Comparison of Heat and Drought Stress Responses among Twelve Tartary Buckwheat (Fagopyrum tataricum) Varieties
Abstract
:1. Introduction
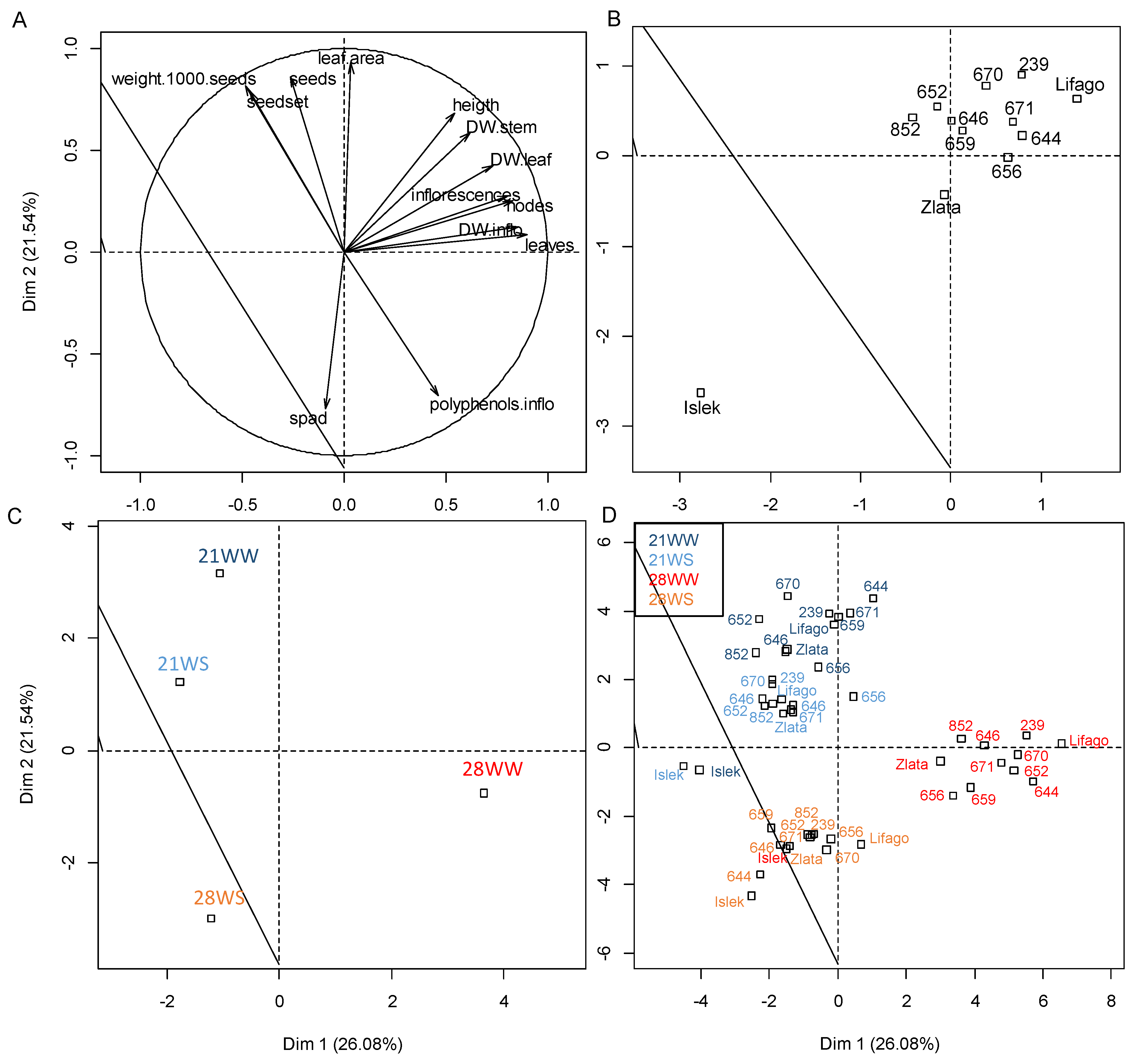
2. Results
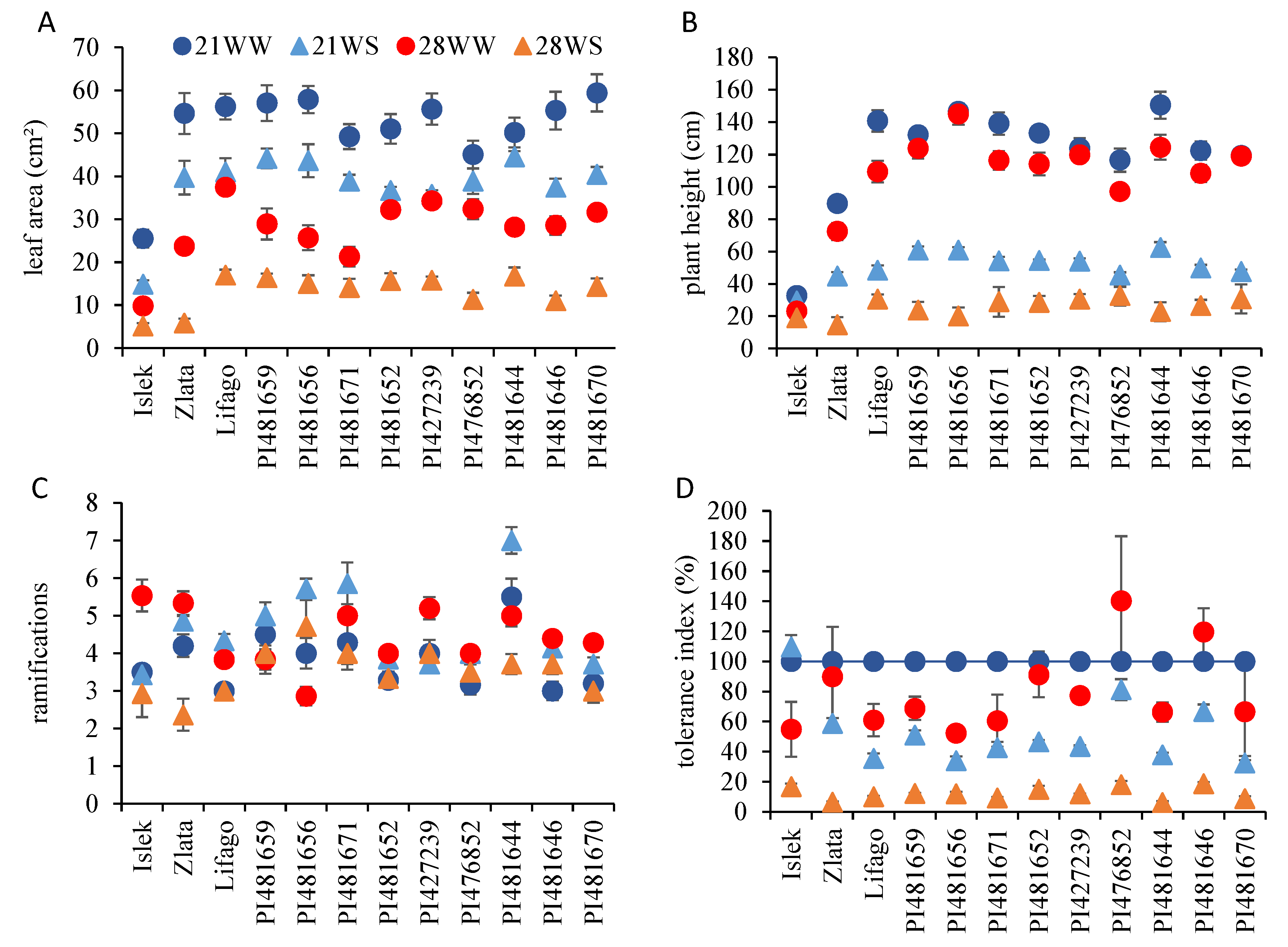
2.1. Vegetative Growth
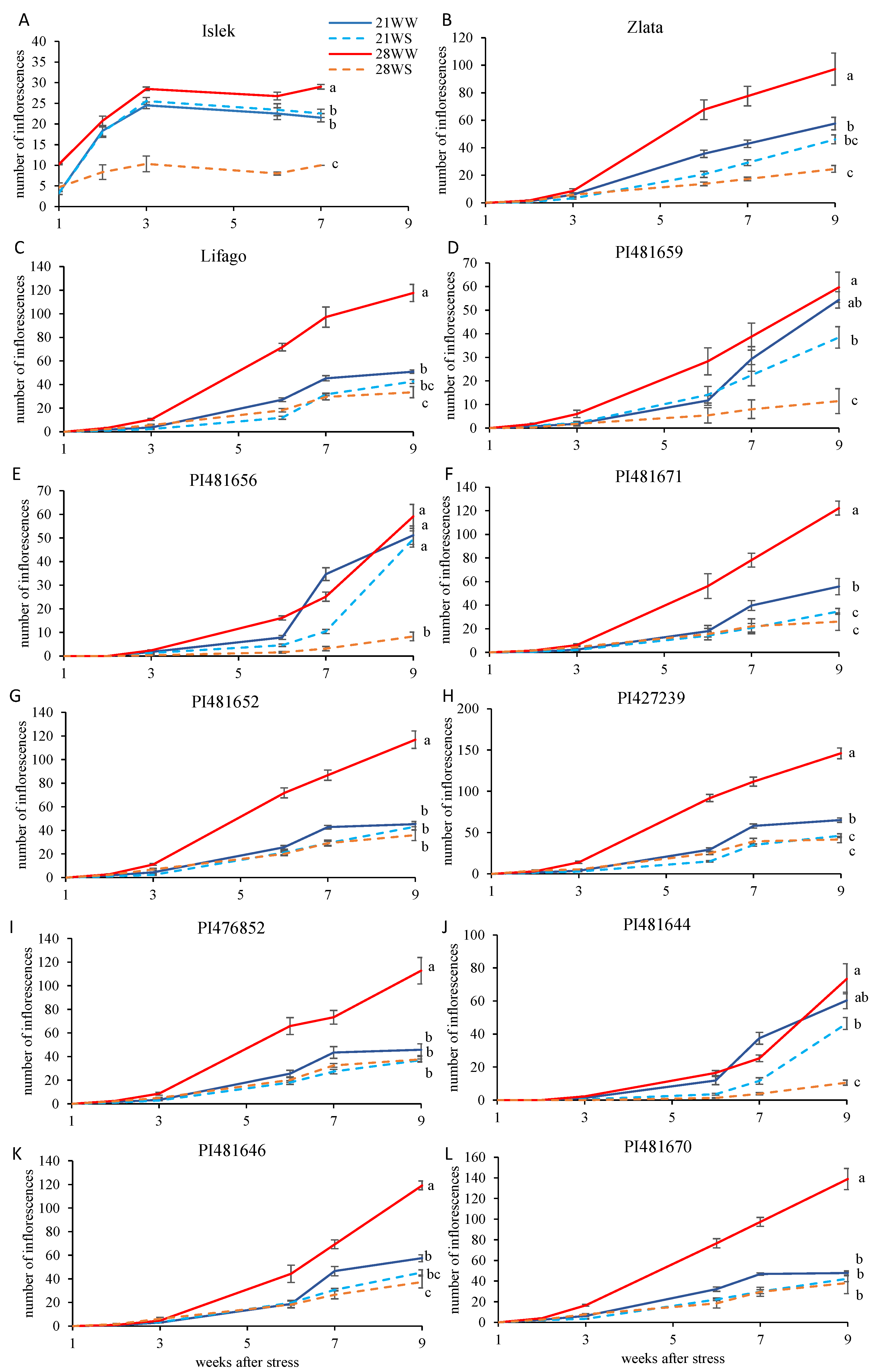
2.2. Reproductive Growth
2.3. Physiological Parameters
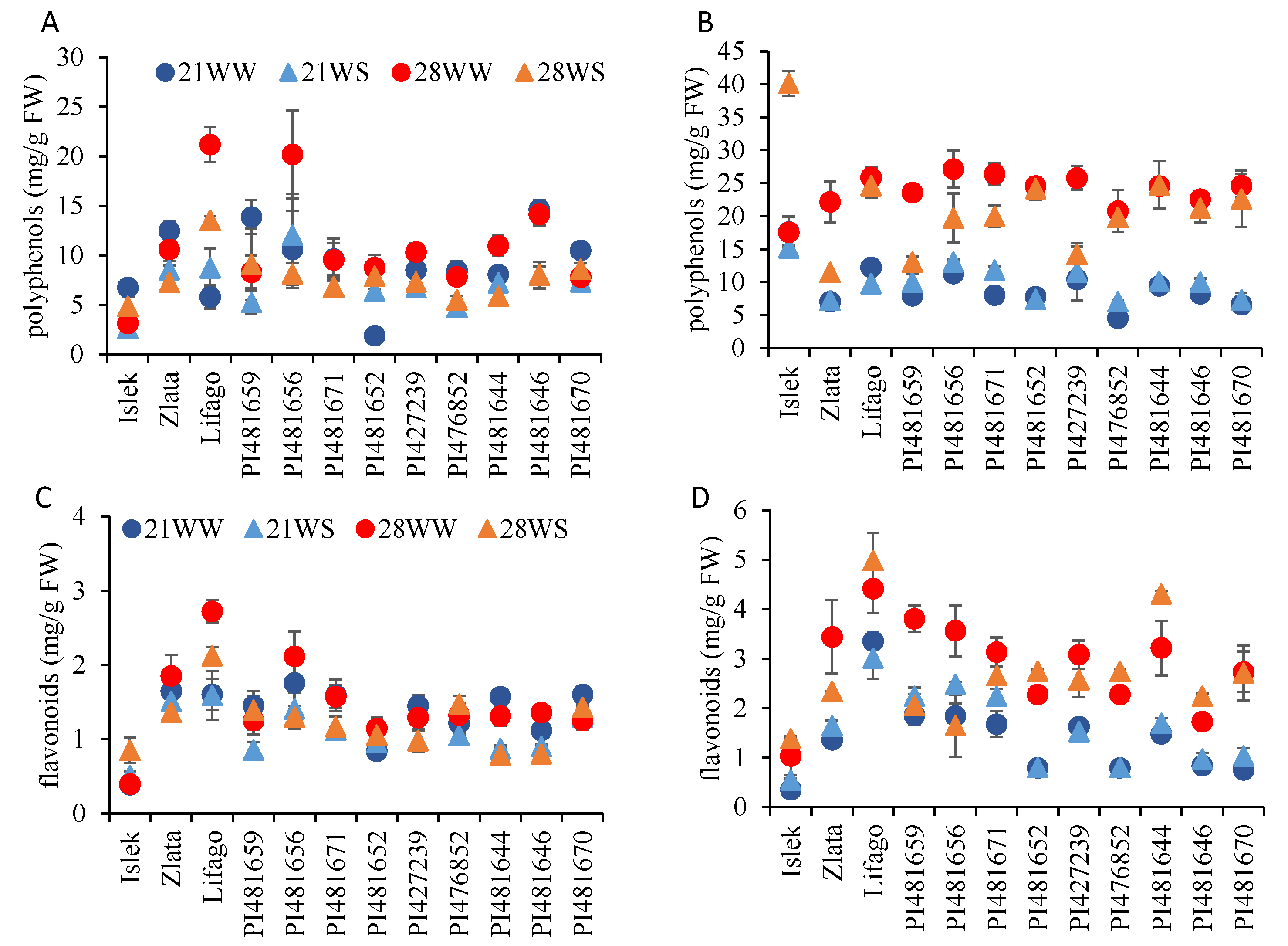
2.4. Antioxidant Production
3. Discussion
4. Materials and Methods
4.1. Plant Culture and Growth Conditions
4.2. Plant Growth Parameters
4.3. Flowering and Seed Parameters
4.4. Physiological Parameters
4.5. Polyphenol and Flavonoid Concentrations
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tollefson, J. IPCC Climate Report: Earth Is Warmer than It’s Been in 125,000 Years. Nature 2021, 596, 171–172. [Google Scholar] [CrossRef]
- Feller, U.; Vaseva, I.I. Extreme Climatic Events: Impacts of Drought and High Temperature on Physiological Processes in Agronomically Important Plants. Front. Environ. Sci. 2014, 2, 39. [Google Scholar] [CrossRef] [Green Version]
- Chaudhry, S.; Sidhu, G.P.S. Climate Change Regulated Abiotic Stress Mechanisms in Plants: A Comprehensive Review. Plant Cell Rep. 2022, 41, 1–31. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global Synthesis of Drought Effects on Cereal, Legume, Tuber and Root Crops Production: A Review. Agric. Water Manag. 2017, 179, 18–33. [Google Scholar] [CrossRef] [Green Version]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing Climate-Resilient Crops: Improving Plant Tolerance to Stress Combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef]
- Sharma, M.; Kumar, P.; Verma, V.; Sharma, R.; Bhargava, B.; Irfan, M. Understanding Plant Stress Memory Response for Abiotic Stress Resilience: Molecular Insights and Prospects. Plant Physiol. Biochem. 2022, 179, 10–24. [Google Scholar] [CrossRef]
- Lamers, J.; van der Meer, T.; Testerink, C. How Plants Sense and Respond to Stressful Environments. Plant Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.K.; Dhanapal, S.; Yadav, B.S. The Dynamic Responses of Plant Physiology and Metabolism during Environmental Stress Progression. Mol. Biol. Rep. 2020, 47, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Rane, J.; Singh, A.K.; Kumar, M.; Boraiah, K.M.; Meena, K.K.; Pradhan, A.; Prasad, P.V.V. The Adaptation and Tolerance of Major Cereals and Legumes to Important Abiotic Stresses. Int. J. Mol. Sci. 2021, 22, 12970. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant Adaptations to the Combination of Drought and High Temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamal, N.M.; Alnor Gorafi, Y.S.; Abdelrahman, M.; Abdellatef, E.; Tsujimoto, H. Stay-Green Trait: A Prospective Approach for Yield Potential, and Drought and Heat Stress Adaptation in Globally Important Cereals. Int. J. Mol. Sci. 2019, 20, 5837. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, E.E.; Webber, H.; Gaiser, T.; Naab, J.; Ewert, F. Heat Stress in Cereals: Mechanisms and Modelling. Eur. J. Agron. 2015, 64, 98–113. [Google Scholar] [CrossRef]
- Jagadish, S.V.K. Heat Stress during Flowering in Cereals—Effects and Adaptation Strategies. New Phytol. 2020, 226, 1567–1572. [Google Scholar] [CrossRef] [Green Version]
- Sardans, J.; Gargallo-Garriga, A.; Urban, O.; Klem, K.; Walker, T.W.N.; Holub, P.; Janssens, I.A.; Peñuelas, J. Ecometabolomics for a Better Understanding of Plant Responses and Acclimation to Abiotic Factors Linked to Global Change. Metabolites 2020, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Hlaváčová, M.; Klem, K.; Rapantová, B.; Novotná, K.; Urban, O.; Hlavinka, P.; Smutná, P.; Horáková, V.; Škarpa, P.; Pohanková, E.; et al. Interactive Effects of High Temperature and Drought Stress during Stem Elongation, Anthesis and Early Grain Filling on the Yield Formation and Photosynthesis of Winter Wheat. Field Crops Res. 2018, 221, 182–195. [Google Scholar] [CrossRef]
- Cheng, A. Review: Shaping a Sustainable Food Future by Rediscovering Long-Forgotten Ancient Grains. Plant Sci. 2018, 269, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.P.; Rahman, H.; Thushar, S.; Singh, R.K. Healthy and Resilient Cereals and Pseudo-Cereals for Marginal Agriculture: Molecular Advances for Improving Nutrient Bioavailability. Front. Genet. 2020, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.; Chaudhary, H.K. Nutraceutical Crop Buckwheat: A Concealed Wealth in the Lap of Himalayas. Crit. Rev. Biotechnol. 2020, 40, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Aubert, L.; Decamps, C.; Jacquemin, G.; Quinet, M. Comparison of Plant Morphology, Yield and Nutritional Quality of Fagopyrum esculentum and Fagopyrum tataricum Grown under Field Conditions in Belgium. Plants 2021, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.C.; Chaudhari, G.V.; Sood, S.; Kant, L.; Pattanayak, A.; Zhang, K.; Fan, Y.; Janovská, D.; Meglič, V.; Zhou, M. Revisiting the Versatile Buckwheat: Reinvigorating Genetic Gains through Integrated Breeding and Genomics Approach. Planta 2019, 250, 783–801. [Google Scholar] [CrossRef]
- Joshi, D.C.; Sood, S.; Hosahatti, R.; Kant, L.; Pattanayak, A.; Kumar, A.; Yadav, D.; Stetter, M.G. From Zero to Hero: The Past, Present and Future of Grain Amaranth Breeding. Theor. Appl. Genet. 2018, 131, 1807–1823. [Google Scholar] [CrossRef]
- Hunt, H.V.; Shang, X.; Jones, M.K. Buckwheat: A Crop from Outside the Major Chinese Domestication Centres? A Review of the Archaeobotanical, Palynological and Genetic Evidence. Veg. Hist. Archaeobotany 2018, 27, 493–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koval, D.; Plocková, M.; Kyselka, J.; Skřivan, P.; Sluková, M.; Horáčková, Š. Buckwheat Secondary Metabolites: Potential Antifungal Agents. J. Agric. Food Chem. 2020, 68, 11631–11643. [Google Scholar] [CrossRef]
- Kreft, I.; Zhou, M.; Golob, A.; Germ, M.; Likar, M.; Dziedzic, K.; Luthar, Z. Breeding Buckwheat for Nutritional Quality. Breed. Sci. 2020, 70, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacquemart, A.-L.; Cawoy, V.; Kinet, J.-M.; Ledent, J.-F.; Quinet, M. Is Buckwheat (Fagopyrum esculentum Moench) Still a Valuable Crop Today? Eur. J. Plant Sci. Biotechnol. 2012, 6, 1–10. [Google Scholar]
- Ahmed, A.; Khalid, N.; Ahmad, A.; Abbasi, N.A.; Latif, M.S.Z.; Randhawa, M.A. Phytochemicals and Biofunctional Properties of Buckwheat: A Review. J. Agric. Sci. 2014, 152, 349–369. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive Value of Pseudocereals and Their Increasing Use as Functional Gluten-Free Ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Zhu, F. Chemical Composition and Health Effects of Tartary Buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Christa, K.; Soral-Śmietana, M. Buckwheat Grains and Buckwheat Products—Nutritional and Prophylactic Value of Their Components—A Review. Czech J. Food Sci. 2008, 26, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal Grains: Nutritional Value, Health Benefits and Current Applications for the Development of Gluten-Free Foods. Food Chem. Toxicol. 2020, 137, 111178. [Google Scholar] [CrossRef]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from Garden: Bioactive Compounds of Buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-Y.; Wang, B.; Schoen, D.J.; Huang, S.-Q. Transitions from Distyly to Homostyly Are Associated with Floral Evolution in the Buckwheat Genus (Fagopyrum). Am. J. Bot. 2017, 104, 1232–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, E. 54. Buckwheat—The World’s Most Biodiversity-Friendly Crop? Biodiversity 2017, 18, 108–123. [Google Scholar] [CrossRef]
- Zhang, K.; He, M.; Fan, Y.; Zhao, H.; Gao, B.; Yang, K.; Li, F.; Tang, Y.; Gao, Q.; Lin, T. Resequencing of Global Tartary Buckwheat Accessions Reveals Multiple Domestication Events and Key Loci Associated with Agronomic Traits. Genome Biol. 2020, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhao, H.; Sun, L.; Wu, W.; Li, C.; Wu, Q. Genome-Wide Identification of MAPK Gene Family Members in Fagopyrum tataricum and Their Expression during Development and Stress Responses. BMC Genom. 2022, 23, 96. [Google Scholar] [CrossRef]
- Ruan, J.; Zhou, Y.; Yan, J.; Zhou, M.; Woo, S.-H.; Weng, W.; Cheng, J.; Zhang, K. Tartary Buckwheat: An Under-Utilized Edible and Medicinal Herb for Food and Nutritional Security. Food Rev. Int. 2020, 38, 440–454. [Google Scholar] [CrossRef]
- Zou, L.; Wu, D.; Ren, G.; Hu, Y.; Peng, L.; Zhao, J.; Garcia-Perez, P.; Carpena, M.; Prieto, M.A.; Cao, H.; et al. Bioactive Compounds, Health Benefits, and Industrial Applications of Tartary Buckwheat (Fagopyrum tataricum). Crit. Rev. Food Sci. Nutr. 2021, 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, J.; Wang, S.; Zhou, Y. Effect of High Hydrostatic Pressure Treatment on the Formation and in Vitro Digestion of Tartary Buckwheat Starch/Flavonoid Complexes. Food Chem. 2022, 382, 132324. [Google Scholar] [CrossRef]
- Li, J.; Gong, Y.; Li, J.; Fan, L. In Vitro Inhibitory Effects of Polyphenols from Tartary Buckwheat on Xanthine Oxidase: Identification, Inhibitory Activity, and Action Mechanism. Food Chem. 2022, 379, 132100. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fu, G.; Xu, Z.; Dong, B.; Li, R.; Wan, Y.; Jiang, G.; Liu, C. In Vitro Nutrition Properties of Whole Tartary Buckwheat Straight Noodles and Its Amelioration on Type 2 Diabetic Rats. Food Biosci. 2022, 46, 101525. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Ma, B.; Gao, Q.; Du, H.; Han, Y.; Li, Y.; Cao, Y.; Qi, M.; Zhu, Y.; et al. The Tartary Buckwheat Genome Provides Insights into Rutin Biosynthesis and Abiotic Stress Tolerance. Mol. Plant 2017, 10, 1224–1237. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-C.; Chang, L.-C.; Wang, M.-L.; Guo, C.-L.; Chung, M.-C.; Jauh, G.-Y. Identification and Exploration of Pollen Tube Small Proteins Encoded by Pollination-Induced Transcripts. Plant Cell Physiol. 2011, 52, 1546–1559. [Google Scholar] [CrossRef]
- Wan, Y. Water Deficit and Recovery-Induced Changes in Growth, Photosynthetic Characteristics, Antioxidant Enzymes and Yield of Two Tartary Buckwheat Genotypes. IJAB 2021, 25, 483–491. [Google Scholar] [CrossRef]
- Aubert, L.; Konrádová, D.; Kebbas, S.; Barris, S.; Quinet, M. Comparison of High Temperature Resistance in Two Buckwheat Species Fagopyrum esculentum and Fagopyrum tataricum. J. Plant Physiol. 2020, 251, 153222. [Google Scholar] [CrossRef]
- Aubert, L.; Konrádová, D.; Barris, S.; Quinet, M. Different Drought Resistance Mechanisms between Two Buckwheat Species Fagopyrum esculentum and Fagopyrum tataricum. Physiol. Plant. 2020, 172, 577–586. [Google Scholar] [CrossRef]
- Blanchard-Gros, R.; Bigot, S.; Martinez, J.-P.; Lutts, S.; Guerriero, G.; Quinet, M. Comparison of Drought and Heat Resistance Strategies among Six Populations of Solanum chilense and Two Cultivars of Solanum lycopersicum. Plants 2021, 10, 1720. [Google Scholar] [CrossRef]
- Golob, A.; Stibilj, V.; Kreft, I.; Germ, M. The Feasibility of Using Tartary Buckwheat as a Se-Containing Food Material. J. Chem. 2015, 2015, 246042. [Google Scholar] [CrossRef] [Green Version]
- Cepková, P.H.; Janovská, D.; Stehno, Z. Assessment of Genetic Diversity of Selected Tartary and Common Buckwheat Accessions. Span. J. Agric. Res. 2009, 7, 844–854. [Google Scholar] [CrossRef] [Green Version]
- Sadok, W.; Lopez, J.R.; Smith, K.P. Transpiration Increases under High-Temperature Stress: Potential Mechanisms, Trade-Offs and Prospects for Crop Resilience in a Warming World. Plant Cell Environ. 2021, 44, 2102–2116. [Google Scholar] [CrossRef] [PubMed]
- Fabjan, N.; Rode, J.; Kosir, I.J.; Wang, Z.; Zhang, Z.; Kreft, I. Tartary Buckwheat (Fagopyrum tataricum Gaertn.) as a Source of Dietary Rutin and Quercitrin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef]
- Lee, L.-S.; Choi, E.-J.; Kim, C.-H.; Sung, J.-M.; Kim, Y.-B.; Seo, D.-H.; Choi, H.-W.; Choi, Y.-S.; Kum, J.-S.; Park, J.-D. Contribution of Flavonoids to the Antioxidant Properties of Common and Tartary Buckwheat. J. Cereal Sci. 2016, 68, 181–186. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant Tolerance to High Temperature in a Changing Environment: Scientific Fundamentals and Production of Heat Stress-Tolerant Crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [Green Version]
- Michiyama, H.; Arikuni, M.; Hirano, T. Effect of Air Temperature on the Growth, Flowering and Ripening in Common Buckwheat. In Proceedings of the 8th ISB, Chunchon, Korea, 30 August–2 September 2001; pp. 138–142. [Google Scholar]
- Cawoy, V.; Ledent, J.-F.; Kinet, J.-M.; Jacquemart, A.-L. Floral Biology of Common Buckwheat (Fagopyrum esculentum Moench). Eur. J. Plant Sci. Biotechnol. 2009, 3, 1–9. [Google Scholar]
- Lachmann, S.; Adachi, T. Studies on the Influence of Photoperiod and Temperature on Floral Traits in Buckwheat (Fagopyrum esculentum Moench) under Controlled Stress Conditions. Plant Breed. 1990, 105, 248–253. [Google Scholar] [CrossRef]
- Michiyama, H.; Oizumi, K.; Takano, R.; Hirano, T.; Suzuki, T.; Morishita, T. Effect of Day Length and Temperature on the Growth, Flowering and Seed-Setting in a Rice-Tartary Buckwheat Line. In Proceedings of the 13th ISB, Cheongju, Korea, 9–11 September 2016; pp. 109–115. [Google Scholar]
- Płażek, A.; Słomka, A.; Kopeć, P.; Dziurka, M.; Hornyák, M.; Sychta, K.; Pastuszak, J.; Dubert, F. Effects of High Temperature on Embryological Development and Hormone Profile in Flowers and Leaves of Common Buckwheat (Fagopyrum esculentum Moench). Int. J. Mol. Sci. 2019, 20, 1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Słomka, A.; Michno, K.; Dubert, F.; Dziurka, M.; Kopeć, P.; Płażek, A. Embryological Background of Low Seed Set in Distylous Common Buckwheat (Fagopyrum esculentum Moench) with Biased Morph Ratios, and Biostimulant-Induced Improvement of It. Crop Pasture Sci. 2017, 68, 680–690. [Google Scholar] [CrossRef]
- Xiao-Jiao, X.; Yong-Qing, Z.; Xing-Xing, M.; Tian, Y.; Ping-Ping, L.; Wen-Yan, Z.; Ru, W. Effects of phosphorus application depth on the growth and root distribution of tartary buckwheat in infertile soil under water stress. J. Plant Nutr. Fertil. 2020, 26, 1481–1491. [Google Scholar] [CrossRef]
- Xiang, D.; Ma, C.; Song, Y.; Wu, Q.; Wu, X.; Sun, Y.; Zhao, G.; Wan, Y. Post-Anthesis Photosynthetic Properties Provide Insights into Yield Potential of Tartary Buckwheat Cultivars. Agronomy 2019, 9, 149. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Chen, Q.; Rong, Y.; Tang, B.; Zhu, L.; Ren, R.; Shi, T.; Chen, Q. Transcriptome Analysis Revealed Gene Regulatory Network Involved in PEG-Induced Drought Stress in Tartary Buckwheat (Fagopyrum tartaricum). PeerJ 2021, 9, e11136. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Wei, W.; Ouyang, J.Y.; Le, L.; Zhao, G.; Peng, L.; Wan, Y. Nitrogen Alleviates Seedling Stage Drought Stress Response on Growth and Yield of Tartary Buckwheat. Int. J. Agric. Biol. 2020, 24, 1167–1177. [Google Scholar]
- Kumar, S.; Gupta, D.; Nayyar, H. Comparative Response of Maize and Rice Genotypes to Heat Stress: Status of Oxidative Stress and Antioxidants. Acta Physiol. Plant. 2012, 34, 75–86. [Google Scholar] [CrossRef]
- Raja, V.; Qadir, S.U.; Alyemeni, M.N.; Ahmad, P. Impact of Drought and Heat Stress Individually and in Combination on Physio-Biochemical Parameters, Antioxidant Responses, and Gene Expression in Solanum lycopersicum. 3 Biotech 2020, 10, 208. [Google Scholar] [CrossRef]
- Zhou, R.; Kong, L.; Wu, Z.; Rosenqvist, E.; Wang, Y.; Zhao, L.; Zhao, T.; Ottosen, C.-O. Physiological Response of Tomatoes at Drought, Heat and Their Combination Followed by Recovery. Physiol. Plant. 2019, 165, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Descamps, C.; Quinet, M.; Baijot, A.; Jacquemart, A.-L. Temperature and Water Stress Affect Plant-Pollinator Interactions in Borago Officinalis (Boraginaceae). Ecol. Evol. 2018, 8, 3443–3456. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Global Warming and Sexual Plant Reproduction. Trends Plant Sci. 2009, 14, 30–36. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Staggenborg, S.A.; Ristic, Z.; Ahuja, L.R.; Reddy, V.R.; Saseendran, S.A.; Yu, Q. Impacts of Drought and/or Heat Stress on Physiological, Developmental, Growth, and Yield Processes of Crop Plants. In Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes; Ahuja, L., Reddy, V., Saseendran, S., Yu, Q., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 2008; Volume 1, pp. 301–355. [Google Scholar]
- Sinha, R.; Fritschi, F.B.; Zandalinas, S.I.; Mittler, R. The Impact of Stress Combination on Reproductive Processes in Crops. Plant Sci. 2021, 311, 111007. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]



| Parameter * | Variety | Temp | Water | Var × Temp | Var × Water | Temp × Water | Var × Temp × Water |
|---|---|---|---|---|---|---|---|
| Leaves | F = 5.58 *** | F = 454.3 *** | F = 677.6 *** | F = 14.8 *** | F = 4.8 *** | F = 501.3 *** | F = 3.8 *** |
| Height | F = 90.14 *** | F = 244.1 *** | F = 3246 *** | F = 2.5 ** | F = 50.6 *** | F = 18.4 *** | F = 2.6 ** |
| Leaf area | F = 53.0 *** | F = 1552.9 *** | F = 586.4 *** | F = 5.2 *** | F = 3.7 *** | F = 31.5 *** | F = 2.3 * |
| Ramification | F = 7.30 *** | F = 7.7 ** | F = 4.8 * | F = 3.7 *** | F = 691.6 *** | F = 61.6 *** | F = 2.5 ** |
| Tolerance index | F = 8.2 *** | F = 212.6 *** | F = 690.3 *** | F = 4.3 *** | F = 4.0 *** | F = 24.2 *** | F = 6.4 *** |
| Leaf DW | F = 25.7 *** | F = 1.2 NS | F = 165.6 *** | F = 1.8 * | F = 4.3 *** | F = 200.8 *** | F = 3.5 *** |
| Stem DW | F = 36.1 *** | F = 55.6 *** | F = 1098.4 *** | F = 3.1 ** | F = 12.1 *** | F = 75.9 *** | F = 2.4 * |
| Inflorescences | F = 13.7 *** | F = 110.8 *** | F = 646.5 *** | F = 15.2 *** | F = 3.6 *** | F = 368.9 *** | F = 2.6 ** |
| Node first inflo | F = 34.6 *** | F = 4.8 * | F = 0.03 NS | F = 0.9 NS | F = 0.7 NS | F = 0.9 NS | F = 2.1 * |
| Days to inflo | F = 55.7 *** | F = 0.3 NS | F = 22.5 *** | F = 3.6 *** | F = 3.6 *** | F = 12.0 *** | F = 2.6 ** |
| Flowers/inflo | F = 14.3 *** | F = 203.2 *** | F = 0.02 NS | F = 17.4 *** | F = 1.9 * | F = 4.9 * | F = 1.1 NS |
| Days to seeds | F = 61.0 *** | F = 1611.1 *** | F = 12.6 *** | F = 13.2 *** | F = 2.3 ** | F = 0.03 NS | F = 0.61 NS |
| Seed set | F = 4.3 *** | F = 2777 *** | F = 3.2 NS | F = 4.3 *** | F = 2.7 ** | F = 3.2 NS | F = 2.7 * |
| Seeds/plant | F = 88.7 *** | F = 2404.5 *** | F = 1545.0 *** | F = 92.1 *** | F = 37.3 *** | F = 81.3 *** | F = 22.9 *** |
| 1000-seed weight | F = 27.8 *** | - | F = 92.7 *** | - | F = 17.7 *** | - | - |
| Variety | Leaf Dry Weight (g) | Stem Dry Weight (g) | ||||||
|---|---|---|---|---|---|---|---|---|
| 21WW | 21WS | 28WW | 28WS | 21WW | 21WS | 28WW | 28WS | |
| Islek | 0.14 ± 0.01 ab | 0.13 ± 0.03 ab | 0.26 ± 0.04 b | 0.08 ± 0.02 a | 0.24 ± 0.03 b | 0.17 ± 0.02 a | 0.17 ± 0.01 a | 0.03 ± 0.01 c |
| Zlata | 0.68 ± 0.05 ab | 0.57 ± 0.05 ab | 1.09 ± 0.19 a | 0.11 ± 0.01 a | 1.02 ± 0.06 a | 0.46 ± 0.08 ab | 1.21 ± 0.22 a | 0.06 ± 0.01 b |
| Lifago | 0.49 ± 0.10 a | 0.71 ± 0.09 ab | 1.15 ± 0.24 b | 0.35 ± 0.02 a | 2.72 ± 0.28 b | 0.82 ± 0.11 a | 2.35 ± 0.17 b | 0.20 ± 0.01 c |
| PI481659 | 0.52 ± 0.15 ab | 0.84 ± 0.02 ab | 1.22 ± 0.11 a | 0.37 ± 0.01 b | 2.33 ± 0.19 b | 0.82 ± 0.10 a | 1.95 ± 0.13 b | 0.22 ± 0.02 c |
| PI481656 | 0.62 ± 0.05 a | 0.76 ± 0.08 b | 0.71 ± 0.11 b | 0.39 ± 0.06 a | 2.74 ± 0.08 b | 0.60 ± 0.08 a | 1.86 ± 0.05 d | 0.22 ± 0.04 c |
| PI481671 | 1.03 ± 0.15 a | 0.78 ± 0.08 ab | 0.88 ± 0.09 a | 0.39 ± 0.01 b | 3.04 ± 0.35 b | 0.75 ± 0.12 a | 2.42 ± 0.43 b | 0.19 ± 0.02 a |
| PI481652 | 0.45 ± 0.01 ab | 0.66 ± 0.03 a | 0.99 ± 0.05 c | 0.31 ± 0.09 b | 1.87 ± 0.3 b | 0.52 ± 0.03 a | 2.39 ± 0.3 b | 0.21 ± 0.03 a |
| PI427239 | 1.15 ± 0.18 b | 0.66 ± 0.07 a | 1.13 ± 0.03 b | 0.37 ± 0.03 a | 2.05 ± 0.4 b | 0.64 ± 0.07 a | 2.65 ± 0.02 b | 0.18 ± 0.02 c |
| PI496852 | 0.28 ± 0.07 a | 0.62 ± 0.05 a | 1.17 ± 0.16 b | 0.22 ± 0.05 a | 1.03 ± 0.22 a | 0.71 ± 0.11 ab | 2.09 ± 0.37 c | 0.18 ± 0.04 b |
| PI481644 | 1.04 ± 0.10 a | 0.73 ± 0.05 ab | 1.55 ± 0.16 c | 0.28 ± 0.06 b | 3.08 ± 0.3 b | 0.85 ± 0.1 a | 2.24 ± 0.03 b | 0.10 ± 0.02 c |
| PI481646 | 0.44 ± 0.07 a | 0.63 ± 0.04 ab | 0.91 ± 0.17 b | 0.31 ±0.02 a | 1.24 ± 0.09 b | 0.68 ± 0.11 a | 2.39 ± 0.10 d | 0.18 ± 0.04 c |
| PI481670 | 0.69 ± 0.23 ab | 0.72 ± 0.09 ab | 1.08 ± 0.14 b | 0.28 ± 0.07 a | 2.42 ± 0.46 b | 0.44 ± 0.03 a | 2.60 ± 0.83 b | 0.23 ± 0.04 a |
| Parameter * | Variety | Temp | Water | Var × Temp | Var × Water | Temp × Water | Var × Temp × Water |
|---|---|---|---|---|---|---|---|
| CCI | F = 47.9 *** | F = 137.1 *** | F = 1995.7 *** | F = 7.4 *** | F = 23.8 *** | F = 133.2 *** | F = 12.2 *** |
| ϕPSII | F = 1.2 NS | F = 1.8 NS | F = 1.6 NS | F = 1.6 NS | F = 1.2 NS | F = 2.9 NS | F = 1.8 NS |
| NPQ | F = 1.1 NS | F = 14.7 *** | F = 0.2 NS | F = 1.4 NS | F = 0.9 NS | F = 1.3 NS | F = 1.1 NS |
| Ai | F = 1.5 NS | F = 9.8 ** | F = 0.1 NS | F = 2.9 ** | F = 1.9 * | F = 0.4 NS | F = 1.1 NS |
| Ei | F = 2.9 *** | F = 148.4 *** | F = 85.9 *** | F = 5.6 *** | F = 2.1 * | F = 73.0 *** | F = 3.7 *** |
| gs | F = 2.8 ** | F = 28.3 *** | F = 43.4 *** | F = 5.1 *** | F = 2.5 *** | F = 46.3 *** | F = 3.8 *** |
| Polyphenols (leaf) | F = 14.8 *** | F = 8.4 ** | F = 45.1 *** | F = 3.6 *** | F = 3.1 ** | F = 1.3 NS | F = 2.0* |
| Polyphenols (inflo) | F = 9.3 *** | F = 783.5 *** | F = 1.5NS | F = 3.7 *** | F = 3.2 ** | F = 13.2 *** | F = 6.7 *** |
| Flavonoids (leaf) | F = 27.3 *** | F = 6.7 * | F = 31.6 *** | F = 2.3 * | F = 3.6 *** | F = 0.4 NS | F = 1.7 NS |
| Flavonoids (inflo) | F = 47.9 *** | F = 420.7 *** | F = 1.2 NS | F = 4.6 *** | F = 2.6 ** | F = 11.7 *** | F = 4.6 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aubert, L.; Quinet, M. Comparison of Heat and Drought Stress Responses among Twelve Tartary Buckwheat (Fagopyrum tataricum) Varieties. Plants 2022, 11, 1517. https://doi.org/10.3390/plants11111517
Aubert L, Quinet M. Comparison of Heat and Drought Stress Responses among Twelve Tartary Buckwheat (Fagopyrum tataricum) Varieties. Plants. 2022; 11(11):1517. https://doi.org/10.3390/plants11111517
Chicago/Turabian StyleAubert, Lauranne, and Muriel Quinet. 2022. "Comparison of Heat and Drought Stress Responses among Twelve Tartary Buckwheat (Fagopyrum tataricum) Varieties" Plants 11, no. 11: 1517. https://doi.org/10.3390/plants11111517






