Adaptative Responses of Common and Tartary Buckwheat to Different Altitudes
Abstract
1. Introduction
2. Results and Discussion
2.1. Soil Properties, Weather Conditions and Physiological Parameters of Buckwheats
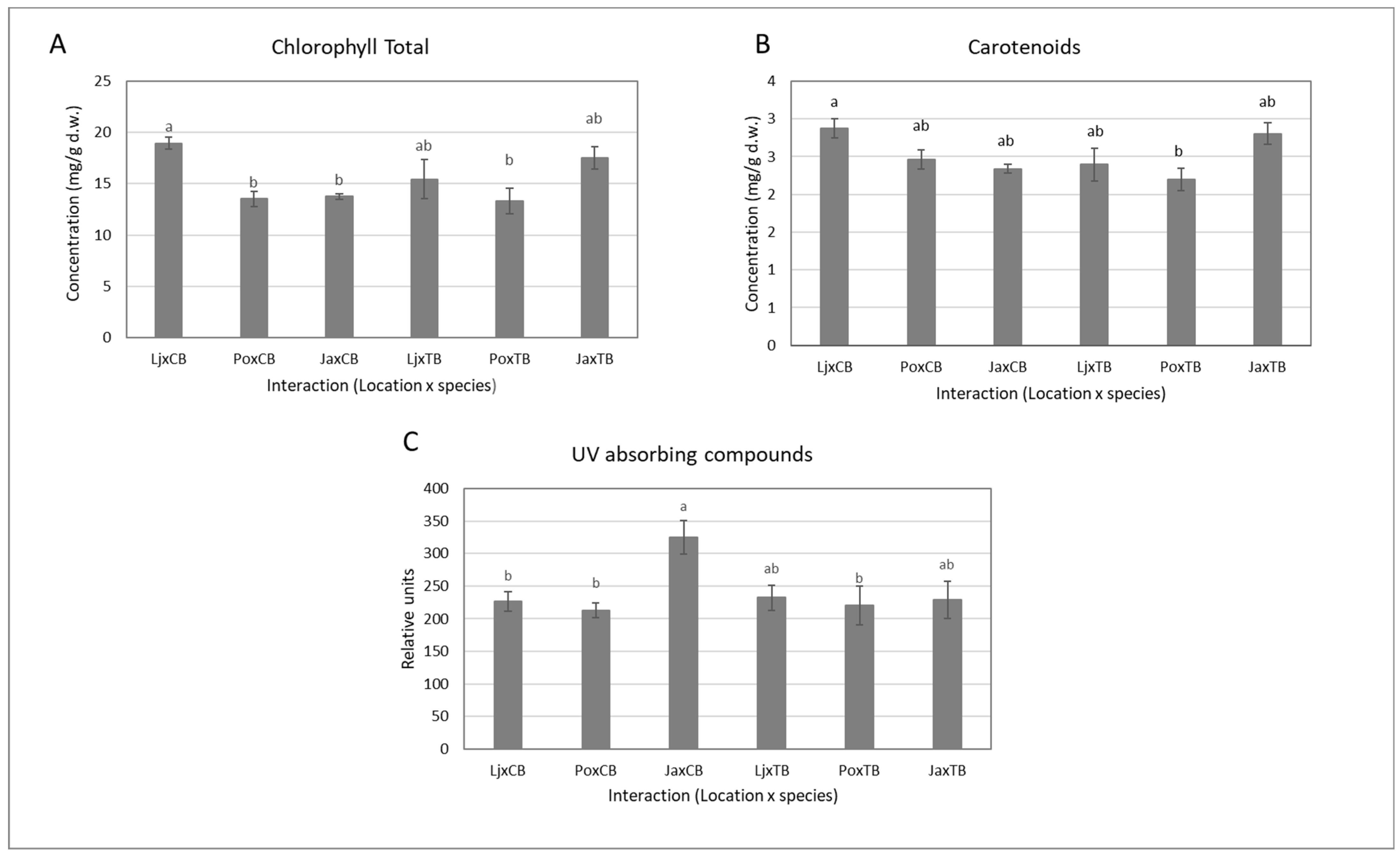
2.2. Biochemical Characteristics
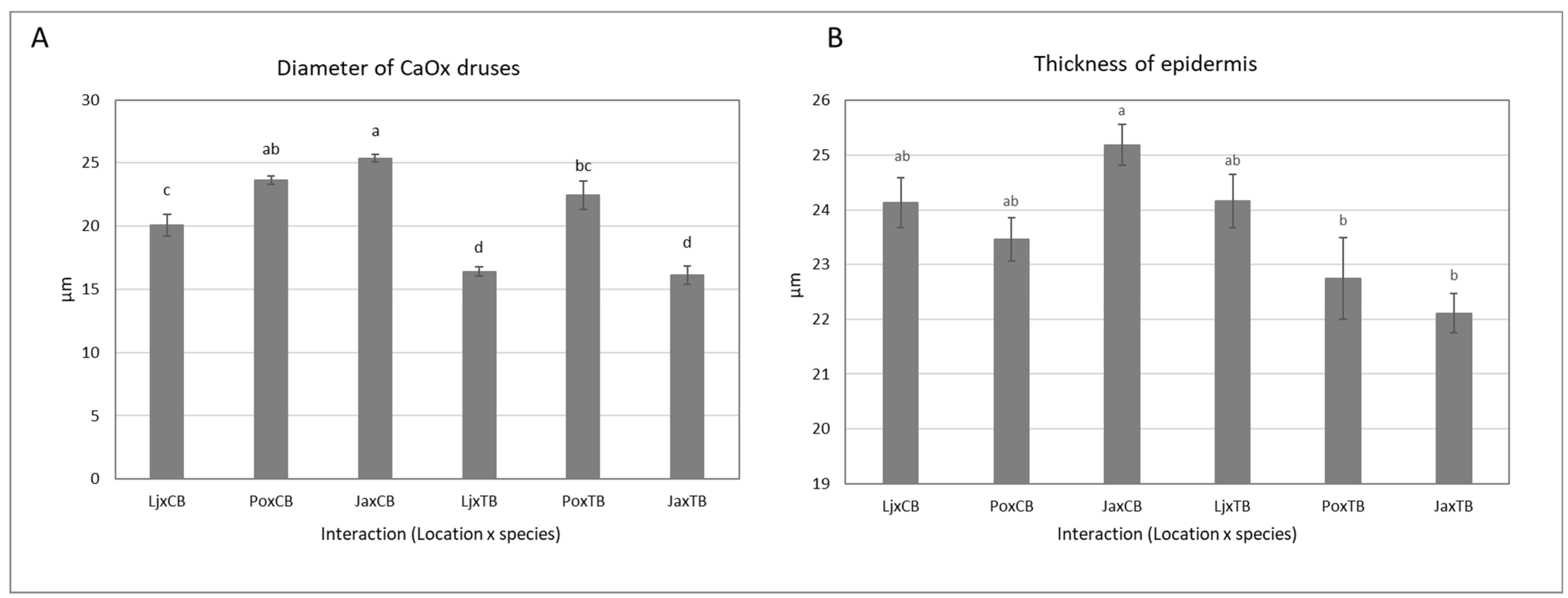
2.3. Morphological Characteristics
3. Materials and Methods
3.1. Experimental Plan
3.2. Biochemical Parameters
3.3. Physiological Parameters
3.4. Morphological Parameters
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogel, E.; Donat, M.G.; Alexander, L.V.; Meinshausen, M.; Ray, D.K.; Karoly, D.; Meinshausen, N.; Frieler, K. The Effects of Climate Extremes on Global Agricultural Yields. Environ. Res. Lett. 2019, 14, 054010. [Google Scholar] [CrossRef]
- Rosbakh, S.; Margreiter, V.; Jelcic, B. Seedlings of Alpine Species Do Not Have Better Frost-Tolerance than Their Lowland Counterparts. Alp. Bot. 2020, 130, 179–185. [Google Scholar] [CrossRef]
- Körner, C. Plant ecology at high elevations. In Alpine Plant Life; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–22. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Robberecht, R.; Billings, W.D. A Steep Latitudinal Gradient of Solar Ultraviolet-B Radiation in the Arctic-Alpine Life Zone. Ecology 1980, 61, 600–611. [Google Scholar] [CrossRef]
- Blumthaler, M.; Ambach, W.; Huber, M. Altitude Effect of Solar UV Radiation Dependent on Albedo, Turbidity, and Solar Elevation. Meteorol. Zeitschrift 1993, 2, 116–120. [Google Scholar] [CrossRef]
- Booij-James, I.S.; Dube, S.K.; Jansen, M.A.K.; Edelman, M.; Mattoo, A.K. Ultraviolet-B Radiation Impacts Light-Mediated Turnover of the Photosystem II Reaction Center Heterodimer in Arabidopsis Mutants Altered in Phenolic Metabolism. Plant Physiol. 2000, 124, 1275–1283. [Google Scholar] [CrossRef]
- Yao, X.; Jianzhou, C.; Xueli, H.; Binbin, L.; Jingmin, L.; Zhaowei, Y. Effects of Selenium on Agronomical Characters of Winter Wheat Exposed to Enhanced Ultraviolet-B. Ecotoxicol. Environ. Saf. 2013, 92, 320–326. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, L.; Qi, D. Variation in Leaf Traits at Different Altitudes Reflects the Adaptive Strategy of Plants to Environmental Changes. Ecol. Evol. 2020, 10, 8166–8175. [Google Scholar] [CrossRef]
- Halbritter, A.H.; Fior, S.; Keller, I.; Billeter, R.; Edwards, P.J.; Holderegger, R.; Karrenberg, S.; Pluess, A.R.; Widmer, A.; Alexander, J.M. Trait Differentiation and Adaptation of Plants along Elevation Gradients. J. Evol. Biol. 2018, 31, 784–800. [Google Scholar] [CrossRef]
- Nakata, P.A. Plant Calcium Oxalate Crystal Formation, Function, and Its Impact on Human Health. Front. Biol. 2012, 7, 254–266. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. Calcium Oxalate in Plants: Formation and Function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef]
- Gal, A.; Brumfeld, V.; Weiner, S.; Addadi, L.; Oron, D. Certain Biominerals in Leaves Function as Light Scatterers. Adv. Mater. 2012, 24, OP77–OP83. [Google Scholar] [CrossRef] [PubMed]
- Golob, A.; Stibilj, V.; Nečemer, M.; Kump, P.; Kreft, I.; Hočevar, A.; Gaberščik, A.; Germ, M. Calcium Oxalate Druses Affect Leaf Optical Properties in Selenium-Treated Fagopyrum tataricum. J. Photochem. Photobiol. B Biol. 2018, 180, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Halbrecq, B.; Romedenne, P.; Ledent, J.F. Evolution of Flowering, Ripening and Seed Set in Buckwheat (Fagopyrum esculentum Moench): Quantitative Analysis. Eur. J. Agron. 2005, 23, 209–224. [Google Scholar] [CrossRef]
- Ohnishi, O. Population Genetics of Cultivated Common Buckwheat, Fagopyrum esculentum Moench, VII, Allezyme Variability in Japan, Korea and China. Jpn. J. Genet. 1988, 63, 507–522. [Google Scholar] [CrossRef][Green Version]
- Ohnishi, O. Search for the Wild Ancestor of Buckwheat III. The Wild Ancestor of Cultivated Common Buckwheat, and of Tatary Buckwheat. Econ. Bot. 1998, 52, 123–133. [Google Scholar] [CrossRef]
- Zhang, K.; He, M.; Fan, Y.; Zhao, H.; Gao, B.; Yang, K.; Li, F.; Tang, Y.; Gao, Q.; Lin, T.; et al. Resequencing of Global Tartary Buckwheat Accessions Reveals Multiple Domestication Events and Key Loci Associated with Agronomic Traits. Genome Biol. 2021, 22, 23. [Google Scholar] [CrossRef]
- Germ, M.; Gaberšick, A. The Effect of Environmental Factors on Buckwheat. Mol. Breed. Nutr. Asp. Buckwheat 2016, 273–281. [Google Scholar] [CrossRef]
- Germ, M.; Kreft, I.; Osvald, J. Influence of UV-B Exclusion and Selenium Treatment on Photochemical Efficiency of Photosystem II, Yield and Respiratory Potential in Pumpkins (Cucurbita pepo L.). Plant Physiol. Biochem. 2005, 43, 445–448. [Google Scholar] [CrossRef]
- Gaberščik, A.; Vončina, M.; Trošt, T.; Germ, M.; Olof Björn, L. Growth and Production of Buckwheat (Fagopyrum esculentum) Treated with Reduced, Ambient, and Enhanced UV-B Radiation. J. Photochem. Photobiol. B Biol. 2002, 66, 30–36. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Björn, L.O.; Bornman, J.F.; Flint, S.D.; Kulandaivelu, G.; Teramura, A.H.; Tevini, M. Effects of Increased Solar Ultraviolet Radiation on Terrestrial Ecosystems. J. Photochem. Photobiol. B Biol. 1998, 46, 40–52. [Google Scholar] [CrossRef]
- Roblek, M.; Germ, M.; Gaberščik, A. Morphological and Biochemical Variations in St. John’s Wort, Hypericum Perforatum L., Growing over Altitudinal and UV-B Radiation Gradients. Period. Biol. 2008, 110, 57–61. [Google Scholar]
- Golob, A.; Kavčič, J.; Stibilj, V.; Gaberščik, A.; Vogel-Mikuš, K.; Germ, M. The Effect of Selenium and UV Radiation on Leaf Traits and Biomass Production in Triticum aestivum L. Ecotoxicol. Environ. Saf. 2017, 136, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Golob, A.; Kugovnik, A.; Kreft, I.; Gaberščik, A.; Germ, M. The Interactions between UV Radiation, Drought and Selenium in Different Buckwheat Species. Acta Biol. Slov. 2019, 62, 57–66. [Google Scholar]
- Yao, Y.; Xuan, Z.; Li, Y.; He, Y.; Korpelainen, H.; Li, C. Effects of Ultraviolet-B Radiation on Crop Growth, Development, Yield and Leaf Pigment Concentration of Tartary Buckwheat (Fagopyrum tataricum) under Field Conditions. Eur. J. Agron. 2006, 25, 215–222. [Google Scholar] [CrossRef]
- Suchar, V.A.; Robberecht, R. Integration and Scaling of UV-B Radiation Effects on Plants: From Molecular Interactions to Whole Plant Responses. Ecol. Evol. 2016, 6, 4866–4884. [Google Scholar] [CrossRef]
- White, J.W.; Montes, C. Variation in Parameters Related to Leaf Thickness in Common Bean (Phaseolus vulgaris L.). F. Crop. Res. 2005, 91, 7–21. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. In Current Protocols in Food Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2001; Volume 1, pp. F4.3.1–F4.3.8. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Extraction of phtosynthetic Tissues: Chlorophylls and carotenoids. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2001; Volume 1, pp. F4.2.1–F4.2.6. [Google Scholar]
- Lindoo, S.J.; Caldwell, M.M. Ultraviolet-B Radiation-Induced Inhibition of Leaf Expansion and Promotion of Anthocyanin Production. Plant Physiol. 1978, 61, 278–282. [Google Scholar] [CrossRef]
- Caldwell, M.M. Solar Ultraviolet Radiation as an Ecological Factor for Alpine Plants. Ecol. Monogr. 1968, 38, 243–268. [Google Scholar] [CrossRef]
- Schreiber, U.; Kühl, M.; Klimant, I.; Reising, H. Measurement of Chlorophyll Fluorescence within Leaves Using a Modified PAM Fluorometer with a Fiber-Optic Microprobe. Photosynth. Res. 1996, 47, 103–109. [Google Scholar] [CrossRef]
| Ljubljana | Podbeže | Javorje | |
|---|---|---|---|
| Soil type | clay loam | loam–clay loam | loam–silt loam |
| Soil moisture (%) | 17.5 | 15.4 | 14.7 |
| EC (µS/cm) | 80.8 | 75.9 | 180.2 |
| C total (%) | 2.8 | 3.1 | 8.4 |
| N (%) | 0.21 | 0.3 | 0.73 |
| C org/N | 12.4 | 10.0 | 11.4 |
| Si (mg/g) | 212 | 162 | 164 |
| P (mg/g) | 1.65 | 1.75 | 1.15 |
| S (mg/g) | 0.24 | 0.38 | 0.36 |
| Cl (mg/g) | 0.64 | 2.31 | 0.96 |
| K (mg/g) | 16.4 | 15.4 | 26.9 |
| Ca (mg/g) | 10.2 | 10.8 | 7.3 |
| Average T June (°C) | 19.4 | 17.4 | 9.7 |
| Average T July (°C) | 21.7 | 19.6 | 12.1 |
| Rainfall June (mm) | 142.7 | 189.8 | 170.9 |
| Rainfall July (mm) | 155.2 | 68.0 | 308.0 |
| Model Parameter | Chlorophylls (mg/g d.w.) | Carotenoids (mg/g d.w.) | Anthocyanins (rel. u/g d.w.) | UV Abs. Comp. (rel u/g d.w.) | |
|---|---|---|---|---|---|
| Location | Lj | 17.2 ± 1.0 a | 2.64 ± 0.14 a | 0.18 ± 0.01 a | 229 ± 11 ab |
| Po | 13.4 ± 0.7 b | 2.42 ± 0.10 a | 0.12 ± 0.01 b | 217 ± 16 b | |
| Ja | 15.6 ± 0.7 ab | 2.57 ± 0.10 a | 0.17 ± 0.01 a | 277 ± 22 a | |
| Pr > F(Location) | 0.005 | 0.306 | 0.001 | 0.012 | |
| Species | CB | 15.4 ± 0.6 a | 2.56 ± 0.08 a | 0.15 ± 0.01 | 255 ± 15 a |
| TB | 15.4 ± 0.9 a | 2.46 ± 0.10 a | 0.16 ± 0.01 | 227 ± 15 a | |
| Pr > F(Species) | 0.973 | 0.762 | 0.463 | 0.148 | |
| Location x Species | Pr > F(Location x Species) | 0.007 | 0.007 | 0.382 | 0.045 |
| Model Parameter | Leaf Thickness (μm) | Thickness of Palisade Tissue (μm) | Epidermis Thickness (μm) | Density of CaOx Druses (No/mm2) | 2r of CaOx Druses (μm) | |
|---|---|---|---|---|---|---|
| Location | Lj | 156 ± 3 b | 56 ± 1 b | 24.1 ± 0.3 a | 21.7 ± 0.7 a | 18.2 ± 0.6 c |
| Po | 168 ± 5 a | 66 ± 2 a | 23.1 ± 0.4 a | 17.3 ± 0.9 b | 23.0 ± 0.6 a | |
| Ja | 150 ± 4 b | 51 ± 2 c | 23.6 ± 0.5 a | 17.4 ± 1.0 b | 20.8 ± 1.3 b | |
| Pr > F (Location) | 0.0002 | <0.0001 | 0.112 | <0.0001 | <0.0001 | |
| Species | CB | 168 ± 3 a | 61 ± 2 a | 24.3 ± 0.4 a | 20.8 ± 0.6 a | 23.0 ± 0.6 a |
| TB | 148 ± 3 b | 54 ± 2 b | 23.0 ± 0.3 b | 16.8 ± 0.9 b | 18.3 ± 0.7 b | |
| Pr > F (Species) | <0.0001 | 0.0002 | 0.003 | <0.0001 | <0.0001 | |
| Location x Species | Pr > F (Location x Species) | 0.125 | 0.238 | 0.007 | 0.602 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golob, A.; Luzar, N.; Kreft, I.; Germ, M. Adaptative Responses of Common and Tartary Buckwheat to Different Altitudes. Plants 2022, 11, 1439. https://doi.org/10.3390/plants11111439
Golob A, Luzar N, Kreft I, Germ M. Adaptative Responses of Common and Tartary Buckwheat to Different Altitudes. Plants. 2022; 11(11):1439. https://doi.org/10.3390/plants11111439
Chicago/Turabian StyleGolob, Aleksandra, Neja Luzar, Ivan Kreft, and Mateja Germ. 2022. "Adaptative Responses of Common and Tartary Buckwheat to Different Altitudes" Plants 11, no. 11: 1439. https://doi.org/10.3390/plants11111439
APA StyleGolob, A., Luzar, N., Kreft, I., & Germ, M. (2022). Adaptative Responses of Common and Tartary Buckwheat to Different Altitudes. Plants, 11(11), 1439. https://doi.org/10.3390/plants11111439







