Environmental Gradients Shaping the Freshwater Bryophyte Communities of Croatia (Western Balkans)
Abstract
1. Introduction
2. Results
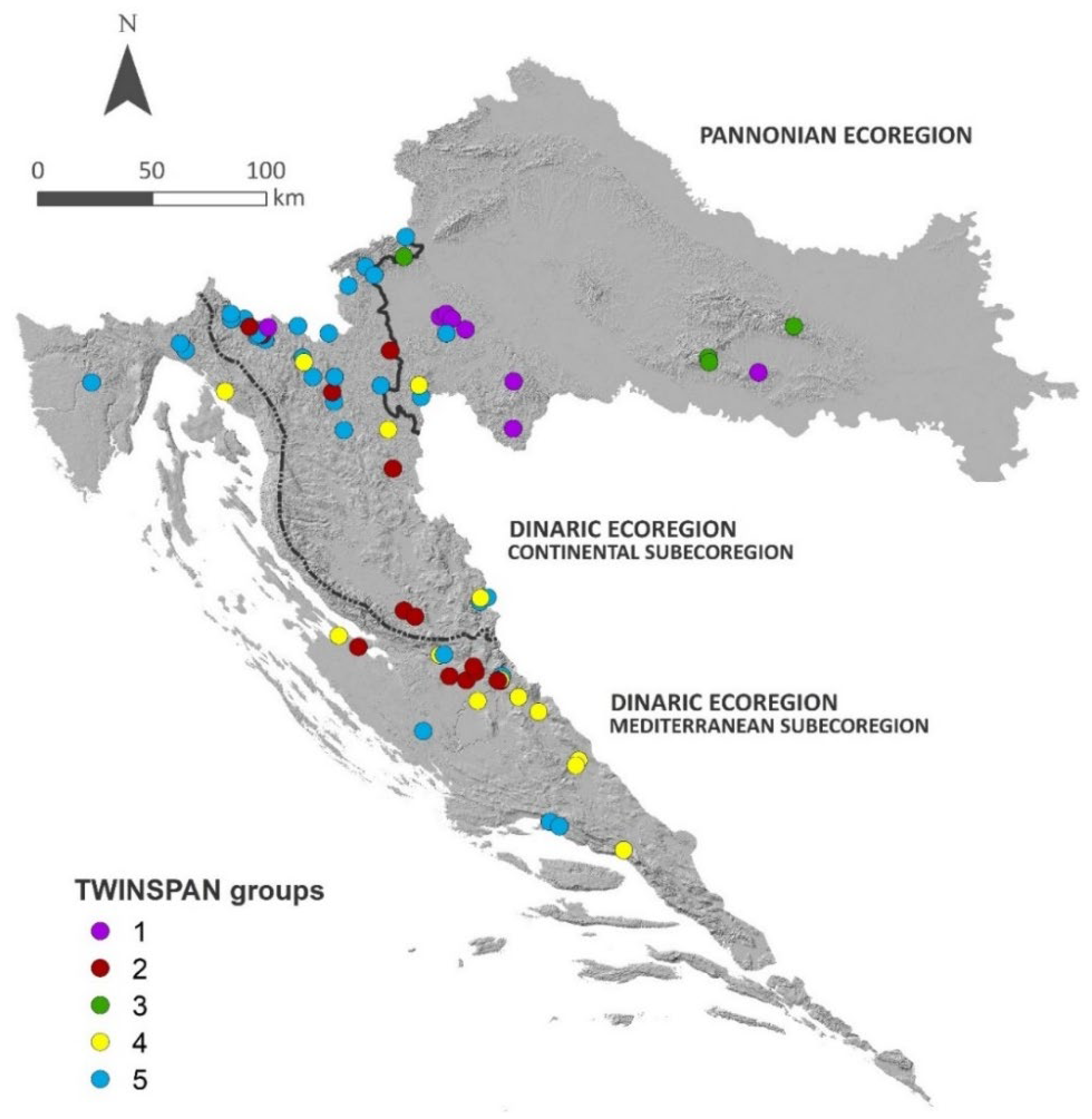
2.1. Community Groups
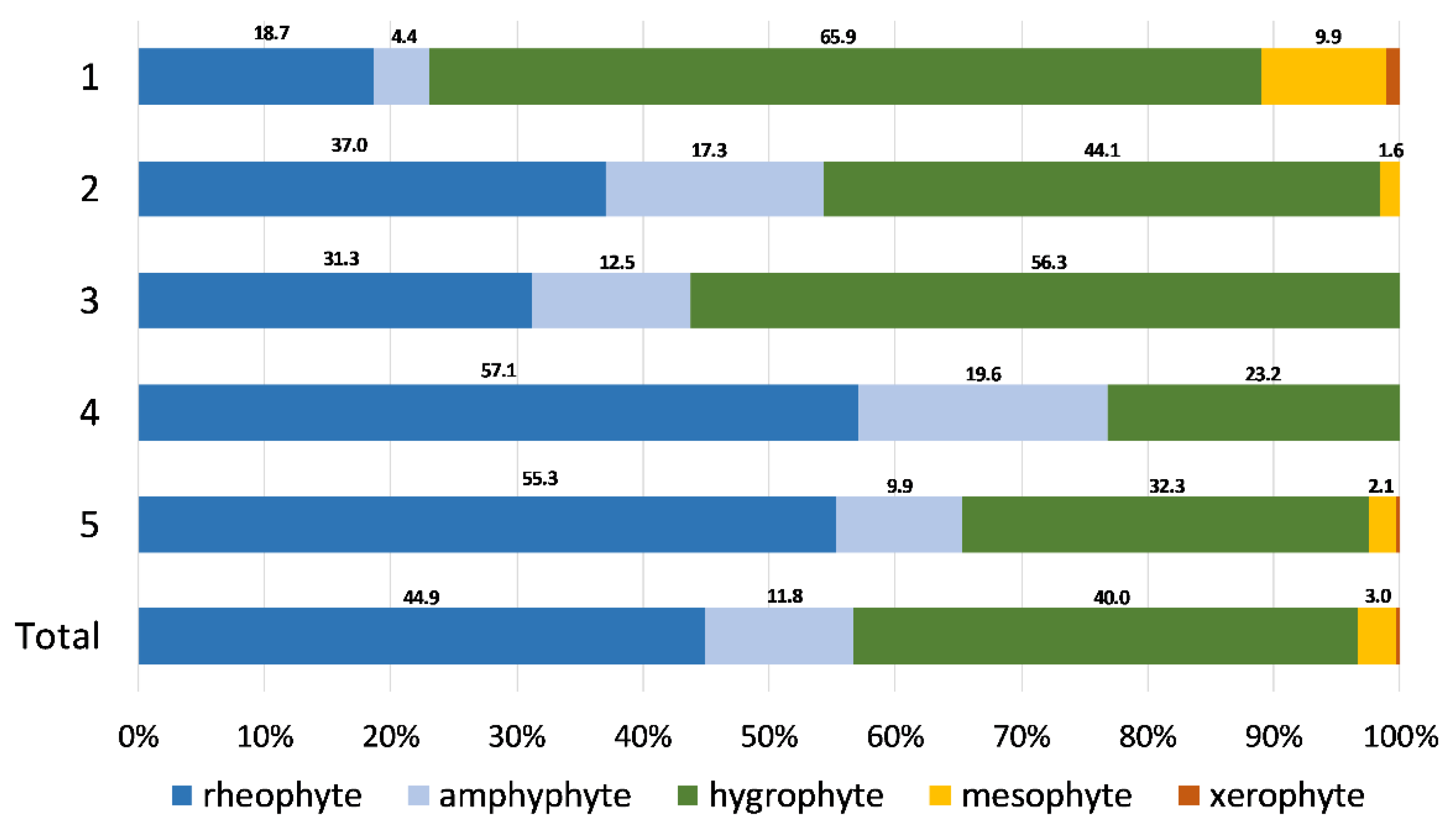
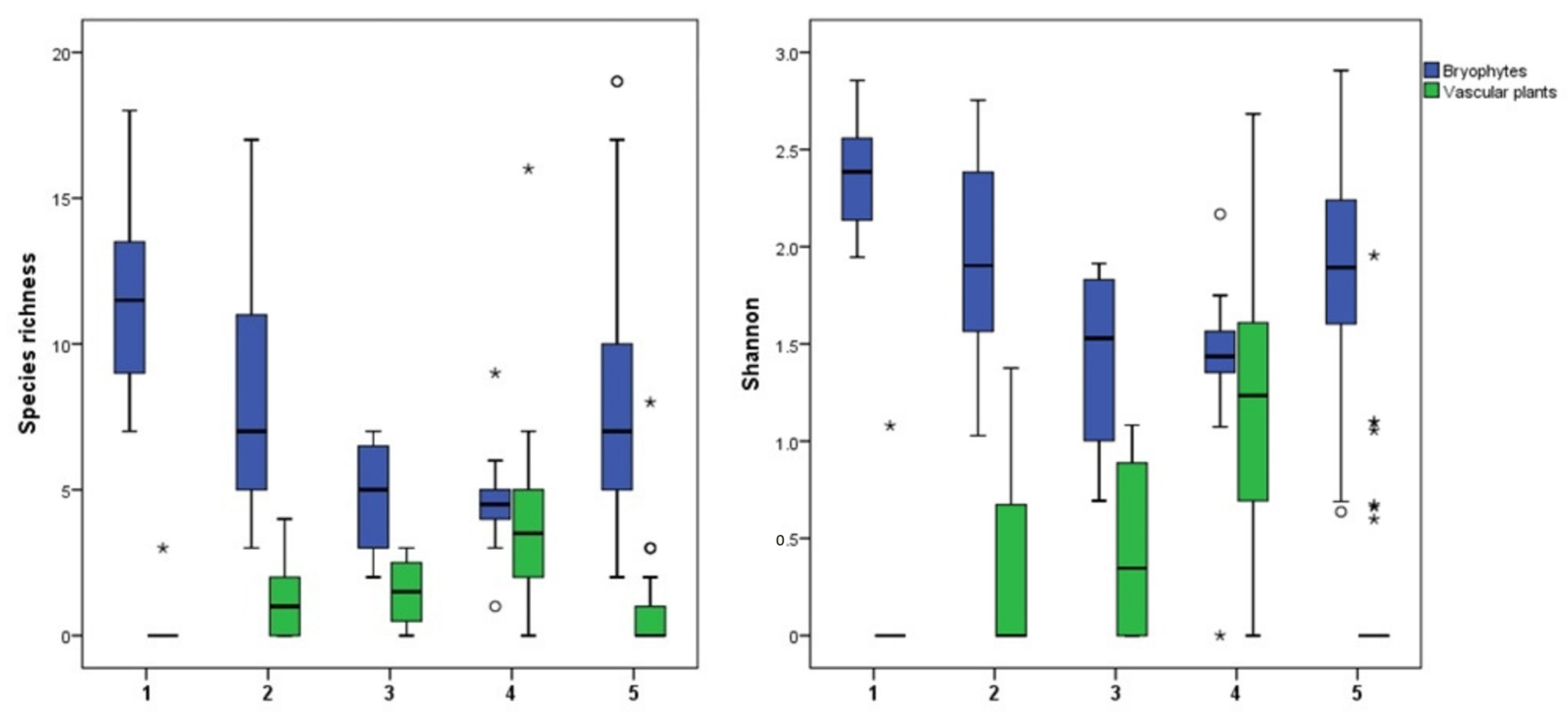
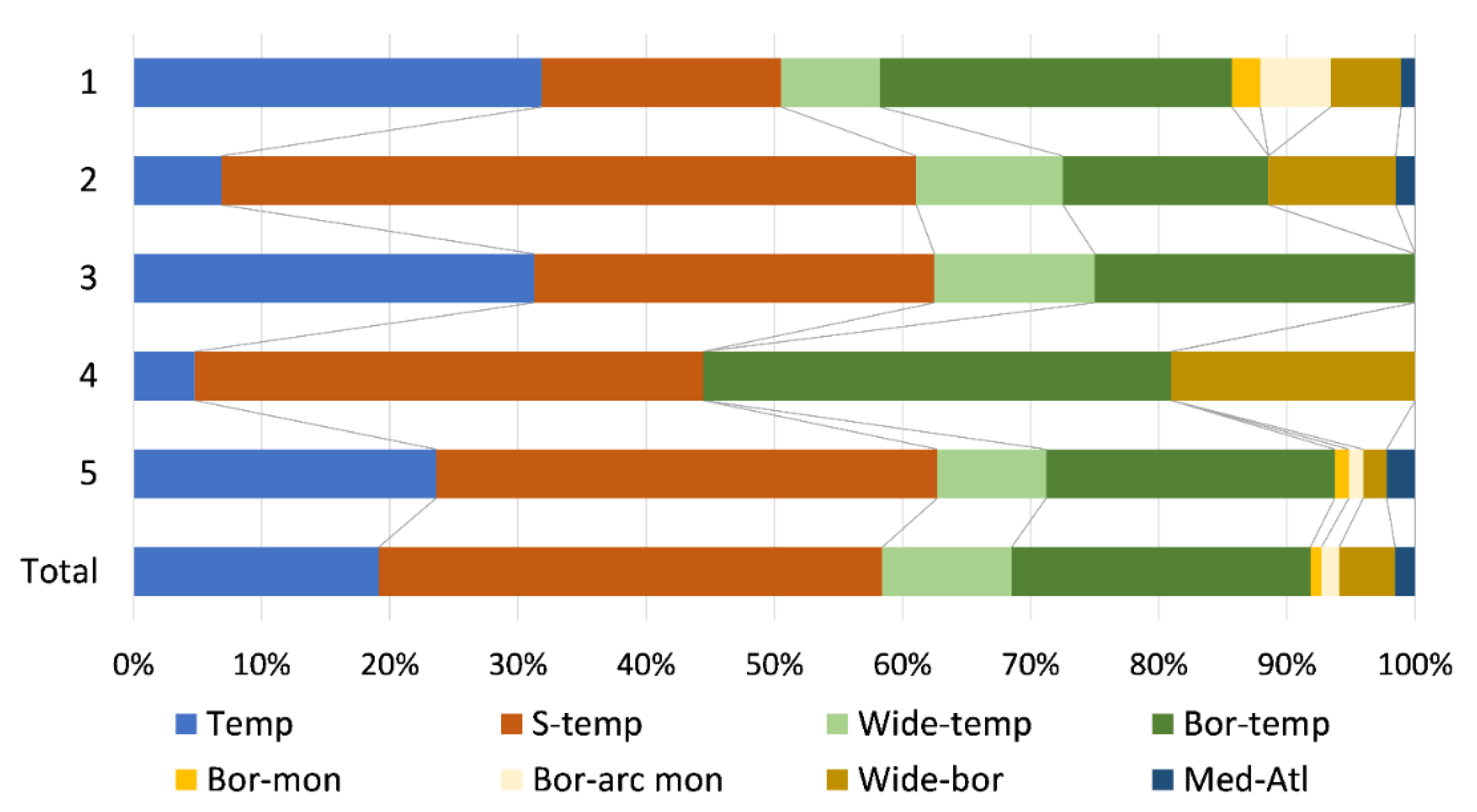
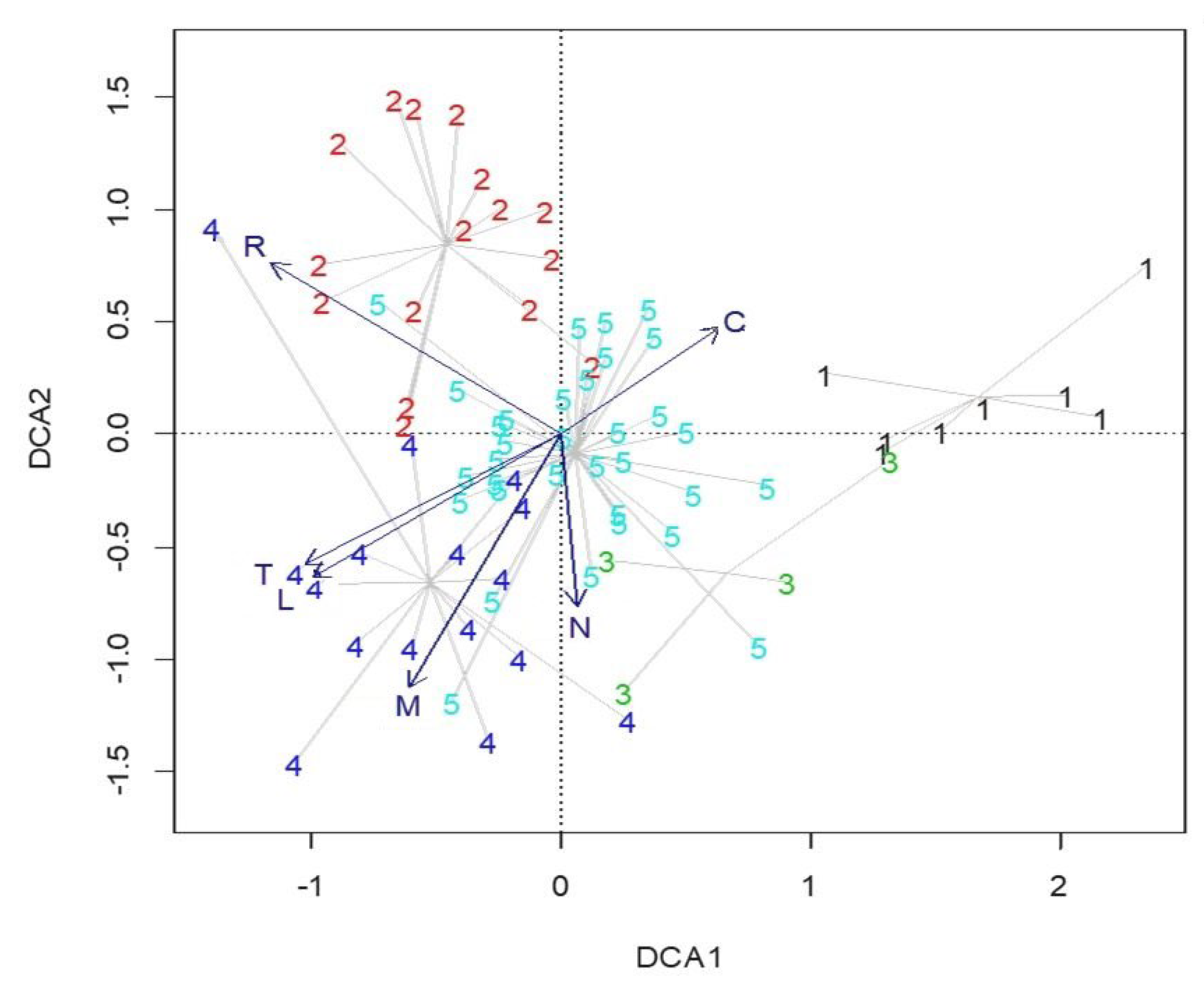
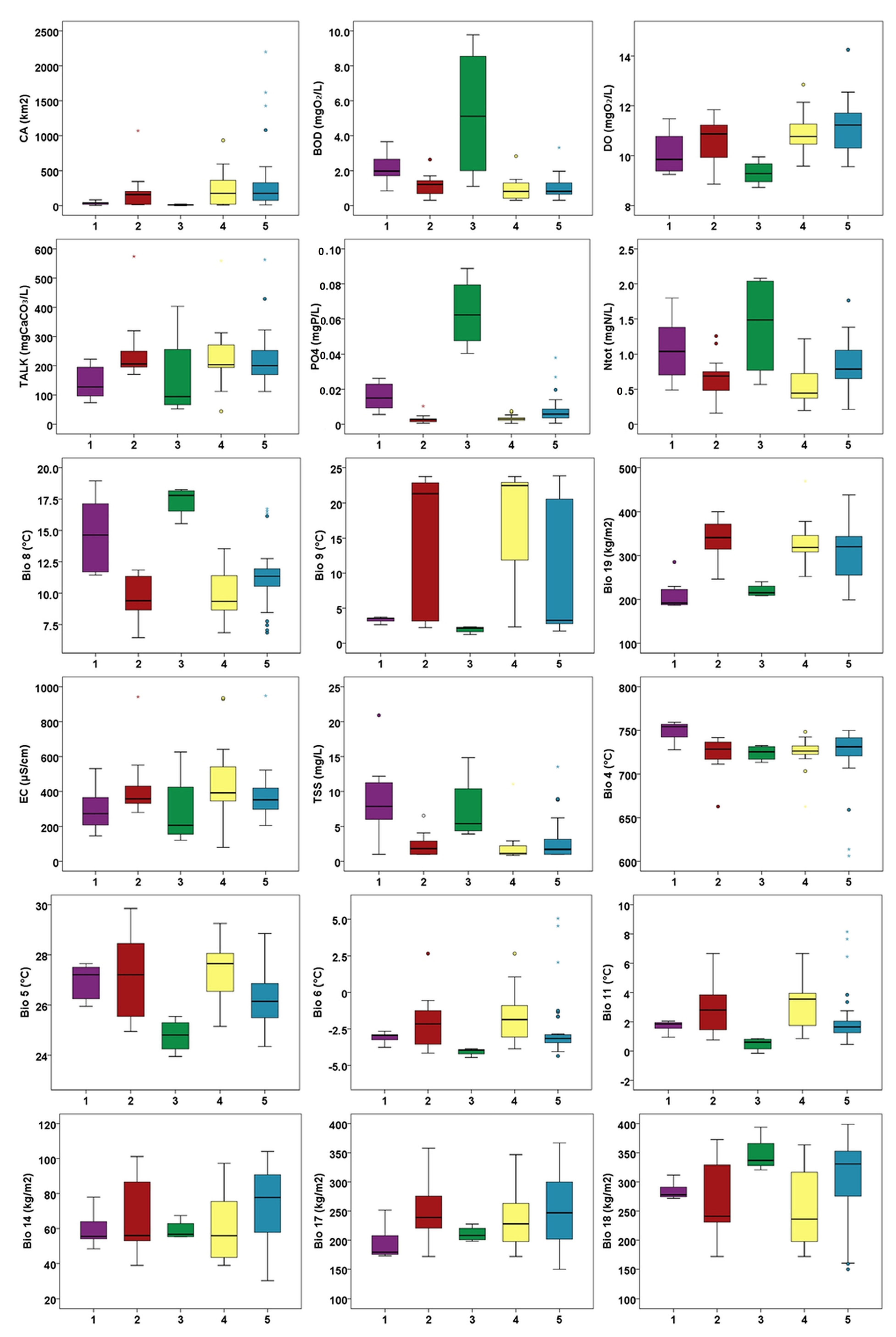
2.2. Environmental Gradients
3. Discussion
4. Materials and Methods
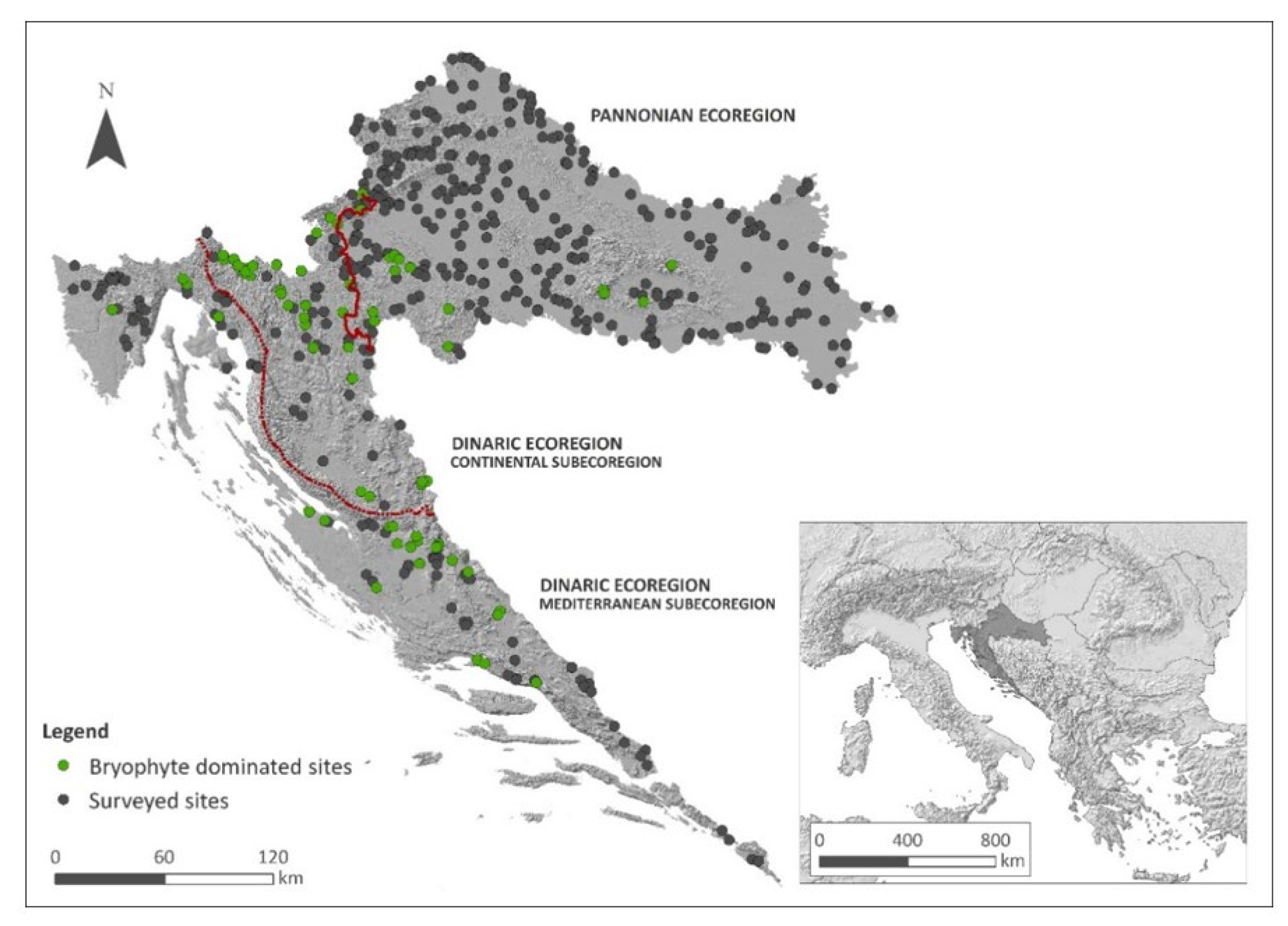
4.1. Study Area
4.2. Macrophyte Vegetation Sampling
4.3. Environmental Data Sampling and Collection
| Environmental Variable | Abbreviation | |
|---|---|---|
| Water physicochemical parameters | Water temperature | T (°C) |
| Water pH | pH | |
| Electrical conductivity | EC (μS/cm) | |
| Total suspended solids | TSS (mg/L) | |
| Dissolved oxygen | DO (mgO₂/L) | |
| Total alkalinity | TALK (mgCaCO₃/L) | |
| Biochemical oxygen demand | BOD (mgO₂/L) | |
| Water chemical parameters | Ammonium | NH₄+ (mgN/L) |
| Nitrites | NO2− (mgN/L) | |
| Nitrates | NO3− (mgN/L) | |
| Total nitrogen | Ntot (mgN/L) | |
| Orthophosphates | PO43− (mgP/L) | |
| Total phosphorus | Ptot (mgP/L) | |
| Physiographical variables | Altitude | Alt (m a.s.l.) |
| Catchment area | CA (km2) | |
| Distance from the source | DFS (m) | |
| Climatic variables | Mean annual air temperature | Bio1 (°C) |
| Mean diurnal air temperature range | Bio2 (°C) | |
| Isothermality | Bio3 (°C) | |
| Temperature seasonality | Bio4 (°C) | |
| Max temperature of the warmest month | Bio5 (°C) | |
| Min temperature of the coldest month | Bio6 (°C) | |
| Temperature annual range | Bio7 (°C) | |
| Mean temperature of wettest quarter | Bio8 (°C) | |
| Mean temperature of driest quarter | Bio9 (°C) | |
| Mean temperature of warmest quarter | Bio10 (°C) | |
| Mean temperature of coldest quarter | Bio11 (°C) | |
| Annual precipitation | Bio12 (kg/m2) | |
| Precipitation of wettest month | Bio13 (kg/m2) | |
| Precipitation of driest month | Bio14 (kg/m2) | |
| Precipitation seasonality | Bio15 (kg/m2) | |
| Precipitation of wettest quarter | Bio16 (kg/m2) | |
| Precipitation of driest quarter | Bio17 (kg/m2) | |
| Precipitation of warmest quarter | Bio18 (kg/m2) | |
| Precipitation of coldest quarter | Bio19 (kg/m2) |
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Synoptic Table with Percentage Frequency and Modified Fidelity Index φ-Coefficients
| TWINSPAN Group | 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Relevés | 8 | 16 | 4 | 15 | 33 | |||||
| Oxyrrhynchium hians (Hedw.) Loeske | 100 | 94.6 | . | --- | . | --- | . | --- | 9 | --- |
| Pellia neesiana (Gottsche) Limpr. | 50 | 66.7 | . | --- | . | --- | . | --- | . | --- |
| Conocephalum salebrosum Szweyk., Buczk. & Odrzyk. | 62 | 63 | . | --- | . | --- | . | --- | 18 | 2.8 |
| Fissidens taxifolius Hedw. | 50 | 61.3 | 6 | --- | . | --- | . | --- | . | --- |
| Chiloscyphus pallescens (Ehrh.) Dumort. | 50 | 58.7 | . | --- | . | --- | 7 | --- | 3 | --- |
| Plagiomnium undulatum (Hedw.) T. J. Kop. | 50 | 57 | . | --- | . | --- | . | --- | 12 | --- |
| Dichodontium pellucidum (Hedw.) Schimp. | 38 | 51 | . | --- | . | --- | . | --- | 6 | --- |
| Pohlia melanodon (Brid.) A. J. Shaw | 38 | 41.5 | 19 | --- | . | --- | . | --- | . | --- |
| Hypnum cupressiforme Hedw. | 25 | 45.9 | . | --- | . | --- | . | --- | . | --- |
| Plagiomnium ellipticum (Brid.) T. J. Kop. | 25 | 42.2 | --- | . | --- | . | --- | 3 | --- | |
| Dichodontium flavescens (Dicks.) Lindb. | 25 | 36.1 | . | --- | . | --- | . | --- | 9 | --- |
| Didymodon tophaceus (Brid.) Lisa | . | --- | 50 | 64 | . | --- | . | --- | 3 | --- |
| Eucladium verticillatum (With.) Bruch & Schimp. | . | --- | 44 | 59.1 | . | --- | . | --- | 3 | --- |
| Apopellia endiviifolia (Dicks.) Nebel & D. Quandt | . | --- | 75 | 49.6 | . | --- | 40 | --- | 33 | --- |
| Fissidens crassipes Wilson ex Bruch & Schimp. | 12 | --- | 69 | 48.8 | . | --- | . | --- | 48 | 25.7 |
| Funaria hygrometrica Hedw. | . | --- | 25 | 42.2 | . | --- | . | --- | 3 | --- |
| Rhynchostegium riparioides (Hedw.) Cardot | 75 | --- | 81 | --- | 75 | --- | 60 | --- | 94 | 20.1 |
| Cratoneuron filicinum (Hedw.) Spruce | 50 | --- | 62 | --- | 50 | --- | 73 | --- | 61 | --- |
| Ptychostomum pseudotriquetrum (Hedw.) J. R. Spence & H. P. Ramsay ex Holyoak & N. Pedersen | 50 | --- | 50 | 31.8 | . | --- | 7 | --- | 9 | --- |
| Leptodictyum riparium (Hedw.) Warnst. | 50 | 33.2 | . | --- | . | --- | 13 | --- | 48 | 31.3 |
| Cinclidotus fontinaloides (Hedw.) P. Beauv. | . | --- | 56 | --- | . | --- | 33 | --- | 64 | 35.8 |
| Fontinalis antipyretica Hedw. | 25 | --- | 44 | --- | . | --- | 67 | --- | 61 | --- |
| Brachythecium rutabulum (Hedw.) Schimp. | 38 | 24.1 | . | --- | 50 | 40.1 | . | --- | 6 | --- |
| Fissidens pusillus (Wilson) Milde | 38 | 23.9 | . | --- | 50 | 40.0 | 7 | --- | . | --- |
| Veronica beccabunga L. | . | --- | . | --- | 50 | 66.7 | . | --- | . | --- |
| Persicaria dubia (Stein) Fourr. | . | --- | . | --- | 50 | 66.7 | . | --- | . | --- |
| Oxyrrhynchium speciosum (Brid.) Warnst. | 25 | --- | . | --- | 50 | 47.4 | . | --- | 3 | --- |
| Marchantia polymorpha L. | 12 | --- | 12 | --- | 50 | --- | 7 | --- | 33 | 12.3 |
| Hygroamblystegium tenax (Hedw.) Jenn. | . | --- | . | --- | 25 | . | --- | 3 | --- | |
| Brachythecium rivulare Schimp. | 12 | --- | . | --- | 25 | --- | . | --- | 27 | 21.3 |
| Juncus buffonius L. | . | --- | . | --- | 25 | --- | . | --- | . | --- |
| Mentha aquatica L. | . | --- | 6 | --- | 25 | --- | 60 | 52.6 | 3 | --- |
| Chiloscyphus polyanthos (L.) Corda | 38 | --- | 6 | --- | . | --- | 47 | 29.2 | 21 | --- |
| Berula erecta (Huds.) Coville | . | --- | 6 | --- | . | --- | 47 | 52 | 9 | --- |
| Sparganium erectum L. | . | --- | . | --- | . | --- | 27 | 47.5 | . | --- |
| Agrostis stolonifera L. | . | --- | 25 | --- | 25 | --- | 33 | --- | 15 | --- |
| Vaucheria A.P.de Candolle sp. | . | --- | . | --- | 25 | --- | 13 | --- | 9 | --- |
| Lythrum salicaria L. | 12 | --- | . | --- | 25 | --- | 20 | --- | 6 | --- |
| Rorippa sylvestris (L.) Besser | . | --- | . | --- | 25 | --- | 13 | --- | 3 | --- |
| Cinclidotus riparius (Host ex Brid.) Arn. | 12 | --- | 25 | --- | . | --- | 7 | --- | 64 | 51.2 |
| Cinclidotus aquaticus (Hedw.) Bruch & Schimp. | . | --- | 19 | --- | . | --- | 40 | --- | 58 | 40.6 |
| Cladophora glomerata (Linnaeus) Kützing | . | --- | 19 | --- | 25 | --- | 13 | --- | 33 | 19.8 |
| Hygroamblystegium varium (Hedw.) Mönk. | 25 | --- | 6 | --- | . | --- | . | --- | 6 | --- |
| Heribaudiella fluviatilis (Areschoug) Svedelius | 25 | --- | . | --- | . | --- | 7 | --- | 6 | --- |
| Mnium spinulosum Bruch & Schimp. | 12 | --- | . | --- | . | --- | . | --- | . | --- |
| Fissidens bryoides Hedw. | 12 | --- | . | --- | . | --- | . | --- | . | --- |
| Plagiomnium elatum (Bruch et Schimp.) T. J. Kop. | 12 | --- | . | --- | . | --- | . | --- | . | --- |
| Brachythecium mildeanum (Schimp.) Schimp | 12 | --- | . | --- | . | --- | . | --- | . | --- |
| Hygroamblystegium humile (P.Beauv.) Vanderp., Goffinet & Hedenäs | 12 | --- | . | --- | . | --- | . | --- | . | --- |
| Bryum ruderale Crundw. & Nyholm | 12 | --- | . | --- | . | --- | . | --- | . | --- |
| Leersia oryzoides (L.) Sw. | 12 | --- | . | --- | . | --- | . | --- | . | --- |
| Scirpoides holoschoenus (L.) Soják L. | . | --- | 19 | 31.1 | . | --- | 7 | --- | . | --- |
| Batrachospermum Roth sp. | . | --- | 19 | 31.1 | . | --- | 7 | --- | . | --- |
| Calliergonella 24ectinate (Hedw.) Loeske | . | --- | 19 | --- | . | --- | . | --- | 9 | --- |
| Spirogyra Link. Sp. | . | --- | 19 | --- | . | --- | . | --- | 9 | --- |
| Jungermannia atrovirens Dumort. | 12 | --- | 25 | --- | . | --- | 7 | --- | 6 | --- |
| Chara vulgaris L. | . | --- | 19 | 35.3 | . | --- | . | --- | 3 | --- |
| Dicranella varia (Hedw.) Schimp. | . | --- | 12 | 32 | . | --- | . | --- | . | --- |
| Palustriella 24ectinate (Hedw.) Ochyra | . | --- | 12 | 22.6 | . | --- | 7 | --- | . | --- |
| Palustriella falcata (Brid.) Hedenäs | . | --- | 12 | 9.3 | . | --- | 13 | 10.8 | 12 | 8.6 |
| Oenanthe fistulosa L. | . | --- | 12 | --- | . | --- | 13 | --- | . | --- |
| Didymodon fallax (Hedw.) R. H. Zander | . | --- | 12 | --- | . | --- | . | --- | 15 | --- |
| Deschampsia cespitosa (L.) P. Beauv. | . | --- | 6 | --- | . | --- | 7 | --- | 9 | --- |
| Hygrohypnum luridum (Hedw.) Jenn. | . | --- | 6 | --- | . | --- | . | --- | 9 | --- |
| Oxyrrhynchium schleicheri (R. Hedw.) Röll | . | --- | 6 | --- | . | --- | . | --- | . | --- |
| Riccia fluitans L. | . | --- | 6 | --- | . | --- | . | --- | . | --- |
| Veronica longifolia L. | . | --- | 6 | --- | . | --- | . | --- | . | --- |
| Fissidens adianthoides Hedw. | 12 | --- | 6 | --- | . | --- | . | --- | . | --- |
| Gymnostomum aeruginosum Sm. | . | --- | 6 | --- | . | --- | . | --- | . | --- |
| Philonotis marchica (Hedw.) Brid. | . | --- | 6 | --- | . | --- | . | --- | . | --- |
| Bryum dichotomum Hedw. | . | --- | 6 | --- | . | --- | . | --- | . | --- |
| Alisma lanceolatum With. | . | --- | 6 | --- | . | --- | 7 | --- | . | --- |
| Mentha longifolia (L.) L. | . | --- | 6 | --- | . | --- | 7 | --- | . | --- |
| Iris pseudacorus L. | . | --- | 6 | --- | . | --- | 7 | --- | . | --- |
| Bolboschoenus maritimus (L.) Palla | . | --- | 6 | --- | . | --- | . | --- | . | --- |
| Juncus compressus Jacq. | . | --- | 6 | --- | . | --- | . | --- | . | --- |
| Brachythecium salebrosum (Hoffm. Ex F.Weber & D.Mohr) Schimp. | . | --- | 6 | --- | . | --- | . | --- | . | --- |
| Poa palustris L. | . | --- | 6 | --- | . | --- | . | --- | . | --- |
| Fissidens arnoldii R. Ruthe | . | --- | 6 | --- | . | --- | . | --- | . | --- |
| Trichostomum crispulum Bruch | . | --- | 6 | --- | . | --- | . | --- | . | --- |
| Lemanea fluviatilis (Linnaeus) C.Agardh | . | --- | 6 | --- | . | --- | . | --- | 6 | --- |
| Bangia atropurpurea (Mertens ex Roth) C.Agardh | . | --- | 6 | --- | . | --- | . | --- | 6 | --- |
| Drepanocladus aduncus (Hedw.) Warnst. | . | --- | 6 | --- | . | --- | . | --- | 3 | --- |
| Audouinella hermannii (Roth) Duby | . | --- | 6 | --- | . | --- | . | --- | 3 | --- |
| Ranunculus trichophyllus Chaix | . | --- | . | --- | . | --- | 20 | 6 | --- | |
| Lysimachia vulgaris L. | . | --- | . | --- | . | --- | 13 | 33.1 | . | --- |
| Lysimachia nummularia L. | . | --- | . | --- | . | --- | 13 | 33.1 | . | --- |
| Caltha palustris L. | . | --- | . | --- | . | --- | 13 | 33.1 | . | --- |
| Scirpus sylvaticus L. | . | --- | . | --- | . | --- | 13 | 33.1 | . | --- |
| Schoenoplectus lacustris (L.) Palla | . | --- | 6 | --- | . | --- | 13 | --- | . | --- |
| Juncus 24ectinate24s L. | . | --- | 6 | --- | . | --- | 13 | --- | 6 | --- |
| Nostoc Vaucher ex Bornet & Flahault sp. | . | --- | 6 | --- | . | --- | 13 | --- | 3 | --- |
| Veronica anagallis-aquatica L. | . | --- | 6 | --- | . | --- | 13 | --- | 3 | --- |
| Zygnema C.Agardh sp. | 12 | --- | . | --- | . | --- | 13 | --- | 3 | --- |
| Lycopus europaeus L. | . | --- | . | --- | . | --- | 13 | --- | 3 | --- |
| Hygroamblystegium fluviatile (Hedw.) Loeske | . | --- | . | --- | . | --- | 7 | --- | . | --- |
| Alisma plantago-aquatica L. | 12 | --- | . | --- | . | --- | 7 | --- | . | --- |
| Myosotis scorpioides L. | . | --- | . | --- | . | --- | 7 | --- | . | --- |
| Samolus valerandi L. | . | --- | . | --- | . | --- | 7 | --- | . | --- |
| Juncus inflexus L. | . | --- | . | --- | . | --- | 7 | --- | . | --- |
| Ranunculus repens L. | . | --- | . | --- | . | --- | 7 | --- | . | --- |
| Rorippa amphibia (L.) Besser | . | --- | . | --- | . | --- | 7 | --- | . | --- |
| Helosciadium repens (Jacq.) W. D. J. Koch | . | --- | . | --- | . | --- | 7 | --- | . | --- |
| Thamnobryum alopecurum (Hedw.) Gangulee | 12 | --- | . | --- | . | --- | . | --- | 12 | --- |
| Fissidens fontanus (Bach.Pyl.) Steud. | . | --- | . | --- | . | --- | . | --- | 9 | --- |
| Didymodon spadiceus (Mitt.) Limpr. | . | --- | . | --- | . | --- | . | --- | 9 | --- |
| Rhynchostegiella teneriffae (Mont.) Dirkse et Bouman | 12 | --- | . | --- | . | --- | . | --- | 9 | --- |
| Potamogeton nodosus Poir. | . | --- | . | --- | . | --- | . | --- | 9 | --- |
| Didymodon luridus Hornsch. | . | --- | . | --- | . | --- | . | --- | 6 | --- |
| Hildenbrandia rivularis (Liebmann) J.Agardh | . | --- | . | --- | . | --- | . | --- | 6 | --- |
| Rhizoclonium hieroglyphicum (C.Agardh) Kützing | 12 | --- | . | --- | . | --- | . | --- | 6 | --- |
| Mnium marginatum (Dicks.) P.Beauv. | . | --- | . | --- | . | --- | . | --- | 6 | --- |
| Rhynchostegiella curviseta (Brid.) Limpr. | . | --- | . | --- | . | --- | . | --- | 3 | 15.6 |
| Lemanea rigida (Sirodot) De Toni | . | --- | . | --- | . | --- | . | --- | 3 | 15.6 |
| Potamogeton perfoliatus L. | . | --- | . | --- | . | --- | . | --- | 3 | --- |
| Chaetophora lobata Schrank | . | --- | . | --- | . | --- | . | --- | 3 | --- |
| Barbula unguiculata Hedw. | 12 | --- | . | --- | . | --- | . | --- | 3 | --- |
| Stuckenia 25ectinate (L.) Börner | . | --- | . | --- | . | --- | . | --- | 3 | --- |
| Carex acuta L. | . | --- | . | --- | . | --- | . | --- | 3 | --- |
| Rhizomnium punctatum (Hedw.) T.J.Kop. | 12 | --- | . | --- | . | --- | . | --- | 3 | --- |
| Tribonema viridae Pascher | . | --- | . | --- | . | --- | . | --- | 3 | --- |
| Didymodon insulanus (De Not.) M.O.Hill | . | --- | . | --- | . | --- | . | --- | 3 | --- |
| Myosoton aquaticum (L.) Moench | . | --- | . | --- | . | --- | . | --- | 3 | --- |
| Fontinalis hypnoides Hartm. Var. duriaei (Schimp.) Kindb. | . | --- | . | --- | . | --- | . | --- | 3 | --- |
| Mougeotia C.Agardh sp. | . | --- | . | --- | . | --- | . | --- | 3 | --- |
| Galium palustre L. | . | --- | . | --- | . | --- | . | --- | 3 | --- |
| Myriophyllum spicatum L. | . | --- | . | --- | . | --- | . | --- | 3 | --- |
| Chara contraria A.Braun ex Kützing | . | --- | . | --- | . | --- | . | --- | 3 | --- |
| Lophocolea bidentata (L.) Dumort. | . | --- | . | --- | . | --- | . | --- | 3 | --- |
| Lemanea fucina Bory | . | --- | . | --- | . | --- | . | --- | 3 | --- |
Appendix B
| TWINSPAN Group | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| ALT (m a.s.l.) | |||||
| mean ± SE | 153.8 ± 20.6 | 282.3 ± 39.5 | 385.2 ± 56.2 | 231.1 ± 30.5 | 218 ± 18.7 |
| min–max | 102.3–227.9 | 93.0–564.0 | 290.1–538.8 | 31.4–376.6 | 0.3–384.2 |
| DFS (km) | |||||
| mean ± SE | 13.1 ± 2.5 | 19.9 ± 7.9 | 4.4 ± 0.8 | 6.6 ± 2.5 | 21.1 ± 5.1 |
| min–max | 1.4–21.5 | 3.6–116.0 | 3.2–6.5 | 0.2–33.8 | 0.04–118.2 |
| CA (km2) | |||||
| mean ± SE | 36.2 ± 8.5 | 198.5 ± 72.1 | 9.8 ± 4.2 | 230.7 ± 71.8 | 361.8 ± 93.2 |
| min–max | 2.7–79.8 | 13.3–1070.7 | 0.7–20.8 | 8.8–933.1 | 10.5–2199.4 |
| T (°C) | |||||
| mean ± SE | 12.3 ± 0.5 | 12.1 ± 0.6 | 14 ± 1.6 | 11.39 ± 0.45 | 12.01 ± 0.39 |
| min–max | 0.1–28.4 | 2.8–25.6 | 2.9–24.2 | 4.4–20.3 | 1.2–25.3 |
| pH | |||||
| mean ± SE | 7.70 ± 0.15 | 8.03 ± 0.05 | 8.08 ± 0.12 | 7.87 ± 0.04 | 7.99 ± 0.04 |
| min–max | 6.50–9.30 | 7.10–8.80 | 7.62–8.60 | 6.90–8.40 | 7.16–9.00 |
| EC (μS/cm) | |||||
| mean ± SE | 296.56 ± 43.34 | 416.29 ± 45.69 | 290.11 ± 114.12 | 459.71 ± 60.55 | 372.31 ± 24.36 |
| min–max | 69.00–722.00 | 129.00–1041.00 | 71.00–654.00 | 61.00–1049.00 | 8.00–1057.00 |
| TSS (mg/L) | |||||
| mean ± SE | 9.03 ± 2.07 | 2.19 ± 0.42 | 7.38 ± 2.52 | 2.12 ± 0.66 | 2.97 ± 0.53 |
| min–max | <1.60–50.00 | <0.53–17.2. | <1.60–33.00 | <0.53–74.00 | <0.53–69.00 |
| TALK (mgCaCO₃/L) | |||||
| mean ± SE | 141.91 ± 20.2 | 247.13 ± 27.69 | 161.19 ± 81.57 | 236.96 ± 29.35 | 213.24 ± 15.39 |
| min–max | 36.70–327.00 | 130.10–631.00 | 14.00–479.00 | 32.00–624.00 | 44.20–636.00 |
| DO (mgO₂/L) | |||||
| mean ± SE | 10.09 ± 0.29 | 10.63 ± 0.23 | 9.31 ± 0.25 | 10.88 ± 0.22 | 11.07 ± 0.15 |
| min–max | 5.46–14.55 | 6.95–14.00 | 2.72–13.16 | 7.50–14.01 | 4.90–16.90 |
| BOD (mgO₂/L) | |||||
| mean ± SE | 2.15 ± 0.30 | 1.17 ± 0.16 | 5.28 ± 1.99 | 0.97 ± 0.17 | 1.02 ± 0.10 |
| min–max | <0.50–7.83 | <0.09–4.60 | 0.81–16.47 | <0.10–5.64 | <0.10–14.3 |
| NH₄+ (mgN/L) | |||||
| mean ± SE | 0.126 ± 0.050 | 0.010 ± 0.003 | 0.0730 ± 0.0250 | 0.077 ± 0.057 | 0.026 ± 0.008 |
| min–max | <0.003–<0.880 | <0.0008–0.1480 | <0.005–0.300 | <0.003–3.420 | <0.0008–1.277 |
| NO2− (mgN/L) | |||||
| mean ± SE | 0.0215 ± 0.0135 | 0.0050 ± 0.0029 | 0.0189 ± 0.01411 | 0.0041 ± 0.0016 | 0.0063 ± 0.0018 |
| min–max | 0.0010–1.3030 | 0.0005–0.2210 | 0.0015–0.1100 | 0.0005–0.0790 | 0.0005–0.0900 |
| NO3− (mgN/L) | |||||
| mean ± SE | 0.527 ± 0.114 | 0.427 ± 0.069 | 0.848 ± 0.224 | 0.424 ± 0.060 | 0.626 ± 0.053 |
| min–max | 0.001–2.615 | 0.017–1.871 | 0.070–2.180 | 0.010–1.400 | 0.010–2.391 |
| Ntot (mgN/L) | |||||
| mean ± SE | 1.067 ± 0.163 | 0.676 ± 0.080 | 1.405 ± 0.376 | 0.563 ± 0.072 | 0.847 ± 0.060 |
| min–max | 0.100–2.632 | 0.110–1.944 | 0.215–2.440 | 0.100–3.565 | 0.190–2.452 |
| PO43− (mgP/L) | |||||
| mean ± SE | 0.0157 ± 0.0029 | 0.0029 ± 0.0007 | 0.0635 ± 0.0104 | 0.0033 ± 0.0005 | 0.0079 ± 0.0014 |
| min–max | <0.0040–<0.0900 | <0.0012–0.0460 | <0.0050–0.7600 | <0.0010–0.0316 | <0.0012–0.2020 |
| Ptot (mgP/L) | |||||
| mean ± SE | 0.0544 ± 0.0087 | 0.0122 ± 0.0028 | 0.1861 ± 0.043 | 0.0154 ± 0,0030 | 0.0232 ± 0.0200 |
| min–max | <0.0030–<0.3200 | <0.0015–0.1150 | 0.0200–0.7600 | <0.002–0.1840 | <0.002–0.3220 |
| bio1 (°C) | |||||
| mean ± SE | 11.58 ± 0.13 | 12.21 ± 0.42 | 9.95 ± 0.28 | 12.60 ± 0.36 | 11.67 ± 0.25 |
| min–max | 10.95–11.95 | 10.25–15.05 | 9.15–10.35 | 10.35–15.05 | 9.85–15.85 |
| bio2 (°C) | |||||
| mean ± SE | 9.18 ± 0.16 | 8.81 ± 0.25 | 8.93 ± 0.17 | 8.77 ± 0.29 | 8.38 ± 0.21 |
| min–max | 8.20–9.60 | 5.90–9.60 | 8.70–9.40 | 5.90–9.90 | 4.70–9.80 |
| bio3 (°C) | |||||
| mean ± SE | 30.53 ± 0.44 | 30.1 ± 0.56 | 31.03 ± 0.38 | 30.08 ± 0.63 | 29.06 ± 0.43 |
| min–max | 27.9–31.7 | 24.2–31.8 | 30.2–31.9 | 24.2–32.9 | 21.6–32.2 |
| bio4 (°C) | |||||
| mean ± SE | 749.30 ± 3.99 | 723.76 ± 5.48 | 724.23 ± 4.48 | 723.81 ± 5.11 | 722.45 ± 6.28 |
| min–max | 727.80–759.30 | 662.90–741.70 | 713.30–732.60 | 662.90–748.30 | 606.20–749.90 |
| bio5 (°C) | |||||
| mean ± SE | 26.94 ± 0.25 | 27.11 ± 0.45 | 24.78 ± 0.34 | 27.39 ± 0.31 | 26.22 ± 0.20 |
| min–max | 25.95–27.65 | 24.95–29.85 | 23.95–25.55 | 25.15–29.25 | 24.35–28.85 |
| bio6 (°C) | |||||
| mean ± SE | −3.07 ± 0.13 | -2.06 ± 0.5 | −4.05 ± 0.14 | −1.68 ± 0.46 | −2.41 ± 0.41 |
| min–max | −3.75–−2.65 | −4.15–2.65 | −4.45–−3.85 | −3.85–2.65 | −4.35–5.05 |
| bio7 (°C) | |||||
| mean ± SE | 30.01 ± 0.17 | 29.17 ± 0.38 | 28.83 ± 0.25 | 29.07 ± 0.40 | 28.63 ± 0.38 |
| min–max | 29.40–30.70 | 24.50–30.40 | 28.40–29.50 | 24.50–30.40 | 21.50–30.50 |
| bio8 (°C) | |||||
| mean ± SE | 14.68 ± 1.13 | 9.59 ± 0.47 | 17.35 ± 0.62 | 9.89 ± 0.47 | 11.36 ± 0.46 |
| min–max | 11.45–18.95 | 6.45–11.85 | 15.55–18.25 | 6.85–13.55 | 6.85–16.75 |
| bio9 (°C) | |||||
| mean ± SE | 3.38 ± 0.13 | 15.36 ± 2.62 | 1.98 ± 0.25 | 17.36 ± 2.34 | 8.50 ± 1.59 |
| min–max | 2.65–3.75 | 2.25–23.75 | 1.25–2.35 | 2.35–23.75 | 1.75–23.85 |
| bio10 (°C) | |||||
| mean ± SE | 21.13 ± 0.16 | 21.59 ± 0.43 | 19.23 ± 0.36 | 21.99 ± 0.35 | 20.93 ± 0.21 |
| min–max | 20.55–21.55 | 19.35–23.75 | 18.25–19.85 | 19.65–23.75 | 18.95–23.85 |
| bio11 (°C) | |||||
| mean ± SE | 1.71 ± 0.13 | 2.82 ± 0.47 | 0.48 ± 0.22 | 3.24 ± 0.42 | 2.21 ± 0.34 |
| min–max | 0.95–2.05 | 0.75–6.65 | −0.15–0.85 | 0.85–6.65 | 0.45–8.15 |
| bio12 (kg/m2) | |||||
| mean ± SE | 1041.98 ± 41.05 | 1357.79 ± 49.47 | 1171.93 ± 52.28 | 1306.52 ± 55.65 | 1334.00 ± 43.27 |
| min–max | 960.80–1304.40 | 1090.00–1697.60 | 1101.30–1323.40 | 1077.80–1913.60 | 972.20–1801.90 |
| bio13 (kg/m2) | |||||
| mean ± SE | 110.90 ± 4.40 | 160.38 ± 5.07 | 133.43 ± 4.03 | 157.63 ± 6.79 | 151.32 ± 5.27 |
| min–max | 101.30–139.20 | 122.50–183.10 | 125.60–144.70 | 124.90–236.30 | 107.70–221.80 |
| bio14 (kg/m2) | |||||
| mean ± SE | 59.14 ± 3.29 | 65.30 ± 5.71 | 59.03 ± 2.87 | 61.23 ± 5.32 | 72.50 ± 3.73 |
| min–max | 48.30–78.00 | 38.90–101.20 | 55.30–67.40 | 38.90–97.30 | 30.20–104.10 |
| bio15 (kg/m2) | |||||
| mean ± SE | 18.54 ± 0.44 | 23.71 ± 1.41 | 21.05 ± 0.51 | 24.67 ± 1.61 | 21.87 ± 0.94 |
| min–max | 15.90–19.80 | 16.60–36.10 | 20.20–22.40 | 16.60–36.10 | 16.40–37.60 |
| bio16 (kg/m2) | |||||
| mean ± SE | 305.74 ± 14.9 | 441.85 ± 15.42 | 351.6 ± 18.67 | 430.73 ± 20.77 | 424.94 ± 15.27 |
| min–max | 279–405.7 | 339.9–524.4 | 323.8–406.6 | 343–675.3 | 299.1–619.7 |
| bio17 (kg/m2) | |||||
| mean ± SE | 193.66 ± 9.79 | 251.63 ± 14.65 | 210.55 ± 6.47 | 237.29 ± 13.50 | 251.38 ± 10.77 |
| min–max | 173.10–251.50 | 171.90–357.90 | 198.30–227.50 | 171.9–346.70 | 149.80–367.00 |
| bio18 (kg/m2) | |||||
| mean ± SE | 283.76 ± 4.78 | 267.39 ± 17.79 | 347.05 ± 16.07 | 251.72 ± 17.43 | 304.32 ± 12.03 |
| min–max | 272.00–311.80 | 171.90–372.80 | 320.80–393.90 | 171.90–363.70 | 149.80–398.80 |
| bio19 (kg/m2) | |||||
| mean ± SE | 209.61 ± 12.13 | 340.66 ± 11.69 | 219.98 ± 7.28 | 331.24 ± 12.60 | 304.98 ± 11.97 |
| min–max | 186.70–285.30 | 246.40–400.10 | 208.80–240.4 | 252.40–469.50 | 199.00–438.20 |
Appendix C
| Environmental Variable | TWINSPAN Group Pairs Compared | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1–2 | 1–3 | 1–4 | 1–5 | 2–3 | 2–4 | 2–5 | 3–4 | 3–5 | 4–5 | |
| T | * | |||||||||
| pH | * | |||||||||
| TSS | * | * | * | * | * | * | ||||
| TALK | * | * | * | |||||||
| DO | * | * | * | * | ||||||
| BOD | * | * | ** | * | * | * | ||||
| NH4+ | * | * | * | * | * | |||||
| NO2− | * | * | * | * | ||||||
| NO3− | * | |||||||||
| Ntot | * | * | * | |||||||
| PO43- | ** | * | ** | * | * | ** | * | * | * | |
| Ptot | ** | * | ** | * | * | * | * | * | ||
| ALT | * | * | * | |||||||
| DFS | * | * | * | * | ||||||
| CA | * | * | * | * | * | |||||
| bio1 | * | * | * | * | * | |||||
| bio2 | * | |||||||||
| bio3 | * | * | ||||||||
| bio4 | * | * | * | * | ||||||
| bio5 | * | * | * | * | * | |||||
| bio6 | * | * | * | * | * | |||||
| bio7 | * | * | * | |||||||
| bio8 | ** | ** | * | * | * | * | * | |||
| bio9 | * | * | * | * | * | * | ||||
| bio10 | * | * | * | * | * | |||||
| bio11 | * | * | * | * | * | |||||
| bio12 | ** | * | * | ** | ||||||
| bio13 | ** | * | ** | ** | * | * | ||||
| bio14 | * | |||||||||
| bio15 | * | * | * | * | ||||||
| bio16 | ** | * | * | ** | * | * | ||||
| bio17 | * | * | * | |||||||
| bio18 | * | * | ||||||||
| bio19 | ** | ** | ** | * | * | * | ||||
References
- Slack, N.G.; Glime, J.M. Niche relationships of mountain stream bryophytes. Bryologist 1985, 88, 7–19. [Google Scholar] [CrossRef]
- Glime, J.M. Habitat and role. In Bryophyte Ecology; Michigan Technological University: Houghton, MI, USA, 2020; Volume 4. [Google Scholar]
- Ceschin, S.; Minciardi, M.R.; Spada, C.D.; Abati, S. Bryophytes of alpine and apennine mountain streams: Floristic features and ecological notes. Cryptogam. Bryol. 2015, 36, 267–283. [Google Scholar] [CrossRef]
- Tremp, H.; Kampmann, D.; Schulz, R. Factors shaping submerged bryophyte communities: A conceptual model for small mountain streams in Germany. Limnologica 2012, 42, 242–250. [Google Scholar] [CrossRef]
- Vitt, D.H.; Glime, J.M. The structural adaptations of aquatic Musci. Lindbergia 1984, 10, 95–110. [Google Scholar]
- Suren, A.M. Bryophyte distribution patterns in relation to macro-, meso-, and micro-scale variables in South Island, New Zealand streams. N. Z. J. Mar. Freshw. Res. 1996, 30, 501–523. [Google Scholar] [CrossRef]
- Glime, J.M. Effects of pollutants on aquatic species. In Bryophytes and Lichens in a Changing Environment; Bates, J.W., Farmer, A.M., Eds.; Clarendon Press: Oxford, UK, 1992; pp. 333–361. [Google Scholar]
- Vieira, C.; Aguiar, F.C.; Portela, A.P.; Monteiro, J.; Raven, P.J.; Holmes, N.T.H.; Cambra, J.; Flor-Arnau, N.; Chauvin, C.; Loriot, S.; et al. Bryophyte communities of Mediterranean Europe: A first approach to model their potential distribution in highly seasonal rivers. Hydrobiologia 2018, 812, 27–43. [Google Scholar] [CrossRef]
- Ferreira, M.T.; Aguiar, F.C. Riparian and aquatic vegetation in Mediterranean-type streams (western Iberia). Limnetica 2006, 25, 411–424. [Google Scholar] [CrossRef]
- Dierßen, K. Distribution, Ecological Amplitude and Phytosociological Characterization of European Bryophytes; Bryophytor; J. Cramer: Berlin, Germany, 2001; ISBN 9783443620288. [Google Scholar]
- Scarlett, P.; O’Hare, M. Community structure of in-stream bryophytes in English and Welsh rivers. Hydrobiologia 2006, 553, 143–152. [Google Scholar] [CrossRef]
- Suren, A.M.; Ormerod, S.J. Aquatic bryophytes in Himalayan streams: Testing a distribution model in a highly heterogeneous environment. Freshw. Biol. 1998, 40, 697–716. [Google Scholar] [CrossRef]
- Gecheva, G.; Pall, K.; Hristeva, Y. Bryophyte communities responses to environmental factors in highly seasonal rivers. Bot. Lett. 2017, 164, 79–91. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Durwael, L. Trophic response curves of aquatic bryophytes in lowland calcareous streams. Bryologist 1999, 102, 720. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Klein, J.P. Aquatic bryophyte assemblages along a gradient of regulation in the river Rhine. Hydrobiologia 1999, 410, 11–16. [Google Scholar] [CrossRef]
- Gecheva, G.; Yurukova, L.; Cheshmedjiev, S.; Ganeva, A. Distribution and bioindication role of aquatic bryophytes in Bulgarian rivers. Biotechnol. Biotechnol. Equip. 2010, 24, 164–170. [Google Scholar] [CrossRef][Green Version]
- Ceschin, S.; Aleffi, M.; Bisceglie, S.; Savo, V.; Zuccarello, V. Aquatic bryophytes as ecological indicators of the water quality status in the Tiber River basin (Italy). Ecol. Indic. 2012, 14, 74–81. [Google Scholar] [CrossRef]
- Vanderpoorten, A. Aquatic bryophytes for a spatio-temporal monitoring of the water pollution of the rivers Meuse and Sambre (Belgium). Environ. Pollut. 1999, 104, 401–410. [Google Scholar] [CrossRef]
- Gecheva, G.; Yurukova, L. Water pollutant monitoring with aquatic bryophytes: A review. Environ. Chem. Lett. 2014, 12, 49–61. [Google Scholar] [CrossRef]
- Muotka, T.; Virtanen, R. The stream as a habitat templet for bryophytes: Species’ distributions along gradients in disturbance and substratum heterogeneity. Freshw. Biol. 1995, 33, 141–160. [Google Scholar] [CrossRef]
- Yurukova, L.D.; Gecheva, G. Biomonitoring in Maritsa River using aquatic bryophytes. J. Environ. Prot. Ecol. 2014, 5, 729–735. [Google Scholar]
- Papp, B.; Ganeva, A.; Natcheva, R. Bryophyte vegetation of Iskur River and its main tributaries. Phytol. Balc. 2006, 12, 181–189. [Google Scholar]
- Sabovljević, M.; Ganeva, A.; Tsakiri, E.; Stefanut, S. Bryology and bryophyte protection in south-eastern Europe. Biol. Conserv. 2001, 101, 73–84. [Google Scholar] [CrossRef]
- Sabovljević, M.; Alegro, A.; Sabovljević, A.; Marka, J.; Vujčić, M. An insight into diversity of the Balkan Peninsula bryophyte flora in the European background. Rev. Ecol. Terre Vie 2011, 66, 399–414. [Google Scholar]
- Pavletić, Z. Istraživanja briofita na travertinskim slapovima rijeke Krke [Research of bryophytes on travertine waterfalls of the Krka River]. Ljetop. Jugosl. Akad. Znan. I Umjet. 1954, 61, 331–351. [Google Scholar]
- Pavletić, Z. Prilozi poznavanju ekologije briofita na slapovima rijeke Krke u Dalmaciji [Contributions to the knowledge of bryophyte ecology on the Krka River waterfalls in Dalmatia]. Rad Jugosl. Akad. Znan. I Umjet. Odjel Za Prir. Nauk. 1957, 312, 95–137. [Google Scholar]
- Pavletić, Z. Ekološki odnosi briofitske vegetacije na slapovima Plitvičkih jezera [Ecological relations of bryophyte vegetation on the Plitvice Lakes waterfalls]. Acta Bot. Croat. 1957, 16, 63–88. [Google Scholar]
- Pavletić, Z. Izvještaj o istraživanjima briofita na slapovima rijeke Une [Report on research of bryophytes on the Una River waterfalls]. Ljetop. Jugosl. Akad. Znan. I Umjet. 1959, 63, 361–366. [Google Scholar]
- Pavletić, Z. Istraživanja briofitske vegetacije na slapovima rijeka Une i Plive ljeti godine 1957 [Research of bryophyte vegetation on the waterfalls of the rivers Una and Pliva in the summer of 1957]. Ljetop. Jugosl. Akad. Znan. I Umjet. 1960, 64, 308–314. [Google Scholar]
- Matoničkin, I.; Pavletić, Z. Životne zajednice na sedrenim slapovima rijeke Une i brzicama pritoke Unca [Biocoenosis of the Una River travertine waterfalls and the rapids of its tributary Unac]. Acta Musei Maced. Sci. Nat. 1959, 6, 77–99. [Google Scholar]
- Matoničkin, I.; Pavletić, Z. Biološke karakteristike sedrenih slapova u našim krškim rijekama [Biological characteristics of travertine waterfalls in our karst rivers]. Geogr. Glas. 1960, 22, 43–56. [Google Scholar]
- Matoničkin, I.; Pavletić, Z. Građa za upoznavanje životnih zajednica u rječici Vrelo kod Dubrovnika [Contribution to te bicoenosis of the rivulet Vrelo near Dubrovnik]. Acta Bot. Croat. 1960, 18–19, 167–176. [Google Scholar]
- Matoničkin, I.; Pavletić, Z. Životni uvjeti na sedrenim slapovima krških rijeka u Jugoslaviji [Biological conditions on the travertine waterfalls of karst rivers in Yugoslavia]. Acta Bot. Croat. 1962, 20–21, 175–198. [Google Scholar]
- Matoničkin, I.; Pavletić, Z. Entwicklung der Lebensgemeinschaften und ihre Bedeutung für Erhaltung der Kalktuffawasserfälle. Arch. Hydrobiol. 1962, 58, 467–473. [Google Scholar]
- Matoničkin, I.; Pavletić, Z. Prethodna ekološko—Biocenološka istraživanja opskrbnih voda Plitvičkih jezera [Preliminary eco-biocenological research of supply waters of the Plitvice Lakes]. Biološki Glas. Period. Biol. 1963, 22, 105–128. [Google Scholar]
- Matoničkin, I.; Pavletić, Z. Sedrene naslage u rijeci Uni i njihova biološka uvjetovanost [Travertine deposits in the Una River and their biological conditionality]. Geogr. Glas. 1963, 25, 105–112. [Google Scholar]
- Pavletić, Z.; Matoničkin, I. Biološka klasifikacija gornjih tijekova krških rijeka [Biological classification of upper reaches of karst rivers]. Acta Bot. Croat. 1965, 24, 151–162. [Google Scholar]
- Matoničkin, I.; Pavletić, Z. Les forms zoogenes du tufs et leur formation dans la region des Lacs de Plitvice en Yougoslavie. Hydrobiologia 1965, 26, 293–300. [Google Scholar] [CrossRef]
- Vieira, C.; Aguiar, F.C.; Ferreira, M.T. The relevance of bryophytes in the macrophyte-based reference conditions in Portuguese rivers. Hydrobiologia 2014, 737, 245–264. [Google Scholar] [CrossRef]
- Lang, P.; Murphy, K.J. Environmental drivers, life strategies and bioindicator capacity of bryophyte communities in high-latitude headwater streams. Hydrobiologia 2012, 679, 1–17. [Google Scholar] [CrossRef]
- Papp, B.; Rajczy, M. Investigations on the condition of bryophyte vegetation of mountain streams in Hungary. J. Hattori Bot. Lab. 1998, 84, 81–90. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Klein, J.P. Variations of aquatic bryophyte assemblages in the Rhine Rift related to water quality. 2. The waterfalls of the Vosges and the Black Forest. J. Bryol. 1999, 21, 109–115. [Google Scholar] [CrossRef]
- Papp, B.; Rajczy, M. The role of Bryophytes as bioindicators of water quality in the River Danube. Int. Ver. Theor. Angew. Limnol. Verh. 1998, 26, 1254–1256. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Klein, J.P.; Stieperaere, H.; Trémolières, M. Variations of aquatic bryophyte assemblages in the Rhine Rift related to water quality. 1. The Alsatian Rhine floodplain. J. Bryol. 1999, 21, 17–23. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Klein, J.P. A comparitive study of the hydrophyte flora from the Alpine Rhine to the Middle Rhine. Application to the conservation of the Upper Rhine aquatic ecosystems. Biol. Conserv. 1999, 87, 163–172. [Google Scholar] [CrossRef]
- Zechmeister, H.; Mucina, L. Vegetation of European springs: High-rank syntaxa of the Montio-Cardaminetea. J. Veg. Sci. 1994, 5, 385–402. [Google Scholar] [CrossRef]
- Couvreur, J.M.; San Martin, G.; Sotiaux, A. Factors Affecting the Presence and the Diversity of Bryophytes in the Petrifying Sources Habitat (7220) in Wallonia and the Brussels-Capital Region, Belgium. Int. J. Agron. 2016, 2016, 1–18. [Google Scholar] [CrossRef]
- Lyons, M.D.; Kelly, D.L. Plant community ecology of petrifying springs (Cratoneurion)—A priority habitat. Phytocoenologia 2017, 47, 13–32. [Google Scholar] [CrossRef]
- Hill, M.O.; Preston, C.D.; Bosanquet, S.D.S.; Roy, D.B. BRYOATT—Attributes of British and Irish Mosses, Liverworts and Hornworts; Centre for Ecology and Hydrology: Huntingdon, UK, 2007; ISBN 978-1-85531-236-4. [Google Scholar]
- Vieira, C.; Séneca, A.; Sérgio, C.; Ferreira, M.T. Bryophyte taxonomic and functional groups as indicators of fine scale ecological gradients in mountain streams. Ecol. Indic. 2012, 18, 98–107. [Google Scholar] [CrossRef]
- Zaninović, K.; Gajić-Čapka, M.; Perčec Tadić, M.; Vučetić, M.; Milković, J.; Bajić, A.; Cindrić, K.; Cvitan, L.; Katušin, Z.; Kaučić, D.; et al. Klimatski atlas Hrvatske/Climate atlas of Croatia 1961–1990, 1971–2000; Državni Hidrometeorološki Zavod: Zagreb, Croatia, 2008; ISBN 978-953-7526-01-6. [Google Scholar]
- Anonymous. River Cetina Watershed and the Adjecent Coastal Area. Environmental and Socio-Economic Profile; United Nations Environment Programme: Nairobi, Kenya, 2000; ISBN 953-6429-34-9. [Google Scholar]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G.; et al. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19, 264. [Google Scholar] [CrossRef]
- Official Gazette 96/19 Uredba o Standardu Kakvoće voda [Regulation on the Water Quality Standard]. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2019_10_96_1879.html (accessed on 2 February 2022).
- Frahm, J.P. Wassermoose als Indikatoren für die Gewässerverschmutzung am Beispiel des Niederrheins. Gewässer Abwasser 1974, 53/54, 91–106. [Google Scholar]
- Sérgio, C.; Vieira, C.; Silva, I. Recent records of Cinclidotus (Musci, Pottiaceae) in Portugal: C. aquaticus (Hedw.) Bruch & Schimp. and C. riparius (Host ex Brid.) Arn. Boletín La Soc. Española Briología 2007, 33–36. [Google Scholar]
- Kochjarová, J.; Šoltés, R.; Hrivnák, R. Cinclidotus aquaticus in Slovakia: Present state and prognoses. Bryonora 2007, 40, 1–6. [Google Scholar]
- Price, M.; Vivien, R. Cinclidotus aquaticus (Hedw.) Bruch & Schimp. (Cinclidotaceae) in the canton of Geneva. Meylania 2010, 45, 26–29. [Google Scholar]
- Vitt, D.H.; Glime, J.M.; Lafarge, E.C. Bryophyte vegetation and habitat gradients of montane streams in western Canada. Hikobia 1986, 9, 367–385. [Google Scholar]
- Vučković, I.; Čanjevac, I.; Plantak, M.; Bočić, N.; Buzjak, N.; Orešić, D.; Pavlek, K.; Vinković, K.; Martinić, I.; Srebočan, M. Sustavno Ispitivanje Hidromorfoloških Elemenata Kakvoće u Rijekama u 2019. i 2020. Godini [Systematic Assessment of Hydromorphological Quality Elements in Rivers in 2019 and 2020]; Elektroprojekt d.d. and Department of Geography, Faculty of Science, Univeristy of Zagreb: Zagreb, Croatia, 2021. [Google Scholar]
- EU Law and Publications. European Community Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Communities 2000, L327, 1–72. [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie, 3rd ed.; Springer: Wien, Austria, 1964; ISBN 978-3-7091-8111-9. [Google Scholar]
- Dierschke, H. Pflanzensoziologie. Grundlagen und Methoden, 1st ed.; Eugen Ulmer: Stuttgart, Germany, 1994; ISBN 3825280780. [Google Scholar]
- Barkman, J.J.; Doing, H.; Segal, S. Kritische Bemerkungen Und Vorschläge Zur Quantitativen Vegetationsanalyse. Acta Bot. Neerl. 1964, 13, 394–419. [Google Scholar] [CrossRef]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. Available online: sweetgum.nybg.org (accessed on 11 February 2022).
- Hodgetts, N.G.; Söderström, L.; Blockeel, T.L.; Caspari, S.; Ignatov, M.S.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An annotated checklist of bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- Domina, G. Euro+Med. Available online: http://ww2.bgbm.org/EuroPlusMed (accessed on 15 March 2022).
- Guiry, M.D.; Guiry, G.M. AlgaeBase. Available online: https://algaebase.org (accessed on 15 March 2022).
- Copernicus Land Monitoring Service EU-DEM v1.0. Available online: https://land.copernicus.eu/%0AImagery-in-situ/eu-dem/eu-dem-v1–0-and-derived-products/eu-dem-v1.0 (accessed on 2 February 2022).
- DGU Topografske Karte Mjerila 1:25 000 (TK25), 1:100 000 (TK100) i 1:200 000 (TK200)—Mrežna Usluga Pregleda za Anonimne Korisnike (WMS). Available online: https://geoportal.dgu.hr/services/tk/wms (accessed on 16 January 2022).
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Hill, M.O. TWINSPAN—A Fortran Program for Arranging Multivariate Data in an Ordered Two-way Table by Classification of The Individuals and Attributes; Cornell University, Department of Ecology and Systematics: Itacha, NY, USA, 1979. [Google Scholar]
- Roleček, J.; Tichý, L.; Zelený, D.; Chytrý, M. Modified TWINSPAN classification in which the hierarchy respects cluster heterogeneity. J. Veg. Sci. 2009, 20, 596–602. [Google Scholar] [CrossRef]
- Tichý, L.; Tichý, L.; Holt, J.; Holt, J. JUICE, program for management, analysis and classification of ecological data. Users Manual. J. Veg. Sci. 2006. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software pacage for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Tichý, L.; Chytrý, M. Statistical determination of diagnostic species for site groups of unequal size. J. Veg. Sci. 2006, 17, 809. [Google Scholar] [CrossRef]
- Hill, M.O.; Preston, C.D. The geographical relationships of British and Irish bryophytes. J. Bryol. 1998, 20, 127–226. [Google Scholar] [CrossRef]
- ter Braak, C.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]




| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| 1 | |||||
| 2 | 0.68 * | ||||
| 3 | 0.58 ** | 0.70 * | |||
| 4 | 0.66 * | 0.36 * | 0.56 ** | ||
| 5 | 0.67 * | 0.33 * | 0.75 * | 0.42 * |
| Pannonian Ecoregion | Dinaric Ecoregion | Total | ||
|---|---|---|---|---|
| Dinaric–Mediterranean Subecoregion | Dinaric–Continental Subecoregion | |||
| Surveyed localities | 331 | 109 | 87 | 527 |
| Bryophyte-dominated localities | 15 | 26 | 35 | 76 |
| TWINSPAN group | ||||
| 1 | 7 | - | 1 | 8 |
| 2 | - | 8 | 8 | 16 |
| 3 | 3 | - | 1 | 4 |
| 4 | 1 | 10 | 4 | 15 |
| 5 | 4 | 8 | 21 | 33 |
| TWINSPAN Groups | Total | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Number of relevés | 8 | 16 | 4 | 15 | 33 | 76 |
| Number of taxa * | 43 | 59 | 18 | 51 | 80 | 130 |
| Bryophyte species | 37 | 35 | 9 | 17 | 45 | 68 |
| Mosses (Bryophyta) | 31 | 29 | 7 | 12 | 38 | 59 |
| pleurocarpous | 14 | 11 | 6 | 7 | 18 | 25 |
| acrocarpous | 17 | 18 | 1 | 5 | 20 | 34 |
| Liverworts | 6 | 6 | 2 | 5 | 7 | 9 |
| leafy | 3 | 2 | 0 | 3 | 4 | 4 |
| thallose | 3 | 4 | 2 | 2 | 3 | 5 |
| Vascular plant species | 3 | 16 | 7 | 28 | 17 | 43 |
| hydrophytes | 0 | 0 | 0 | 1 | 5 | 5 |
| helophytes | 3 | 16 | 7 | 27 | 12 | 38 |
| Macroalgae taxa * | 3 | 8 | 2 | 6 | 18 | 19 |
| Taxa richness | ||||||
| mean ± SE | 12.25 ± 1.33 | 10.56 ± 1.21 | 6.75 ± 1.49 | 9.27 ± 1.02 | 10.24 ± 1.02 | 10.14 ± 0.58 |
| range (min–max) | 7–18 | 4–24 | 3–10 | 4–20 | 4–25 | 3–25 |
| Bryophyte species richness | ||||||
| mean ± SE | 11.38 ± 1.18 | 8.94 ± 0.91 | 4.00 ± 1.08 | 4.4 ± 0.46 | 8.21 ± 0.83 | 7.57 ± 0.50 |
| range (min–max) | 7–16 | 3–17 | 2–7 | 1–9 | 2–20 | 1–20 |
| Group 1—Oxyrrhynchium hians–Chiloscyphus pallescens Community |
| Characteristic species: Oxyrrhynchium hians, Pellia neesiana, Conocephalum salebrosum, Fissidens taxifolius, Chiloscyphus pallescens, Plagiomnium undulatum, Dichodontium pellucidum, Pohlia melanodon, Hypnum cupressiforme, Plagiomnium ellipticum Constant species: Oxyrrhynchium hians, Pellia neesiana, Conocephalum salebrosum, Fissidens taxifolius, Chiloscyphus pallescens, Plagiomnium undulatum, Rhynchostegium riparioides, Cratoneuron filicinum, Ptychostomum pseudotriquetrum, Leptodyctium riparium Distribution: Mainly Pannonian Ecoregion Ecology: Mostly restricted to the small lowland rivers with small catchment areas and under the influence of a temperate climate; in water with high values of orthophosphates, BOD and TSS, as well as low alkalinity due to silicate substrate; occurring on shaded habitats along river stretches flowing through forests. Characterized by high bryophyte richness, a high share of hygrophyte bryophytes growing on river margins and rough mats and turfs in lifeform spectrum. |
| Group 2—Didymodon tophaceus–Apopellia endiviifolia Community |
| Characteristic species: Didymodon tophaceus, Eucladium verticillatum, Apopellia endiviifolia, Fissidens crassipes, Funaria hygrometrica Constant species: Didymodon tophaceus, Apopellia endiviifolia, Fissidens crassipes, Funaria hygrometrica, Rhynchostegium riparioides, Cratoneuron filicinum, Ptychostomum pseudotriquetrum, Cinclidotus fontinaloides Distribution: Dinaric Ecoregion Ecology: Mainly tufa-forming community, occurring in karstic rivers with high alkalinity and pH values reflecting the dominant carbonate bedrock; in clean water with low nutrient content and BOD values and high dissolved oxygen levels. Characteristic for watercourses with considerable seasonality in water flow (intermittent rivers with small catchment areas, under the influence of the Mediterranean climate with dry and hot summers) and cascades in the lower courses of karstic rivers with larger catchment areas. Characterized by a high share of hygrophyte species and turfs in lifeform spectrum. |
| Group 3—Fissidens pusillus–Veronica beccabunga Community |
| Characteristic species: Brachythecium rutabulum, Fissidens pusillus, Veronica beccabunga, Persicaria dubia, Oxyrrhynchium speciosum Constant species: Brachythecium rutabulum, Fissidens pusillus, Veronica beccabunga, Persicaria dubia, Oxyrrhynchium speciosum, Rhynchostegium riparioides, Cratoneuron filicinum, Marchantia polymorpha Distribution: Mainly Pannonian Ecoregion Ecology: Occurring mainly in small, semi-montane watercourses with small catchment areas and under the influence of a temperate climate; in waters with high nutrient levels, BOD and TSS, and lower dissolved oxygen concentrations. Species-poor community, characterized by a higher share of hygrophytes and rough mats in lifeform spectrum, which grow on periodically submerged substrates. |
| Group 4—Berula erecta–Cratoneuron filicinum Community |
| Characteristic species: Mentha aquatica, Berula erecta, Sparganium erectum Constant species: Cratoneuron filicinum, Rhynchostegium riparioides, Fontinalis antipyretica, Mentha aquatica Distribution: Mainly Dinaric Ecoregion, Mediterranean Subecoregion Ecology: Transitional community of karstic rivers with large catchment areas and permanent flow, where vascular species start to outcompete bryophytes. Occurring in clean water with low nutrient content and BOD values, where helophytes occupy the river margins and shallower water, while bryophytes are confined to the riverbed. Characterized by a high share of rheophytes and aquatic trailings in lifeform spectrum, but low overall bryophyte species richness. |
| Group 5—Cinclidotus Community |
| Characteristic species: Cinclidotus riparius, C. aquaticus Constant species: Cinclidotus riparius, C. aquaticus, C. fontinaloides, Rhynchostegium riparioides, Cratoneuron filicinum, Fontinalis antipyretica Distribution: Mainly Dinaric Ecoregion, i.e., its Continental Subecoregion Ecology: The most widespread community, with a wide ecological range; in general, in waters with intermediate values of the water quality parameters (orthophosphates, total nitrogen and biochemical oxygen demand) and climatic variables associated with precipitation and water availability. Mostly in permanent karstic rivers with large catchment areas flowing over carbonate bedrock in neutral to basic water. Species-rich community characterized by a high share of rheophyte species and aquatic trailing in lifeform spectrum. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimac, A.; Alegro, A.; Šegota, V.; Vuković, N.; Koletić, N. Environmental Gradients Shaping the Freshwater Bryophyte Communities of Croatia (Western Balkans). Plants 2022, 11, 1542. https://doi.org/10.3390/plants11121542
Rimac A, Alegro A, Šegota V, Vuković N, Koletić N. Environmental Gradients Shaping the Freshwater Bryophyte Communities of Croatia (Western Balkans). Plants. 2022; 11(12):1542. https://doi.org/10.3390/plants11121542
Chicago/Turabian StyleRimac, Anja, Antun Alegro, Vedran Šegota, Nina Vuković, and Nikola Koletić. 2022. "Environmental Gradients Shaping the Freshwater Bryophyte Communities of Croatia (Western Balkans)" Plants 11, no. 12: 1542. https://doi.org/10.3390/plants11121542
APA StyleRimac, A., Alegro, A., Šegota, V., Vuković, N., & Koletić, N. (2022). Environmental Gradients Shaping the Freshwater Bryophyte Communities of Croatia (Western Balkans). Plants, 11(12), 1542. https://doi.org/10.3390/plants11121542







