3 and a passive cutaneous anaphylaxis (PCA) mouse model Degranulation assay RT-PCR, PCA Western blot analyses | Suppressed the release and expression of allergic and proinflammatory key enzymes (IL-4, TNF-α and PGE2, COX-2, NFKB1, and NFKB2) in a dose-dependent manner | [45] |
| Origins | Cell Lines | Methods | Key Findings | References |
|---|---|---|---|---|
| Synthesized | Mouse model of oligoasthenospermia | Epididymal sperm concentration and motility evaluation Hormone level assessment Real-time PCR Western blot analysis Evaluation of the testicular levels of ROS and MDA | Decreased oxidative stress through glutathione peroxidase 3 drastically reduced oxidative stress (in vivo) by inhibiting the nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway | [19] |
| Purchased | WT and NFE2L2 KO(NFE2L2−/−) mice strain | ERG recording and processing of signals OCT imaging Antioxidant capacity analysis Immunohistochemical staining Confocal imaging | Retained retinal function Decreased accumulation of ubiquitin-tagged proteins Decreased chronic oxidative stress Preserved retinal function and morphology in the NFE2L2 KO disease model Reduced the risk of age-related macular degeneration (AMD) and halted its development | [85] |
| Purchased | In vitro non-enzymatic assays | ORAC-FL assay ABTS assay FRAP assay | Strong antioxidant and free radical scavenging properties | [18] |
| Synthetized | AA model induced in Lewis rats | Oral administration of pinosylvin to AA induced animals Monitoring of the hind paw volume Monitoring of the luminol-enhanced chemiluminescence (CL) of the joint Monitoring of myeloperoxidase (MPO) activity in hind paw joint | Reduced HPV (at days 14 and 28) Reduced joint CL and MPO activity in joint homogenate | [80] |
| Not reported | Human retinal pigment epithelial cells (ARPE-19) | Toxicity assessment Oxidative stress assessment MTT assay Real-time PCR Nrf2 and p62 RNA interference | Improved cell viability against oxidative stress (5 and 10 µM) Validated the importance of Nrf2 and HO-1 in pinosylvin-mediated protection against oxidative stress | [77] |
| Synthesized | Bovine aortic endothelial cells (BAECs) | Measurement of apoptosis Measurement of caspase-3 activity Cell proliferation Western blot analysis Cell migrationAdhesion of THP-1 to BAECs | Activated endothelial nitric oxide synthase Impacted cell proliferation in endothelial cells Stimulated cell migration and tube formation Avoided inflammatory cardiovascular disorders | [1] |
| Synthesized | AA model induced in rats | Formation of reactive oxygen species Western blot analysis Measurement of ATP liberation Flow cytometry Effects of pinosylvin on arthritis | Reduced both extracellular and intracellular oxidant generation in isolated human neutrophils Inhibited PKC activation triggered by phorbol myristate acetate Increased the number of neutrophils in the blood of arthritic rats Improved whole blood chemiluminescence (both spontaneous and PMA-stimulated) Reduced the number of neutrophils and the number of reactive oxygen species in the blood | [81] |
| Not reported | AA model induced in rats | 28 days of oral administration Changes in hind paw volume and arthrogram evaluation γ-glutamyltransferase (GGT) activity assessment Measurement of thiobarbituric acid reactive substances (TBARS) | Decreased the activity of GGT in the spleen Reduced the activity of GGT in joint tissue Exhibited moderate efficacy in preventing oxidative damage | [17] |
| Synthetized | AA model induced in rats | Assessment of hind paw volume Measurement of the C-reactive protein Monocyte chemotactic protein-1 (MCP-1) level measurement Plasma levels of thiobarbituric acid reactive substances (TBARS) and F2-isoprostanes measurement G-glutamyltransferase and lipoxygenase (LOX) activity evaluation | Increased NF-κB activation in the liver and lung, HO-1 expression and LOX activity in the lung, MCP-1 levels in plasma, and F2-isoprostane plasmatic levels Reduced the OS (an increase of HO-1 expression in the lung and reduction in plasmatic TBARS) Decreased the LOX activity in the lung | [16] |
| Origins | Cell Lines | Methods | Key Findings | References |
|---|---|---|---|---|
| Not clear | THP1 and U937 monocytic cell lines | Trypan blue exclusion assay Cell sorting analysis RT-PCR Preparation of cell lysates Western blot analysis Detection of LC3 puncta DNA transfection | Increased (50–100 μmol/L) cell death Caused caspase-3 activation, phosphatidylserine flipping, LC3II accumulation, LC3 puncta, and p62 degradation Induced cell death Caused downregulation of AMP-activated protein kinase (AMPK) α1 | [86] |
| Not reported | Bovine aortic endothelial cells | Apoptosis assay Western blot analysis Flow cytometry analysis Measurement of caspase-3 activity | Increased caspase-3 activity, phosphatidylserine flip-flop, and nuclear fragmentation Activated JNK and endothelial NO synthase | [87] |
| Synthesized | Molt and Raji lymphoblastoid cell lines. | Growth inhibitory action Cell count and viability DNA and protein synthesis assessment | Inhibited cell proliferation Inhibited [3H] thymidine and leucine uptake | [88] |
| Pinus resinosa | A549, DLD-1, and WS1 cells | Cytotoxicity assay | 66 ± 10 < IC50 < 75 ± 14 μM | [89] |
| Synthesized | HCT 116 colorectal cancer cells | Proliferation inhibitory potential testing Cell cycle distribution analysis Western blot analysis RT-PCR Identification of gene expression cDNA microarray Electrophoretic mobility shift assay | Slowed cell growth Slowed cell cycle transition from G1 phase to S phase Decreased the levels of cyclin D1, cyclin E, CDK2, c-Myc, pRb, and p53 Stopped the activation of proteins involved in the FAK/c-Src/ERK signaling pathway and the PI3K/Akt/GSK-3b signaling pathway Inhibited b-nuclear catenin translocation | [21] |
| Synthesized | HT1080 human fibrosarcoma and Balb/c mice | RT-PCR Wound healing assay Colony dispersion assessement In vivo pulmonary metastasis method Gelatin zymography | Inhibited the production of matrix metalloproteinase (MMP)-2, MMP-9, and membrane type 1-MMP Reduced HT1080 cell migration Slowed tumor nodule growth and tumor weight in lung tissue Downregulated MMP-9 and cyclooxygenase-2 (COX-2) expression and ERK1/2 and Akt phosphorylation in lung carcinoma tissues | [5] |
| Not reported | ARPE-19 cells | Toxicity evaluation Oxidative stress assessment Cell viability RT-PCR Nrf2 and p62 RNA interference | Improved cell viability in the face of oxidative stress Increased HO-1 expression No effect on Nrf2 expression Protected against oxidative damage | [77] |
| Purchased | LNCaP-par and LNCaP-abl prostate cancer cells | High-throughput screening (HTS) Cell viability and apoptosis assays Gene expression analysisqPT-PCR | Inhibited androgen signaling and intracellular steroidogenesis in CRPC cells | [23] |
| Purchased | Nasal cavity cancer cells (RPMI 2650) | MTT assay Gap closure assay Cell migration assay Cell invasion assay Western blot analysis Proteome profiler human protease array | Suppressed migration and invasion of NPC039 and NPCBM cells at increasing doses Lowered the protein expression levels of MMP2 and MMP9 Decreased the enzyme activity of MMP2Reduced vimentin and N-cadherin expression (in NPC cells) Increased zonula occludens-1 and E-cadherin expression Inhibited NPC039 and NPCBM cell invasion and migration by modulating the p38, ERK1/2, and JNK1/2 pathways Inhibited NPC cell migration and invasion | [20] |
| Purchased | SCC-9 and HSC-3 cancer cells (tongue squamous) | MTT assay Wound closure assessment Gelatin zymography Cell migration and invasion evaluation Western blot analysis | Decreased the enzymatic activity of MMP-2 and lowered its protein level Raised the expression of TIMP-2 Stopped cancer cell growth in a wound-healing experiment Reduced ERK1/2 protein phosphorylation in SAS and SCC-9 cells | [20] |
| Not reported | Bovine aortic endothelial cells | Apoptosis experiment Western blot analysis Flow cytometry Measurement of caspase-3 activity Hoechst staining | Induced (100 μmol/L) cell death Boosted caspase-3 activation, nuclear condensation, and the “flip-flop” of phosphatidylserine (at high concentrations) Inhibited necrosis Promoted LC3 conversion from LC3-I to LC3-II and p62 degradation Stimulated AMP-activated protein kinase (AMPK) and an AMPK inhibitor Reversed the inhibitory impact of an AMPK inhibitor Induced autophagy via AMPK activation | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakrim, S.; Machate, H.; Benali, T.; Sahib, N.; Jaouadi, I.; Omari, N.E.; Aboulaghras, S.; Bangar, S.P.; Lorenzo, J.M.; Zengin, G.; et al. Natural Sources and Pharmacological Properties of Pinosylvin. Plants 2022, 11, 1541. https://doi.org/10.3390/plants11121541
Bakrim S, Machate H, Benali T, Sahib N, Jaouadi I, Omari NE, Aboulaghras S, Bangar SP, Lorenzo JM, Zengin G, et al. Natural Sources and Pharmacological Properties of Pinosylvin. Plants. 2022; 11(12):1541. https://doi.org/10.3390/plants11121541
Chicago/Turabian StyleBakrim, Saad, Hamza Machate, Taoufiq Benali, Nargis Sahib, Imane Jaouadi, Nasreddine El Omari, Sara Aboulaghras, Sneh Punia Bangar, José Manuel Lorenzo, Gokhan Zengin, and et al. 2022. "Natural Sources and Pharmacological Properties of Pinosylvin" Plants 11, no. 12: 1541. https://doi.org/10.3390/plants11121541
APA StyleBakrim, S., Machate, H., Benali, T., Sahib, N., Jaouadi, I., Omari, N. E., Aboulaghras, S., Bangar, S. P., Lorenzo, J. M., Zengin, G., Montesano, D., Gallo, M., & Bouyahya, A. (2022). Natural Sources and Pharmacological Properties of Pinosylvin. Plants, 11(12), 1541. https://doi.org/10.3390/plants11121541











